Abstract
We conducted a retrospective analysis of Citrobacter spp. surveillance data from acute care hospitals that contributed Citrobacter spp. data to the national surveillance system ANRESIS from January 2010 to December 2022. The incidence of Citrobacter spp. bloodstream infections (BSIs) in Switzerland was calculated, as well as the proportion of Citrobacter spp. isolates from urinary tract samples. We also evaluated the susceptibility of Citrobacter spp. isolates to clinically important antibiotics. From 2010 to 2022, there were 33,958 Citrobacter spp. from patients across 55 acute care hospitals continuously participating in ANRESIS included in this analysis. We observed an annual increase in the number of Citrobacter spp. BSIs, from 2.5 to 4.2 cases per 100,000 patient days (IRR: 1.04, 95% CI: 0.96–1.12). We found a higher incidence among male versus female patients (IRR: 2.47, 95% CI: 1.28–4.74) and in those aged ≥65 years, as compared with younger patients (IRR: 2.26, 95% CI: 1.18–4.32). The proportion of Citrobacter spp. among positive urinary tract samples also increased (from 18.6 to 24.7 per 1000 samples). Among ICU patients, there was a considerable proportion of resistance to third-generation cephalosporins among C. freundii isolates (26.8–44.0%), compared with non-freundii isolates (1.7–6.9%). Citrobacter spp. is gaining clinical importance in Switzerland; further studies are needed to better understand the underlying mechanisms.
1. Introduction
Citrobacter spp., mainly C. freundii, are increasingly recognized as primarily healthcare-associated pathogens causing urinary, abdominal, and respiratory tract infections [1,2]. A recent systematic review of 41 studies found that approximately 85% of Citrobacter spp. infections were hospital-acquired [2]. This review highlighted an upward trend in the incidence of Citrobacter infections since 2010, underscoring the growing challenge these pathogens present within healthcare settings [2].
Further, over the past five years, surveillance studies conducted across several European countries, including Germany, Spain, and Finland, have documented an increase in Citrobacter isolates with clinically relevant resistance genes, including extended-spectrum β-lactamases (ESBLs), Klebsiella pneumoniae carbapenemase (KPC), metallo-β-lactamases (e.g., VIM), and OXA-48 carbapenemases [1,3,4]. Among Citrobacter species, C. freundii may present particular therapeutic challenges through resistance to multiple antibiotics [5].
There is currently limited information available on the epidemiological burden, temporal trends, and resistance patterns of Citrobacter spp. The objective of this study was to describe the clinical and epidemiological features of Citrobacter spp. among hospitalized patients in Switzerland using national surveillance data generated by the Swiss National Centre for Antibiotic Resistance (ANRESIS), which collects routine antibiotic resistance data from microbiology laboratories located across Switzerland [6].
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
We conducted a retrospective analysis of Citrobacter spp. reported to ANRESIS from 1 January 2010 to 31 December 2022. During this period, acute care hospitals contributing data to ANRESIS represented an average of 72% of annual patient days across Switzerland. As the number of acute care hospitals participating in ANRESIS increased during the study period, we restricted the present analysis to data from acute care hospitals that reported at least one Citrobacter spp. to ANRESIS each year during the entire 13-year study period. We included only the first Citrobacter spp. isolate per patient per year reported to ANRESIS.
2.2. Data Sources
Participating hospitals are requested to report to ANRESIS all Citrobacter isolates. Patient-level data reported to ANRESIS included demographic information, specimen collection year and type (blood culture, urinary tract, respiratory tract, gastrointestinal tract, or other), location of patient consultation (inpatient versus outpatient), and type of unit of hospitalization (intensive care unit (ICU) versus non-ICU). The following institutional-level information was also collected: linguistic region of Switzerland (German-speaking versus Latin-speaking, which combined French and Italian-speaking cantons) and type of hospital (university vs. non-university). For each Citrobacter spp. isolate phenotypic antimicrobial susceptibility data were also included.
