Growth, Health, Quality, and Production of Onions (Allium cepa L.) Inoculated with Systemic Biological Products
Abstract
1. Introduction
2. Materials and Methods
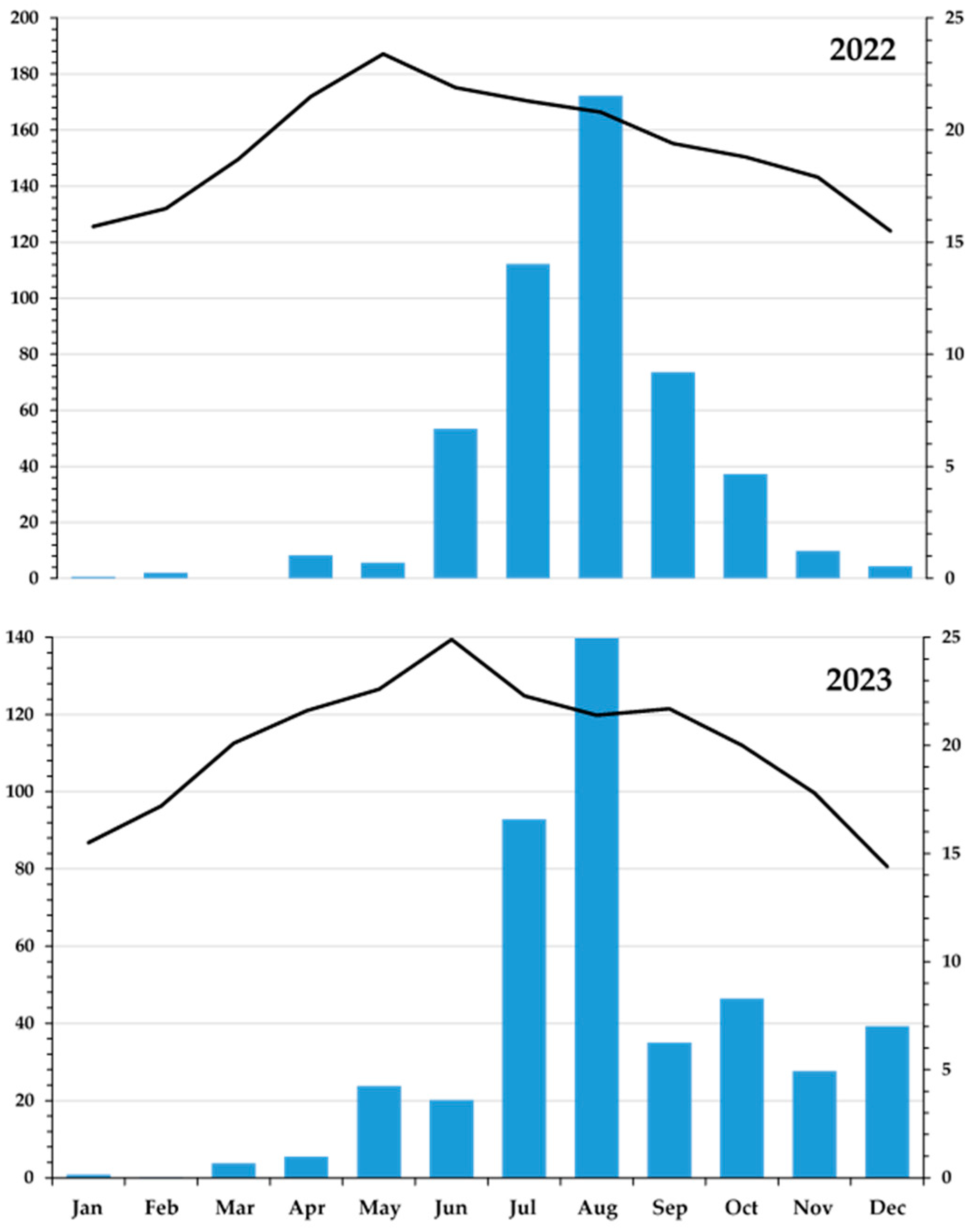
2.1. Study Site
2.2. Experimental Treatments
2.3. Variables Analyzed
2.4. Statistical Analysis
2.5. Verification of the Presence of Beneficial Microorganisms Within Internal Onion Tissues
2.6. DNA Extraction, PCR, and Restriction Analysis for Bacterial Strains
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, H.A.; Mann, L.K. Onion and Their Allies; Leonard Hill (Books) Ltd.: London, UK, 1963; pp. 1–169. [Google Scholar]
- Roldán, E.; Sánchez-Moreno, C.; de Ancos, B.; Cano, P. Characterization of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem. 2008, 108, 907–916. [Google Scholar] [CrossRef]
- Sekara, A.; Pokluda, R.; Del Vacchio, L.; Somma, S.; Caruso, G. Interactions among genotype, environment and agronomic practices on production and quality of storage onion (Allium cepa L.)—A review. Hortic. Sci. 2017, 44, 201–212. [Google Scholar] [CrossRef]
- Uz Daily. Available online: https://www.uzdaily.uz/en/a-new-record-for-onion-cultivation-has-been-set-up-to-150-tons-were-collected-from-each-hectare/ (accessed on 19 February 2025).
