The Reliable Detection of Homocysteine Using a Biosensor Based on Recombinant Cystathionine β-Synthase and Nanoporous Gold
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Heterologous Expression of the CBS Protein
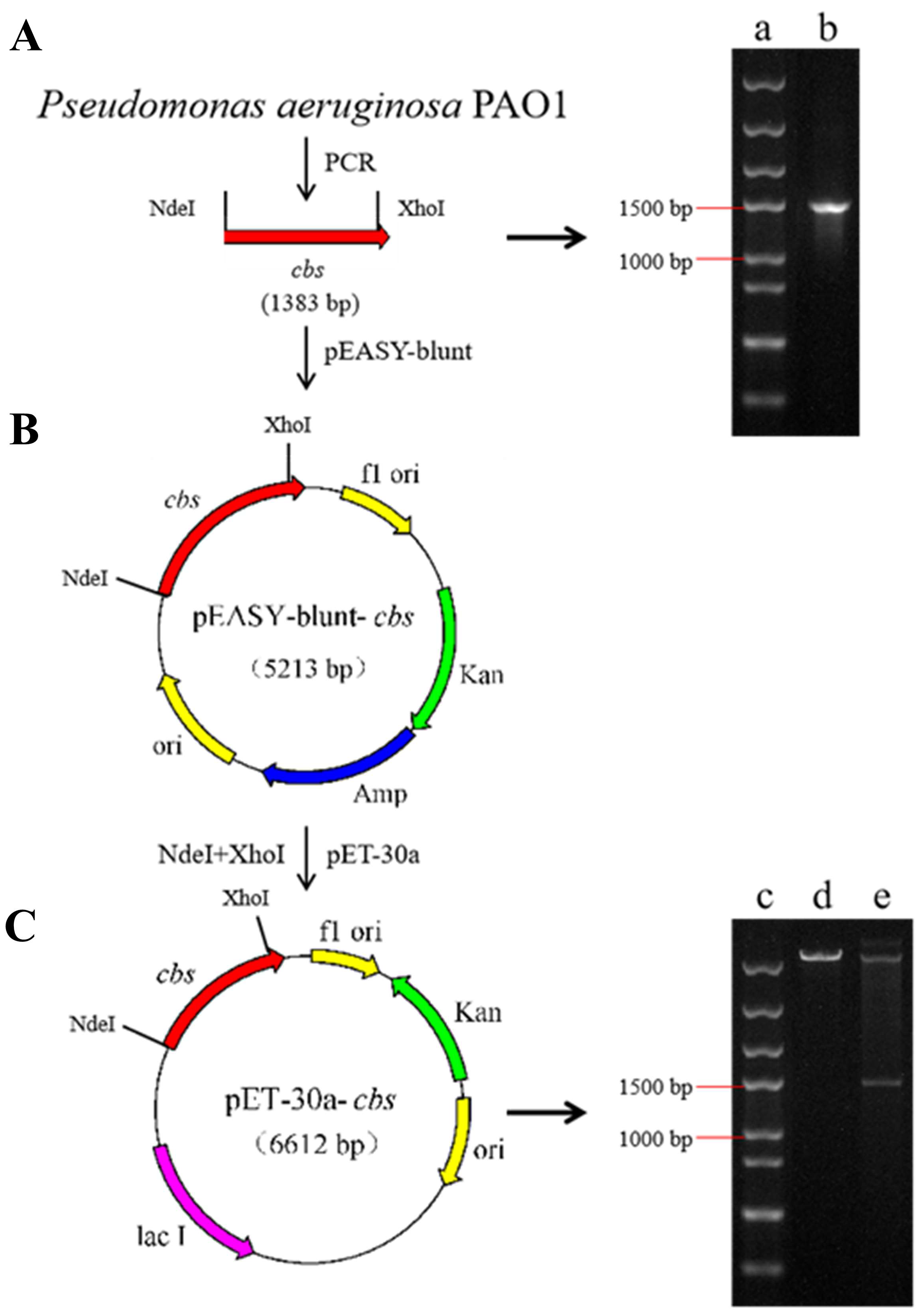
2.2.1. Construction of the Recombinant Expression Strain
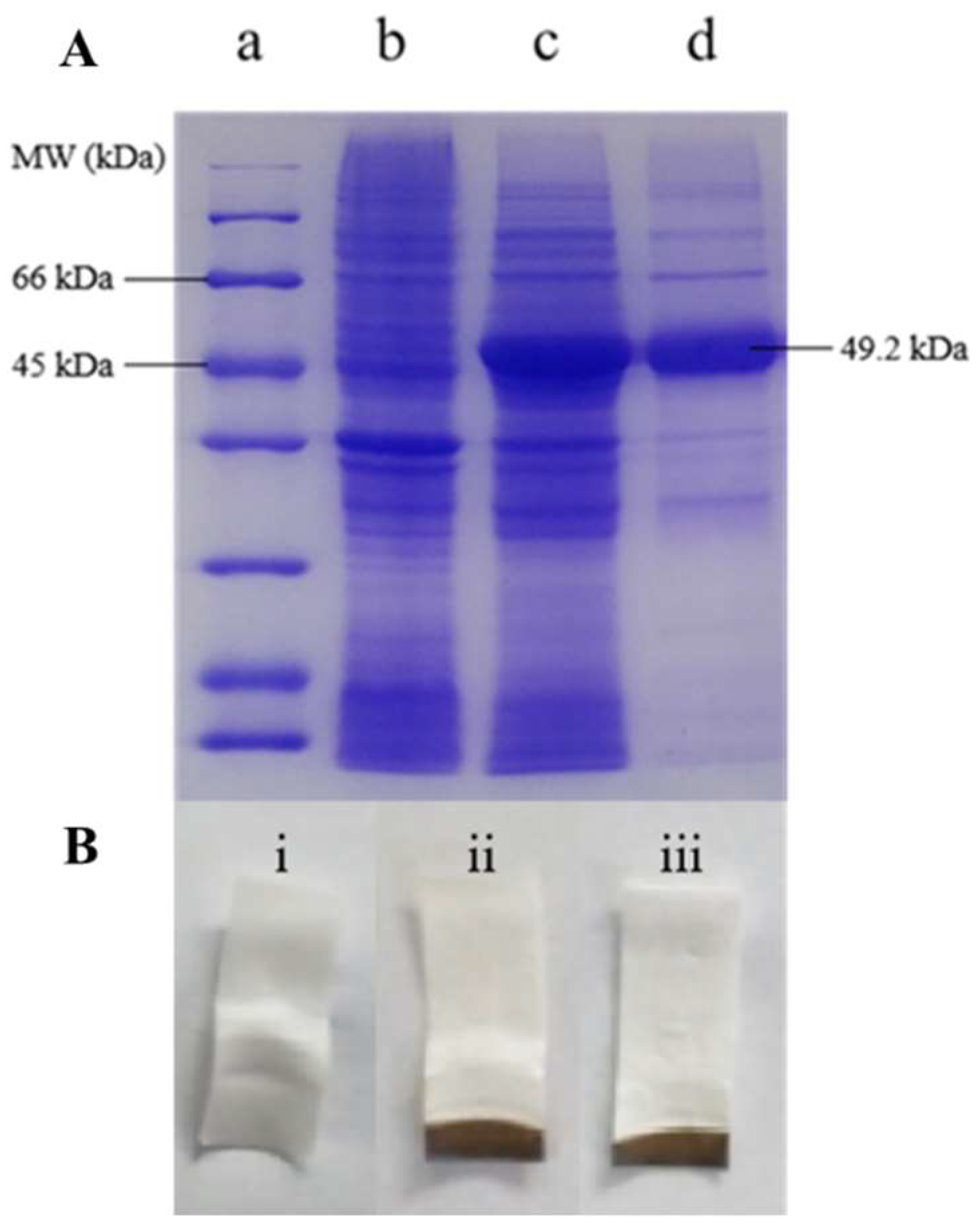
2.2.2. Expression, Purification, and Validation of Enzyme Activity
2.2.3. Investigation of CBS Protein Specificity by HPLC
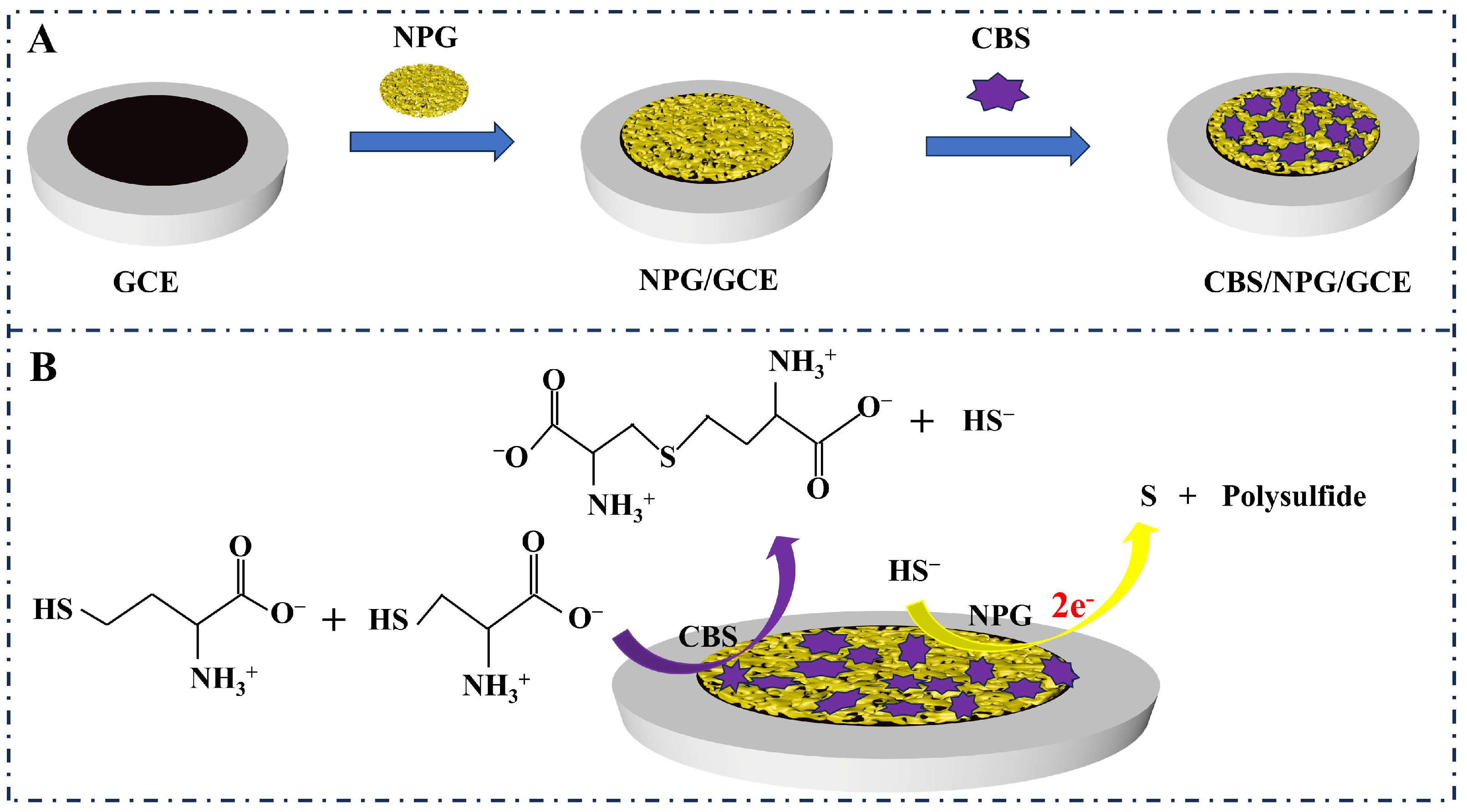
2.3. Preparation and Detection of CBS/NPG/GCE Electrode
3. Results and Discussion
3.1. Heterologous Expression and Activity Verification of the CBS Protein
3.1.1. Construction of Recombinant Cystathionine β-Synthase
3.1.2. Enzymatic Properties of the CBS Protein
3.2. Construction and Characterization of the CBS/NPG/GCE Electrode
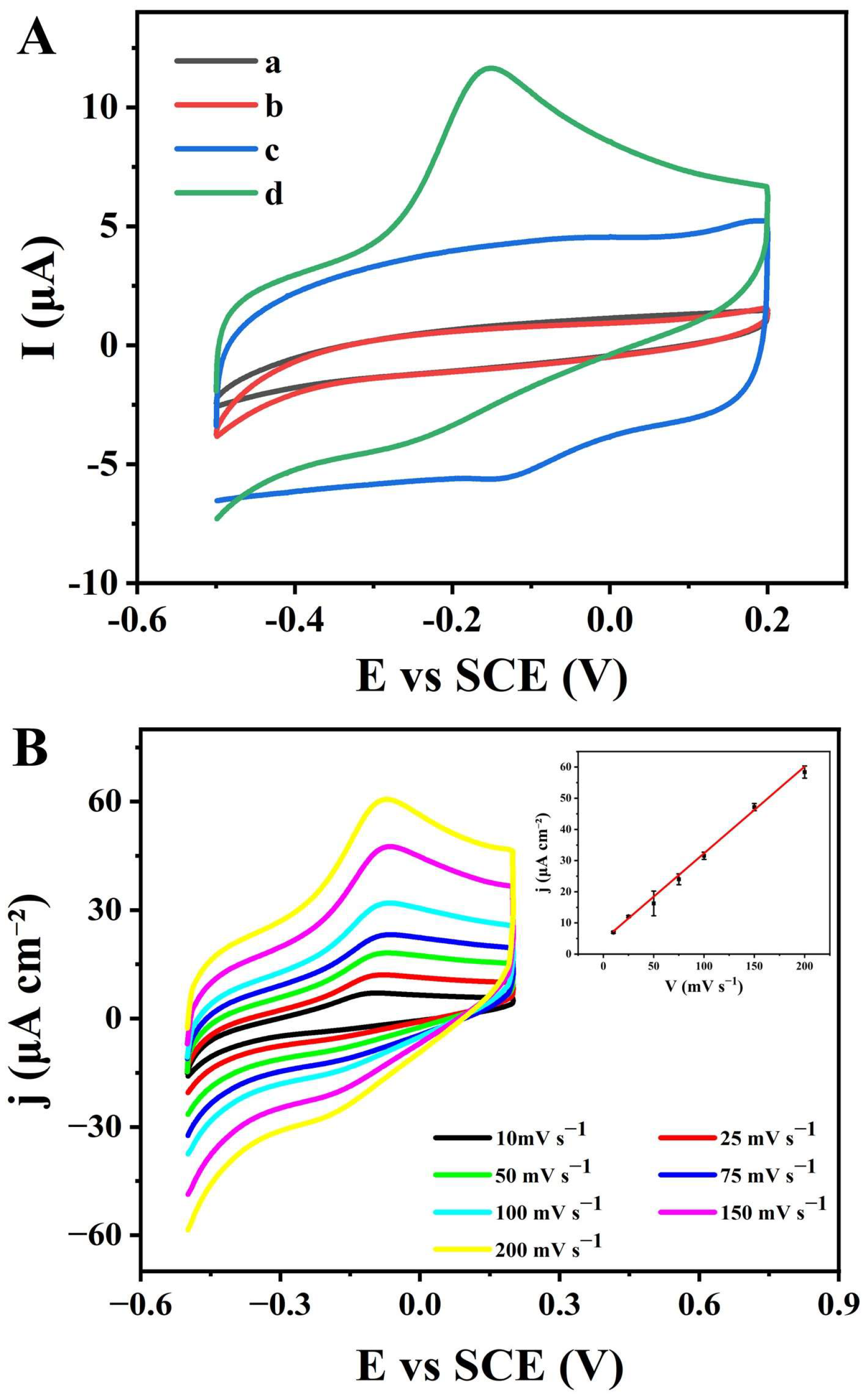
3.2.1. The Detection Principle of the CBS/NPG/GCE Electrode
3.2.2. Catalytic Kinetics of Sulfide at the CBS/NPG/GCE Electrode
3.3. Hcy Detection Using the CBS/NPG/GCE Electrode
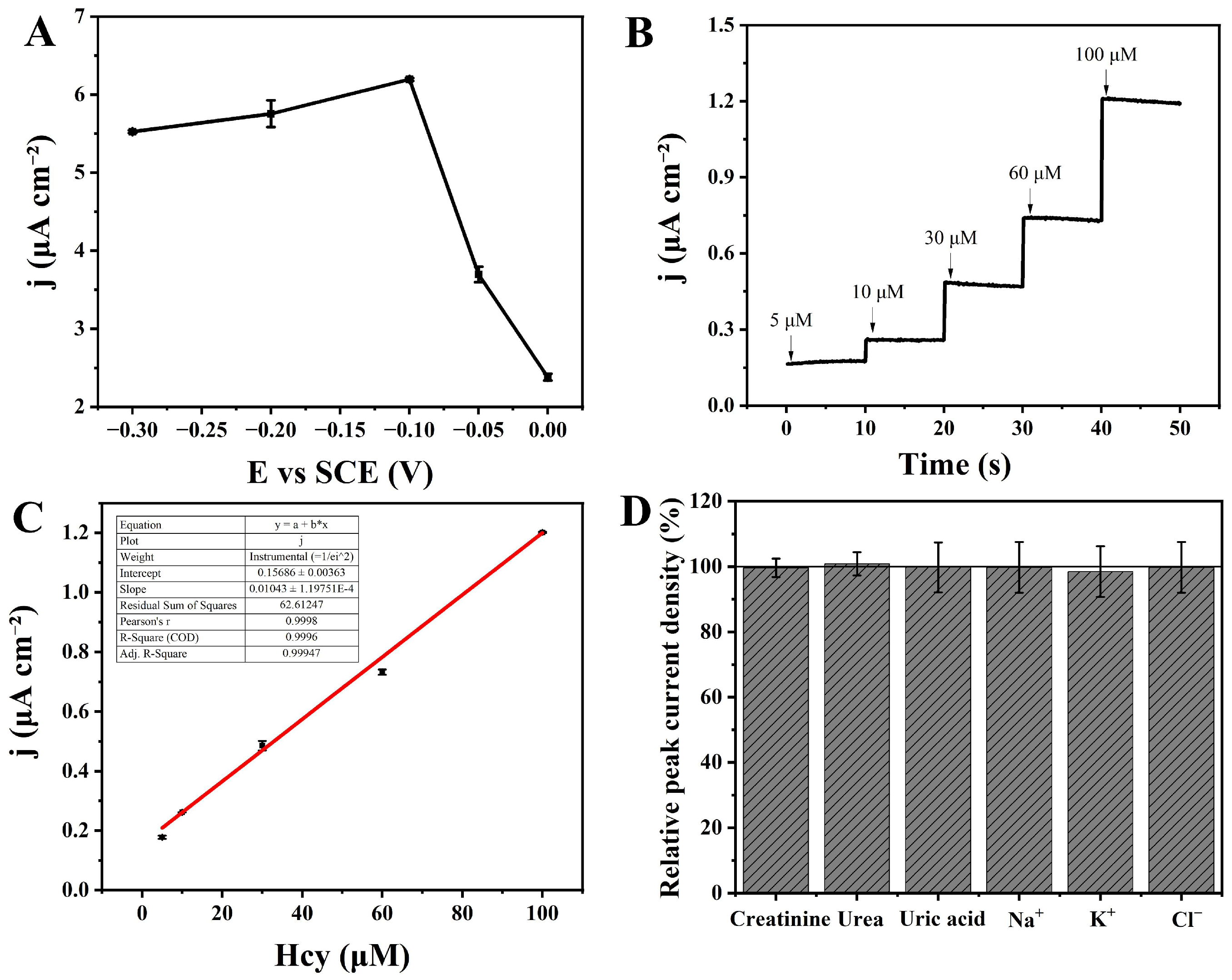
3.3.1. The Applied Potential Optimization for Hcy Detection
3.3.2. The Detection of Hcy by Amperometric i-t Technique
3.3.3. Interference Resistance and Stability of the CBS/NPG/GCE Electrode
3.3.4. Real Sample Detection of Hcy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, G.S.; Bhattacharya, R.; Singh, L.R. Functional Inhibition of Redox Regulated Heme Proteins: A Novel Mechanism towards Oxidative Stress Induced by Homocysteine. Redox Biol. 