A Sustainable Combined Approach to Control the Microbial Bioburden in the School Environment
Abstract
1. Introduction
2. Materials and Methods
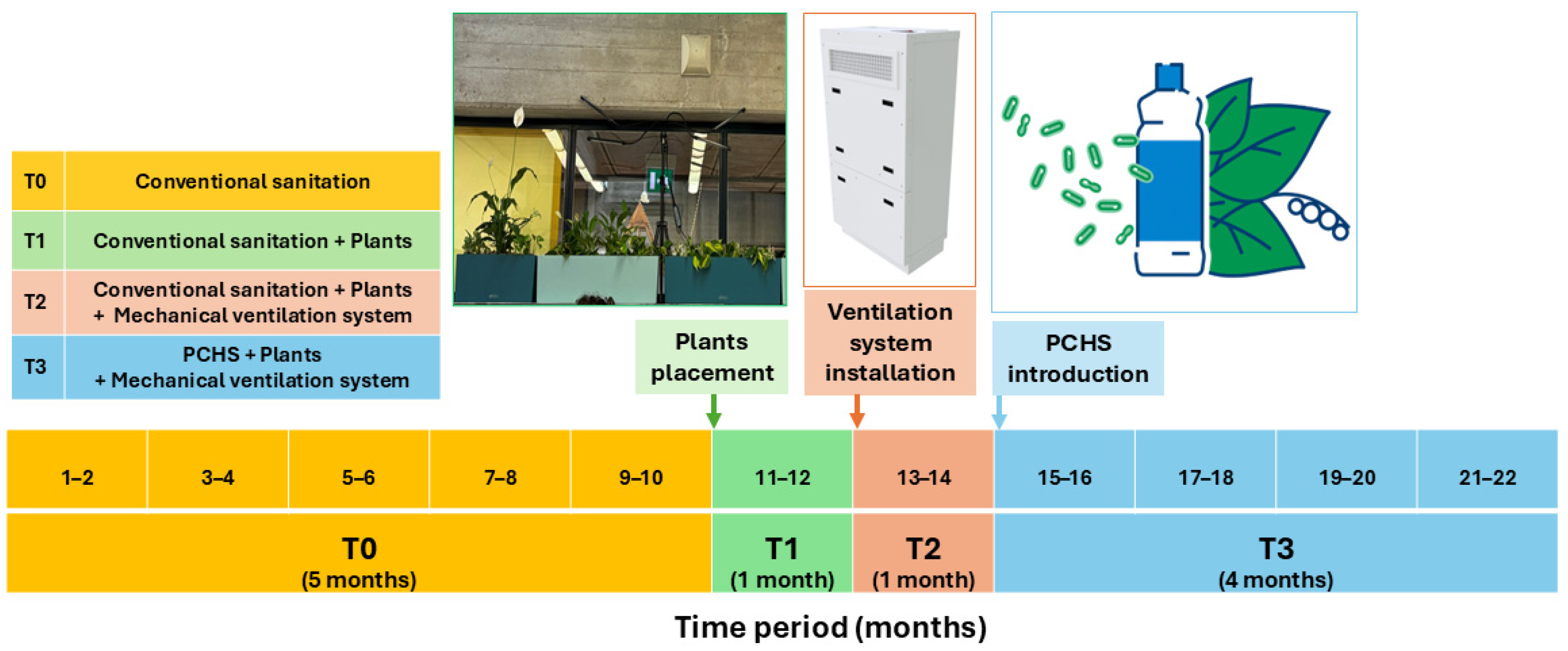
2.1. Study Design
2.2. Green Interventions: Plants, Ventilation, and Probiotic-Based Sanitation
2.3. Environmental Monitoring
2.4. Microbiological Analyses
2.5. Molecular Analyses
2.6. Statistical Analyses
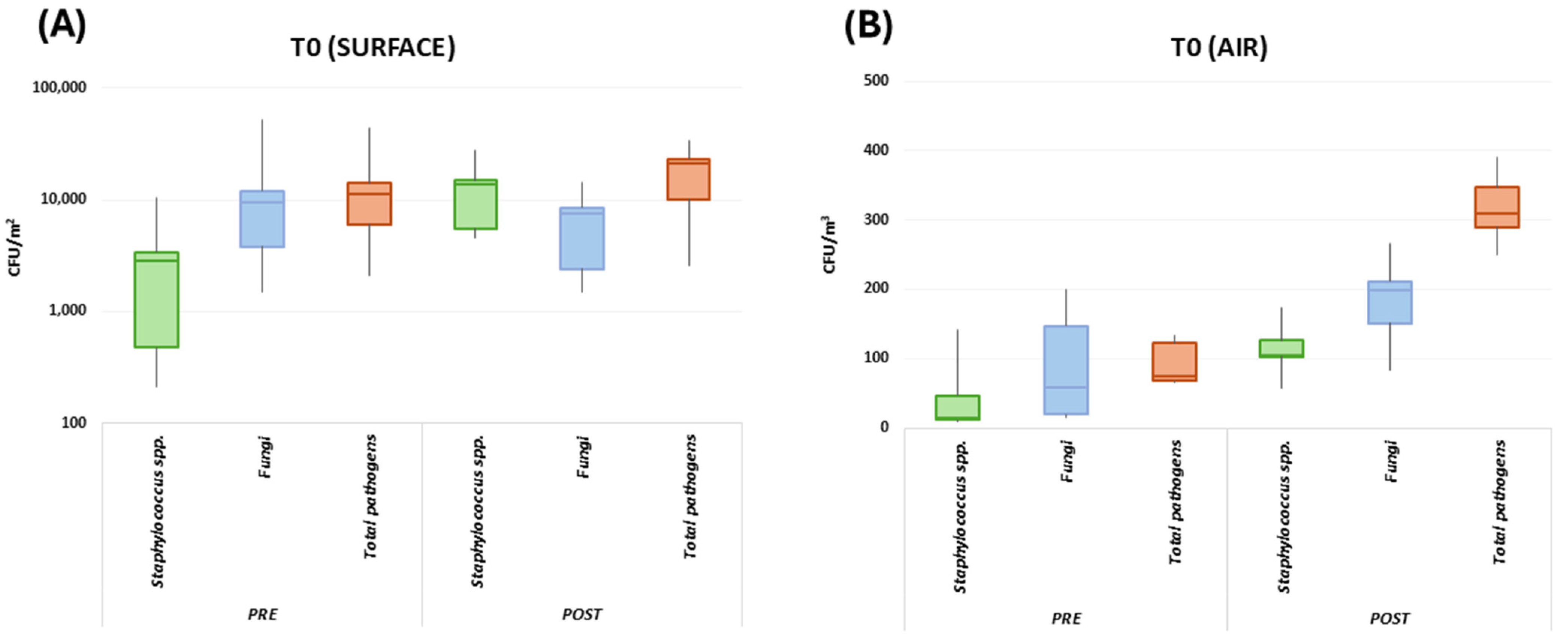
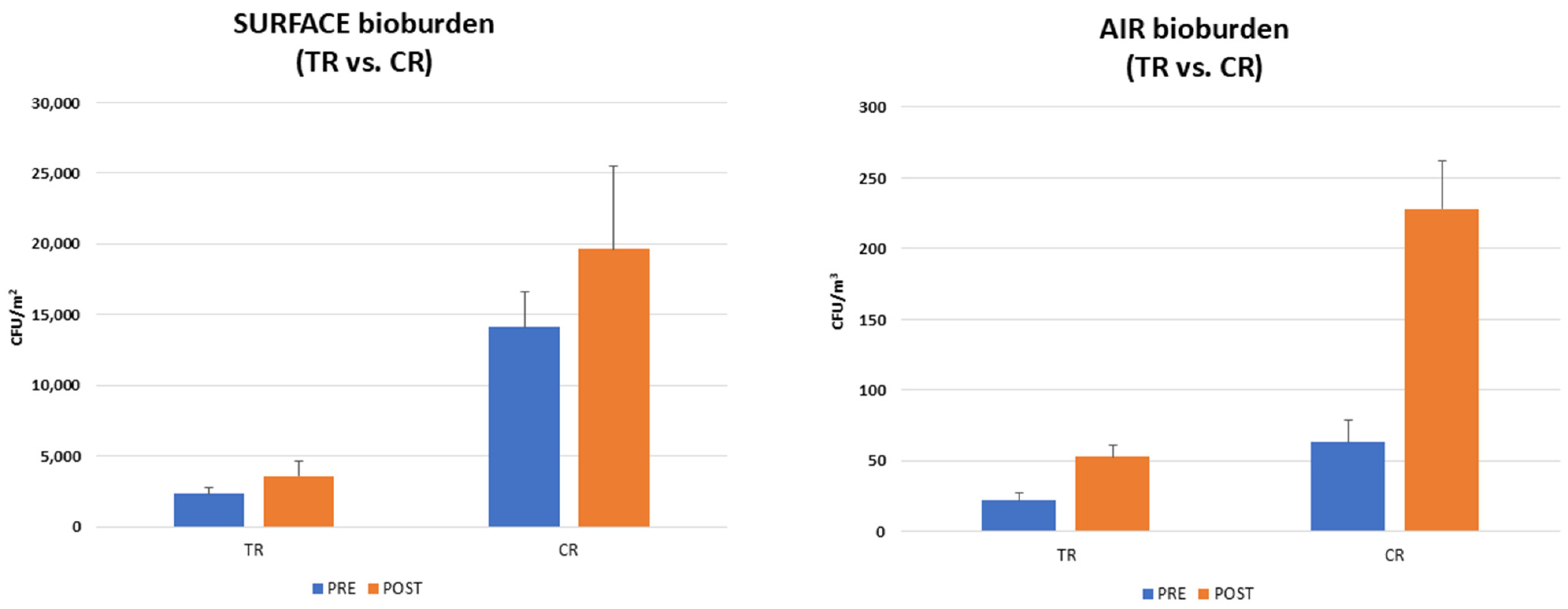
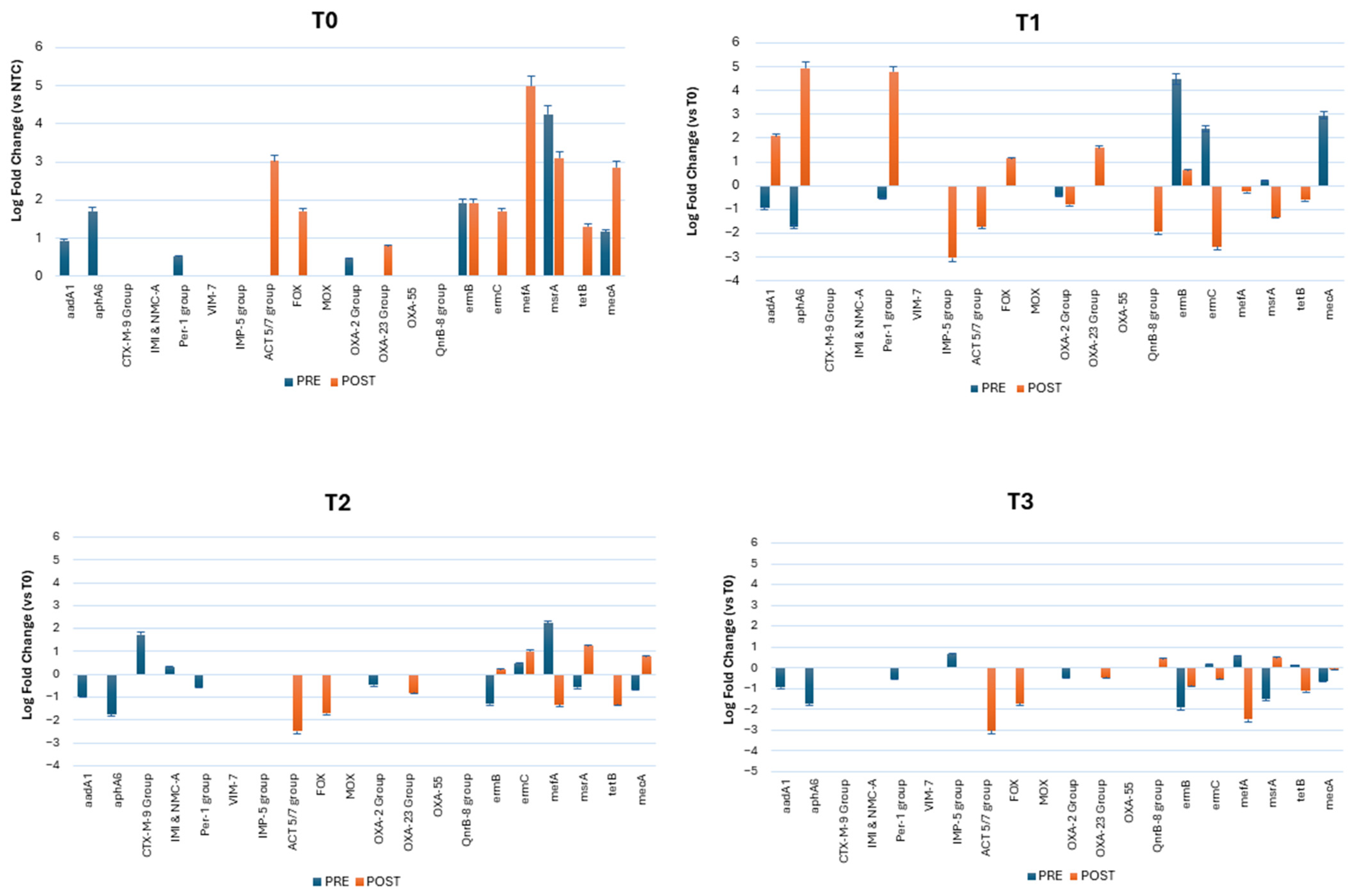
3. Results
3.1. School Environment Bioburden
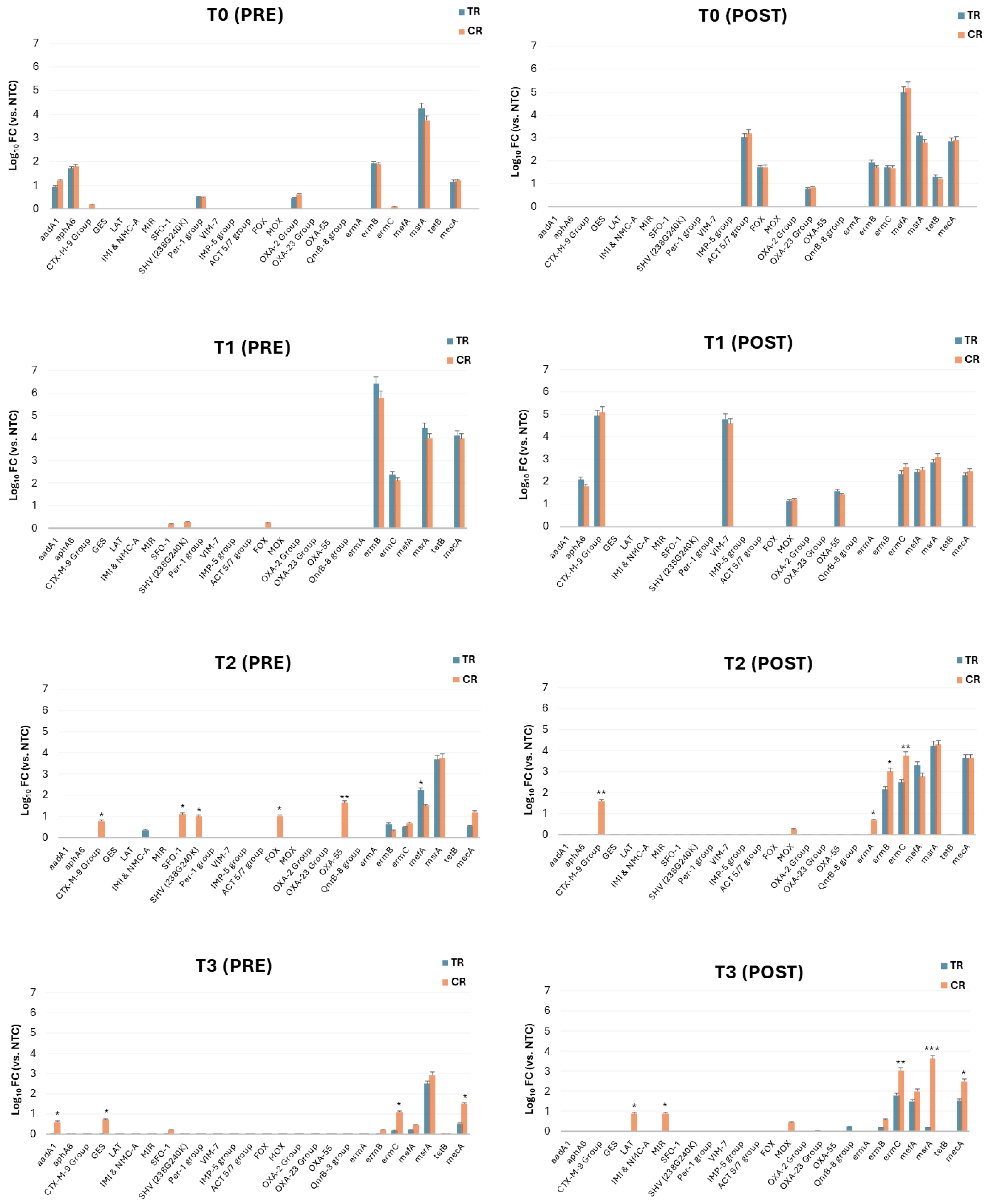
3.2. Resistome Characterization of the Classroom Bioburden
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Microbiomes of the Built Environment; National Academies Press: Washington, DC, USA, 2017; ISBN 978-0-309-44980-9.
- Fu, X.; Ou, Z.; Zhang, M.; Meng, Y.; Li, Y.; Wen, J.; Hu, Q.; Zhang, X.; Norbäck, D.; Deng, Y.; et al. Indoor Bacterial, Fungal and Viral Species and Functional Genes in Urban and Rural Schools in Shanxi Province, China–Association with Asthma, Rhinitis and Rhinoconjunctivitis in High School Students. Microbiome 2021, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Ou, Z.; Sun, Y. Indoor Microbiome and Allergic Diseases: From Theoretical Advances to Prevention Strategies. Eco-Environ. Health 2022, 1, 133–146. [Google Scholar] [CrossRef]
- Li, S.; Yang, Z.; Hu, D.; Cao, L.; He, Q. Understanding Building-Occupant-Microbiome Interactions toward Healthy Built Environments: A Review. Front. Environ. Sci. Eng. 2021, 15, 65. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, Y.; Ou, Z.; Li, Y.; Zhang, M.; Chen, Y.; Zhang, Z.; Chen, X.; Mu, P.; Norbäck, D.; et al. Indoor Microbiome, Air Pollutants and Asthma, Rhinitis and Eczema in Preschool Children—A Repeated Cross-Sectional Study. Environ. Int. 2022, 161, 107137. [Google Scholar] [CrossRef]
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human Occupancy as a Source of Indoor Airborne Bacteria. PLoS ONE 2012, 7, e34867. [Google Scholar] [CrossRef]
- Meadow, J.F.; Altrichter, A.E.; Kembel, S.W.; Moriyama, M.; O’Connor, T.K.; Womack, A.M.; Brown, G.Z.; Green, J.L.; Bohannan, B.J.M. Bacterial Communities on Classroom Surfaces Vary with Human Contact. Microbiome 2014, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Meadow, J.F.; Altrichter, A.E.; Kembel, S.W.; Kline, J.; Mhuireach, G.; Moriyama, M.; Northcutt, D.; O’Connor, T.K.; Womack, A.M.; Brown, G.Z.; et al. Indoor Airborne Bacterial Communities Are Influenced by Ventilation, Occupancy, and Outdoor Air Source. Indoor Air 2014, 24, 41–48. [Google Scholar] [CrossRef]
- Kembel, S.W.; Jones, E.; Kline, J.; Northcutt, D.; Stenson, J.; Womack, A.M.; Bohannan, B.J.M.; Brown, G.Z.; Green, J.L. Architectural Design Influences the Diversity and Structure of the Built Environment Microbiome. ISME J. 2012, 6, 1469–1479. [Google Scholar] [CrossRef]
- Täubel, M.; Castagnoli, E.; Salthammer, T.; Morawska, L.; Salonen, H. The Impact of Cleaning on the Microbiomes of Indoor Surfaces. Indoor Environ. 2024, 1, 100021. [Google Scholar] [CrossRef]
- Esty, B.; Phipatanakul, W. School Exposure and Asthma. Ann. Allergy Asthma Immunol. 2018, 120, 482–487. [Google Scholar] [CrossRef]