- FAO. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 February 2025).
- Statistic Korea. Cultivated Area of Garlic and Onions. 2020. Available online: https://www.korea.kr/briefing/pressReleaseView.do?newsId=156517342#goList (accessed on 6 August 2024).
- Fresh Plaza. Available online: https://www.freshplaza.com/north-america/article/2104408/ (accessed on 19 February 2025).
- Agricultura Moderna. Available online: https://www.amrevista.com/post/el-cultivo-de-cebolla-en-méxico (accessed on 19 February 2025).
- Bayer. Onion Disease Field Guide. Available online: https://www.vegetables.bayer.com/content/dam/bayer-vegetables/multi-country/disease-guides/disease-guide-pdfs/Bayer%20Onion%20Disease%20Guide.pdf (accessed on 19 February 2025).
- Aguado-Santacruz, G.A.; Rascón-Cruz, Q.; Luna-Bulbarela, A. Impacto Económico y Ambiental del Empleo de Fertilizantes Químicos. In Introducción al Uso y Manejo de los Biofertilizantes en la Agricultura; Aguado-Santacruz, G.A., Ed.; INIFAP/SAGARPA: Morelos, México, 2012; pp. 1–22. [Google Scholar]
- Aguado-Santacruz, G.A.; Moreno-Gómez, B.; Dorantes-González, J.R.A.; Bobadilla-Meléndez, M.; Lozano-Contreras, M.G.; Díaz-Valasis, M.; Irizar-Garza, M.B. Mejoramiento de la productividad y sanidad de los cultivos agrícolas mediante el uso de productos biológicos. In Producción y uso de Bioinsumos para la Nutrición Vegetal y Conservación de la Fertilidad del Suelo, 3rd ed.; Reyes-Castillo, A., García-Silva, R., Zetina-Lezama, R., Espinosa-Ramírez, M., Reveles-Hernández, M., Aguado-Santacruz, G.A., Camas-Gómez, R., Báez-Pérez, A., Patishtan-Pérez, J., Eds.; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias: Tecomán, México, 2024; pp. 1–494. [Google Scholar]
- Petrovic, B.; Sȩkara, A.; Pokluda, R. Biofertilizers enhance quality of onion. Agronomy 2020, 10, 1937. [Google Scholar] [CrossRef]
- Aguado-Santacruz, G.A. Uso de Microorganismos como Biofertilizantes. In Introducción al Uso y Manejo de los Biofertilizantes en la Agricultura; Aguado-Santacruz, G.A., Ed.; INIFAP/SAGARPA: Morelos, México, 2012; pp. 35–78. [Google Scholar]
- Aguado-Santacruz, G.A. Productos biológicos sistémicos: La nueva frontera biotecnológica para aumentar el rendimiento y la sanidad de los cultivos agrícolas. In Memorias de la 31a Semana Internacional de Agronomía; Universidad Juárez del Estado de Durango: Venecia, México, 2019. [Google Scholar]
- Aguado-Santacruz, G.A.; Arreola-Tostado, J.M.; Aguirre-Mancilla, C.; García-Moya, E. Use of systemic biofertilizers in sugarcane results in highly reproducible increments in yield and quality of harvests. Heliyon 2024, 10, e28750. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.M.; Brown, B.D.; Shock, C.C.; Horneck, D.A.; Stevens, R.G.; Pelter, G.Q.; Feibert, E.B.G. Nutrient Management for Onions in the Pacific Northwest; Pacific Northwest Publications: Eugene, OR, USA, 2001. [Google Scholar]
- Hanna, M.G.; Elamin, S.M. Effect of different organic fertilizers on growth, yield and total soluble solid of the onion (Allium cepa L.) variety Baftaim-s. J. Agric. Vet. Sci. 2013, 14, 61–67. [Google Scholar]
- Pokluda, R.; Ragasova, L.N.; Jurica, M.; Kalisz, A.; Komorowska, M.; Niemiec, M.; Caruso, G.; Gastol, M.; Sekara, A. The shaping of onion seedlings performance through substrate formulation and co-inoculation with beneficial microorganism consortia. Front. Plant Sci. 2023, 14, 1222557. [Google Scholar] [CrossRef]
- Petrovic, B.; Kopta, T.; Pokluda, R. Effect of biofertilizers on yield and morphological parameters of onion cultivars. Folia Hortic. 2019, 31, 51–59. [Google Scholar] [CrossRef]