For antimicrobial susceptibility testing, isolates were considered susceptible (susceptible or susceptible, increased exposure), resistant, or unknown when the antimicrobial susceptibility testing had not been performed. In each of the selected laboratories, antimicrobial susceptibility testing was performed according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [7,8]. Most laboratories switched from CLSI to EUCAST breakpoints between 2011 and 2013. We assessed Citrobacter spp. susceptibility to clinically important antibiotics, including cefepime, third-generation cephalosporins, carbapenems, and fluoroquinolones among patients in three settings: (1) ICU, (2) non-ICU inpatient wards, and (3) outpatient departments. The results were stratified by Citrobacter species: C. freundii versus non-freundii. A Citrobacter spp. isolate was considered resistant to an antibiotic group if resistant to at least one antibiotic within a given group (cefepime, third-generation cephalosporins, carbapenems, and fluoroquinolones).
2.3. Statistical Analysis
We described the characteristics of Citrobacter spp. included in the analysis. The categorical variables were reported as frequencies and proportions. Differences in the characteristics of patients by Citrobacter species were compared using Pearson’s chi-squared test. The incidence of Citrobacter spp. from blood cultures in Switzerland and per linguistic region was calculated per 100,000 patient days using annual patient-day data for each acute care hospital included in the analysis from the Federal Office of Public Health (FOPH) [9]. The results were then stratified by Citrobacter species, sex, and age category (<18 years, 18–64 years, ≥65 years). We modeled temporal trends in the incidence of Citrobacter spp. bloodstream isolates using negative binomial regression with a log-link function, accounting for overdispersion, and included patient days as an offset variable. The model included year, linguistic region, sex, and age category (<18 years, 18–64 years, ≥65 years) as covariates.
We calculated the proportion of Citrobacter spp. in urinary tract samples across Switzerland and by linguistic region, expressed per 1000 positive urinary tract samples (all bacterial species) submitted to ANRESIS. This approach accounts for annual variations in the total number of urinary tract samples sent to ANRESIS. As above, the results were stratified by Citrobacter species, sex, and age category (<18 years, 18–64 years, and ≥65 years). For all analyses, p-values ≤ 0.05 were considered statistically significant. All statistical analyses were performed with R statistical software version 3.5.2 (R Foundation, Vienna, Austria).
3. Results
3.1. Characteristics of Citrobacter spp. Species
Between 1 January 2010 and 31 December 2022, 33,958 Citrobacter spp. were reported to ANRESIS from 55 acute care hospitals across Switzerland (Figure 1). Characteristics of Citrobacter spp. are presented in Table 1. Among Citrobacter species, the majority were C. koseri (50.6%), followed by C. freundii species (39.9%).
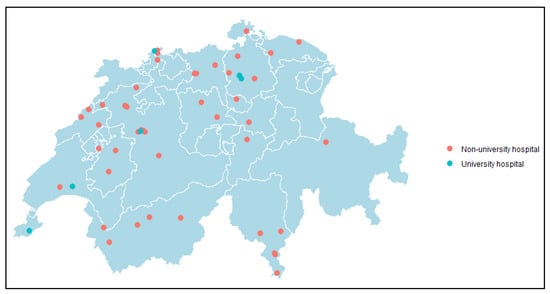
Figure 1.
Map of Switzerland indicating the 55 included acute care hospitals that reported at least one Citrobacter spp. to ANRESIS each year from 1 January 2010 to 31 December 2022. Green circles represent university hospitals; orange circles represent non-university hospitals.

Table 1.
Characteristics of 33,958 Citrobacter spp. from 55 ANRESIS acute-care hospitals, 2010–2022.
