5,6-Dihydro-5,6-Epoxymultiplolide A, Cytosporone C, and Uridine Production by Diaporthe hongkongensis, an Endophytic Fungus from Minquartia guianensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. Fermentation, Extraction, and Isolation of Compounds
2.3. Structural Identification
3. Results
3.1. Structural Identification of the Compounds
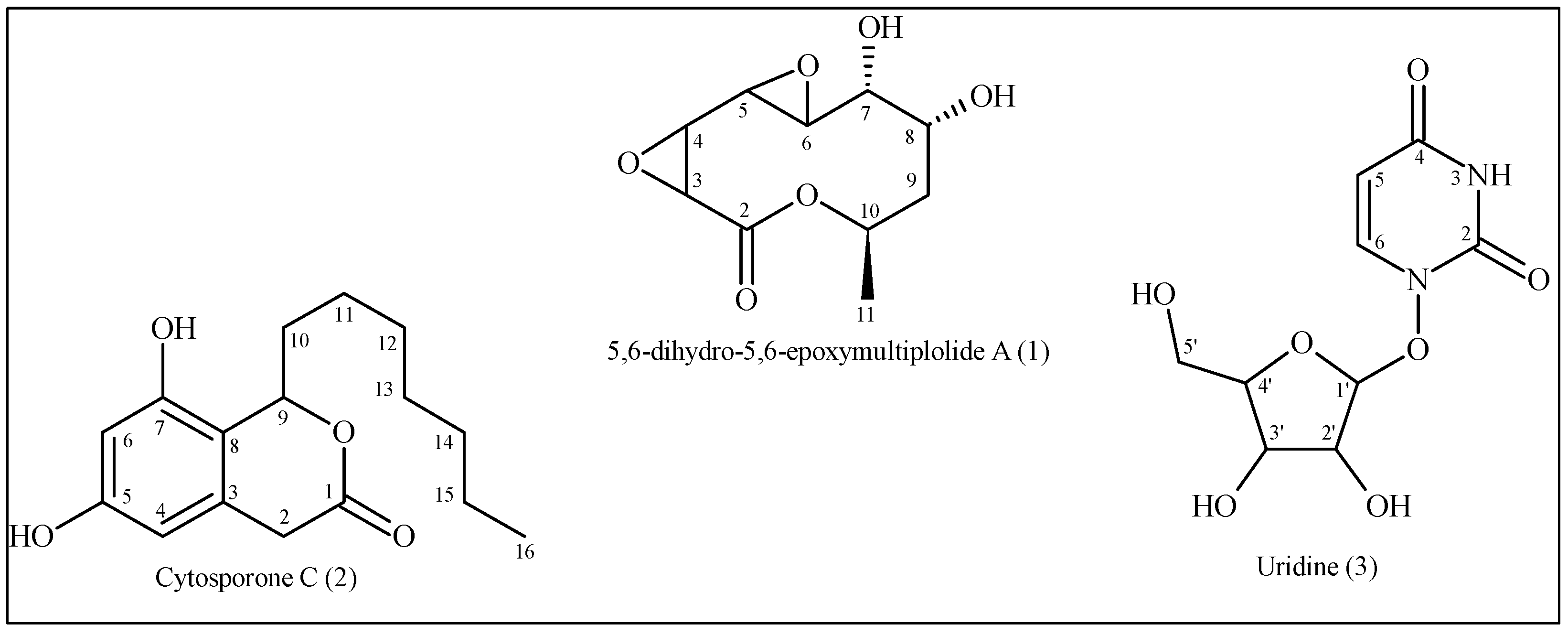
3.1.1. 5,6-Dihydro-5,6-Epoxymultiplolide A (1)
3.1.2. Cytosporone C (2)
3.1.3. Uridine (3)
3.1.4. NMR Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, W.; Khan, B.; Dai, Q.; Lin, J.; Kang, L.; Rajput, N.A.; Yan, W.; Liu, G. Potential of Secondary Metabolites of Diaporthe Species Associated with Terrestrial and Marine Origins. J. Fungi 2023, 9, 453. [Google Scholar] [CrossRef]
- Noriler, S.A.; Savi, D.C.; Aluizio, R.; Cortes, A.M.P.; Possiede, Y.M.; Glienke, C. Bioprospecting and Structure of Fungal Endophyte Communities Found in the Brazilian Biomes, Pantanal, and Cerrado. Front. Microbiol. 2018, 9, 1526. [Google Scholar] [CrossRef]
- Kemkuignou, B.M.; Lambert, C.; Stadler, M.; Fogue, S.K.; Felix, Y.M. Unprecedented Antimicrobial and Cytotoxic Polyketides from Cultures of Diaporthe africana sp. nov. J. Fungi 2023, 9, 781. [Google Scholar] [CrossRef]
- Manichart, N.; Laosinwattana, C.; Somala, N.; Teerarak, M.; Chotsaeng, N. Physiological Mechanism of Action and Partial Separation of Herbicide–Active Compounds from the Diaporthe sp. Extract on Amaranthus tricolor L. Sci. Rep. 2023, 13, 18693. [Google Scholar] [CrossRef]
- Casas, L.L. Bioprospecção de Fungos Endofíticos de Minquartia guianensis Aubl. Master’s Thesis, Universidade do Estado do Amazonas, Manaus, Brazil, 2016; 86p. Available online: https://ri.uea.edu.br/server/api/core/bitstreams/2a4cb6c0-11d4-4b57-99a5-dc9bdaf39583/content (accessed on 4 January 2025).
