Microbiota Dysbiosis in Mytilus chilensis Is Induced by Hypoxia, Leading to Molecular and Functional Consequences
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design (Mussel Acclimatization, Hypoxia Challenge, and Sampling for Microbiological Analysis)
2.2. DNA Isolation and 16S Amplification
2.3. Library Preparation and Nanopore Sequencing
2.4. Data Processing and Taxonomic Assignment
2.5. Community Profiling and Statistical Testing
2.6. Data Processing and Heat Tree Visualization of Microbial Communities
2.7. Linear Discriminant Analysis Effect Size (LEfSe) and Correlation Network Analysis
2.8. Prediction of Metagenomic Functional Potential
2.9. Data Availability
3. Results
3.1. Alpha and Beta Diversity Analysis of M. chilensis Microbiota Under Normoxia and Hypoxia
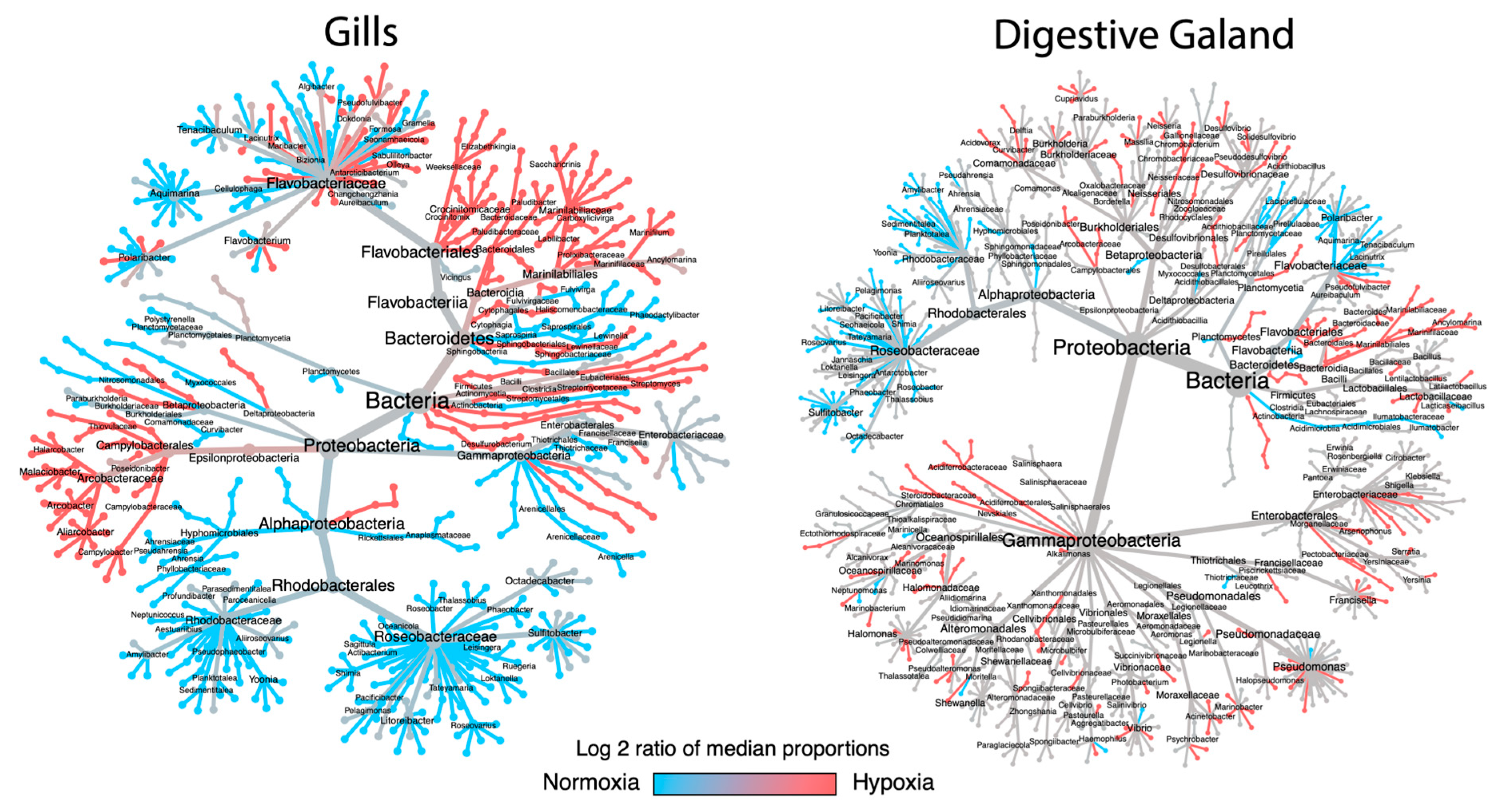
3.2. Taxonomic Shifts in the Microbiota of M. chilensis Under Normoxia and Hypoxia
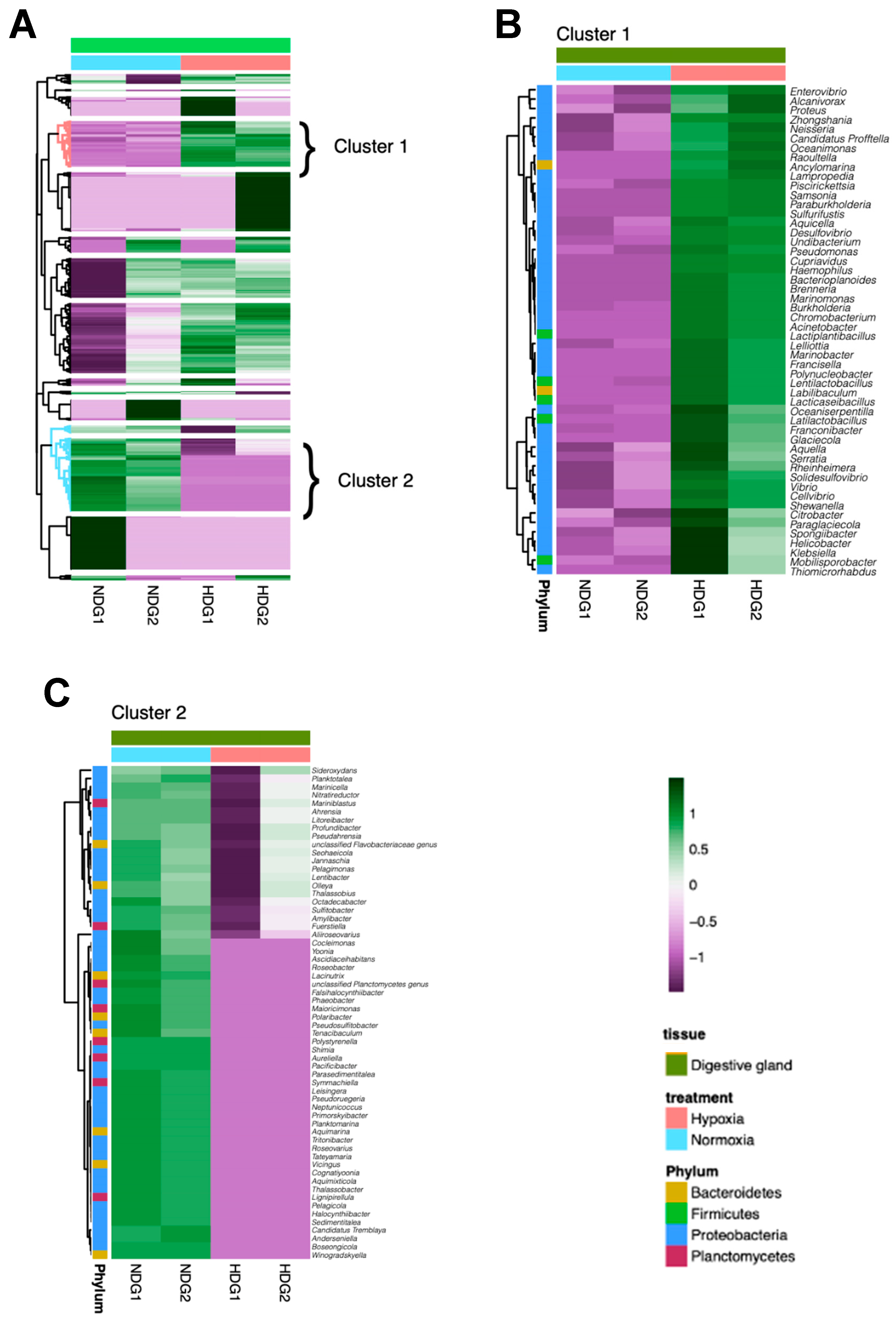
3.3. Analysis of Bacterial Genus Relative Abundance in the Microbiota of M. chilensis Under Normoxia and Hypoxia
3.4. Linear Discriminant Analysis
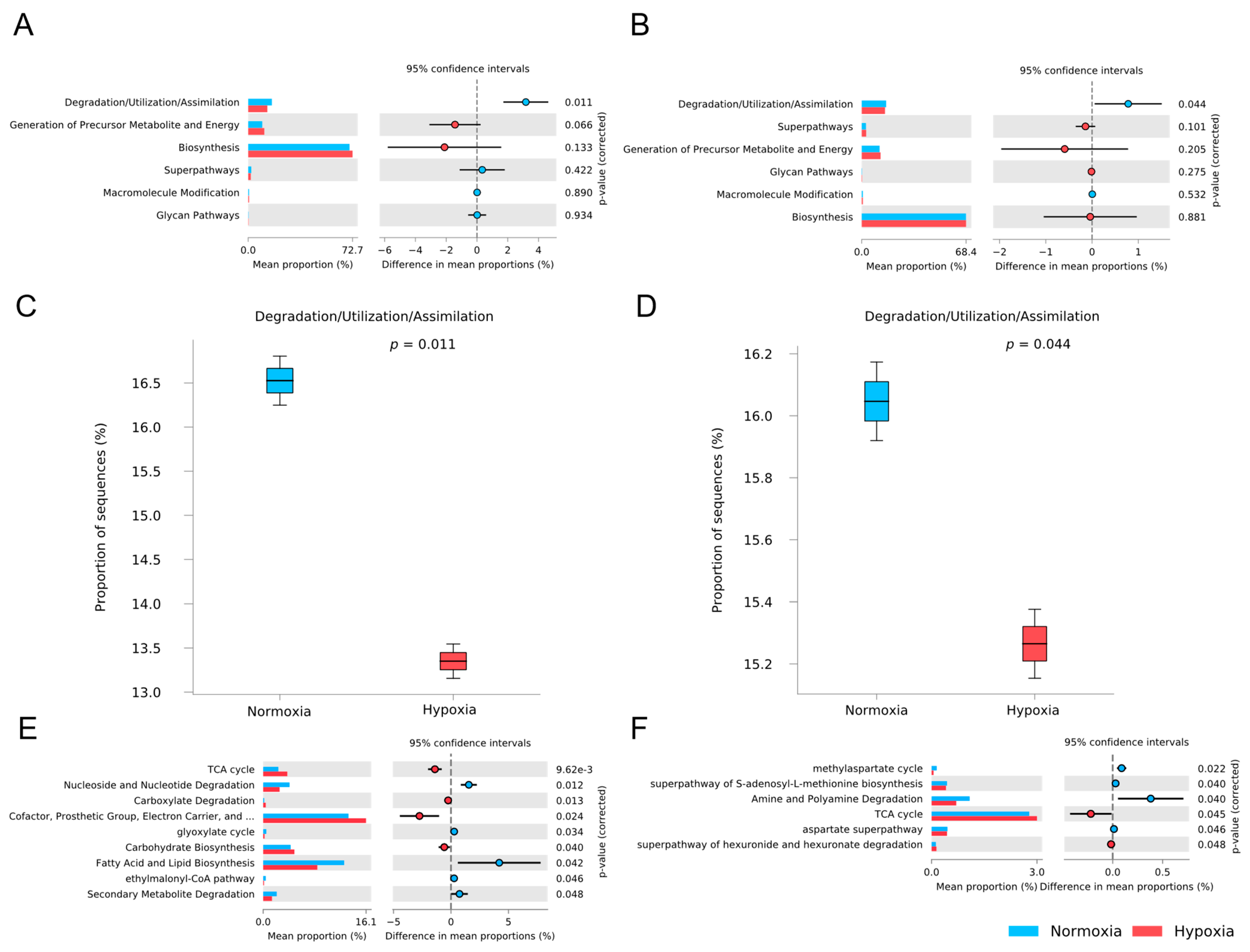
3.5. Functional Potential Prediction of the M. chilensis Microbiota Under Normoxia and Hypoxia
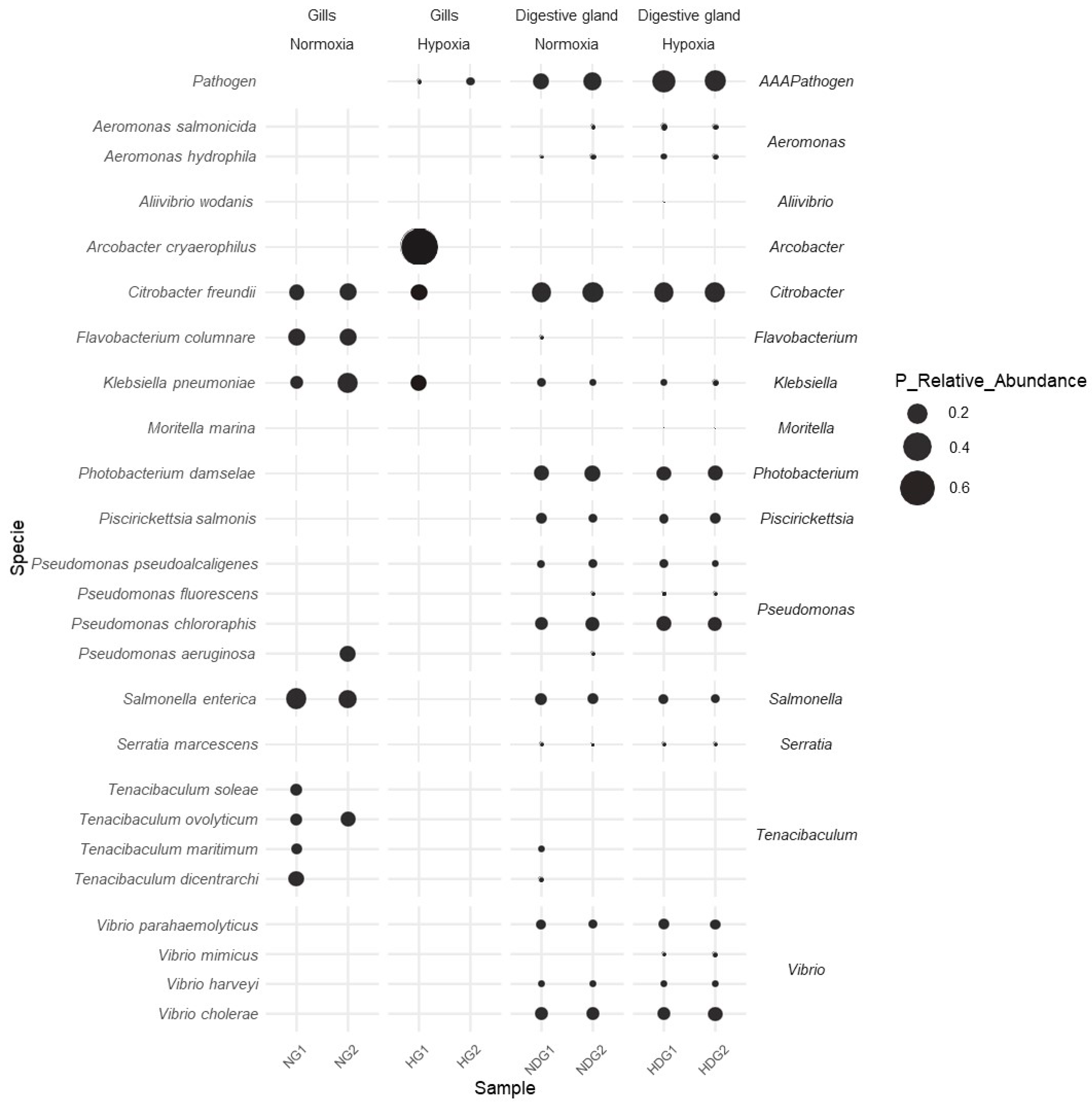
3.6. Dynamics of Bacterial Pathogens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| °C | grados Celsius |
| ANID | Agencia Nacional de Investigación y Desarrollo |
| ANOSIM | Analysis of Similarities |
| CA | California |
| CEBB | Ethics Committee of the Universidad de Concepción |
| C.G.-E. | Cristian Gallardo Escárate |
| D.V.-M | Diego Valenzuela Miranda |
| DO | Dissolved oxygen |
| FDR | false discovery rate |
| FONDAP | Fondo de Financiamiento de Centros de Investigación en Áreas Prioritarias |
| INCAR | Interdisciplinary Center for Aquaculture Research |
| IPIAP | Instituto Público de Investigación de Acuicultura |
| LDA | linear discriminant analysis |
| LDOW | low dissolved oxygen water |
| LEfSe | Linear Discriminant Analysis Effect Size |
| log2 | logarithm base 2 |
| M. chilensis | Mytilus chilensis |
| MA | Massachusetts |
| MDPI | Multidisciplinary Digital Publishing Institute |
| mg/L | milligrams per liter |
| M.M.-R | Milton Montúfar Romero |
| M.F.M.-R | María Fernanda Morales-Rivera |
| n | sample size |
| NCBI | National Center for Biotechnology Information |
| OTUs | operational taxonomic units |
| PCoA | Principal Coordinates Analysis |
| PCR | Polymerase Chain Reaction |
| pH | potential of hydrogen |
| PICRUSt2 | Phylogenetic Investigation of Communities by Reconstruction of Unobserved States |
| Q-score | Quality score |
| rRNA | ribosomal ribonucleic acid |
| SENESCYT | Secretaría de Educación Superior, Ciencia, Tecnología e Innovación |
| SparCC | Sparse Correlations for Compositional Data |
| SRA | Sequence Read Archive |
| STAMP | Statistical Analysis of Metagenomic Profiles |
| TCA | Tricarboxylic Acid Cycle |
| UK | United Kingdom |
| USA | United States of America |
| V.V.-M | Valentina Valenzuela-Muñoz |
References
- Fan, S.; Li, H.; Zhao, R. Effects of normoxic and hypoxic conditions on the immune response and gut microbiota of Bostrichthys sinensis. Aquaculture 2020, 525, 735336. [Google Scholar] [CrossRef]
- Choumiline, K.; Pérez-Cruz, L.; Gray, A.; Bates, S.; Lyons, T. Scenarios of Deoxygenation of the Eastern Tropical North Pacific During the Past Millennium as a Window Into the Future of Oxygen Minimum Zones. Front. Earth Sci. 2019, 7, 237. [Google Scholar] [CrossRef]
- Conley, D.; Carstensen, J.; Vaquer-Sunyer, R.; Duarte, C. Ecosystem thresholds with hypoxia. Hydrobiologia 2009, 629, 21–29. [Google Scholar] [CrossRef]
- Diaz, R.; Rosenberg, R. Spreading Dead Zones and Consequences for Marine Ecosystems. Science 2008, 321, 926–929. [Google Scholar] [CrossRef]
- Hofmann, A.; Peltzer, E.; Walz, P.; Brewer, P. Hypoxia by degrees: Establishing definitions for a changing ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 1212–1226. [Google Scholar] [CrossRef]
- McArley, T.; Hickey, A.; Herbert, N. Hyperoxia increases maximum oxygen consumption and aerobic scope of intertidal fish facing acutely high temperatures. J. Exp. Biol. 2018, 221, jeb189993. [Google Scholar] [CrossRef]
- Moffitt, S.; Moffitt, R.; Sauthoff, W.; Davis, C.; Hewett, K.; Hill, T. Paleoceanographic insights on recent oxygen minimum zone expansion: Lessons for modern oceanography. PLoS ONE 2015, 10, e0115246. [Google Scholar] [CrossRef]
- Sattari, M.; Bagherzadeh, F.; Sharifpour, I.; Kazemi, R. Effect of hypoxia, normoxia and hyperoxia conditions on gill histopathology in two weight groups of beluga (Huso huso). Casp. J. Environ. Sci. 2013, 11, 77–84. [Google Scholar]
- Hernández-Miranda, E.; Quiñones, R.; Aedo, G.; Valenzuela, A.; Mermoud, N.; Román, C.; Yañez, F. A major fish stranding caused by a natural hypoxic event in a shallow bay of the eastern South Pacific Ocean. J. Fish Biol. 2010, 76, 1543–1564. [Google Scholar] [CrossRef]
- Hernández-Miranda, E.; Veas, R.; Anabalón, V.; Quiñones, R. Short-term alteration of biotic and abiotic components of the pelagic system in a shallow bay produced by a strong natural hypoxia event. PLoS ONE 2017, 12, e0179023. [Google Scholar] [CrossRef]
- Hernández-Miranda, E.; Veas, R.; Labra, F.; Salamanca, M.; Quiñones, R. Response of the epibenthic macrofaunal community to a strong upwelling-driven hypoxic event in a shallow bay of the southern Humboldt Current System. Mar. Environ. Res. 2012, 79, 16–28. [Google Scholar] [CrossRef]
- Labra, F.; Hernández-Miranda, E.; Quiñones, R. Dynamic relationships between body size, species richness, abundance, and energy use in a shallow marine epibenthic faunal community. Ecol. Evol. 2015, 5, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Yang, Y.; Pan, G. Oxygen micro-nanobubbles for mitigating eutrophication induced sediment pollution in freshwater bodies. J. Environ. Manag. 2023, 331, 117281. [Google Scholar] [CrossRef] [PubMed]
- De la Maza, L.; Farias, L. The intensification of coastal hypoxia off central Chile: Long term and high frequency variability. Front. Earth Sci. 2023, 10, 929271. [Google Scholar] [CrossRef]
- Khan, F.; Shang, Y.; Chang, X.; Kong, H.; Zuberi, A.; Fang, J.; Liu, W.; Peng, J.; Zhang, X.; Hu, M.; et al. Effects of Ocean Acidification, Hypoxia, and Warming on the Gut Microbiota of the Thick Shell Mussel Mytilus coruscus Through 16S rRNA Gene Sequencing. Front. Mar. Sci. 2021, 8, 736338. [Google Scholar] [CrossRef]
- Andreyeva, A.; Gostyukhina, O.; Kladchenko, E.; Afonnikov, D.; Rasskazov, D.; Lantushenko, A.; Vodiasova, E. Hypoxia exerts oxidative stress and changes in expression of antioxidant enzyme genes in gills of Mytilus galloprovincialis (Lamarck, 1819). Mar. Biol. Res. 2021, 17, 369–379. [Google Scholar] [CrossRef]
- Gu, H.; Shang, Y.; Clements, J.; Dupont, S.; Wang, T.; Wei, S.; Wang, X.; Chen, J.; Huang, W.; Hu, M.; et al. Hypoxia aggravates the effects of ocean acidification on the physiological energetics of the blue mussel Mytilus edulis. Mar. Pollut. Bull. 2019, 149, 110538. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Li, Q.; Li, J.; Lin, D.; Lu, W. Immune toxicity of TiO2 under hypoxia in the green-lipped mussel Perna viridis based on flow cytometric analysis of hemocyte parameters. Sci. Total Environ. 2014, 470, 791–799. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Cheung, S.; Shin, P.; Lu, W.; Li, J. Immune parameter changes of hemocytes in green-lipped mussel Perna viridis exposure to hypoxia and hyposalinity. Aquaculture 2012, 356, 22–29. [Google Scholar] [CrossRef]
- Falfushynska, H.; Piontkivska, H.; Sokolova, I. Effects of intermittent hypoxia on cell survival and inflammatory responses in the intertidal marine bivalves Mytilus edulis and Crassostrea gigas. J. Exp. Biol. 2020, 223, jeb217026. [Google Scholar] [CrossRef]
- Haider, F.; Falfushynska, H.; Timm, S.; Sokolova, I. Effects of hypoxia and reoxygenation on intermediary metabolite homeostasis of marine bivalves Mytilus edulis and Crassostrea gigas. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 242, 110657. [Google Scholar] [CrossRef]
- Sweet, M.; Bulling, M. On the Importance of the Microbiome and Pathobiome in Coral Health and Disease. Front. Mar. Sci. 2017, 4, 9. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, L.; Lai, Y.; Chiang, C. The utility of microbiome (microbiota) and exosomes in dentistry. J. Dent. Sci. 2024, 19, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.; Stanton, C.; Murphy, E. The gut microbiota and the liver. Pathophysiological and clinical implications. J. Hepatol. 2013, 58, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, L.; Sapinho, D.; Maddi, A.; Miotti, L. Scientometric analysis of the term ’microbiota’ in research publications (1999-2017): A second youth of a century-old concept. FEMS Microbiol. Lett. 2019, 366, fnz138. [Google Scholar] [CrossRef]