3.2. Citrobacter spp. Bloodstream Infections (BSIs)
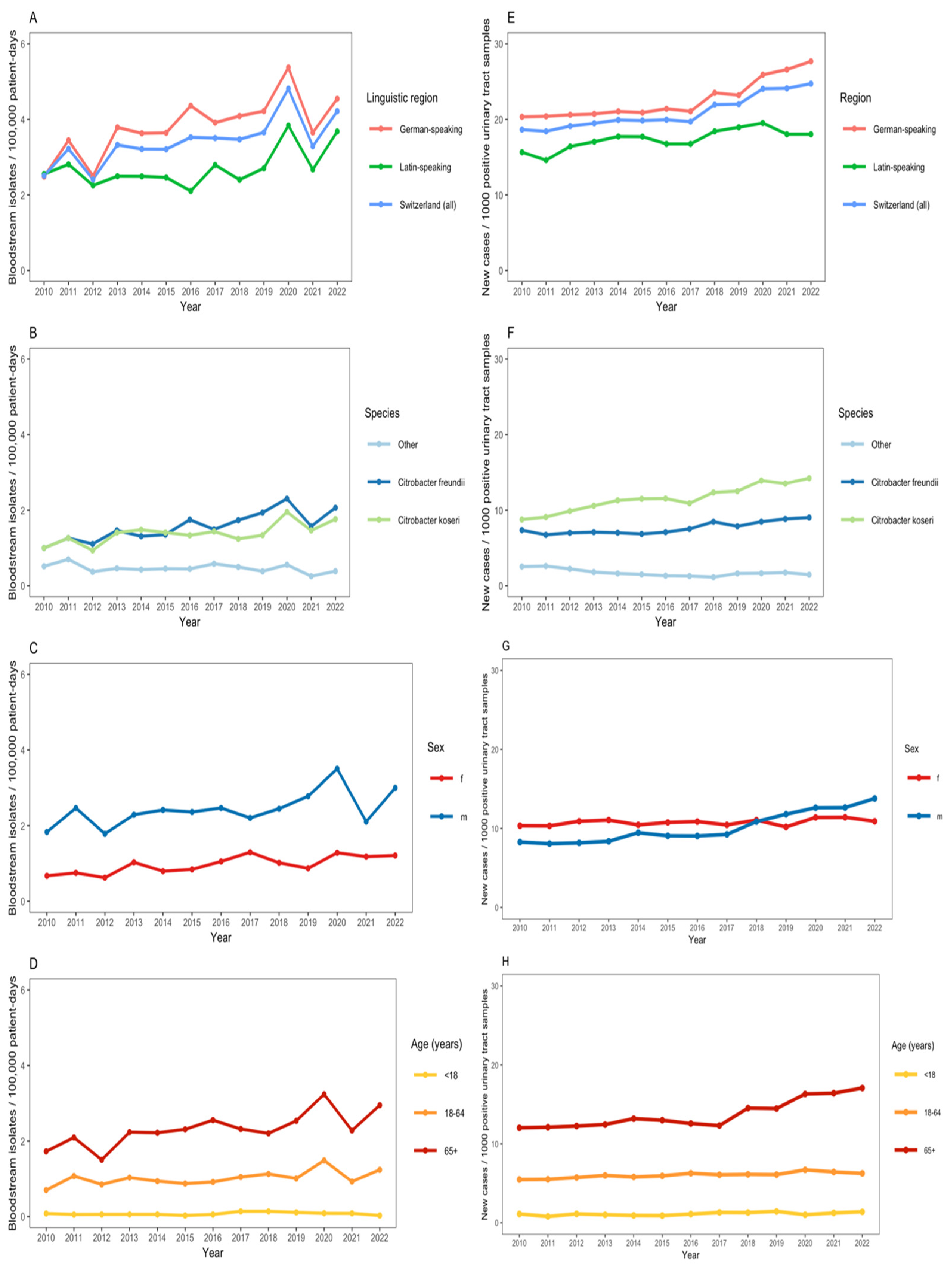
We observed an increase in the annual number of Citrobacter spp. BSIs (from 2.5 cases per 100,000 patient days in 2010 to 4.2 cases in 2022) with a notable increase during the first pandemic COVID-19 waves in 2020 (Figure 2A). C. koseri and C. freundii were the predominant Citrobacter species among bloodstream isolates (Figure 2B). The incidence of Citrobacter spp. BSI was greater in males, as compared with females, and in patients aged ≥65 years (Figure 2C,D). The adjusted negative binomial regression model indicated a non-significant annual increase in the incidence of Citrobacter spp. BSIs of 3.7% (IRR: 1.04, 95% CI: 0.96–1.12). In the same adjusted model, the incidence rate of Citrobacter spp. BSIs in the German-speaking region was 2.41 (95% CI: 1.26–4.61) as compared with the Latin-speaking region; males had more than twice the incidence rate of Citrobacter spp. BSIs compared with females (IRR: 2.47, 95% CI: 1.28–4.74); individuals aged ≥65 years had a significantly higher incidence rate of BSIs compared with the 18–64 age group (IRR: 2.26, 95% CI: 1.18–4.32).