2021, 46, 102080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The Metabolism and Significance of Homocysteine in Nutrition and Health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and Homocysteine as Biomarker of Various Diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef]
- Lehotsky, J.; Petras, M.; Kovalska, M.; Tothova, B.; Drgova, A.; Kaplan, P. Mechanisms Involved in the Ischemic Tolerance in Brain: Effect of the Homocysteine. Cell Mol. Neurobiol. 2015, 35, 7–15. [Google Scholar] [CrossRef]
- Givvimani, S.; Munjal, C.; Narayanan, N.; Aqil, F.; Tyagi, G.; Metreveli, N.; Tyagi, S.C. Hyperhomocysteinemia Decreases Intestinal Motility Leading to Constipation. Am. J. Physiol.-Gastrointest. Liver Physiol. 2012, 303, G281–G290. [Google Scholar] [CrossRef]
- Iacobazzi, V.; Infantino, V.; Castegna, A.; Andria, G. Hyperhomocysteinemia: Related Genetic Diseases and Congenital Defects, Abnormal DNA Methylation and Newborn Screening Issues. Mol. Genet. Metab. 2014, 113, 27–33. [Google Scholar] [CrossRef]
- Perna, A.F.; Ingrosso, D. Atherosclerosis Determinants in Renal Disease: How Much Is Homocysteine Involved? Nephrol. Dial. Transplant. 2016, 31, 860–863. [Google Scholar] [CrossRef]
- Schalinske, K.L.; Smazal, A.L. Homocysteine Imbalance: A Pathological Metabolic Marker. Adv. Nutr. 2012, 3, 755–762. [Google Scholar] [CrossRef]
- Marroncini, G.; Martinelli, S.; Menchetti, S.; Bombardiere, F.; Martelli, F.S. Hyperhomocysteinemia and Disease—Is 10 μmol/L a Suitable New Threshold Limit? Int. J. Mol. Sci. 2024, 25, 12295. [Google Scholar] [CrossRef]