- Lokmer, A.; Wegner, K. Hemolymph microbiome of Pacific oysters in response to temperature, temperature stress and infection. Int. Soc. Microb. Ecol. J. 2015, 9, 670–682. [Google Scholar] [CrossRef]
- Green, T.; Barnes, A. Bacterial diversity of the digestive gland of Sydney rock oysters, Saccostrea glomerata infected with the paramyxean parasite, Marteilia sydneyi. J. Appl. Microbiol. 2010, 109, 613–622. [Google Scholar] [CrossRef]
- Zurel, D.; Benayahu, Y.; Or, A.; Kovacs, A.; Gophna, U. Composition and dynamics of the gill microbiota of an invasive Indo-Pacific oyster in the eastern Mediterranean Sea. Environ. Microbiol. 2011, 13, 1467–1476. [Google Scholar] [CrossRef]
- Dubé, C.; Ky, C.; Planes, S. Microbiome of the Black-Lipped Pearl Oyster Pinctada margaritifera, a Multi-Tissue Description With Functional Profiling. Front. Microbiol. 2019, 10, 1548. [Google Scholar] [CrossRef]
- Voolstra, C.; Ziegler, M. Adapting with Microbial Help: Microbiome Flexibility Facilitates Rapid Responses to Environmental Change. Bioessays 2020, 42, 2000004. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, M.; Cani, P.; Knauf, C. The Gut Microbiome Influences Host Endocrine Functions. Endocr. Rev. 2019, 40, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Soen, Y. Environmental disruption of host-microbe co-adaptation as a potential driving force in evolution. Front. Genet. 2014, 5, 168. [Google Scholar] [CrossRef]
- Suzuki, T.; Ley, R. The role of the microbiota in human genetic adaptation. Science 2020, 370, eaaz6827. [Google Scholar] [CrossRef]
- McLaren, M.; Callahan, B. Pathogen resistance may be the principal evolutionary advantage provided by the microbiome. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190592. [Google Scholar] [CrossRef]
- Zeb, F.; Osaili, T.; Obaid, R.; Naja, F.; Radwan, H.; Ismail, L.; Hasan, H.; Hashim, M.; Alam, I.; Sehar, B.; et al. Gut Microbiota and Time-Restricted Feeding/Eating: A Targeted Biomarker and Approach in Precision Nutrition. Nutrients 2023, 15, 259. [Google Scholar] [CrossRef]
- Masanja, F.; Yang, K.; Xu, Y.; He, G.; Liu, X.; Xu, X.; Jiang, X.; Luo, X.; Mkuye, R.; Deng, Y.; et al. Bivalves and microbes: A mini-review of their relationship and potential implications for human health in a rapidly warming ocean. Front. Mar. Sci. 2023, 10, 1182438. [Google Scholar] [CrossRef]
- Xie, C.; Han, Y.; Dong, M.; Zhang, Y.; Song, H.; Huang, H.; Zhang, H.; Liu, Y.; Wei, L.; Wang, X. Analysis of microbial communities on the coloured mantle surface of three common bivalves. Aquac. Rep. 2024, 37, 102220. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.; Stombaugh, J.; Mackey, L.; Knight, R.; Farber, S.; Rawls, J. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Wos-Oxley, M.; Catalano, S.; Hassan, M.; Li, X.; Qin, J.; Oxley, A. Host Species and Environment Shape the Gut Microbiota of Cohabiting Marine Bivalves. Microb. Ecol. 2023, 86, 1755–1772. [Google Scholar] [CrossRef]
- Valenzuela, T.; Rilling, J.; Larama, G.; Acuna, J.; Campos, M.; Inostroza, N.; Araya, M.; Altamirano, K.; Fujiyoshi, S.; Yarimizu, K.; et al. 16S rRNA-Based Analysis Reveals Differences in the Bacterial Community Present in Tissues of Choromytilus chorus (Mytilidae, Bivalvia) Grown in an Estuary and a Bay in Southern Chile. Diversity 2021, 13, 209. [Google Scholar] [CrossRef]
- Auguste, M.; Lasa, A.; Pallavicini, A.; Gualdi, S.; Vezzulli, L.; Canesi, L. Exposure to TiO2 nanoparticles induces shifts in the microbiota composition of Mytilus galloprovincialis hemolymph. Sci. Total Environ. 2019, 670, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Clinton, S.; Hamp, T.; Oliver, J.; Ringwood, A. Potential impacts of hypoxia and a warming ocean on oyster microbiomes. Mar. Environ. Res. 2018, 139, 27–34. [Google Scholar] [CrossRef]
- Li, Y.; Yang, N.; Liang, X.; Yoshida, A.; Osatomi, K.; Power, D.; Batista, F.; Yang, J. Elevated Seawater Temperatures Decrease Microbial Diversity in the Gut of Mytilus coruscus. Front. Physiol. 2018, 9, 839. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Chen, Y.; Ding, W.; Shao, A.; Liang, X.; Zhu, Y.; Yang, J. Characterization of Gut Microbiome in the Mussel Mytilus galloprovincialis in Response to Thermal Stress. Front. Physiol. 2019, 10, 1086. [Google Scholar] [CrossRef]
- Diaz-Puente, B.; Pita, A.; Uribe, J.; Cuellar-Pinzon, J.; Guinez, R.; Presa, P. A biogeography-based management for Mytilus chilensis: The genetic hodgepodge of Los Lagos versus the pristine hybrid zone of the Magellanic ecotone. Aquat. Conserv.-Mar. Freshw. Ecosyst. 2020, 30, 412–425. [Google Scholar] [CrossRef]
- Santibañez, P.; Romalde, J.; Fuentes, D.; Figueras, A.; Figueroa, J. Health Status of Mytilus chilensis from Intensive Culture Areas in Chile Assessed by Molecular, Microbiological, and Histological Analyses. Pathogens 2022, 11, 494. [Google Scholar] [CrossRef]
- Liu, M.; Li, Q.; Tan, L.; Wang, L.; Wu, F.; Li, L.; Zhang, G. Host-microbiota interactions play a crucial role in oyster adaptation to rising seawater temperature in summer. Environ. Res. 2023, 216, 114585. [Google Scholar] [CrossRef]
- Hughes, D.; Alderdice, R.; Cooney, C.; Kuhl, M.; Pernice, M.; Voolstra, C.; Suggett, D. Coral reef survival under accelerating ocean deoxygenation. Nat. Clim. Change 2020, 10, 296–307. [Google Scholar] [CrossRef]
- Salmond, N.; Wing, S. Sub-lethal and lethal effects of chronic and extreme multiple stressors on a critical New Zealand bivalve under hypoxia. Mar. Ecol. Prog. Ser. 2023, 703, 81–93. [Google Scholar] [CrossRef]
- Iriarte, J.; Pantoja, S.; Daneri, G. Oceanographic Processes in Chilean Fjords of Patagonia: From small to large-scale studies. Prog. Oceanogr. 2014, 129, 1–7. [Google Scholar] [CrossRef]
- Yevenes, M.; Lagos, N.; Farías, L.; Vargas, C. Greenhouse gases, nutrients and the carbonate system in the Reloncaví Fjord (Northern Chilean Patagonia): Implications on aquaculture of the mussel, Mytilus chilensis, during an episodic volcanic eruption. Sci. Total Environ. 2019, 669, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Vargas, C. Hypoxia in Chilean Patagonian Fjords. Prog. Oceanogr. 2014, 129, 62–74. [Google Scholar] [CrossRef]
- Linford, P.; Pérez-Santos, I.; Montes, I.; Dewitte, B.; Buchan, S.; Narváez, D.; Saldías, G.; Pinilla, E.; Garreaud, R.; Díaz, P.; et al. Recent Deoxygenation of Patagonian Fjord Subsurface Waters Connected to the Peru-Chile Undercurrent and Equatorial Subsurface Water Variability. Glob. Biogeochem. Cycles 2023, 37, e2022GB007688. [Google Scholar] [CrossRef]
- Díaz, P.; Pérez-Santos, I.; Basti, L.; Garreaud, R.; Pinilla, E.; Barrera, F.; Tello, A.; Schwerter, C.; Arenas-Uribe, S.; Soto-Riquelme, C.; et al. The impact of local and climate change drivers on the formation, dynamics, and potential recurrence of a massive fish-killing microalgal bloom in Patagonian fjord. Sci. Total Environ. 2023, 865, 161288. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Cifuentes, U.; Pizarro, O.; Djurfeldt, L.; Caceres, M. Seasonal hydrography and surface outflow in a fjord with a deep sill: The Reloncaví fjord, Chile. Ocean Sci. 2016, 12, 533–544. [Google Scholar] [CrossRef]
- Schneider, W.; Pérez-Santos, I.; Ross, L.; Bravo, L.; Seguel, R.; Hernández, F. On the hydrography of Puyuhuapi Channel, Chilean Patagonia. Prog. Oceanogr. 2014, 129, 8–18. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Díaz, P.; Silva, N.; Garreaud, R.; Montero, P.; Henríquez-Castillo, C.; Barrera, F.; Linford, P.; Amaya, C.; Contreras, S.; et al. Oceanography time series reveals annual asynchrony input between oceanic and estuarine waters in Patagonian fjords. Sci. Total Environ. 2021, 798, 149241. [Google Scholar] [CrossRef]
- Soto, D.; León-Muñoz, J.; Garreaud, R.; Quinoñes, R.; Morey, F. Scientific warnings could help to reduce farmed salmon mortality due to harmful algal blooms. Mar. Policy 2021, 132, 104705. [Google Scholar] [CrossRef]
- Linford, P.; Pérez-Santos, I.; Montero, P.; Díaz, P.; Aracena, C.; Pinilla, E.; Barrera, F.; Castillo, M.; Alvera-Azcárate, A.; Alvarado, M.; et al. Oceanographic processes driving low-oxygen conditions inside Patagonian fjords. Biogeosciences 2024, 21, 1433–1459. [Google Scholar] [CrossRef]
- Mardones, J.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the environmental processes responsible for the world’s largest farmed fish-killing harmful algal bloom: Chile, 2016. Sci. Total Environ. 2021, 766, 144383. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Daneri, G.; González, H.; Iriarte, J.; Tapia, F.; Lizárraga, L.; Sanchez, N.; Pizarro, O. Seasonal variability of primary production in a fjord ecosystem of the Chilean Patagonia: Implications for the transfer of carbon within pelagic food webs. Cont. Shelf Res. 2011, 31, 202–215. [Google Scholar] [CrossRef]