- Ketema, R.M.; Araki, A.; Ait Bamai, Y.; Saito, T.; Kishi, R. Lifestyle Behaviors and Home and School Environment in Association with Sick Building Syndrome among Elementary School Children: A Cross-Sectional Study. Environ. Health Prev. Med. 2020, 25, 28. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Norbäck, D.; Yuan, Q.; Li, Y.; Zhu, X.; Hashim, J.H.; Hashim, Z.; Ali, F.; Zheng, Y.-W.; Lai, X.-X.; et al. Indoor Microbiome, Environmental Characteristics and Asthma among Junior High School Students in Johor Bahru, Malaysia. Environ. Int. 2020, 138, 105664. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Blázquez, T.; Cardelli, R.; De Angelis, E.; Puglisi, G.; Escandón, R.; Suárez, R. Air Change Rates and Infection Risk in School Environments: Monitoring Naturally Ventilated Classrooms in a Northern Italian Urban Context. Heliyon 2023, 9, e19120. [Google Scholar] [CrossRef] [PubMed]
- Csobod, E.; Annesi-Maesano, I.; Carrer, P.; Kephalopoulos, S.; Madureira, J.; Rudnai, P.; De Oliveira Fernandes, E.; Barrero, J.; Beregszászi, T.; Hyvarinen, A.; et al. SINPHONIE—Schools Indoor Pollution & Health Observatory Network in Europe—Final. Report; EUR 26738; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Chiesa, G.; Vigliotti, M. Comparing Mechanical Ventilation Control Strategies for Indoor Air Quality: Monitoring and Simulation Results of a School Building in Northern Italy. Energy Build. 2024, 322, 114665. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A Review of Indoor Microbial Growth across Building Materials and Sampling and Analysis Methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Adams, R.I.; Leppänen, H.; Karvonen, A.M.; Jacobs, J.; Borràs-Santos, A.; Valkonen, M.; Krop, E.; Haverinen-Shaughnessy, U.; Huttunen, K.; Zock, J.; et al. Microbial Exposures in Moisture-damaged Schools and Associations with Respiratory Symptoms in Students: A Multi-country Environmental Exposure Study. Indoor Air 2021, 31, 1952–1966. [Google Scholar] [CrossRef] [PubMed]
- Gunn, C.; Vahdati, M.; Shahrestani, M. Green Walls in Schools—The Potential Well-Being Benefits. Build. Environ. 2022, 224, 109560. [Google Scholar] [CrossRef]
- Mahnert, A.; Moissl-Eichinger, C.; Berg, G. Microbiome Interplay: Plants Alter Microbial Abundance and Diversity within the Built Environment. Front. Microbiol. 2015, 6, 887. [Google Scholar] [CrossRef]
- Chiesi, L.; Costa, P.; Ciaravella, F.; Galmarini, B. Re-Naturalizing the Built Environment. Plants, Architecture, and Pedagogy in Contemporary Green Schools. Front. Sustain. Cities 2024, 6, 1397159. [Google Scholar] [CrossRef]
- Pichlhöfer, A.; Sesto, E.; Hollands, J.; Korjenic, A. Health-Related Benefits of Different Indoor Plant Species in a School Setting. Sustainability 2021, 13, 9566. [Google Scholar] [CrossRef]
- Shaughnessy, R.; Hernandez, M.; Haverinen-Shaughnessy, U. Effects of Classroom Cleaning on Student Health: A Longitudinal Study. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Lindenmayer, J.M.; Schoenfeld, S.; O’Grady, R.; Carney, J.K. Methicillin-Resistant Staphylococcus Aureus in a High School Wrestling Team and the Surrounding Community. Arch. Intern. Med. 1998, 158, 895. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, Q.; Jaradat, Z.; Khanfar, M.; Alnohoud, R.; Alzu’bi, M.; Makahleh, S.; Abulaila, S. Methicillin-Resistant Staphylococcus Aureus Contamination of High-Touched Surfaces in a University Campus. J. Appl. Microbiol. 2022, 132, 4486–4500. [Google Scholar] [CrossRef]
- van Tonder, A.J.; McCullagh, F.; McKeand, H.; Thaw, S.; Bellis, K.; Raisen, C.; Lay, L.; Aggarwal, D.; Holmes, M.; Parkhill, J.; et al. Colonization and Transmission of Staphylococcus Aureus in Schools: A Citizen Science Project. Microb. Genom. 2023, 9, 000993. [Google Scholar] [CrossRef] [PubMed]