- Takahashi, W.Y.; Galvão, C.W.; Cassán, F.D.; Urrea-Valencia, S.; Stremel, A.C.; Stets, M.I.; Kremer, M.A.S.; Jesus, E.C.; Etto, R. M Tracking maize colonization and growth promotion by Azospirillum reveals strain-specific behavior and the influence of inoculation method. Plant Physiol. Biochem. 2024, 215, 108979. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Bethke, J.A.; Cowles, R.S. Systemic insecticides and their use in ornamental plant systems. Floric. Ornamental Biotechnol. 2011, 5, 1–9. [Google Scholar]
- Hall, L.; Beckie, H.; Wolf, T.M. How Herbicides Work: Biology to Application; Alberta Agriculture and Rural Development, Alberta Government: Edmonton, AB, Canada, 1999. [Google Scholar]
- Vaishnav, A.; Shukla, A.K.; Sharma, A.; Kumar, R.; Choudhary, D.K. Endophytic bacteria in plant salt stress tolerance: Current and future prospects. J. Plant Growth Regul. 2018, 38, 650–668. [Google Scholar] [CrossRef]
- Singh, K.R.; Singh, P.; Guo, D.-J.; Sharma, A.; Li, D.-P.; Li, X.; Verma, K.K.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; et al. Root-derived endophytic diazotrophic bacteria Pantoea cypripedii AF1 and Kosakonia arachidis EF1 promote nitrogen assimilation and growth in sugarcane. Front. Microbiol. 2021, 12, 774707. [Google Scholar] [CrossRef]
- INEGI. Compendio de Información Geográfica Municipal: Celaya, Guanajuato; Instituto Nacional de Estadística y Geografía: Celaya, México, 2010. [Google Scholar]
- Osuna, C.F.J.; Ramírez, R.S. Manual para Cultivar Cebolla con Fertirriego y riego por Gravedad en el Estado de Morelos; Folleto técnico No. 12; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP)—Centro de Investigación Regional Pacífico Sur Campo Experimental Zacatepec: Morelos, México, 2013. [Google Scholar]
- Aguado-Santacruz, G.A.; Cabrera-Ponce, J.L.; Ramírez-Chávez, E.; Rascón-Cruz, Q.; Herrera-Estrella, L.; Olalde-Portugal, V. Establishment, characterization and plant regeneration from highly chlorophyllous embryogenic cell cultures of blue grama grass, Bouteloua gracilis (H.B.K.) Lag ex. Steud. Plant Cell Rep. 2001, 20, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Barret, M. Modified method for the determination of pyruvic acid with dinitrophenyl hydrazine in the assessment of onion pungency. J. Sci. Food Agric. 2003, 83, 1210–1213. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 1st ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1974; pp. 1–620. [Google Scholar]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Cazorla, F.M.; Romero, D.; Pérez-García, A.; Lugtenberg, B.J.J.; de Vicente, A.; Bloemberg, G. Isolation and characterization of antagonistic Bacillus subtilis strains from the avocado rhizoplane displaying biocontrol activity. J. Appl. Microbiol. 2007, 103, 1950–1959. [Google Scholar] [CrossRef] [PubMed]
- Ferrera-Cerrato, R.; González-Chávez, M.; Rodríguez-Mendoza, M.D.L.N. Manual de Agromicrobiología, 1st ed.; Trillas: México City, México, 1993; p. 142. [Google Scholar]
- Johnsen, K.; Nielsen, P. Diversity of Pseudomonas strains isolated with King’s B and Gould’s S1 agar determined by repetitive extragenic palindromic-polymerase chain reaction, 16S rDNA sequencing and Fourier transform infrared spectroscopy characterization. FEMS Microbiol. Lett. 1999, 173, 155–162. [Google Scholar] [CrossRef]
- Elad, Y.; Chet, I.; Henis, Y. A selective medium for improving quantitative isolation of Trichoderma spp. from soil. Phytoparasitica 1981, 9, 59–67. [Google Scholar] [CrossRef]