- Daneri, G.; Montero, P.; Lizárraga, L.; Torres, R.; Iriarte, J.L.; Jacob, B.; González, H.E.; Tapia, F.J. Primary Productivity and heterotrophic activity in an enclosed marine area of central Patagonia (Puyuhuapi channel; 44° S, 73° W). Biogeosciences Discuss. 2012, 2012, 5929–5968. [Google Scholar] [CrossRef]
- Adzigbli, L.; Sokolov, E.; Ponsuksili, S.; Sokolova, I. Tissue- and substrate-dependent mitochondrial responses to acute hypoxia-reoxygenation stress in a marine bivalve (Crassostrea gigas). J. Exp. Biol. 2022, 225, jeb243304. [Google Scholar] [CrossRef]
- Sokolov, E.; Markert, S.; Hinzke, T.; Hirschfeld, C.; Becher, D.; Ponsuksili, S.; Sokolova, I. Effects of hypoxia-reoxygenation stress on mitochondrial proteome and bioenergetics of the hypoxia-tolerant marine bivalve Crassostrea gigas. J. Proteom. 2019, 194, 99–111. [Google Scholar] [CrossRef]
- Steffen, J.; Falfushynska, H.; Piontkivska, H.; Sokolova, I. Molecular Biomarkers of the Mitochondrial Quality Control Are Differently Affected by Hypoxia-Reoxygenation Stress in Marine Bivalves Crassostrea gigas and Mytilus edulis. Front. Mar. Sci. 2020, 7, 19. [Google Scholar] [CrossRef]
- Amorim, K.; Piontkivska, H.; Zettler, M.; Sokolov, E.; Hinzke, T.; Nair, A.; Sokolova, I. Transcriptional response of key metabolic and stress response genes of a nuculanid bivalve, Lembulus bicuspidatus from an oxygen minimum zone exposed to hypoxia-reoxygenation. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2021, 256, 110617. [Google Scholar] [CrossRef]
- Falfushynska, H.; Sokolov, E.; Piontkivska, H.; Sokolova, I. The Role of Reversible Protein Phosphorylation in Regulation of the Mitochondrial Electron Transport System During Hypoxia and Reoxygenation Stress in Marine Bivalves. Front. Mar. Sci. 2020, 7, 467. [Google Scholar] [CrossRef]
- Liu, T.; Lu, Y.; Sun, M.; Shen, H.; Niu, D. Effects of acute hypoxia and reoxygenation on histological structure, antioxidant response, and apoptosis in razor clam Sinonovacula constricta. Fish Shellfish. Immunol. 2024, 145, 109310. [Google Scholar] [CrossRef]
- Adzigbli, L.; Ponsuksili, S.; Sokolova, I. Mitochondrial responses to constant and cyclic hypoxia depend on the oxidized fuel in a hypoxia-tolerant marine bivalve Crassostrea gigas. Sci. Rep. 2024, 14, 9658. [Google Scholar] [CrossRef]
- Ivanina, A.; Sokolova, I. Effects of intermittent hypoxia on oxidative stress and protein degradation in molluscan mitochondria. J. Exp. Biol. 2016, 219, 3794–3802. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, H.; Chen, J.; Liao, Y.; Mkuye, R.; Deng, Y.; Du, X. Integrated transcriptomic and metabolomic analysis reveals the response of pearl oyster (Pinctada fucata martensii) to long-term hypoxia. Mar. Environ. Res. 2023, 191, 106133. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Hao, R.; Du, X.; Deng, Y. Molecular characterization of OSR1 in Pinctada fucata martensii and association of allelic variants with growth traits. Aquaculture 2020, 516, 734617. [Google Scholar] [CrossRef]
- Li, X.; Shi, H.; Xia, H.; Zhou, Y.; Qiu, Y. Seasonal hypoxia and its potential forming mechanisms in the Mirs Bay, the northern South China Sea. Cont. Shelf Res. 2014, 80, 1–7. [Google Scholar] [CrossRef]
- Calvete, C.; Sobarzo, M. Quantification of the surface brackish water layer and frontal zones in southern Chilean fjords between Boca del Guafo (43°30′S) and Estero Elefantes (46°30′S). Cont. Shelf Res. 2011, 31, 162–171. [Google Scholar] [CrossRef]
- Cáceres, M.; Valle-Levinson, A.; Sepúlveda, H.; Holderied, K. Transverse variability of flow and density in a Chilean fjord. Cont. Shelf Res. 2002, 22, 1683–1698. [Google Scholar] [CrossRef]
- Montúfar-Romero, M.; Valenzuela-Muñoz, V.; Valenzuela-Miranda, D.; Gallardo-Escárate, C. Hypoxia in the Blue Mussel Mytilus chilensis Induces a Transcriptome Shift Associated with Endoplasmic Reticulum Stress, Metabolism, and Immune Response. Genes 2024, 15, 658. [Google Scholar] [CrossRef]
- Sun, B.; Hu, M.; Lan, X.; Waiho, K.; Lv, X.; Xu, C.; Wang, Y. Nano-titanium dioxide exacerbates the harmful effects of perfluorooctanoic acid on the health of mussels. Environ. Int. 2024, 187, 108681. [Google Scholar] [CrossRef]
- Mardones, M.; Mardones-Toledo, D.; Büchner-Miranda, J.; Salas-Yanquin, L.; Gray, M.; Cubillos, V.; Montory, J.; Chaparro, O. Food acquisition by the intertidal filter feeder bivalve Perumytilus purpuratus: Can the gill explain a differential performance between smaller individuals and the larger ones? J. Exp. Mar. Biol. Ecol. 2024, 571, 151982. [Google Scholar] [CrossRef]
- Haque, M.; Eom, H.; Nam, S.; Shin, Y.; Rhee, J. Chlorothalonil induces oxidative stress and reduces enzymatic activities of Na+/K+-ATPase and acetylcholinesterase in gill tissues of marine bivalves. PLoS ONE 2019, 14, e0214236. [Google Scholar] [CrossRef]
- Sforzini, S.; Moore, M.; Oliveri, C.; Volta, A.; Jha, A.; Banni, M.; Viarengo, A. Role of mTOR in autophagic and lysosomal reactions to environmental stressors in molluscs. Aquat. Toxicol. 2018, 195, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Otegui, M.; Fiori, S.; Menechella, A.; Dos Santos, E.; Gimenez, J. Histological characterization and morphological alterations in gill and digestive gland in non-native bivalve from the Province of Buenos Aires: Spatial and seasonal evaluation. Zool. Anz. 2024, 312, 11–19. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, W.; Shin, Y.; Lee, D.; Kim, Y.; Kim, J.; Rhee, J. Microcystin-LR bioconcentration induces antioxidant responses in the digestive gland of two marine bivalves Crassostrea gigas and Mytilus edulis. Aquat. Toxicol. 2017, 188, 119–129. [Google Scholar] [CrossRef]
- Borkovic-Mitic, S.; Pavlovic, S.; Perendija, B.; Despotovic, S.; Gavric, J.; Gacic, Z.; Saicic, Z. Influence of some metal concentrations on the activity of antioxidant enzymes and concentrations of vitamin E and SH-groups in the digestive gland and gills of the freshwater bivalve Unio tumidus from the Serbian part of Sava River. Ecol. Indic. 2013, 32, 212–221. [Google Scholar] [CrossRef]
- Tang, B.; Riisgård, H. Relationship between oxygen concentration, respiration and filtration rate in blue mussel Mytilus edulis. J. Oceanol. Limnol. 2018, 36, 395–404. [Google Scholar] [CrossRef]
- Porter, E.; Porter, F. A Strain Gauge Monitor (SGM) for Continuous Valve Gape Measurements in Bivalve Molluscs in Response to Laboratory Induced Diel-cycling Hypoxia and pH. JoVE-J. Vis. Exp. 2018, 138, 57404. [Google Scholar] [CrossRef]
- Sun, S.; Yang, M.; Fu, H.; Ge, X.; Zou, J. Altered intestinal microbiota induced by chronic hypoxia drives the effects on lipid metabolism and the immune response of oriental river prawn Macrobrachium nipponense. Aquaculture 2020, 526, 735431. [Google Scholar] [CrossRef]
- Valenzuela-Miranda, D.; Valenzuela-Muñoz, V.; Benavente, B.; Muñoz-Trorcoso, M.; Nuñez-Acuña, G.; Gallardo-Escárate, C. The Atlantic salmon microbiome infected with the sea louse Caligus rogercresseyi reveals tissue-specific functional dysbiosis. Aquaculture 2024, 580, 740328. [Google Scholar] [CrossRef]
- Ciuffreda, L.; Rodríguez-Pérez, H.; Flores, C. Nanopore sequencing and its application to the study of microbial communities. Comput. Struct. Biotechnol. J. 2021, 19, 1497–1511. [Google Scholar] [CrossRef]
- Bonenfant, Q.; Noé, L.; Touzet, H. Porechop_ABI: Discovering unknown adapters in Oxford Nanopore Technology sequencing reads for downstream trimming. Bioinform. Adv. 2023, 3, vbac085. [Google Scholar] [CrossRef]
- Curry, K.; Wang, Q.; Nute, M.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. Emu: Species-level microbial community profiling of full-length 16S rRNA Oxford Nanopore sequencing data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Beckerman, A.; Childs, D.; Petchey, O. Data Management, Manipulation, and Exploration with Dplyr; Oxford University Press: New York, NY, USA, 2017; pp. 57–77. [Google Scholar]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive statistical, functional and integrative analysis of microbiome data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Foster, Z.; Sharpton, T.; Grünwald, N. Metacoder: An R package for visualization and manipulation of community taxonomic diversity data. PLOS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.; Maffei, V.; Zaneveld, J.; Yurgel, S.; Brown, J.; Taylor, C.; Huttenhower, C.; Langille, M. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Yang, C.; Mai, J.; Cao, X.; Burberry, A.; Cominelli, F.; Zhang, L. ggpicrust2: An R package for PICRUSt2 predicted functional profile analysis and visualization. Bioinformatics 2023, 39, btad470. [Google Scholar] [CrossRef]