- Vandini, A.; Temmerman, R.; Frabetti, A.; Caselli, E.; Antonioli, P.; Balboni, P.G.; Platano, D.; Branchini, A.; Mazzacane, S. Hard Surface Biocontrol in Hospitals Using Microbial-Based Cleaning Products. PLoS ONE 2014, 9, e108598. [Google Scholar] [CrossRef]
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental Impacts of the Widespread Use of Chlorine-Based Disinfectants during the COVID-19 Pandemic. Environ. Sci. Pollut. Res. 2022, 29, 85742–85760. [Google Scholar] [CrossRef]
- Al-Ghalith, G.A.; Knights, D. Bygiene: The New Paradigm of Bidirectional Hygiene. Yale J. Biol. Med. 2015, 88, 359–365. [Google Scholar]
- Al-Marzooq, F.; Al Bayat, S.; Sayyar, F.; Ishaq, H.; Nasralla, H.; Koutaich, R.; Kawas, S. Al Can Probiotic Cleaning Solutions Replace Chemical Disinfectants in Dental Clinics? Eur. J. Dent. 2018, 12, 532–539. [Google Scholar] [CrossRef]
- Klassert, T.E.; Zubiria-Barrera, C.; Neubert, R.; Stock, M.; Schneegans, A.; López, M.; Driesch, D.; Zakonsky, G.; Gastmeier, P.; Slevogt, H.; et al. Comparative Analysis of Surface Sanitization Protocols on the Bacterial Community Structures in the Hospital Environment. Clin. Microbiol. Infect. 2022, 28, 1105–1112. [Google Scholar] [CrossRef]
- Afinogenova, A.G.; Kraeva, L.A.; Afinogenov, G.E.; Veretennikov, V.V. Probiotic-Based Sanitation as Alternatives to Chemical Disinfectants. Russ. J. Infect. Immun. 2018, 7, 419–424. [Google Scholar] [CrossRef]
- Kleintjes, W.G.; Prag, R.; Ebrahim, M.; Kotzee, E.P. The Effect of Probiotics for Environmental Cleaning on Hospital-Acquired Infection in a Burn Centre: The Results of a Non-Randomised Controlled Prospective Study. S. Afr. J. Plast. Reconstr. Aesthetic Surg. Burn. 2020, 3, 33. [Google Scholar] [CrossRef]
- Caselli, E.; Brusaferro, S.; Coccagna, M.; Arnoldo, L.; Berloco, F.; Antonioli, P.; Tarricone, R.; Pelissero, G.; Nola, S.; La Fauci, V.; et al. Reducing Healthcare-Associated Infections Incidence by a Probiotic-Based Sanitation System: A Multicentre, Prospective, Intervention Study. PLoS ONE 2018, 13, e0199616. [Google Scholar] [CrossRef]
- D’Accolti, M.; Soffritti, I.; Bini, F.; Mazziga, E.; Cason, C.; Comar, M.; Volta, A.; Bisi, M.; Fumagalli, D.; Mazzacane, S.; et al. Shaping the Subway Microbiome through Probiotic-Based Sanitation during the COVID-19 Emergency: A Pre–Post Case–Control Study. Microbiome 2023, 11, 64. [Google Scholar] [CrossRef]
- Caselli, E.; Arnoldo, L.; Rognoni, C.; D’Accolti, M.; Soffritti, I.; Lanzoni, L.; Bisi, M.; Volta, A.; Tarricone, R.; Brusaferro, S.; et al. Impact of a Probiotic-Based Hospital Sanitation on Antimicrobial Resistance and HAI-Associated Antimicrobial Consumption and Costs: A Multicenter Study. Infect. Drug Resist. 2019, 12, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; D’Accolti, M.; Soffritti, I.; Lanzoni, L.; Bisi, M.; Volta, A.; Berloco, F.; Mazzacane, S. An Innovative Strategy for the Effective Reduction of MDR Pathogens from the Nosocomial Environment. In Advances in Microbiology, Infectious Diseases and Public Health; Advance in Experimental Medicine and Biology; Springer, Cham, Switzerland, 2019; Volume 1249, pp. 79–91. [CrossRef]
- Caselli, E.; D’Accolti, M.; Soffritti, I.; Piffanelli, M.; Mazzacane, S. Spread of Mcr-1-Driven Colistin Resistance on Hospital Surfaces, Italy. Emerg. Infect. Dis. 2018, 24, 1752–1753. [Google Scholar] [CrossRef]