- Stachniuk, J.; Kubalczyk, P.; Furmaniak, P.; Głowacki, R. A Versatile Method for Analysis of Saliva, Plasma and Urine for Total Thiols Using HPLC with UV Detection. Talanta 2016, 155, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, P.E.; Morgan, S.L.; Vaughn, W.H. Modification of a High-Performance Liquid Chromatographic Method for Assay of Homocysteine in Human Plasma. J. Chromatogr. B Biomed. Appl. 1993, 617, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, Y.; Hasegawa, H.; Tagoku, K.; Hashimoto, T. Simultaneous Determination of Methionine and Total Homocysteine in Human Plasma by Gas Chromatography–Mass Spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2001, 758, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Magera, M.J.; Lacey, J.M.; Casetta, B.; Rinaldo, P. Method for the Determination of Total Homocysteine in Plasma and Urine by Stable Isotope Dilution and Electrospray Tandem Mass Spectrometry. Clin. Chem. 1999, 45, 1517–1522. [Google Scholar] [CrossRef]
- Dou, C.; Xia, D.; Zhang, L.; Chen, X.; Flores, P.; Datta, A.; Yuan, C. Development of a Novel Enzymatic Cycling Assay for Total Homocysteine. Clin. Chem. 2005, 51, 1987–1989. [Google Scholar] [CrossRef]
- Shipchandler, M.T.; Moore, E.G. Rapid, Fully Automated Measurement of Plasma Homocyst(e)Ine with the Abbott IMx Analyzer. Clin. Chem. 1995, 41, 991–994. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Twite, D.; Shih, J.; Holets-McCormack, S.R.; Gunter, E.W. Method Comparison for Total Plasma Homocysteine between the Abbott IMx Analyzer and an HPLC Assay with Internal Standardization. Clin. Chem. 1999, 45, 152–153. [Google Scholar] [CrossRef]