- Caspi, R.; Billington, R.; Ferrer, L.; Foerster, H.; Fulcher, C.; Keseler, I.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 2016, 44, D471–D480. [Google Scholar] [CrossRef]
- Parks, D.; Tyson, G.; Hugenholtz, P.; Beiko, R. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Todgham, A.; Stillman, J. Physiological Responses to Shifts in Multiple Environmental Stressors: Relevance in a Changing World. Integr. Comp. Biol. 2013, 53, 539–544. [Google Scholar] [CrossRef]
- Earhart, M.; Blanchard, T.; Harman, A.; Schulte, P. Hypoxia and High Temperature as Interacting Stressors: Will Plasticity Promote Resilience of Fishes in a Changing World? Biol. Bull. 2022, 243, 149–170. [Google Scholar] [CrossRef]
- Howard, R.; Schul, M.; Bravo, L.; Altieri, A.; Meyer, J. Shifts in the coral microbiome in response to in situ experimental deoxygenation. Appl. Environ. Microbiol. 2023, 89, e00577-23. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C. Unraveling Interactions between the Microbiome and the Host Immune System To Decipher Mechanisms of Disease. mSystems 2018, 3, 00183-17. [Google Scholar] [CrossRef]
- Clavel, T.; Gomes-Neto, J.; Lagkouvardos, I.; Ramer-Tait, A. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunol. Rev. 2017, 279, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, Y.; Xiong, K.; Tu, Z.; Waiho, K.; Yang, C.; Deng, Y.; Li, S.; Fang, J.; Hu, M.; et al. Combined effect of salinity and hypoxia on digestive enzymes and intestinal microbiota in the oyster Crassostrea hongkongensis. Environ. Pollut. 2023, 331, 121921. [Google Scholar] [CrossRef]
- Hemraj, D.; Falkenberg, L.; Cheung, K.; Man, L.; Carini, A.; Russell, B. Acidification and hypoxia drive physiological trade-offs in oysters and partial loss of nutrient cycling capacity in oyster holobiont. Front. Ecol. Evol. 2023, 11, 1083315. [Google Scholar] [CrossRef]
- Allam, B.; Espinosa, E. Bivalve immunity and response to infections: Are we looking at the right place? Fish Shellfish. Immunol. 2016, 53, 4–12. [Google Scholar] [CrossRef]
- González, R.; Henríquez-Castillo, C.; Lohrmann, K.; Romero, M.; Ramajo, L.; Schmitt, P.; Brokordt, K. The Gill Microbiota of Argopecten purpuratus Scallop Is Dominated by Symbiotic Campylobacterota and Upwelling Intensification Differentially Affects Their Abundance. Microorganisms 2022, 10, 2330. [Google Scholar] [CrossRef]
- Dor-Roterman, Y.; Benayahu, Y.; Reshef, L.; Gophna, U. Host-Microbiome Interactions in a Changing Sea: The Gill Microbiome of an Invasive Oyster under Drastic Temperature Changes. Microorganisms 2024, 12, 197. [Google Scholar] [CrossRef]
- Assié, A.; Borowski, C.; van der Heijden, K.; Raggi, L.; Geier, B.; Leisch, N.; Schimak, M.; Dubilier, N.; Petersen, J. A specific and widespread association between deep-sea Bathymodiolus mussels and a novel family of Epsilonproteobacteria. Environ. Microbiol. Rep. 2016, 8, 805–813. [Google Scholar] [CrossRef]
- Brown, E.; Sadarangani, M.; Finlay, B. The role of the immune system in governing host-microbe interactions in the intestine. Nat. Immunol. 2013, 14, 660–667. [Google Scholar] [CrossRef]
- Shade, A.; Handelsman, J. Beyond the Venn diagram: The hunt for a core microbiome. Environ. Microbiol. 2012, 14, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ramos, M.; Mansell, T. Leveraging quorum sensing to manipulate microbial dynamics. Curr. Opin. Biomed. Eng. 2021, 19, 100306. [Google Scholar] [CrossRef]
- Soto-Aceves, M.; Diggle, S.; Greenberg, E. Microbial Primer: LuxR- LuxI Quorum Sensing. Microbiology 2023, 169, 001343. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.; Shukla, A.; Patel, D. Quorum Sensing and Quorum Quenching: Two sides of the same coin. Physiol. Mol. Plant Pathol. 2023, 123, 101927. [Google Scholar] [CrossRef]
- Chan, Y.; Li, A.; Gopalakrishnan, S.; Shin, P.; Wu, R.; Pointing, S.; Chiu, J. Interactive effects of hypoxia and polybrominated diphenyl ethers (PBDEs) on microbial community assembly in surface marine sediments. Mar. Pollut. Bull. 2014, 85, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Umezawa, Y.; Kondo, R.; Wada, M. Effects of bottom-water hypoxia on sediment bacterial community composition in a seasonally hypoxic enclosed bay (Omura Bay, West Kyushu, Japan). FEMS Microbiol. Ecol. 2018, 94, fiy053. [Google Scholar] [CrossRef]
- Seibel, B.; Häfker, N.; Trübenbach, K.; Zhang, J.; Tessier, S.; Pörtner, H.; Rosa, R.; Storey, K. Metabolic suppression during protracted exposure to hypoxia in the jumbo squid, Dosidicus gigas, living in an oxygen minimum zone. J. Exp. Biol. 2014, 217, 2555–2568. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- Pohlenz, C.; Gatlin, D. Interrelationships between fish nutrition and health. Aquaculture 2014, 431, 111–117. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome is Associated With Favorable Responses to Anti-Programmed Death 1 Immunotherapy in Chinese Patients With NSCLC. J. Thorac. Oncol. 2019, 14, 1378–1389. [Google Scholar] [CrossRef]
- Lenis, Y.; Elmetwally, M.; Maldonado-Estrada, J.; Bazer, F. Physiological importance of polyamines. Zygote 2017, 25, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, K.; Barone, S.; Soleimani, M. Polyamines and Their Metabolism: From the Maintenance of Physiological Homeostasis to the Mediation of Disease. Med. Sci. 2022, 10, 38. [Google Scholar] [CrossRef]
- Egan, S.; Gardiner, M. Microbial Dysbiosis: Rethinking Disease in Marine Ecosystems. Front. Microbiol. 2016, 7, 991. [Google Scholar] [CrossRef]
- Pierce, M.; Ward, J. Gut Microbiomes of the Eastern Oyster (Crassostrea virginica) and the Blue Mussel (Mytilus edulis): Temporal Variation and the Influence of Marine Aggregate-Associated Microbial Communities. mSphere 2019, 4, 00730-19. [Google Scholar] [CrossRef]
- Trabal, N.; Mazón-Suástegui, J.; Vázquez-Juárez, R.; Asencio-Valle, F.; Morales-Bojórquez, E.; Romero, J. Molecular Analysis of Bacterial Microbiota Associated with Oysters (Crassostrea gigas and Crassostrea corteziensis) in Different Growth Phases at Two Cultivation Sites. Microb. Ecol. 2012, 64, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Fernández, N.; Mazón-Suástegui, J.; Vázquez-Juárez, R.; Ascencio-Valle, F.; Romero, J. Changes in the composition and diversity of the bacterial microbiota associated with oysters (Crassostrea corteziensis, Crassostrea gigas and Crassostrea sikamea) during commercial production. FEMS Microbiol. Ecol. 2014, 88, 69–83. [Google Scholar] [CrossRef]
- Utermann, C.; Parrot, D.; Breusing, C.; Stuckas, H.; Staufenberger, T.; Blümel, M.; Labes, A.; Tasdemir, D. Combined genotyping, microbial diversity and metabolite profiling studies on farmed Mytilus spp. from Kiel Fjord. Sci. Rep. 2018, 8, 7983. [Google Scholar] [CrossRef]
- Li, J.; Ni, J.; Li, J.; Wang, C.; Li, X.; Wu, S.; Zhang, T.; Yu, Y.; Yan, Q. Comparative study on gastrointestinal microbiota of eight fish species with different feeding habits. J. Appl. Microbiol. 2014, 117, 1750–1760. [Google Scholar] [CrossRef]
- Auguste, M.; Lasa, A.; Balbi, T.; Pallavicini, A.; Vezzulli, L.; Canesi, L. Impact of nanoplastics on hemolymph immune parameters and microbiota composition in Mytilus galloprovincialis. Mar. Environ. Res. 2020, 159, 105017. [Google Scholar] [CrossRef]
- Santibáñez, P.; Romalde, J.; Maldonado, J.; Fuentes, D.; Figueroa, J. First characterization of the gut microbiome associated with Mytilus chilensis collected at a mussel farm and from a natural environment in Chile. Aquaculture 2022, 548, 737644. [Google Scholar] [CrossRef]
- van der Meer, D.; van den Thillart, G.; Witte, F.; de Bakker, M.; Besser, J.; Richardson, M.; Spaink, H.; Leito, J.; Bagowski, C. Gene expression profiling of the long-term adaptive response to hypoxia in the gills of adult zebrafish. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 289, R1512–R1519. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, H.; Lin, C. Effects of hypoxia on ionic regulation, glycogen utilization and antioxidative ability in the gills and liver of the aquatic air-breathing fish Trichogaster microlepis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 179, 25–34. [Google Scholar] [CrossRef]
- Yang, L.; Lv, L.; Liu, H.; Wang, M.; Sui, Y.; Wang, Y. Effects of Ocean Acidification and Microplastics on Microflora Community Composition in the Digestive Tract of the Thick Shell Mussel Mytilus coruscus Through 16S RNA Gene Sequencing. Bull. Environ. Contam. Toxicol. 2021, 107, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yu, Z.; Yang, M.; Zhang, T.; Wang, H. Analysis of microbial abundance and community composition in esophagus and intestinal tract of wild veined rapa whelk (Rapana venosa) by 16S rRNA gene sequencing. J. Gen. Appl. Microbiol. 2018, 64, 158–166. [Google Scholar] [CrossRef]