- Araújo, K.S.; Alves, J.L.; Pereira, O.L.; de Queiroz, M.V. Five New Species of Endophytic Penicillium from Rubber Trees in the Brazilian Amazon. Braz. J. Microbiol. 2024, 55, 3051–3074. [Google Scholar] [CrossRef]
- Rodrigues, L.C.; Rebouças, N.P.B.; Santos, A.L.C.; Garcia, D.L.F. Potencial Terapêutico da espécie Minquartia guianensis (Aubl.): Uma breve revisão. Rev. Fitos 2024, 18, e1597. [Google Scholar] [CrossRef]
- Lima, J.L.L.; Chagas, E.C.O. Coulaceae in Flora e Funga do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brazil, 2020. Available online: https://floradobrasil.jbrj.gov.br/consulta/ficha.html?idDadosListaBrasil=618588 (accessed on 3 January 2025).
- Cursino, L.M.C.; Nunez, C.V.; Paula, R.C.; Nascimento, M.F.A.; Santos, P.A. Triterpenes from Minquartia guianensis (Olacaceae) and in vitro antimalarial activity. Quím Nova [Internet] 2012, 35, 2165–2168. [Google Scholar] [CrossRef]
- Alexandre, S.A.; Espinar, M.T.F.; Nunez, C.V. Triterpenes, steroids and phenolic isolated from Minquartia guianensis Aubl. (Coulaceae) and antibacterial activity. Concilium 2023, 23, 883–895. [Google Scholar] [CrossRef]
- Toppo, P.; Kagatay, L.L.; Gurung, A.; Singla, P.; Chakraborty, R.; Roy, S.; Mathur, P. Endophytic fungi mediate production of bioactive secondary metabolites via modulation of genes involved in key metabolic pathways and their contribution in different biotechnological sector. 3 Biotech 2023, 13, 191. [Google Scholar] [CrossRef]
- Majid, M.; Ganai, B.A.; Wani, A.H. Antifungal, Antioxidant Activity, and GC-MS Profiling of Diaporthe amygdali GWS39: A First Report Endophyte from Geranium wallichianum. Curr. Microbiol. 2024, 82, 40. [Google Scholar] [CrossRef]
- Tanapichatsakul, C.; Monggoot, S.; Gentekaki, E.; Pripdeevech, P. Antibacterial and Antioxidant Metabolites of Diaporthe spp. Isolated from Flowers of Melodorum fruticosum. Curr. Microbiol. 2018, 75, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xing, S.; Wei, X.; Lu, J.; Zhao, G.; Ma, X.; Dai, Z.; Liang, X.; Huang, W.; Liu, Y.; et al. 12-O-Deacetyl-Phomoxanthone A Inhibits Ovarian Tumor Growth and Metastasis by Downregulating PDK4. Biomed. Pharmacother. 2024, 175, 116736. [Google Scholar] [CrossRef] [PubMed]
- Farinella, V.F.; Kawafune, E.S.; Tangerina, M.M.P.; Domingos, H.V.; Lotufo, L.V.C.; Ferreira, M.J.P. OSMAC Strategy Integrated with Molecular Networking for Accessing Griseofulvin Derivatives from Endophytic Fungi of Moquiniastrum polymorphum (Asteraceae). Molecules 2021, 26, 7316. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, E.M.P. Produtos Naturais de Bignonia magnifica W. Bull. (Bignoniaceae) e a Prospecção Metabólica dos Seus Fungos Endofíticos. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2023; 170p. [Google Scholar] [CrossRef]
- Tan, Q.; Yan, X.; Lin, X.; Huang, Y.; Zheng, Z.; Song, S.; Lu, C.; Shen, Y. Chemical Constituents of the Endophytic Fungal Phomopsis sp. NXZ-05 of Camptotheca acuminata. Helv. Chim. Acta 2007, 90, 1811–1817. [Google Scholar] [CrossRef]
- Brady, S.F.; Wagenaar, M.M.; Singh, M.P.; Janso, J.F.; Clardy, J. The Cytosporones, New Octaketide Antibiotics Isolated from an Endophytic Fungus. Org. Lett. 2000, 2, 4043–4046. [Google Scholar] [CrossRef]