- Caselli, E.; D’Accolti, M.; Vandini, A.; Lanzoni, L.; Camerada, M.T.; Coccagna, M.; Branchini, A.; Antonioli, P.; Balboni, P.G.; Di Luca, D.; et al. Impact of a Probiotic-Based Cleaning Intervention on the Microbiota Ecosystem of the Hospital Surfaces: Focus on the Resistome Remodulation. PLoS ONE 2016, 11, e0148857. [Google Scholar] [CrossRef]
- Cason, C.; D’Accolti, M.; Campisciano, G.; Soffritti, I.; Ponis, G.; Mazzacane, S.; Maggiore, A.; Risso, F.M.; Comar, M.; Caselli, E. Microbial Contamination in Hospital Environment Has the Potential to Colonize Preterm Newborns’ Nasal Cavities. Pathogens 2021, 10, 615. [Google Scholar] [CrossRef]
- Cason, C.; D’Accolti, M.; Soffritti, I.; Mazzacane, S.; Comar, M.; Caselli, E. Next-Generation Sequencing and PCR Technologies in Monitoring the Hospital Microbiome and Its Drug Resistance. Front. Microbiol. 2022, 13, 969863. [Google Scholar] [CrossRef]
- D’Accolti, M.; Soffritti, I.; Bini, F.; Mazziga, E.; Mazzacane, S.; Caselli, E. Pathogen Control in the Built Environment: A Probiotic-Based System as a Remedy for the Spread of Antibiotic Resistance. Microorganisms 2022, 10, 225. [Google Scholar] [CrossRef]
- D’Accolti, M.; Soffritti, I.; Mazzacane, S.; Caselli, E. Fighting AMR in the Healthcare Environment: Microbiome-Based Sanitation Approaches and Monitoring Tools. Int. J. Mol. Sci. 2019, 20, 1535. [Google Scholar] [CrossRef]
- Hollingshead, S.; Vapnek, D. Nucleotide Sequence Analysis of a Gene Encoding a Streptomycin/Spectinomycin Adenyltransferase. Plasmid 1985, 13, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Aris, P.; Boroumand, M.A.; Douraghi, M. Amikacin Resistance Due to the AphA6 Gene in Multi-Antibiotic Resistant Acinetobacter Baumannii Isolates Belonging to Global Clone 1 from Iran. BMC Microbiol. 2019, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zeng, Z.; Chen, S.; Ma, J.; He, L.; Liu, Y.; Deng, Y.; Lei, T.; Zhao, J.; Liu, J.-H. High Prevalence of BlaCTX-M Extended-Spectrum β-Lactamase Genes in Escherichia Coli Isolates from Pets and Emergence of CTX-M-64 in China. Clin. Microbiol. Infect. 2010, 16, 1475–1481. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeong, S.H. Nomenclature of GES-Type Extended-Spectrum β-Lactamases. Antimicrob. Agents Chemother. 2005, 49, 2148–2150. [Google Scholar] [CrossRef]
- Walther-Rasmussen, J.; Høiby, N. Class A Carbapenemases. J. Antimicrob. Chemother. 2007, 60, 470–482. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Inoue, M. Characterization of SFO-1, a Plasmid-Mediated Inducible Class A β-Lactamase from Enterobacter cloacae. Antimicrob. Agents Chemother. 1999, 43, 307–313. [Google Scholar] [CrossRef]
- Aly, M.M.; Abu Alsoud, N.M.; Elrobh, M.S.; Al Johani, S.M.; Balkhy, H.H. High Prevalence of the PER-1 Gene among Carbapenem-Resistant Acinetobacter Baumannii in Riyadh, Saudi Arabia. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Toleman, M.A.; Rolston, K.; Jones, R.N.; Walsh, T.R. VIM-7, an Evolutionarily Distinct Metallo-β-Lactamase Gene in a Pseudomonas Aeruginosa Isolate from the United States. Antimicrob. Agents Chemother. 2004, 48, 329–332. [Google Scholar] [CrossRef]
- Brízio, A.; Conceição, T.; Pimentel, M.; Da Silva, G.; Duarte, A. High-Level Expression of IMP-5 Carbapenemase Owing to Point Mutation in the -35 Promoter Region of Class 1 Integron among Pseudomonas Aeruginosa Clinical Isolates. Int. J. Antimicrob. Agents 2006, 27, 27–31. [Google Scholar] [CrossRef]