- Lu, G.; Wang, F.; Yu, Z.; Lu, M.; Wang, Y.; Liu, C.; Xue, Z.; Wu, Y.; Wang, L.; Song, L. Bacterial communities in gills and intestines of yesso scallop (Patinopecten yessoensis) and its habitat waters in Changhai (Dalian, China). ISJ-Invertebr. Surviv. J. 2017, 14, 340–351. [Google Scholar]
- Li, Z.; Li, L.; Sokolova, I.; Shang, Y.; Huang, W.; Khor, W.; Fang, J.; Wang, Y.; Hu, M. Effects of elevated temperature and different crystal structures of TiO2 nanoparticles on the gut microbiota of mussel Mytilus coruscus. Mar. Pollut. Bull. 2024, 199, 115979. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, J.; He, Z.; Li, Z.; Zhao, B.; Mu, Y.; Lee, J.; Chu, Z. Effects of fulvic acid on growth performance and intestinal health of juvenile loath Paramisgurnus dabryanus (Sauvage). Fish Shellfish. Immunol. 2017, 62, 47–56. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, W.; Hu, G.; Qiu, L.; Meng, S.; Song, C.; Fan, L.; Zhao, Z.; Bing, X.; Chen, J. Gut microbiota analysis of juvenile genetically improved farmed tilapia (Oreochromis niloticus) by dietary supplementation of different resveratrol concentrations. Fish Shellfish. Immunol. 2018, 77, 200–207. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, R.; Mo, J.; Yang, S.; Gu, N.; Wu, Z.; Babu, V.; Li, J.; Huang, Y.; Lin, L. Effects of brewer’s yeast hydrolysate on the growth performance and the intestinal bacterial diversity of largemouth bass (Micropterus salmoides). Aquaculture 2018, 484, 139–144. [Google Scholar] [CrossRef]
- Dishaw, L.; Flores-Torres, J.; Lax, S.; Gemayel, K.; Leigh, B.; Melillo, D.; Mueller, M.; Natale, L.; Zucchetti, I.; De Santis, R.; et al. The Gut of Geographically Disparate Ciona intestinalis Harbors a Core Microbiota. PLoS ONE 2014, 9, e93386. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, B.; Jiang, Q.; Ke, C. Changes in gut-associated flora and bacterial digestive enzymes during the development stages of abalone (Haliotis diversicolor). Aquaculture 2012, 338, 147–153. [Google Scholar] [CrossRef]
- King, G.; Judd, C.; Kuske, C.; Smith, C. Analysis of Stomach and Gut Microbiomes of the Eastern Oyster (Crassostrea virginica) from Coastal Louisiana, USA. PLoS ONE 2012, 7, e51475. [Google Scholar] [CrossRef] [PubMed]
- Rungrassamee, W.; Klanchui, A.; Maibunkaew, S.; Chaiyapechara, S.; Jiravanichpaisal, P.; Karoonuthaisiri, N. Characterization of Intestinal Bacteria in Wild and Domesticated Adult Black Tiger Shrimp (Penaeus monodon). PLoS ONE 2014, 9, e91853. [Google Scholar] [CrossRef]
- Givens, C.; Burnett, K.; Burnett, L.; Hollibaugh, J. Microbial communities of the carapace, gut, and hemolymph of the Atlantic blue crab, Callinectes sapidus. Mar. Biol. 2013, 160, 2841–2851. [Google Scholar] [CrossRef]
- Hakim, J.; Koo, H.; Dennis, L.; Kumar, R.; Ptacek, T.; Morrow, C.; Lefkowitz, E.; Powell, M.; Bej, A.; Watts, S. An abundance of Epsilonproteobacteria revealed in the gut microbiome of the laboratory cultured sea urchin, Lytechinus variegatus. Front. Microbiol. 2015, 6, 1047. [Google Scholar] [CrossRef]
- Musella, M.; Wathsala, R.; Tavella, T.; Rampelli, S.; Barone, M.; Palladino, G.; Biagi, E.; Brigidi, P.; Turroni, S.; Franzellitti, S.; et al. Tissue-scale microbiota of the Mediterranean mussel (Mytilus galloprovincialis) and its relationship with the environment. Sci. Total Environ. 2020, 717, 137209. [Google Scholar] [CrossRef]
- Griffin, T.; Baer, J.; Ward, J. Direct Comparison of Fecal and Gut Microbiota in the Blue Mussel (Mytilus edulis) Discourages Fecal Sampling as a Proxy for Resident Gut Community. Microb. Ecol. 2021, 81, 180–192. [Google Scholar] [CrossRef]
- Deegan, L.; Peterson, B.; Portier, R. Stable isotopes and cellulase activity as evidence for detritus as a food source for juvenile Gulf menhaden. Estuaries 1990, 13, 14–19. [Google Scholar] [CrossRef]
- McKee, L.; La Rosa, S.; Westereng, B.; Eijsink, V.; Pope, P.; Larsbrink, J. Polysaccharide degradation by the Bacteroidetes: Mechanisms and nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. [Google Scholar] [CrossRef]
- Shahbaz, U. Chitin, Characteristic, Sources, and Biomedical Application. Curr. Pharm. Biotechnol. 2020, 21, 1433–1443. [Google Scholar] [CrossRef]
- Brinkmann, S.; Spohn, M.; Schäberle, T. Bioactive natural products from Bacteroidetes. Nat. Prod. Rep. 2022, 39, 1045–1065. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Asoh, S.; Hiranuma, H.; Ohsawa, I.; Iio, K.; Satou, A.; Ishikura, M.; Ohta, S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J. Nutr. Biochem. 2010, 21, 381–389. [Google Scholar] [CrossRef]
- Fischbach, M.; Walsh, C. Antibiotics for Emerging Pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef]
- Sathe, P.; Laxman, K.; Myint, M.; Dobretsov, S.; Richter, J.; Dutta, J. Bioinspired nanocoatings for biofouling prevention by photocatalytic redox reactions. Sci. Rep. 2017, 7, 3624. [Google Scholar] [CrossRef]
- Fimlaid, K.; Shen, A. Diverse mechanisms regulate sporulation sigma factor activity in the Firmicutes. Curr. Opin. Microbiol. 2015, 24, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Vesth, T.; Ozen, A.; Andersen, S.; Kaas, R.; Lukjancenko, O.; Bohlin, J.; Nookaew, I.; Wassenaar, T.; Ussery, D. Veillonella, Firmicutes: Microbes disguised as Gram negatives. Stand. Genomis Sci. 2013, 9, 431–448. [Google Scholar] [CrossRef]
- Xiao, S.; Jiang, S.; Qian, D.; Duan, J. Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder. Appl. Microbiol. Biotechnol. 2020, 104, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Sun, F.; Wang, C.; Zhang, L.; Zhang, X. Assessment of the effect of Enteromorpha prolifera on bacterial community structures in aquaculture environment. PLoS ONE 2017, 12, e0179792. [Google Scholar] [CrossRef]
- Zhao, G.; He, H.; Wang, H.; Liang, Y.; Guo, C.; Shao, H.; Jiang, Y.; Wang, M. Variations in Marine Bacterial and Archaeal Communities during an Ulva prolifera Green Tide in Coastal Qingdao Areas. Microorganisms 2022, 10, 1204. [Google Scholar] [CrossRef]
- Fernández-Gómez, B.; Richter, M.; Schüler, M.; Pinhassi, J.; Acinas, S.; González, J.; Pedrós-Alió, C. Ecology of marine Bacteroidetes: A comparative genomics approach. ISME J. 2013, 7, 1026–1037. [Google Scholar] [CrossRef]
- Bergauer, K.; Fernandez-Guerra, A.; Garcia, J.; Sprenger, R.; Stepanauskas, R.; Pachiadaki, M.; Jensen, O.; Herndl, G. Organic matter processing by microbial communities throughout the Atlantic water column as revealed by metaproteomics. Proc. Natl. Acad. Sci. USA 2018, 115, E400–E408. [Google Scholar] [CrossRef] [PubMed]
- Newell, R. Ecosystem influences of natural and cultivated populations of suspension-feeding bivalve molluscs: A review. J. Shellfish. Res. 2004, 23, 51–61. [Google Scholar]
- Zehr, J.; Jenkins, B.; Short, S.; Steward, G. Nitrogenase gene diversity and microbial community structure: A cross-system comparison. Environ. Microbiol. 2003, 5, 539–554. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cai, C.; Yang, C.; Zheng, Z.; Wang, Q.; Du, X.; Deng, Y. Effect of protein sources in formulated diets on the growth, immune response, and intestinal microflora of pearl oyster Pinctada fucata martensii. Aquac. Rep. 2020, 16, 100253. [Google Scholar] [CrossRef]
- Amin, S.; Parker, M.; Armbrust, E. Interactions between Diatoms and Bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Buchan, A.; LeCleir, G.; Gulvik, C.; González, J. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef]
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J. Algae as an important environment for bacteria—Phylogenetic relationships among new bacterial species isolated from algae. Phycologia 2013, 52, 14–24. [Google Scholar] [CrossRef]
- Shin, N.; Whon, T.; Bae, J. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Bryukhanov, A.; Korneeva, V.; Dinarieva, T.; Karnachuk, O.; Netrusov, A.; Pimenov, N. Components of antioxidant systems in the cells of aerotolerant sulfate-reducing bacteria of the genus Desulfovibrio (strains A2 and TomC) isolated from metal mining waste. Microbiology 2016, 85, 649–657. [Google Scholar] [CrossRef]
- Schoenborn, L.; Abdollahi, H.; Tee, W.; Dyall-Smith, M.; Janssen, P. A member of the delta subgroup of proteobacteria from a pyogenic liver abscess is a typical sulfate reducer of the genus Desulfovibrio. J. Clin. Microbiol. 2001, 39, 787–790. [Google Scholar] [CrossRef]
- Finster, K.; Kjeldsen, K. Desulfovibrio oceani subsp oceani sp nov., subsp nov and Desulfovibrio oceani subsp galateae subsp nov., novel sulfate-reducing bacteria isolated from the oxygen minimum zone off the coast of Peru. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2010, 97, 221–229. [Google Scholar] [CrossRef]