- Inoue, T.; Kirchhoff, J.R. Determination of Thiols by Capillary Electrophoresis with Amperometric Detection at a Coenzyme Pyrroloquinoline Quinone Modified Electrode. Anal. Chem. 2002, 74, 1349–1354. [Google Scholar] [CrossRef]
- Chen Gang, Z.L.W.J. Miniaturized Capillary Electrophoresis System with a Carbon Nanotube Microelectrode for Rapid Separation and Detection of Thiols. Talanta 2004, 64, 1018–1023. [Google Scholar] [CrossRef]
- Wen, X.-H.; Zhao, X.-F.; Peng, B.-F.; Yuan, K.-P.; Li, X.-X.; Zhu, L.-Y.; Lu, H.-L. Facile Preparation of an Electrochemical Aptasensor Based on Au NPs/Graphene Sponge for Detection of Homocysteine. Appl. Surf. Sci. 2021, 556, 149735. [Google Scholar] [CrossRef]
- Gholami-Orimi, F.; Taleshi, F.; Biparva, P.; Karimi-Maleh, H.; Beitollahi, H.; Ebrahimi, H.R.; Shamshiri, M.; Bagheri, H.; Fouladgar, M.; Taherkhani, A. Voltammetric Determination of Homocysteine Using Multiwall Carbon Nanotube Paste Electrode in the Presence of Chlorpromazine as a Mediator. J. Anal. Methods Chem. 2012, 2012, 902184. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, J.-H.; Lu, H.-T.; Zhang, Q. Electrochemistry and Voltammetric Determination of L-Tryptophan and L-Tyrosine Using a Glassy Carbon Electrode Modified with a Nafion/TiO2-Graphene Composite Film. Microchim. Acta 2011, 173, 241–247. [Google Scholar] [CrossRef]