- Lopes, M.A.; Takasaki, K.; Bostwick, D.E.; Helentjaris, T.; Larkins, B.A. Identification of two opaque2 modifier loci in quality protein maize. Mol. Gen. Genet. 1995, 247, 603–613. [Google Scholar] [CrossRef]
- Cordeiro, E.C.N.; Mógor, Á.F.; de Oliveira Amatussi, J.; Mógor, G.; de Lara, G.B.; Marques, H.M.C. Microalga biofertilizer triggers metabolic changes improving onion growth and yield. Horticulturae 2022, 8, 223. [Google Scholar] [CrossRef]
- Gemin, L.G.; Mógor, A.F.; Amatussi, J.D.O.; Mógor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Čolo, J.; Hajnal-Jafari, T.I.; Đurić, S.; Stamenov, D.; Hamidović, S. Plant growth promotion rhizobacteria in onion production. Polish J. Microbiol. 2014, 63, 83–88. [Google Scholar] [CrossRef]
- Singh, V.K.; Kumari, S.A.; Chaudhary, V.K.; Shree, S. Role of biofertilizer and chemical fertilizer for sustainable onion (Allium cepa L.) production. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2034–2040. [Google Scholar] [CrossRef]
- Arunachalam, T.; Gade, K.; Mahadule, P.A.; Soumia, P.S.; Govindasamy, V.; Gawande, S.J.; Mahajan, V. Optimizing plant growth, nutrient uptake, and yield of onion through the application of phosphorus solubilizing bacteria and endophytic fungi. Front. Microbiol. 2024, 15, 1442912. [Google Scholar] [CrossRef]
- Manna, D.; Ghosal, A.; Adhikary, R.; Maity, T.K. Influence of bio-fertilizers on growth, yield and quality of onion (Allium cepa L.) Cv. Sukhsagar. Environ. Ecol. 2014, 32, 728–730. [Google Scholar]
- Kadam, A.A.; Kulkarni, S.A. Effect of bio-fertilizers on growth, yield and quality of onion (Allium cepa). Environ. Ind. Manag. Econ. Agric. Rural. Urban Dev. Sustain. Potential 2023, 1, 120–123. [Google Scholar]
- Verma, N.; Jaiswal, R.K.; Ali, S.A.; Kumar, D.R. Impact of biofertilizers on growth and yield attributes of onion (Allium cepa L.) var. AFLR. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 931–938. [Google Scholar] [CrossRef]
- Sarhan, M.G.R.; Bashandy, S.O. Enhancing onion yield, quality, storability and profitability by using FYM, copper and bio-fertilizer. Egypt. J. Soil Sci. 2021, 61, 323–335. [Google Scholar] [CrossRef]
- Singh, V.V.; Mauriya, S.K.; Pal, S.; Ram, R.B.; Yadav, S.P. Effect of bio-fertilizers on growth, yield and quality traits of onion (Allium cepa L.). Int. Res. J. Pure Appl. Chem. 2020, 21, 18–22. [Google Scholar] [CrossRef]
- AVRDC. AVRDC Report 1999; Asian Vegetable Research and Development Center: Tainan, Taiwan, 2000; p. 152. [Google Scholar]
- Kiran, P.R.; Aradwad, P.; Kumar, T.V.A.; Nayana, N.P.; Ramya, C.S.; Sahoo, M.; Sumit, B.U.; Yadav, R.; Kar, A.; Mani, I. A comprehensive review on recent advances in postharvest treatment, storage, and quality evaluation of onion (Allium cepa): Current status, and challenges. Future Postharvest Food 2024, 1, 124–157. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Q.; Wang, Z.; Wang, L.; Li, X.; Fan, Z.; Zhang, Y.; Li, J.; Gao, X.; Shi, J.; et al. Effects of nitrogen fertilizer on photosynthetic characteristics, biomass, and yield of wheat under different shading conditions. Agronomy 2021, 11, 1989. [Google Scholar] [CrossRef]
- Yu, W.; Hayat, K.; Ma, J.; Fan, X.; Yang, Y.; Zhang, Z.; Yu, Q.; Qian, M.; Lin, H. Effect of antibiotic perturbation on nitrous oxide emissions: An in-depth analysis. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1612–1632. [Google Scholar] [CrossRef]