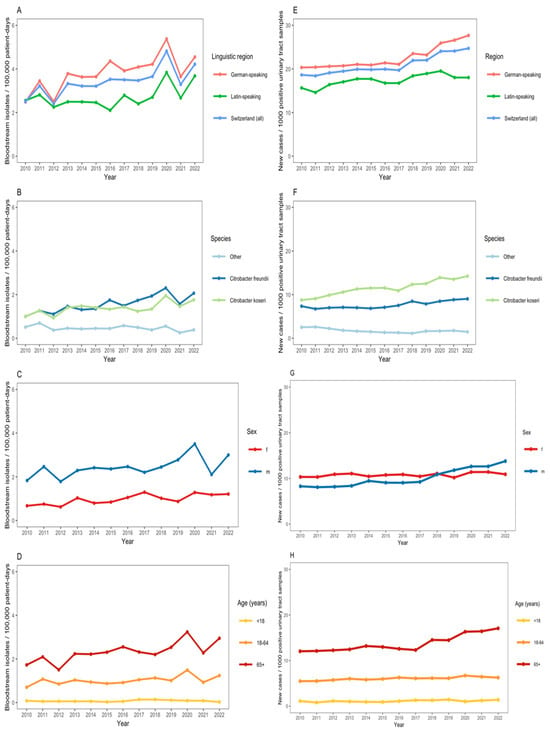
Figure 2.
(A–D): Incidence of Citrobacter bacteremia per 100,000 patient-days across 55 ANRESIS acute care hospitals in Switzerland, 2010–2022, N = 1590, stratified by linguistic region (A), Citrobacter species (B), sex (C), and age group (D). (E–H): Proportion of Citrobacter spp. from urinary tract samples per 1000 positive urinary tract samples sent to ANRESIS across 55 acute care hospitals in Switzerland, 2010–2022, N = 19,699, stratified by linguistic region (E), Citrobacter species (F), sex (G) and age group (H).
3.3. Citrobacter spp. from Urinary Tract Samples
There was also an increase in the annual proportion of Citrobacter spp. per 1000 positive urinary tract samples submitted to ANRESIS (from 18.6 per 1000 positive urinary tract samples in 2010 to 24.7 in 2022) (Figure 2E). The annual proportion of Citrobacter spp. among positive urinary tract samples was greater in the German-speaking region of Switzerland than in the Latin-speaking region (Figure 2E). C. koseri and C. freundii were the predominant Citrobacter species among positive urinary tract samples (Figure 2F). From 2017 onwards, there was a notable increase in the proportion of Citrobacter spp. among male patients and in those ≥65 years (Figure 2G,H).