- Guan, L.; Beig, M.; Wang, L.; Navidifar, T.; Moradi, S.; Motallebi Tabaei, F.; Teymouri, Z.; Abedi Moghadam, M.; Sedighi, M. Global Status of Antimicrobial Resistance in Clinical Enterococcus Faecalis Isolates: Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 80. [Google Scholar] [CrossRef]
- Gonzalez Leiza, M.; Perez-Diaz, J.C.; Ayala, J.; Casellas, J.M.; Martinez-Beltran, J.; Bush, K.; Baquero, F. Gene Sequence and Biochemical Characterization of FOX-1 from Klebsiella Pneumoniae, a New AmpC-Type Plasmid-Mediated Beta-Lactamase with Two Molecular Variants. Antimicrob. Agents Chemother. 1994, 38, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, L.S.; Tzelepi, E.; Mentis, A.F. Nucleotide Sequence of a Plasmid-Mediated Cephalosporinase Gene (BlaLAT-1) Found in Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 1994, 38, 2207–2209. [Google Scholar] [CrossRef]
- Papanicolaou, G.A.; Medeiros, A.A.; Jacoby, G.A. Novel Plasmid-Mediated Beta-Lactamase (MIR-1) Conferring Resistance to Oxyimino- and Alpha-Methoxy Beta-Lactams in Clinical Isolates of Klebsiella Pneumoniae. Antimicrob. Agents Chemother. 1990, 34, 2200–2209. [Google Scholar] [CrossRef]
- Oguri, T.; Furuyama, T.; Okuno, T.; Ishii, Y.; Tateda, K.; Bonomo, R.A.; Shimizu-Ibuka, A. Crystal Structure of Mox-1, a Unique Plasmid-Mediated Class C β-Lactamase with Hydrolytic Activity towards Moxalactam. Antimicrob. Agents Chemother. 2014, 58, 3914–3920. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Maurya, A.; Talukdar, A.; Dhar (Chanda), D.; Chakravarty, A. Genetic Environment of OXA-2 Beta-Lactamase Producing Gram-Negative Bacilli from a Tertiary Referral Hospital. Indian J. Med. Res. 2015, 141, 368. [Google Scholar] [CrossRef]
- Smith, C.A.; Antunes, N.T.; Stewart, N.K.; Toth, M.; Kumarasiri, M.; Chang, M.; Mobashery, S.; Vakulenko, S.B. Structural Basis for Carbapenemase Activity of the OXA-23 β-Lactamase from Acinetobacter Baumannii. Chem. Biol. 2013, 20, 1107–1115. [Google Scholar] [CrossRef]
- Héritier, C.; Poirel, L.; Nordmann, P. Genetic and Biochemical Characterization of a Chromosome-Encoded Carbapenem-Hydrolyzing Ambler Class D β-Lactamase from Shewanella Algae. Antimicrob. Agents Chemother. 2004, 48, 1670–1675. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Baghchesaraei, H.; Peymani, A. Plasmid-Mediated Quinolone-Resistance (Qnr) Genes in Clinical Isolates of Escherichia Coli Collected from Several Hospitals of Qazvin and Zanjan Provinces, Iran. Osong Public Health Res. Perspect. 2016, 7, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Malhotra-Kumar, S.; Mazzariol, A.; Van Heirstraeten, L.; Lammens, C.; de Rijk, P.; Cornaglia, G.; Goossens, H. Unusual Resistance Patterns in Macrolide-Resistant Streptococcus Pyogenes Harbouring Erm(A). J. Antimicrob. Chemother. 2009, 63, 42–46. [Google Scholar] [CrossRef]
- Min, Y.-H.; Kwon, A.-R.; Yoon, E.-J.; Shim, M.-J.; Choi, E.-C. Translational Attenuation and MRNA Stabilization as Mechanisms of Erm (B) Induction by Erythromycin. Antimicrob. Agents Chemother. 2008, 52, 1782–1789. [Google Scholar] [CrossRef]
- Shivakumar, A.G.; Dubnau, D. Characterization of a Plasmid-Specified Ribosome Methylase Associated with Macrolide Resistance. Nucleic Acids Res. 1981, 9, 2549–2562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daly, M.M.; Doktor, S.; Flamm, R.; Shortridge, D. Characterization and Prevalence of MefA, MefE, and the Associated Msr (D) Gene in Streptococcus Pneumoniae Clinical Isolates. J. Clin. Microbiol. 2004, 42, 3570–3574. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux-Mediated Antimicrobial Resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Warburton, P.J.; Ciric, L.; Lerner, A.; Seville, L.A.; Roberts, A.P.; Mullany, P.; Allan, E. TetAB(46), a Predicted Heterodimeric ABC Transporter Conferring Tetracycline Resistance in Streptococcus Australis Isolated from the Oral Cavity. J. Antimicrob. Chemother. 2013, 68, 17–22. [Google Scholar] [CrossRef]