- Auria-Luna, F.; Foss, F.W.; Molina-Canteras, J.; Velazco-Cabral, I.; Marauri, A.; Larumbe, A.; Aparicio, B.; Vázquez, J.L.; Alberro, N.; Arrastia, I.; et al. Supramolecular Chemistry in Solution and Solid–Gas Interfaces: Synthesis and Photophysical Properties of Monocolor and Bicolor Fluorescent Sensors for Barium Tagging in Neutrinoless Double Beta Decay. RSC Appl. Interfaces 2025, 2, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.Z.; Kaczmarek, M. Artificial Intelligence in IR Thermal Imaging and Sensing for Medical Applications. Sensors 2025, 25, 891. [Google Scholar] [CrossRef]
- Guo, L.; Zhao, Y.; Huang, Q.; Huang, J.; Tao, Y.; Chen, J.; Li, H.-Y.; Liu, H. Electrochemical Protein Biosensors for Disease Marker Detection: Progress and Opportunities. Microsyst. Nanoeng. 2024, 10, 65. [Google Scholar] [CrossRef]
- Sajeevan, A.; Sukumaran, R.A.; Panicker, L.R.; Kotagiri, Y.G. Trends in Ready-to-Use Portable Electrochemical Sensing Devices for Healthcare Diagnosis. Microchimica. Acta 2025, 192, 80. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, J.; Liao, S.-J.; Zhou, P.-C.; Shen, Y.-T.; Yu, W.; Li, W.; Shen, A.-G. Simultaneous SERS Sensing of Cysteine and Homocysteine in Blood Based on the CBT-Cys Click Reaction: Toward Precisive Diagnosis of Schizophrenia. Anal. Chem. 2024, 96, 5331–5339. [Google Scholar] [CrossRef]
- Singh, S.; Padovani, D.; Leslie, R.A.; Chiku, T.; Banerjee, R. Relative Contributions of Cystathionine β-Synthase and γ-Cystathionase to H2S Biogenesis via Alternative Trans-Sulfuration Reactions. J. Biol. Chem. 2009, 284, 22457–22466. [Google Scholar] [CrossRef]