- Walczak, D.; Sikorski, A.; Grzywacz, D.; Nowacki, A.; Liberek, B. Characteristic 1H NMR Spectra of β-D-Ribofuranosides and Ribonucleosides: Factors driving furanose ring conformations. RSC Adv. 2022, 12, 29223–29239. [Google Scholar] [CrossRef]
- Tomm, H.A.; Ucciferri, L.; Ross, A.C. Advances in Microbial Culturing Conditions to Activate Silent Biosynthetic Gene Clusters for Novel Metabolite Production. J. Ind. Microbiol. Biotechnol. 2019, 46, 1381–1400. [Google Scholar] [CrossRef]
- Muchlisyiyah, J.; Shamsudin, R.; Kadir Basha, R.; Shukri, R.; How, S.; Niranjan, K.; Onwude, D. Parboiled Rice Processing Method, Rice Quality, Health Benefits, Environment, and Future Perspectives: A Review. Agriculture 2023, 13, 1390. [Google Scholar] [CrossRef]
- Geris, R.; Jesus, V.E.T.; Silva, A.F.; Malta, M. Exploring Culture Media Diversity to Produce Fungal Secondary Metabolites and Cyborg Cells. Chem. Biodivers. 2024, 21, e202302066. [Google Scholar] [CrossRef]
- Alexandre, A.S. Bioprospecção de Diaporthe hongkongensis Fungo Endofítico Isolado De Minquartia guianensis (Olacaceae). Master’s Thesis, Universidade do Estado do Amazonas, Manaus, Brazil, 2018; 86p. Available online: https://pos.uea.edu.br/data/area/titulado/download/74-15.pdf (accessed on 8 January 2025).
- Xu, S.; Li, M.; Hu, Z.; Shao, Y.; Ying, J.; Zhang, H. The Potential Use of Fungal Co-Culture Strategy for Discovery of New Secondary Metabolites. Microorganisms 2023, 11, 464. [Google Scholar] [CrossRef]
- González, J.B.; Castellanos, M.R.T. Solid-State Fermentation: Special Physiology of Fungi. In Fungal Metabolites. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Kukmar, V.; Ahluwalia, V.; Saran, S.; Kumar, J.; Patel, A.K.; Singhania, R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: Challenges and solutions. Bioresour. Technol. 2021, 303, 124566. [Google Scholar] [CrossRef]
- Soccol, C.R.; Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.S. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Yu, W.; Pei, R.; Zhou, J. Molecular regulation of fungal secondary metabolism. World J. Microbiol. Biotechnol. 2023, 39, 204. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Sakinah, A.M.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef] [PubMed]
- Boonphong, S.; Kittakoop, P.; Isaka, M.; Pittayakhajonwut, N.; Thebtaranonth, Y. Multiplolides A and B, New Antifungal 10-Membered Lactones from Xylaria multiplex. J. Nat. Prod. 2001, 64, 965–967. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Yang, X.Q.; Wan, C.P.; Wang, B.Y.; Yin, H.Y.; Shi, L.J.; Wu, Y.M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. Potential antihyperlipidemic polyketones from endophytic Diaporthe sp. JC-J7 in Dendrobium nobile. RSC Adv. 2018, 8, 41810–41817. [Google Scholar] [CrossRef]
- Kaur, B.; Singh, P. Epoxides: Developability as Active Pharmaceutical Ingredients and Biochemical Probes. Bioorg. Chem. 2022, 125, 105862. [Google Scholar] [CrossRef]
- Aparicio, J.F.; Fouces, R.; Mendes, M.V.; Oliveira, N.; Martín, J.F. A complex Multienzyme System Encoded by Five Polyketide Synthase Genes is Involved in the Biosynthesis of the 26-membered Polyene Macrolide Pimaricin in Streptomyces natalensis. Chem. Biol. 2000, 7, 895–905. [Google Scholar] [CrossRef]