- Utsui, Y.; Yokota, T. Role of an Altered Penicillin-Binding Protein in Methicillin- and Cephem-Resistant Staphylococcus Aureus. Antimicrob. Agents Chemother. 1985, 28, 397–403. [Google Scholar] [CrossRef]
- Stabile, L.; Dell’Isola, M.; Russi, A.; Massimo, A.; Buonanno, G. The Effect of Natural Ventilation Strategy on Indoor Air Quality in Schools. Sci. Total Environ. 2017, 595, 894–902. [Google Scholar] [CrossRef]
- Bringslimark, T.; Hartig, T.; Patil, G.G. Psychological Benefits of Indoor Plants in Workplaces: Putting Experimental Results into Context. HortScience 2007, 42, 581–587. [Google Scholar] [CrossRef]
- Pegas, P.N.; Alves, C.A.; Nunes, T.; Bate-Epey, E.F.; Evtyugina, M.; Pio, C.A. Could Houseplants Improve Indoor Air Quality in Schools? J. Toxicol. Environ. Health A 2012, 75, 1371–1380. [Google Scholar] [CrossRef]
- Nabi, G.; Wang, Y.; Hao, Y.; Khan, S.; Wu, Y.; Li, D. Massive Use of Disinfectants against COVID-19 Poses Potential Risks to Urban Wildlife. Environ. Res. 2020, 188, 109916. [Google Scholar] [CrossRef]
- Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.A.; Ahmed, N.; et al. The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics 2021, 11, 35. [Google Scholar] [CrossRef]
- Kampf, G. Biocidal Agents Used for Disinfection Can Enhance Antibiotic Resistance in Gram-Negative Species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef]
- D’Accolti, M.; Soffritti, I.; Bini, F.; Mazziga, E.; Arnoldo, L.; Volta, A.; Bisi, M.; Antonioli, P.; Laurenti, P.; Ricciardi, W.; et al. Potential Use of a Combined Bacteriophage–Probiotic Sanitation System to Control Microbial Contamination and AMR in Healthcare Settings: A Pre-Post Intervention Study. Int. J. Mol. Sci. 2023, 24, 6535. [Google Scholar] [CrossRef]
- Park, J.-H.; Lemons, A.R.; Roseman, J.; Green, B.J.; Cox-Ganser, J.M. Bacterial Community Assemblages in Classroom Floor Dust of 50 Public Schools in a Large City: Characterization Using 16S RRNA Sequences and Associations with Environmental Factors. Microbiome 2021, 9, 15. [Google Scholar] [CrossRef]
- Yang, J.I.L.; Lee, B.G.; Park, J.-H.; Yeo, M.-K. Airborne Fungal and Bacterial Microbiome in Classrooms of Elementary Schools during the COVID-19 Pandemic Period: Effects of School Disinfection and Other Environmental Factors. Indoor Air 2022, 32, e13107. [Google Scholar] [CrossRef]
- Mousavi, B.; Hedayati, M.T.; Hedayati, N.; Ilkit, M.; Syedmousavi, S. Aspergillus Species in Indoor Environments and Their Possible Occupational and Public Health Hazards. Curr. Med. Mycol. 2016, 2, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Salonen, H.; Duchaine, C.; Mazaheri, M.; Clifford, S.; Lappalainen, S.; Reijula, K.; Morawska, L. Airborne Viable Fungi in School Environments in Different Climatic Regions—A Review. Atmos. Environ. 2015, 104, 186–194. [Google Scholar] [CrossRef]
- Sadrizadeh, S.; Yao, R.; Yuan, F.; Awbi, H.; Bahnfleth, W.; Bi, Y.; Cao, G.; Croitoru, C.; de Dear, R.; Haghighat, F.; et al. Indoor Air Quality and Health in Schools: A Critical Review for Developing the Roadmap for the Future School Environment. J. Build. Eng. 2022, 57, 104908. [Google Scholar] [CrossRef]