- Yadav, P.K.; Martinov, M.; Vitvitsky, V.; Seravalli, J.; Wedmann, R.; Filipovic, M.R.; Banerjee, R. Biosynthesis and Reactivity of Cysteine Persulfides in Signaling. J. Am. Chem. Soc. 2016, 138, 289–299. [Google Scholar] [CrossRef]
- Wei, C.; Li, X.; Xu, F.; Tan, H.; Li, Z.; Sun, L.; Song, Y. Metal Organic Framework-Derived Anthill-like Cu@carbon Nanocomposites for Nonenzymatic Glucose Sensor. Anal. Methods 2014, 6, 1550. [Google Scholar] [CrossRef]
- Xu, H.; Xia, C.; Wang, S.; Han, F.; Akbari, M.K.; Hai, Z.; Zhuiykov, S. Electrochemical Non-Enzymatic Glucose Sensor Based on Hierarchical 3D Co3O4/Ni Heterostructure Electrode for Pushing Sensitivity Boundary to a New Limit. Sens. Actuators. B Chem. 2018, 267, 93–103. [Google Scholar] [CrossRef]
- Özcan, L.; Şahin, Y.; Türk, H. Non-Enzymatic Glucose Biosensor Based on Overoxidized Polypyrrole Nanofiber Electrode Modified with Cobalt(II) Phthalocyanine Tetrasulfonate. Biosens. Bioelectron. 2008, 24, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899. [Google Scholar] [CrossRef]
- Xiao, X.; Si, P.; Magner, E. An Overview of Dealloyed Nanoporous Gold in Bioelectrochemistry. Bioelectrochemistry 2016, 109, 117–126. [Google Scholar] [CrossRef]
- Kim, S.H. Nanoporous Gold: Preparation and Applications to Catalysis and Sensors. Curr. Appl. Phys. 2018, 18, 810–818. [Google Scholar] [CrossRef]
- Stine, K.J. Enzyme Immobilization on Nanoporous Gold: A Review. Biochem. Insights 2017, 10, 117862641774860. [Google Scholar] [CrossRef]
- van der Zalm, J.; Chen, S.; Huang, W.; Chen, A. Review—Recent Advances in the Development of Nanoporous Au for Sensing Applications. J. Electrochem. Soc. 2020, 167, 037532. [Google Scholar] [CrossRef]