- Pereira, P.; He, Q.; Xavier, A.; Zhou, J.; Pereira, I.; Louro, R. Transcriptional response of Desulfovibrio vulgaris Hildenborough to oxidative stress mimicking environmental conditions. Arch. Microbiol. 2008, 189, 451–461. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramel, F.; Amrani, A.; Pieulle, L.; Lamrabet, O.; Voordouw, G.; Seddiki, N.; Brèthes, D.; Company, M.; Dolla, A.; Brasseur, G. Membrane-bound oxygen reductases of the anaerobic sulfate-reducing Desulfovibrio vulgaris Hildenborough: Roles in oxygen defence and electron link with periplasmic hydrogen oxidation. Microbiology 2013, 159, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Cypionka, H. Oxygen respiration by Desulfovibrio species. Annu. Rev. Microbiol. 2000, 54, 827–848. [Google Scholar] [CrossRef]
- Abdollahi, H.; Wimpenny, J. Effects of Oxygen on the Growth of Desulfovibrio desulfuricans. J. Gen. Microbiol. 1990, 136, 1025–1030. [Google Scholar] [CrossRef]
- Lopez-Sánchez, R.; Rebollar, E.; Gutierrez-Rios, R.; Garciarrubio, A.; Juarez, K.; Segovia, L. Metagenomic analysis of carbohydrate-active enzymes and their contribution to marine sediment biodiversity. World J. Microbiol. Biotechnol. 2024, 40, 95. [Google Scholar] [CrossRef]
- Eppinger, M.; Baar, C.; Raddatz, G.; Huson, D.; Schuster, S. Comparative analysis of four Campylobacterales. Nat. Rev. Microbiol. 2004, 2, 872–885. [Google Scholar] [CrossRef]
- Gupta, R. Molecular signatures (unique proteins and conserved indels) that are specific for the epsilon proteobacteria (Campylobacterales). BMC Genom. 2006, 7, 167. [Google Scholar] [CrossRef]
- Van Goethem, M.; Makhalanyane, T.; Cowan, D.; Valverde, A. Cyanobacteria and Alphaproteobacteria May Facilitate Cooperative Interactions in Niche Communities. Front. Microbiol. 2017, 8, 2099. [Google Scholar] [CrossRef]
- Lu, Y.; Cheung, S.; Koh, X.; Xia, X.; Jing, H.; Lee, P.; Kao, S.; Gan, J.; Dai, M.; Liu, H. Active degradation-nitrification microbial assemblages in the hypoxic zone in a subtropical estuary. Sci. Total Environ. 2023, 904, 166694. [Google Scholar] [CrossRef]
- Waite, D.; Vanwonterghem, I.; Rinke, C.; Parks, D.; Zhang, Y.; Takai, K.; Sievert, S.; Simon, J.; Campbell, B.; Hanson, T.; et al. Comparative Genomic Analysis of the Class Epsilonproteobacteria and Proposed Reclassification to Epsilonbacteraeota (phyl. nov.). Front. Microbiol. 2017, 8, 682. [Google Scholar] [CrossRef] [PubMed]
- On, S. Taxonomy of Campylobacter, Arcobacter, Helicobacter and related bacteria:: Current status, future prospects and immediate concerns. J. Appl. Microbiol. 2001, 90, 1S–15S. [Google Scholar] [CrossRef] [PubMed]
- Gazdag, O.; Takács, T.; Ködöböcz, L.; Krett, G.; Szili-Kovács, T. Alphaproteobacteria communities depend more on soil types than land managements. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2019, 69, 147–154. [Google Scholar] [CrossRef]
- Bagagnan, S.; Guerin-Rechdaoui, S.; Marconi, A.; Rocher, V.; Giusti-Miller, S.; Moilleron, R.; Jusselme, M. Overview of microbial communities in the surface water of the Seine River to understand their response to climate change and human activities. Aquat. Ecol. 2024, 58, 1067–1089. [Google Scholar] [CrossRef]
- Gao, F.; Li, F.; Tan, J.; Yan, J.; Sun, H. Bacterial Community Composition in the Gut Content and Ambient Sediment of Sea Cucumber Apostichopus japonicus Revealed by 16S rRNA Gene Pyrosequencing. PLoS ONE 2014, 9, e100092. [Google Scholar] [CrossRef]
- Phoma, S.; Vikram, S.; Jansson, J.; Ansorge, I.; Cowan, D.; Van de Peer, Y.; Makhalanyane, T. Agulhas Current properties shape microbial community diversity and potential functionality. Sci. Rep. 2018, 8, 10542. [Google Scholar] [CrossRef]
- Papale, M.; Rizzo, C.; Caruso, G.; Amalfitano, S.; Maimone, G.; Miserocchi, S.; La Ferla, R.; Aspholm, P.; Decembrini, F.; Azzaro, F.; et al. Ice Melt-Induced Variations of Structural and Functional Traits of the Aquatic Microbial Community along an Arctic River (Pasvik River, Norway). Water 2021, 13, 2297. [Google Scholar] [CrossRef]
- Voloshina, E.; Kosiakova, N.; Prokhorenko, I. Lipopolysaccharide from Rhodobacter capsulatus Counteracts the Effects of Toxic Lipopolysaccharides and Inhibits the Release of TNF-α, IL-6, and IL-1β in Human Whole Blood. Biol. Membr. 2013, 30, 357–363. [Google Scholar] [CrossRef]
- Song, Z.; Li, K.; Li, K. Acute effects of the environmental probiotics Rhodobacter sphaeroides on intestinal bacteria and transcriptome in shrimp Penaeus vannamei. Fish Shellfish. Immunol. 2024, 145, 109316. [Google Scholar] [CrossRef]
- Yoon, J. Thetidibacter halocola gen. nov., sp. nov., a novel member within the family Roseobacteraceae isolated from seawater. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2023, 116, 631–641. [Google Scholar] [CrossRef]
- Nedashkovskaya, O.; Otstavnykh, N.; Balabanova, L.; Bystritskaya, E.; Kim, S.; Zhukova, N.; Tekutyeva, L.; Isaeva, M. Rhodoalgimonas zhirmunskyi gen. nov., sp. nov., a Marine Alphaproteobacterium Isolated from the Pacific Red Alga Ahnfeltia tobuchiensis: Phenotypic Characterization and Pan-Genome Analysis. Microorganisms 2023, 11, 2463. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, W.; Zhong, Y.; Guo, L.; Zhou, P.; Xu, X. Thiosulfatihalobacter marinus gen. nov. sp. nov., a novel member of the family Roseobacteraceae, isolated from the West Pacific Ocean. Int. J. Syst. Evol. Microbiol. 2022, 72, 005286. [Google Scholar] [CrossRef]
- Dutta, D.; Kaushik, A.; Kumar, D.; Bag, S. Foodborne Pathogenic Vibrios: Antimicrobial Resistance. Front. Microbiol. 2021, 12, 638331. [Google Scholar] [CrossRef]
- Zago, V.; Veschetti, L.; Patuzzo, C.; Malerba, G.; Lleo, M. Resistome, Mobilome and Virulome Analysis of Shewanella algae and Vibrio spp. Strains Isolated in Italian Aquaculture Centers. Microorganisms 2020, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Ager-Wick, E.; Kumar, J.; Karunasagar, I.; Karunasagar, I.; Peng, B.; Evensen, O.; Sorum, H.; Munang’andu, H. Aeromonas species isolated from aquatic organisms, insects, chicken, and humans in India show similar antimicrobial resistance profiles. Front. Microbiol. 2022, 13, 1008870. [Google Scholar] [CrossRef]
- Ng, W.; Shum, H.; To, K.; Sridhar, S. Emerging Infections Due to Shewanella spp.: A Case Series of 128 Cases Over 10 Years. Front. Med. 2022, 9, 850938. [Google Scholar] [CrossRef]
- Zong, Z. Nosocomial peripancreatic infection associated with Shewanella xiamenensis. J. Med. Microbiol. 2011, 60, 1387–1390. [Google Scholar] [CrossRef]
- Antonelli, A.; Di Palo, D.; Galano, A.; Becciani, S.; Montagnani, C.; Pecile, P.; Galli, L.; Rossolini, G. Intestinal carriage of Shewanella xiamenensis simulating carriage of OXA-48-producing Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2015, 82, 1–3. [Google Scholar] [CrossRef]
- Janda, J.; Abbott, S. The genus Shewanella: From the briny depths below to human pathogen. Crit. Rev. Microbiol. 2014, 40, 293–312. [Google Scholar] [CrossRef]
- Bello-López, J.; Cabrero-Martínez, O.; Ibáñez-Cervantes, G.; Hernández-Cortez, C.; Pelcastre-Rodríguez, L.; Gonzalez-Avila, L.; Castro-Escarpulli, G. Horizontal Gene Transfer and Its Association with Antibiotic Resistance in the Genus Aeromonas spp. Microorganisms 2019, 7, 363. [Google Scholar] [CrossRef]
- Lerminiaux, N.; Cameron, A. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Huang, Z.; Xiao, Y.; Wang, D. Shewanella infection in humans: Epidemiology, clinical features and pathogenicity. Virulence 2022, 13, 1515–1532. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.; Abulreesh, H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Pavón, A.; Riquelme, D.; Jaña, V.; Iribarren, C.; Manzano, C.; Lopez-Joven, C.; Reyes-Cerpa, S.; Navarrete, P.; Pavez, L.; García, K. The High Risk of Bivalve Farming in Coastal Areas With Heavy Metal Pollution and Antibiotic-Resistant Bacteria: A Chilean Perspective. Front. Cell. Infect. Microbiol. 2022, 12, 867446. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Baron, S.; Barraud, O. Aeromonas: The multifaceted middleman in the One Health world. Curr. Opin. Microbiol. 2022, 65, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; LaMartina, E.; Lewis, J.; Dahl, A.; Nadig, N.; Szabo, A.; Newton, R.; Skwor, T. One Health and Global Health View of Antimicrobial Susceptibility through the “Eye” of Aeromonas: Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents 2023, 62, 106848. [Google Scholar] [CrossRef]
- Yang, C.; Guo, M.; Yang, H.; Wen, Y.; Zhu, Z.; Wang, T.; Zhu, J.; Chen, L.; Du, H. blaKPC-24-Harboring Aeromonas veronii from the Hospital Sewage Samples in China. Microbiol. Spectr. 2022, 10, e00555-22. [Google Scholar] [CrossRef]
- Grilo, M.; Pereira, A.; Sousa-Santos, C.; Robalo, J.; Oliveira, M. Climatic Alterations Influence Bacterial Growth, Biofilm Production and Antimicrobial Resistance Profiles in Aeromonas spp. Antibiotics 2021, 10, 1008. [Google Scholar] [CrossRef]