- Ikeda, H.; Òmura, S. Biosynthesis, Regulation, and Genetics of Macrolide Production. In: Macrolide Antibiotics: Chemistry, Biochemistry, and Practice. Elsevier 2003, 2, 285–326. [Google Scholar] [CrossRef]
- Hur, J.Y.; Jeong, E.; Kim, Y.C.; Lee, S.R. Strategies for Natural Product Discovery by Unlocking Cryptic Biosynthetic Gene Clusters in Fungi. Separations 2023, 10, 333. [Google Scholar] [CrossRef]
- Tsunematsu, Y. Genomics-directed activation of cryptic natural product pathways deciphers codes for biosynthesis and molecular function. J. Nat. Med. 2021, 75, 261–274. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Du, S.T.; Xiao, J.; Wang, D.C.; Han, W.B.; Zhang, Q.; Gao, J.M. Isolation and Characterization of Antifungal Metabolites from the Melia azedarach-Associated Fungus Diaporthe eucalyptorum. J. Agric. Food Chem. 2020, 68, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Kumar, A. Secondary metabolites from endophytic fungi: Production, methods of analysis, and diverse pharmaceutical potential. Symbiosis 2023, 90, 111–125. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, W.; Chen, T. Cytosporones W and X: Two Mutually Converting Epimers from a Mangrove Endophytic Fungus Diaporthe sp. ZJHJYZ-1. ASC Omega 2023, 29, 26628–26634. [Google Scholar] [CrossRef]
- Jeremy, B.; Nida, M.; Whittney, N.B. Epigenetic Tailoring for the Production of Anti-Infective Cytosporones from the Marine Fungus Leucostoma persoonii. Mar. Drugs 2012, 10, 762–774. [Google Scholar] [CrossRef]
- Zamberlam, C.E.M.; Meza, A.; Leite, C.B. Total synthesis and allelopathic activity of cytosporones A–C. J. Brazil Chem. Soc. 2012, 23, 124–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Xie, C.; Fang, J. Uridine Metabolism and Its Role in Glucose, Lipid, and Amino Acid Homeostasis. Biomed. Res. Int. 2020, 1, 7091718. [Google Scholar] [CrossRef]
- Khezri, M.K.; Turkkan, A.; Koc, C.; Salman, B.; Levent, P.; Cakir, A.; Kafa, I.M.; Cansev, M.; Bekar, A. Uridine Treatment Improves Nerve Regeneration and Functional Recovery in a Rat Model of Sciatic Nerve Injury. Turk. Neurosurg. 2022, 32, 935–943. [Google Scholar] [CrossRef]
- Baumel, B.S.; Doraiswamy, P.M.; Sabbagh, M.; Wurtman, R. Potential Neuroregenerative and Neuroprotective Effects of Uridine/Choline-Enriched Multinutrient Dietary Intervention for Mild Cognitive Impairment: A Narrative Review. Neurol. Ther. 2021, 10, 43–60. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Deng, Y.; Gao, L. Uridine and its role in metabolic diseases, tumors, and neurodegenerative diseases. Front Physiol. 2024, 15, 1360891. [Google Scholar] [CrossRef]
- Arbour, C.A.; Imperiali, B. Uridine natural products: Challenging Targets and Inspiration for Novel Small Molecule Inhibitors. Bioorg. Med. Chem. 2020, 28, 115661. [Google Scholar] [CrossRef]
- Munia, N.S.; Alanazi, M.M.; Bakri, Y.E.; Alanazi, A.S.; Mukhrish, Y.E. Uridine Derivatives: Synthesis, Biological Evaluation, and In Silico Studies as Antimicrobial and Anticancer Agents. Medicina 2023, 59, 1107. [Google Scholar] [CrossRef]
- Garg, R.; Kumar, R.; Srivastava, R. Exploring nucleoside analogs: Key targets in the viral life cycle—Advancing strategies against SARS-CoV-2. Med. Chem. Res. 2024, 33, 869–884. [Google Scholar] [CrossRef]