- He, J. Methodological Study and Clinical Application of Simultaneous Determination of Plasma Homocysteine and Its Related Thiols by High Performance Liquid Chromatography. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2005. [Google Scholar]
- Meng, F.; Yan, X.; Liu, J.; Gu, J.; Zou, Z. Nanoporous Gold as Non-Enzymatic Sensor for Hydrogen Peroxide. Electrochim. Acta 2011, 56, 4657–4662. [Google Scholar] [CrossRef]
- Alfonso, D.R. First-Principles Studies of H2S Adsorption and Dissociation on Metal Surfaces. Surf. Sci. 2008, 602, 2758–2768. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, H.; Sun, H.; Gao, R.; Liu, H.; Wang, X.; Xu, P.; Xun, L. Nanoporous Gold-Based Microbial Biosensor for Direct Determination of Sulfide. Biosens. Bioelectron. 2017, 98, 29–35. [Google Scholar] [CrossRef]
- Bian, C.; Wang, H.; Zhang, X.; Xiao, S.; Liu, Z.; Wang, X. Sensitive Detection of Low-Concentration Sulfide Based on the Synergistic Effect of RGO, Np-Au, and Recombinant Microbial Cell. Biosens. Bioelectron. 2020, 151, 111985. [Google Scholar] [CrossRef] [PubMed]
| Reaction System | Reaction Temperature (°C) | Reaction Time | ||
|---|---|---|---|---|
| The strain colony | 95 | 10 min | ||
| 20 μM cbs-NdeI-F 2 μL | 95 | 30 s |  | 30 cycles |
| 20 μM cbs-XhoI-R 2 μL | 65 | 30 s | ||
| FastPfu DNA Polymerase 4 μL | 72 | 40 s | ||
| 5×FastPfu Buffer 40 μL | 72 | 10 min | ||
| 10 mM dNTPs 4 μL | 4 | 10 min | ||
| ddH2O 148 μL | ||||