- Spann, T.M.; Schumann, A.W. The role of plant nutrients in disease development with emphasis on Citrus and Huanglongbing. Proc. Fla. State Hort. Soc. 2009, 122, 169–171. [Google Scholar]
| Component | % |
|---|---|
| Protein | 9.3 |
| Polysaccharides | 8.2 |
| Carbohydrates | 9.3 |
| Phosphorous | 0.7 |
| Potassium | 1.2 |
| Iron | 1.9 |
| Calcium | 0.5 |
| Magnesium | 0.5 |
| Bacterial Species | |||
|---|---|---|---|
| P. fluorescens | A. brasilense | B. subtilis | |
| Amplified fragments from ITS region | 692 654 614 | 668 | 455 265 |
| Restriction fragments generated with Dde I | 195 138 105 | 190 122 88 | 208 |
| Variable | Days After Transplantation | |||||
|---|---|---|---|---|---|---|
| 60 | 90 | 120 | ||||
| Control | Inoculated | Control | Inoculated | Control | Inoculated | |
| Plant height (cm) | 37.1 a | 50.1 b | 38.9 a | 78.8 b | 83.1 a | 100.1 b |
| Plant fresh weight (g) | 239.8 a | 379.6 b | 399.9 a | 512.6 b | 467.9 a | 781.1 b |
| No. leaves | 6.2 a | 9.8 b | 7.1 a | 10.6 b | 10.2 a | 15.8 b |
| Bulb fresh weight (g) | 68.9 a | 113.0 b | 188.7 a | 289.3 b | 357.9 a | 519.4 b |
| Chlorophyll (µg∙g FW−1) | 111.2 a | 137.7 b | 121.6 a | 137.2 b | 178.3 a | 220.2 b |
| Variable | Days After Transplantation | |||||
|---|---|---|---|---|---|---|
| 60 | 90 | 120 | ||||
| Control | Inoculated | Control | Inoculated | Control | Inoculated | |
| Plant height (cm) | 47.3 a | 56.1 b | 60.3 a | 86.2 b | 78.9 a | 99.8 b |
| Plant weight (g) | 279.8 a | 444.3 b | 411.1 a | 554.8 b | 486.6 a | 811.3 b |
| No. leaves | 7.2 a | 12.0 b | 8.9 a | 14.8 b | 11.6 a | 17.2 b |
| Bulb weight (g) | 82.4 a | 115.5 b | 200.8 a | 330.8 b | 366.8 a | 572.8 b |
| Chlorophyll (µg∙g FW−1) | 109.1 a | 138.0 b | 122.3 a | 157.9 b | 163.3 a | 231.4 b |
| Variable | 2022 | 2023 | ||
|---|---|---|---|---|
| Control | Inoculated | Control | Inoculated | |
| Yield (t∙ha−1) | 33.8 a | 48.2 b | 41.9 a | 61.3 b |
| Total soluble solids (°Brix) | 10.21 a | 14.15 b | 10.30 a | 14.62 b |
| Pyruvic acid content (μmol/g) | 2.24 a | 3.78 b | 2.45 a | 4.22 b |
| Fungal incidence (%) | 16.1 a | 9.5 b | 18.7 a | 9.1 b |
| Microorganism | Frequency (%) |
|---|---|
| Azospirillum brasilense | 100 |
| Bacillus subtilis | 100 |
| Pseudomonas fluorescens | 100 |
| Trichoderma hamatum | 39 |
| T. harzianum | 55 |
| T. koningii | 53 |
| T. viridae | 52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Benicio, G.M.; Aguirre-Mancilla, C.L.; Arreola-Tostado, J.M.; Aguado-Santacruz, G.A. Growth, Health, Quality, and Production of Onions (Allium cepa L.) Inoculated with Systemic Biological Products. Microorganisms 2025, 13, 797. https://doi.org/10.3390/microorganisms13040797
Gutiérrez-Benicio GM, Aguirre-Mancilla CL, Arreola-Tostado JM, Aguado-Santacruz GA. Growth, Health, Quality, and Production of Onions (Allium cepa L.) Inoculated with Systemic Biological Products. Microorganisms. 2025; 13(4):797. https://doi.org/10.3390/microorganisms13040797
Chicago/Turabian StyleGutiérrez-Benicio, Glenda Margarita, César Leobardo Aguirre-Mancilla, Jesús Manuel Arreola-Tostado, and Gerardo Armando Aguado-Santacruz. 2025. "Growth, Health, Quality, and Production of Onions (Allium cepa L.) Inoculated with Systemic Biological Products" Microorganisms 13, no. 4: 797. https://doi.org/10.3390/microorganisms13040797
APA StyleGutiérrez-Benicio, G. M., Aguirre-Mancilla, C. L., Arreola-Tostado, J. M., & Aguado-Santacruz, G. A. (2025). Growth, Health, Quality, and Production of Onions (Allium cepa L.) Inoculated with Systemic Biological Products. Microorganisms, 13(4), 797. https://doi.org/10.3390/microorganisms13040797