- Barik, S. Inhibition of Viral RNA-Dependent RNA Polymerases by Nucleoside Inhibitors: An Illustration of the Unity and Diversity of Mechanisms. Int. J. Mol. Sci. 2022, 23, 12649. [Google Scholar] [CrossRef]
- Amaro, R.L.; Buey, R.M.; Revuelta, J.L. Increased production of inosine and guanosine by means of metabolic engineering of the purine pathway in Ashbya gossypii. Microb. Cell Factories 2015, 14, 58. [Google Scholar] [CrossRef]
- Zhang, K.; Qin, M.; Hou, Y. Efficient production of guanosine in Escherichia coli by combinatorial metabolic engineering. Microb. Cell Factories 2024, 23, 182. [Google Scholar] [CrossRef]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal Secondary Metabolism—From Biochemistry to Genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of Fungal Secondary Metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and Natural Functions of Antibiotics Produced by Beneficial and Plant Pathogenic Bacteria. Ann. Rev. Phytopath 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Netzker, T.; Fischer, J.; Weber, J.; Mattern, D.J.; König, C.C.; Valiante, V.; Brakhage, A.A. Microbial communication leading to the activation of silent fungal secondary metabolite gene clusters. Front. Microbiol. 2015, 6, 299. [Google Scholar] [CrossRef]
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef]
- Rolfes, R.J. Regulation of purine nucleotide biosynthesis: In yeast and beyond. Biochem. Soc. Trans. 2006, 34, 786–790. [Google Scholar] [CrossRef]
- Mousa, W.K.; Raizada, M.N. Biodiversity of genes encoding anti-microbial traits within plant-associated microbes. Front. Plant Sci. 2015, 6, 231. [Google Scholar] [CrossRef]
- Kusari, S.; Singh, S.; Jayabaskaran, C. Biotechnological potential of plant-associated endophytic fungi: Hope versus hype. Trends Biotechnol. 2018, 36, 898–908. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Bills, G.F.; Gloer, J.B. Biologically active secondary metabolites from the fungi. Microbiol. Spectr. 2016, 4, 1–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandre, A.d.S.; Casas, L.L.; Silva, D.R.d.; Nunez, C.V. 5,6-Dihydro-5,6-Epoxymultiplolide A, Cytosporone C, and Uridine Production by Diaporthe hongkongensis, an Endophytic Fungus from Minquartia guianensis. Microorganisms 2025, 13, 792. https://doi.org/10.3390/microorganisms13040792
Alexandre AdS, Casas LL, Silva DRd, Nunez CV. 5,6-Dihydro-5,6-Epoxymultiplolide A, Cytosporone C, and Uridine Production by Diaporthe hongkongensis, an Endophytic Fungus from Minquartia guianensis. Microorganisms. 2025; 13(4):792. https://doi.org/10.3390/microorganisms13040792
Chicago/Turabian StyleAlexandre, Andrei da Silva, Luana Lopes Casas, David Ribeiro da Silva, and Cecilia Veronica Nunez. 2025. "5,6-Dihydro-5,6-Epoxymultiplolide A, Cytosporone C, and Uridine Production by Diaporthe hongkongensis, an Endophytic Fungus from Minquartia guianensis" Microorganisms 13, no. 4: 792. https://doi.org/10.3390/microorganisms13040792
APA StyleAlexandre, A. d. S., Casas, L. L., Silva, D. R. d., & Nunez, C. V. (2025). 5,6-Dihydro-5,6-Epoxymultiplolide A, Cytosporone C, and Uridine Production by Diaporthe hongkongensis, an Endophytic Fungus from Minquartia guianensis. Microorganisms, 13(4), 792. https://doi.org/10.3390/microorganisms13040792