| Reaction System | Cys Added to the Reaction System (µM) | CBS Protein Volume (mg mL−1) | Cys Remaining in the Reaction System (µM) | RSD (%) |
|---|---|---|---|---|
| #1 | 200 | 0.5 | 202 ± 16 | +1.00 |
| #2 | 250 | 0.5 | 261.70 ± 0.15 | +4.68 |
| #3 | 300 | 0.5 | 299 ± 6 | −0.20 |
| Sample | Spiked Hcy (µM) | Detected by CBS/NPG/GCE (µM) | Recovery Rate (%) | RSD (%) |
|---|---|---|---|---|
| #1 | 10 | 9.7 ± 0.6 | 97.19 | −2.81 |
| #2 | 30 | 29.8 ± 1.3 | 99.34 | −0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Gao, Y.; Zhang, L.; Cai, T.; Liu, R.; Wang, X. The Reliable Detection of Homocysteine Using a Biosensor Based on Recombinant Cystathionine β-Synthase and Nanoporous Gold. Microorganisms 2025, 13, 559. https://doi.org/10.3390/microorganisms13030559
Huang Z, Gao Y, Zhang L, Cai T, Liu R, Wang X. The Reliable Detection of Homocysteine Using a Biosensor Based on Recombinant Cystathionine β-Synthase and Nanoporous Gold. Microorganisms. 2025; 13(3):559. https://doi.org/10.3390/microorganisms13030559
Chicago/Turabian StyleHuang, Zihan, Yan Gao, Lei Zhang, Ting Cai, Ruijun Liu, and Xia Wang. 2025. "The Reliable Detection of Homocysteine Using a Biosensor Based on Recombinant Cystathionine β-Synthase and Nanoporous Gold" Microorganisms 13, no. 3: 559. https://doi.org/10.3390/microorganisms13030559
APA StyleHuang, Z., Gao, Y., Zhang, L., Cai, T., Liu, R., & Wang, X. (2025). The Reliable Detection of Homocysteine Using a Biosensor Based on Recombinant Cystathionine β-Synthase and Nanoporous Gold. Microorganisms, 13(3), 559. https://doi.org/10.3390/microorganisms13030559