- Gufe, C.; Hodobo, T.; Mbonjani, B.; Majonga, O.; Marumure, J.; Musari, S.; Jongi, G.; Makaya, P.; Machakwa, J. Antimicrobial Profiling of Bacteria Isolated from Fish Sold at Informal Market in Mufakose, Zimbabwe. Int. J. Microbiol. 2019, 2019, 8759636. [Google Scholar] [CrossRef]
- Montezzi, L.; Campana, E.; Corrêa, L.; Justo, L.; Paschoal, R.; da Silva, I.; Souza, M.; Drolshagen, M.; Picao, R. Occurrence of carbapenemase-producing bacteria in coastal recreational waters. Int. J. Antimicrob. Agents 2015, 45, 174–177. [Google Scholar] [CrossRef]
- Rahman, M.; Akter, S.; Ashrafudoulla, M.; Chowdhury, M.; Mahamud, A.; Park, S.; Ha, S. Insights into the mechanisms and key factors influencing biofilm formation by Aeromonas hydrophila in the food industry: A comprehensive review and bibliometric analysis. Food Res. Int. 2024, 175, 113671. [Google Scholar] [CrossRef]
- Sherik, M.; Eves, R.; Guo, S.; Lloyd, C.; Klose, K.; Davies, P. Sugar-binding and split domain combinations in repeats-in-toxin adhesins from Vibrio cholerae and Aeromonas veronii mediate cell-surface recognition and hemolytic activities. mBio 2024, 15, e02291-23. [Google Scholar] [CrossRef] [PubMed]
- Semwal, A.; Kumar, A.; Kumar, N. A review on pathogenicity of Aeromonas hydrophila and their mitigation through medicinal herbs in aquaculture. Heliyon 2023, 9, e14088. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, X.; Liu, Y.; Lu, C. Detection of three virulence genes alt, ahp and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J. Appl. Microbiol. 2011, 110, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hsu, G.; Hsu, B.; Yang, P.; Kuo, Y.; Wang, J.; Hussain, B.; Huang, S. Prevalence, virulence-gene profiles, antimicrobial resistance, and genetic diversity of human pathogenic Aeromonas spp. from shellfish and aquatic environments. Environ. Pollut. 2021, 287, 117361. [Google Scholar] [CrossRef]
- Roger, F.; Marchandin, H.; Jumas-Bilak, E.; Kodjo, A.; Lamy, B.; ColBVH, S.G. Multilocus genetics to reconstruct aeromonad evolution. BMC Microbiol. 2012, 12, 62. [Google Scholar] [CrossRef]
- Majeed, S.; De Silva, L.; Kumarage, P.; Heo, G. Occurrence of potential virulence determinants in Aeromonas spp. isolated from different aquatic environments. J. Appl. Microbiol. 2023, 134, lxad031. [Google Scholar] [CrossRef]
- Dien, L.; Ngo, T.; Nguyen, T.; Kayansamruaj, P.; Salin, K.; Mohan, C.; Rodkhum, C.; Dong, H. Non-antibiotic approaches to combat motile Aeromonas infections in aquaculture: Current state of knowledge and future perspectives. Rev. Aquac. 2022, 15, 333–366. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Graf, J. Spatial and temporal population dynamics of a naturally occurring two-species microbial community inside the digestive tract of the medicinal leech. Appl. Environ. Microbiol. 2007, 73, 1984–1991. [Google Scholar] [CrossRef]
- Kari, Z.; Wee, W.; Sukri, S.; Harun, H.; Reduan, M.; Khoo, M.; Doan, H.; Goh, K.; Wei, L. Role of phytobiotics in relieving the impacts of Aeromonas hydrophila infection on aquatic animals: A mini-review. Front. Vet. Sci. 2022, 9, 1023784. [Google Scholar] [CrossRef]
- Milligan, E.; Calarco, J.; Davis, B.; Keenum, I.; Liguori, K.; Pruden, A.; Harwood, V. A Systematic Review of Culture-Based Methods for Monitoring Antibiotic-Resistant Acinetobacter, Aeromonas, and Pseudomonas as Environmentally Relevant Pathogens in Wastewater and Surface Water. Curr. Environ. Health Rep. 2023, 10, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.; de Oliveira, W.; Correia, M.; Fontes, A.; Coelho, L. Aeromonas and Human Health Disorders: Clinical Approaches. Front. Microbiol. 2022, 13, 868890. [Google Scholar] [CrossRef]
- Piotrowska, M.; Popowska, M. Insight into the mobilome of Aeromonas strains. Front. Microbiol. 2015, 6, 494. [Google Scholar] [CrossRef]
- Chen, F.; Yu, T.; Yin, Z.; Wang, P.; Lu, X.; He, J.; Zheng, Y.; Zhou, D.; Gao, B.; Mu, K. Uncovering the hidden threat: The widespread presence of chromosome-borne accessory genetic elements and novel antibiotic resistance genetic environments in Aeromonas. Virulence 2023, 14, 2271688. [Google Scholar] [CrossRef]
- Tekedar, H.; Kumru, S.; Blom, J.; Perkins, A.; Griffin, M.; Abdelhamed, H.; Karsi, A.; Lawrence, M. Comparative genomics of Aeromonas veronii: Identification of a pathotype impacting aquaculture globally. PLoS ONE 2019, 14, e0221018. [Google Scholar] [CrossRef]
- Subirats, J.; Sànchez-Melsió, A.; Borrego, C.; Balcázar, J.; Simonet, P. Metagenomic analysis reveals that bacteriophages are reservoirs of antibiotic resistance genes. Int. J. Antimicrob. Agents 2016, 48, 163–167. [Google Scholar] [CrossRef]
- Ott, B.; Cruciger, M.; Dacks, A.; Rio, R. Hitchhiking of host biology by beneficial symbionts enhances transmission. Sci. Rep. 2014, 4, 5825. [Google Scholar] [CrossRef]
- Bomar, L.; Graf, J. Investigation into the Physiologies of Aeromonas veronii in vitro and Inside the Digestive Tract of the Medicinal Leech Using RNA-seq. Biol. Bull. 2012, 223, 155–166. [Google Scholar]
- McFall-Ngai, M. Negotiations between animals and bacteria: The ’diplomacy’ of the squid-vibrio symbiosis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 126, 471–480. [Google Scholar]
- Braschler, T.; Merino, S.; Tomás, J.; Graf, J. Complement resistance is essential for colonization of the digestive tract of Hirudo medicinalis by Aeromonas strains. Appl. Environ. Microbiol. 2003, 69, 4268–4271. [Google Scholar] [CrossRef]
- Maltz, M.; LeVarge, B.; Graf, J. Identification of iron and heme utilization genes in Aeromonas and their role in the colonization of the leech digestive tract. Front. Microbiol. 2015, 6, 763. [Google Scholar] [CrossRef] [PubMed]
- Bücker, R.; Krug, S.; Rosenthal, R.; Günzel, D.; Fromm, A.; Zeitz, M.; Chakraborty, T.; Fromm, M.; Epple, H.; Schulzke, J. Aerolysin From Aeromonas hydrophila Perturbs Tight Junction Integrity and Cell Lesion Repair in Intestinal Epithelial HT-29/B6 Cells. J. Infect. Dis. 2011, 204, 1283–1292. [Google Scholar] [CrossRef]
- von Graevenitz, A. The role of Aeromonas in diarrhea: A review. Infection 2007, 35, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Janda, J. Recent Advances in the Study of the Taxonomy, Pathogenicity, and Infectious Syndromes Associated with the Genus Aeromonas. Clin. Microbiol. Rev. 1991, 4, 397–410. [Google Scholar] [CrossRef]
- Nelson, M.; Graf, J. Bacterial symbioses of the medicinal leech Hirudo verbana. Gut Microbes 2012, 3, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, K.; Gohritz, A.; Busch, K.; Spies, M.; Vogt, P. Hirudo medicinalis-leech applications in plastic and reconstructive microsurgery—A literature review. Handchir. Mikkrochirurgie Plast. Chir. 2007, 39, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Vaelli, P.; Theis, K.; Williams, J.; O’Connell, L.; Foster, J.; Eisthen, H. The skin microbiome facilitates adaptive tetrodotoxin production in poisonous newts. eLife 2020, 9, e53898. [Google Scholar] [CrossRef]
- Zhang, F.; Dashti, N.; Hynes, R.; Smith, D. Plant growth-promoting rhizobacteria and soybean [Glycine max (L) Merr] growth and physiology at suboptimal root zone temperatures. Ann. Bot. 1997, 79, 243–249. [Google Scholar] [CrossRef]
- Lichty, K.; Loughran, R.; Ushijima, B.; Richards, G.; Boyd, E. Osmotic stress response of the coral and oyster pathogen Vibrio coralliilyticus: Acquisition of catabolism gene clusters for the compatible solute and signaling molecule myo-inositol. Appl. Environ. Microbiol. 2024, 90, e00920-24. [Google Scholar] [CrossRef]
- Dempsey, A.; Kitting, C.; Rosson, R. Bacterial variability among individual penaeid shrimp digestive tracts. Crustaceana 1989, 56, 267–278. [Google Scholar] [CrossRef]
- Indergand, S.; Graf, J. Ingested blood contributes to the specificity of the symbiosis of Aeromonas veronii biovar sobria and Hirudo medicinalis, the medicinal leech. Appl. Environ. Microbiol. 2000, 66, 4735–4741. [Google Scholar]
- Graf, J. Lessons from Digestive-Tract Symbioses Between Bacteria and Invertebrates. Annu. Rev. Microbiol. 2016, 70, 375–393. [Google Scholar] [CrossRef]
- Silver, A.; Graf, J. Innate and procured immunity inside the digestive tract of the medicinal leech. ISJ-Invertebr. Surviv. J. 2011, 8, 173–178. [Google Scholar]
- Nyholm, S.; Graf, J. Knowing your friends: Invertebrate innate immunity fosters beneficial bacterial symbioses. Nat. Rev. Microbiol. 2012, 10, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Mardeni, J.; McClure, E.; Beka, L.; Graf, J. Host Matters: Medicinal Leech Digestive-Tract Symbionts and Their Pathogenic Potential. Front. Microbiol. 2016, 7, 1569. [Google Scholar] [CrossRef]