- Song, Z.; Chen, L.; Sun, S.; Yang, G.; Yu, G. Unveiling the Airborne Microbial Menace: Novel Insights into Pathogenic Bacteria and Fungi in Bioaerosols from Nursery Schools to Universities. Sci. Total Environ. 2024, 929, 172694. [Google Scholar] [CrossRef]
- Prussin, A.J.; Marr, L.C. Sources of Airborne Microorganisms in the Built Environment. Microbiome 2015, 3, 78. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Stephens, B. Microbiology of the Built Environment. Nat. Rev. Microbiol. 2018, 16, 661–670. [Google Scholar] [CrossRef]
- Votintseva, A.A.; Fung, R.; Miller, R.R.; Knox, K.; Godwin, H.; Wyllie, D.H.; Bowden, R.; Crook, D.W.; Walker, A.S. Prevalence of Staphylococcus Aureus Protein A (Spa) Mutants in the Community and Hospitals in Oxfordshire. BMC Microbiol. 2014, 14, 63. [Google Scholar] [CrossRef] [PubMed]
- Stanforth, B.; Krause, A.; Starkey, C.; Ryan, T.J. Prevalence of Community-Associated Methicillin-Resistant Staphylococcus Aureus in High School Wrestling Environments. J. Environ. Health 2010, 72, 12–16. [Google Scholar]
- Małecka-Adamowicz, M.; Koim-Puchowska, B.; Dembowska, E.A. Diversity of Bioaerosols in Selected Rooms of Two Schools and Antibiotic Resistance of Isolated Staphylococcal Strains (Bydgoszcz, Poland): A Case Study. Atmosphere 2020, 11, 1105. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, J.; Zhang, T.; He, S.; Li, Y.; Zhang, W.; Ye, X.; Yao, Z. Environmental Contamination Prevalence, Antimicrobial Resistance and Molecular Characteristics of Methicillin-Resistant Staphylococcus Aureus and Staphylococcus Epidermidis Isolated from Secondary Schools in Guangzhou, China. Int. J. Environ. Res. Public Health 2020, 17, 623. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, J.M.; Kwak, K.; Dowling, P.; VanOsdol, T.; Barnes, C. Health Effects of Indoor Fungi. Ann. Allergy Asthma Immunol. 2005, 94, 313–320. [Google Scholar] [CrossRef]
- Mahnert, A.; Haratani, M.; Schmuck, M.; Berg, G. Enriching Beneficial Microbial Diversity of Indoor Plants and Their Surrounding Built Environment with Biostimulants. Front. Microbiol. 2018, 9, 2985. [Google Scholar] [CrossRef]
- Niza, I.L.; Bueno, A.M.; Gameiro da Silva, M.; Broday, E.E. Air Quality and Ventilation: Exploring Solutions for Healthy and Sustainable Urban Environments in Times of Climate Change. Results Eng. 2024, 24, 103157. [Google Scholar] [CrossRef]
- Ballerini, V.; Coccagna, M.; Volta, A.; Droghetti, L.; Rossi di Schio, E.; Valdiserri, P.; Mazzacane, S. The Role of Mechanical Ventilation on Indoor Air Quality in Schools: An Experimental Comprehensive Analysis. Buildings 2025, 15, 869. [Google Scholar] [CrossRef]
- BS EN 16798-1:2019; Energy Performance of Buildings—Ventilation for Buildings—Part 1: Indoor Environmental Input Parameters for Design and Assessment of Energy Performance of Buildings Addressing Indoor Air Quality, Thermal Environment, Lighting and Acoustics. British Standards Institution: London, UK, 2019.
- Caselli, E. Hygiene: Microbial Strategies to Reduce Pathogens and Drug Resistance in Clinical Settings. Microb. Biotechnol. 2017, 10, 1079–1083. [Google Scholar] [CrossRef]
- Urdaci, M.C.; Pinchuk, I. Antimicrobial Activity of Bacillus Probiotics. In Bacterial Spore Formers—Probiotics and Emerging Applications; 2004; pp. 1–12. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20043171459 (accessed on 25 February 2025).
- Zhang, M.; Tang, H.; Chen, Y.; Chen, Z.; Xu, Y.; Fu, X.; Sun, Y.; Zhao, Z. Impact of Environmental Characteristics on Children’s Gut Microbiota—A Pilot Study in Assessing the Role of Indoor Microbiome and Metabolites. Environ. Res. 2023, 234, 116114. [Google Scholar] [CrossRef]
- Lee, B.G.; Yang, J.I.; Kim, E.; Geum, S.W.; Park, J.; Yeo, M. Investigation of Bacterial and Fungal Communities in Indoor and Outdoor Air of Elementary School Classrooms by 16S RRNA Gene and ITS Region Sequencing. Indoor Air 2021, 31, 1553–1562. [Google Scholar] [CrossRef]
- Park, J.-H.; Lemons, A.R.; Croston, T.L.; Roseman, J.; Green, B.J.; Cox-Ganser, J.M. More Diverse School Microbiota May Provide Better Protection against Respiratory Infections for School Staff. Build. Environ. 2025, 271, 112657. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Shama, A.; Norbäck, D.; Chen, Q.; Xia, Y.; Zhang, X.; Sun, Y. Exploring the Role of Indoor Microbiome and Environmental Characteristics in Rhinitis Symptoms among University Students. Front. Microbiomes 2024, 3, 1277177. [Google Scholar] [CrossRef]
- Dockx, Y.; Täubel, M.; Bijnens, E.M.; Witters, K.; Valkonen, M.; Jayaprakash, B.; Hogervorst, J.; Nawrot, T.S.; Casas, L. Indoor Green Can Modify the Indoor Dust Microbial Communities. Indoor Air 2022, 32, e13011. [Google Scholar] [CrossRef] [PubMed]
- Dockx, Y.; Täubel, M.; Bijnens, E.M.; Witters, K.; Valkonen, M.; Jayaprakash, B.; Hogervorst, J.; Nawrot, T.S.; Casas, L. Residential Green Space Can Shape the Indoor Microbial Environment. Environ. Res. 2021, 201, 111543. [Google Scholar] [CrossRef]
- Soininen, L.; Roslund, M.I.; Nurminen, N.; Puhakka, R.; Laitinen, O.H.; Hyöty, H.; Sinkkonen, A.; Cerrone, D.; Grönroos, M.; Hui, N.; et al. Indoor Green Wall Affects Health-Associated Commensal Skin Microbiota and Enhances Immune Regulation: A Randomized Trial among Urban Office Workers. Sci. Rep. 2022, 12, 6518. [Google Scholar] [CrossRef]
- Danielski, I.; Svensson, Å.; Weimer, K.; Lorentzen, L.; Warne, M. Effects of Green Plants on the Indoor Environment and Wellbeing in Classrooms—A Case Study in a Swedish School. Sustainability 2022, 14, 3777. [Google Scholar] [CrossRef]
- Daisey, J.M.; Angell, W.J.; Apte, M.G. Indoor Air Quality, Ventilation and Health Symptoms in Schools: An Analysis of Existing Information. Indoor Air 2003, 13, 53–64. [Google Scholar] [CrossRef]
- Langiano, E.; Ferrara, M.; Falese, L.; Lanni, L.; Diotaiuti, P.; Di Libero, T.; De Vito, E. Assessment of Indoor Air Quality in School Facilities: An Educational Experience of Pathways for Transversal Skills and Orientation (PCTO). Sustainability 2024, 16, 6612. [Google Scholar] [CrossRef]


| Features | Measures |
|---|---|
| Length | 8.47 m |
| Width | 5.75 m |
| Ceiling height | 3.23 m |
| Maximum height | 5.15 m |
| Floor area | 48.7 m2 |
| Volume | 170 m3 |
| ARGs | Antibiotic/Gene Function | Reference |
|---|---|---|
| aadA1 | Aminoglycoside | Hollingshead et al., 1985 [44] |
| aphA6 | Aminoglycoside | Aris et al., 2019 [45] |
| CTX-M-9 Group | Class A beta-lactamase | Sun et al., 2010 [46] |
| GES | Class A beta-lactamase | Lee et al., 2005 [47] |
| IMI & NMC-A | Class A beta-lactamase | Walther-Rasmussen et al., 2007 [48] |
| SFO-1 | Class A beta-lactamase | Matsumoto et al., 1999 [49] |
| SHV (238G240K) | Class A beta-lactamase | Caselli et al., 2016 [39] |
| Per-1 group | Class A beta-lactamase | Aly et al., 2016 [50] |
| VIM-7 | Class B beta-lactamase | Toleman et al., 2004 [51] |
| IMP-5 group | Class B beta-lactamase | Brízio et al., 2006 [52] |
| ACT 5/7 group | Class C beta-lactamase | Guan et al., 2024 [53] |
| FOX | Class C beta-lactamase | Gonzalez Leiza et al., 1994 [54] |
| LAT | Class C beta-lactamase | Tzouvelekis et al., 1994 [55] |
| MIR | Class C beta-lactamase | Papanicolaou et al., 1990 [56] |
| MOX | Class C beta-lactamase | Oguri et al., 2014 [57] |
| OXA-2 Group | Class D beta-lactamase | Bhattacharjee et al., 2015 [58] |
| OXA-23 Group | Class D beta-lactamase | Smith et al., 2013 [59] |
| OXA-55 | Class D beta-lactamase | Héritier et al., 2004 [60] |
| QnrB-8 group | Fluoroquinolone | Rezazadeh et al., 2016 [61] |
| ermA | Macrolide lincosamide streptogramin_b | Malhotra-Kumar et al., 2009 [62] |
| ermB | Macrolide lincosamide streptogramin_b | Min et al., 2008 [63] |
| ermC | Macrolide lincosamide streptogramin_b | Shivakumar et al., 1981 [64] |
| mefA | Macrolide lincosamide streptogramin_b | Daly et al., 2004 [65] |
| msrA | Macrolide lincosamide streptogramin_b | Poole et al., 2005 [66] |
| tetB | Tetracycline efflux pump | Warburton et al., 2013 [67] |
| mecA | Methicillin | Utsui et al., 1985 [68] |
| Setting | Study Aim | Analysis Methods | Primary Outcomes | References |
|---|---|---|---|---|
| Elementary schools (Korea) | Microbial monitoring | NGS (16S rRNA/ITS) |
| Lee et al., 2021 [96] |
| Elementary schools (USA) | Impact of indoor microbiome on resp. infections | NGS (16S rRNA/ITS) |
| Park et al., 2025 [97] |
| Dormitory rooms (China) | Impact of indoor microbiome on rhinitis | NGS (16S rRNA) |
| Fu et al., 2024 [98] |
| 155 Households (Belgium) | Impact of plants on indoor microbiome | NGS (16S rRNA/ITS); qPCR |
| Dockx et al., 2022 [99] |
| 176 Living rooms (Belgium) | Impact of outdoor green space on indoor microbiome | NGS (16S rRNA/ITS); qPCR |
| Dockx et al., 2021 [100] |
| Offices (Finland) | Impact of green walls on skin microbiota and immunity | NGS (16S rRNA) |
| Soininen et al., 2022 [101] |
| Secondary school (Sweden) | Impact of indoor plants on environment | Measurement of indoor physical parameters (sensors) |
| Danielski et al., 2022 [102] |
| Several schools | Review of indoor air quality and health | Correlation of ventilation, CO2, and CFU counts |
| Daisey et al., 2003 [103] |
| High school (Italy) | Environmental and microbial monitoring | Measurement of CO2 and CFU counts in indoor air |
| Langiano et al., 2024 [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Accolti, M.; Soffritti, I.; Mazziga, E.; Bini, F.; Bisi, M.; Volta, A.; Mazzacane, S.; Caselli, E. A Sustainable Combined Approach to Control the Microbial Bioburden in the School Environment. Microorganisms 2025, 13, 791. https://doi.org/10.3390/microorganisms13040791
D’Accolti M, Soffritti I, Mazziga E, Bini F, Bisi M, Volta A, Mazzacane S, Caselli E. A Sustainable Combined Approach to Control the Microbial Bioburden in the School Environment. Microorganisms. 2025; 13(4):791. https://doi.org/10.3390/microorganisms13040791
Chicago/Turabian StyleD’Accolti, Maria, Irene Soffritti, Eleonora Mazziga, Francesca Bini, Matteo Bisi, Antonella Volta, Sante Mazzacane, and Elisabetta Caselli. 2025. "A Sustainable Combined Approach to Control the Microbial Bioburden in the School Environment" Microorganisms 13, no. 4: 791. https://doi.org/10.3390/microorganisms13040791
APA StyleD’Accolti, M., Soffritti, I., Mazziga, E., Bini, F., Bisi, M., Volta, A., Mazzacane, S., & Caselli, E. (2025). A Sustainable Combined Approach to Control the Microbial Bioburden in the School Environment. Microorganisms, 13(4), 791. https://doi.org/10.3390/microorganisms13040791