- Mukherjee, M.; Zaiden, N.; Teng, A.; Hu, Y.; Cao, B. Shewanella biofilm development and engineering for environmental and bioenergy applications. Curr. Opin. Chem. Biol. 2020, 59, 84–92. [Google Scholar] [CrossRef]
- Lemaire, O.; Méjean, V.; Iobbi-Nivol, C. The Shewanella genus: Ubiquitous organisms sustaining and preserving aquatic ecosystems. FEMS Microbiol. Rev. 2020, 44, 155–170. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F. Insights on nitrate respiration by Shewanella. Front. Mar. Sci. 2015, 1, 80. [Google Scholar] [CrossRef]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- Visick, K.; Stabb, E.; Ruby, E. A lasting symbiosis: How Vibrio fischeri finds a squid partner and persists within its natural host. Nat. Rev. Microbiol. 2021, 19, 654–665. [Google Scholar] [CrossRef]
- Brauge, T.; Mougin, J.; Ells, T.; Midelet, G. Sources and contamination routes of seafood with human pathogenic Vibrio spp.: A Farm-to-Fork approach. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13283. [Google Scholar] [CrossRef]
- Mancini, M.; Alessiani, A.; Donatiello, A.; Didonna, A.; D’Attoli, L.; Faleo, S.; Occhiochiuso, G.; Carella, F.; Di Taranto, P.; Pace, L.; et al. Systematic Survey of Vibrio spp. and Salmonella spp. in Bivalve Shellfish in Apulia Region (Italy): Prevalence and Antimicrobial Resistance. Microorganisms 2023, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.; Cubillejo, I.; Nag, D.; Withey, J. Advances in cholera research: From molecular biology to public health initiatives. Front. Microbiol. 2023, 14, 1178538. [Google Scholar] [CrossRef]
- Dubert, J.; Barja, J.; Romalde, J. New Insights into Pathogenic Vibrios Affecting Bivalves in Hatcheries: Present and Future Prospects. Front. Microbiol. 2017, 8, 762. [Google Scholar] [CrossRef]
- Beaz-Hidalgo, R.; Balboa, S.; Romalde, J.; Figueras, M. Diversity and pathogenecity of Vibrio species in cultured bivalve molluscs. Environ. Microbiol. Rep. 2010, 2, 34–43. [Google Scholar] [CrossRef]
- Tercero-Alburo, J.; González-Márquez, H.; Bonilla-González, E.; Quiñones-Ramírez, E.; Vázquez-Salinas, C. Identification of capsule, biofilm, lateral flagellum, and type IV pili in Vibrio mimicus strains. Microb. Pathog. 2014, 76, 77–83. [Google Scholar] [CrossRef]
- Muñoz, D.; de Marín, C.; Marval, H.; Martínez, C. Identification of Bacteria of the Genus Vibrio Associated to Zones of Bivalve Mollusks Extraction, Sucre State, Venezuela. Rev. Científica-Fac. Cienc. Vet. 2012, 22, 459–467. [Google Scholar]
- Pruzzo, C.; Gallo, G.; Canesi, L. Persistence of vibrios in marine bivalves: The role of interactions with haemolymph components. Environ. Microbiol. 2005, 7, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Qin, Z.; Feng, Y.; Geng, Y.; Huang, X.; Ouyang, P.; Chen, D.; Guo, H.; Deng, H.; Fang, J.; et al. Sialic acid catabolism contributes to Vibrio mimicus virulence. Aquaculture 2023, 574, 739660. [Google Scholar] [CrossRef]
- Zhao, J.; Manno, D.; Hawari, J. Psychrilyobacter atlanticus gen. nov., sp nov., a marine member of the phylum Fusobacteria that produces H2 and degrades nitramine explosives under low temperature conditions. Int. J. Syst. Evol. Microbiol. 2009, 59, 491–497. [Google Scholar] [CrossRef]
- Diaz, R.; Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioral responses of benthic macrofauna. Oceanogr. Mar.Biol. Annu. Rev. 1995, 33, 245–303. [Google Scholar]
- Olivier, A.; Jones, L.; Le Vay, L.; Christie, M.; Wilson, J.; Malham, S. A global review of the ecosystem services provided by bivalve aquaculture. Rev. Aquac. 2020, 12, 3–25. [Google Scholar] [CrossRef]
- Yadav, S.; Koenen, M.; Bale, N.; Damsté, J.; Villanueva, L. The physiology and metabolic properties of a novel, low-abundance Psychrilyobacter species isolated from the anoxic Black Sea shed light on its ecological role. Environ. Microbiol. Rep. 2021, 13, 899–910. [Google Scholar] [CrossRef]
- Boutin, S.; Bernatchez, L.; Audet, C.; Derôme, N. Network Analysis Highlights Complex Interactions between Pathogen, Host and Commensal Microbiota. PLoS ONE 2013, 8, e84772. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Nagano, Y.; Fujiwara, Y.; Hatada, Y.; Nogi, Y. Aquimarina macrocephali sp. nov., isolated from sediment adjacent to sperm whale carcasses. Int. J. Syst. Evol. Microbiol. 2010, 60, 2298–2302. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- El-Saadony, M.; Alagawany, M.; Patra, A.; Kar, I.; Tiwari, R.; Dawood, M.; Dhama, K.; Abdel-Latif, H. The functionality of probiotics in aquaculture: An overview. Fish Shellfish. Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Quinn, R.; Hazra, S.; Smolowitz, R.; Chistoserdov, A. Real-time PCR assay for Aquimarina macrocephali subsp homaria and its distribution in shell disease lesions of Homarus americanus, Milne-Edwards, 1837, and environmental samples. J. Microbiol. Methods 2017, 139, 61–67. [Google Scholar] [CrossRef]
- Park, S.; Choe, H.; Baik, K.; Seong, C. Aquimarina mytili sp nov., isolated from the gut microflora of a mussel, Mytilus coruscus, and emended description of Aquimarina macrocephali. Int. J. Syst. Evol. Microbiol. 2012, 62, 1974–1979. [Google Scholar] [CrossRef]
- Jadeja, N.; Worrich, A. From gut to mud: Dissemination of antimicrobial resistance between animal and agricultural niches. Environ. Microbiol. 2022, 24, 3290–3306. [Google Scholar] [CrossRef]
- Subirats, J.; Triadó-Margarit, X.; Mandaric, L.; Acuña, V.; Balcázar, J.; Sabater, S.; Borrego, C. Wastewater pollution differently affects the antibiotic resistance gene pool and biofilm bacterial communities across streambed compartments. Mol. Ecol. 2017, 26, 5567–5581. [Google Scholar] [CrossRef] [PubMed]
- Bentzon-Tilia, M.; Sonnenschein, E.; Gram, L. Monitoring and managing microbes in aquaculture—Towards a sustainable industry. Microb. Biotechnol. 2016, 9, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Chiarri, M.; Guo, X.; Tanguy, A.; He, Y.; Proestou, D. The use of -omic tools in the study of disease processes in marine bivalve mollusks. J. Invertebr. Pathol. 2015, 131, 137–154. [Google Scholar] [CrossRef]
- Habteweld, H.; Asfaw, T. Novel Dietary Approach with Probiotics, Prebiotics, and Synbiotics to Mitigate Antimicrobial Resistance and Subsequent Out Marketplace of Antimicrobial Agents: A Review. Infect. Drug Resist. 2023, 16, 3191–3211. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.; Stiemsma, L.; Amenyogbe, N.; Brown, E.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Montagnani, C.; Dantan, L.; Nicolas, N.; Travers, M.; Duperret, L.; Charrière, G.; Toulza, E.; Mitta, G.; Cosseau, C.; et al. Cross-talk and mutual shaping between the immune system and the microbiota during an oyster’s life. Philos. Trans. R. Soc. B-Biol. Sci. 2024, 379, 20230065. [Google Scholar] [CrossRef]
- Majnik, A.; Lane, R. The relationship between early-life environment, the epigenome and the microbiota. Epigenomics 2015, 7, 1173–1184. [Google Scholar] [CrossRef]
- Camara, M.; Griffith, S.; Evans, S. Can selective breeding reduce the heavy metals content of pacific oysters (Crassostrea gigas), and are there trade-offs with growth or survival? J. Shellfish. Res. 2005, 24, 979–986. [Google Scholar]
- Dupont, S.; Lokmer, A.; Corre, E.; Auguet, J.; Petton, B.; Toulza, E.; Montagnani, C.; Tanguy, G.; Pecqueur, D.; Salmeron, C.; et al. Oyster hemolymph is a complex and dynamic ecosystem hosting bacteria, protists and viruses. Anim. Microbiome 2020, 2, 12. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montúfar-Romero, M.; Valenzuela-Miranda, D.; Valenzuela-Muñoz, V.; Morales-Rivera, M.F.; Gallardo-Escárate, C. Microbiota Dysbiosis in Mytilus chilensis Is Induced by Hypoxia, Leading to Molecular and Functional Consequences. Microorganisms 2025, 13, 825. https://doi.org/10.3390/microorganisms13040825
Montúfar-Romero M, Valenzuela-Miranda D, Valenzuela-Muñoz V, Morales-Rivera MF, Gallardo-Escárate C. Microbiota Dysbiosis in Mytilus chilensis Is Induced by Hypoxia, Leading to Molecular and Functional Consequences. Microorganisms. 2025; 13(4):825. https://doi.org/10.3390/microorganisms13040825
Chicago/Turabian StyleMontúfar-Romero, Milton, Diego Valenzuela-Miranda, Valentina Valenzuela-Muñoz, María F. Morales-Rivera, and Cristian Gallardo-Escárate. 2025. "Microbiota Dysbiosis in Mytilus chilensis Is Induced by Hypoxia, Leading to Molecular and Functional Consequences" Microorganisms 13, no. 4: 825. https://doi.org/10.3390/microorganisms13040825
APA StyleMontúfar-Romero, M., Valenzuela-Miranda, D., Valenzuela-Muñoz, V., Morales-Rivera, M. F., & Gallardo-Escárate, C. (2025). Microbiota Dysbiosis in Mytilus chilensis Is Induced by Hypoxia, Leading to Molecular and Functional Consequences. Microorganisms, 13(4), 825. https://doi.org/10.3390/microorganisms13040825