3.4. Citrobacter spp. Antibiotic Susceptibility
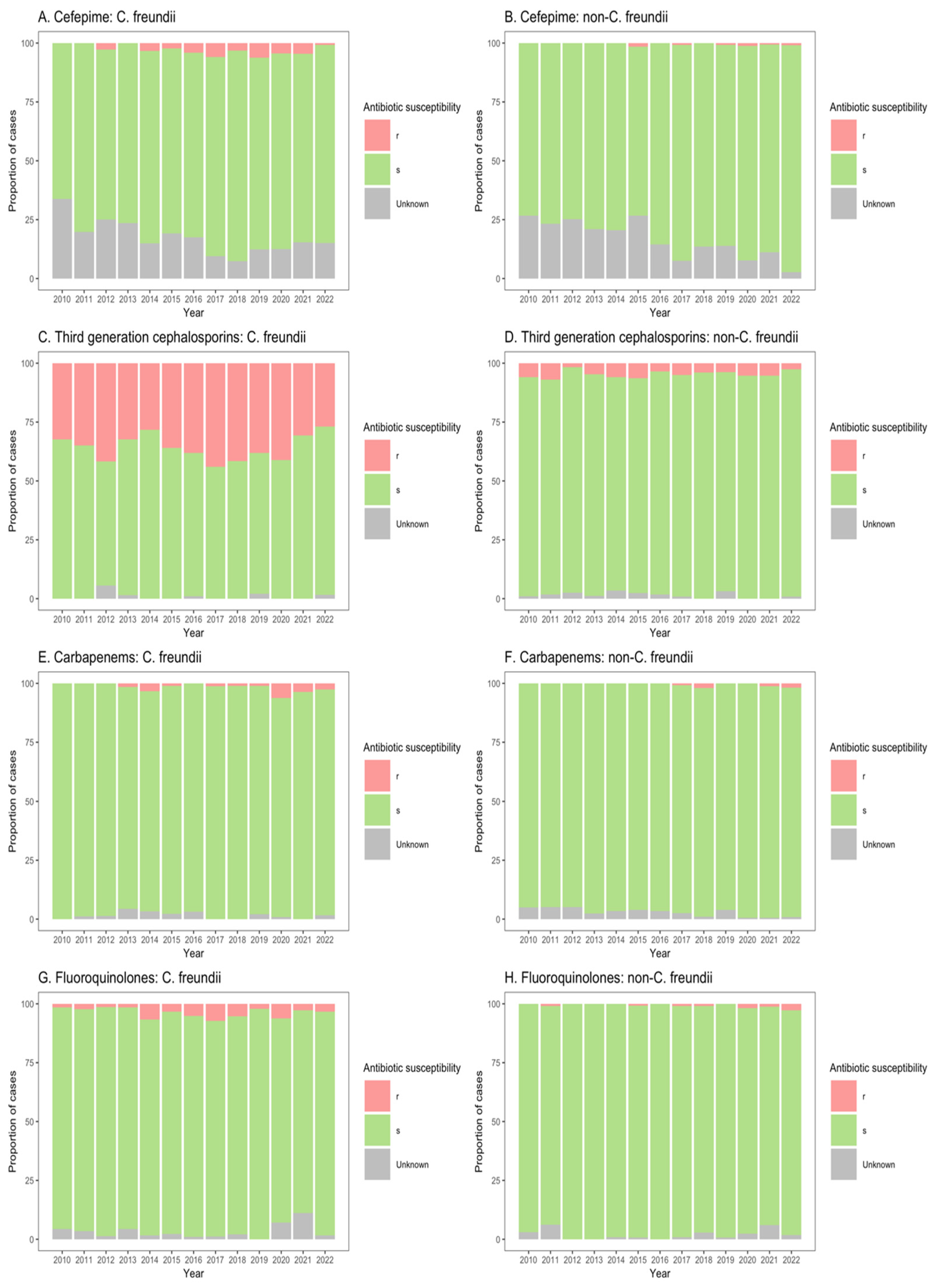
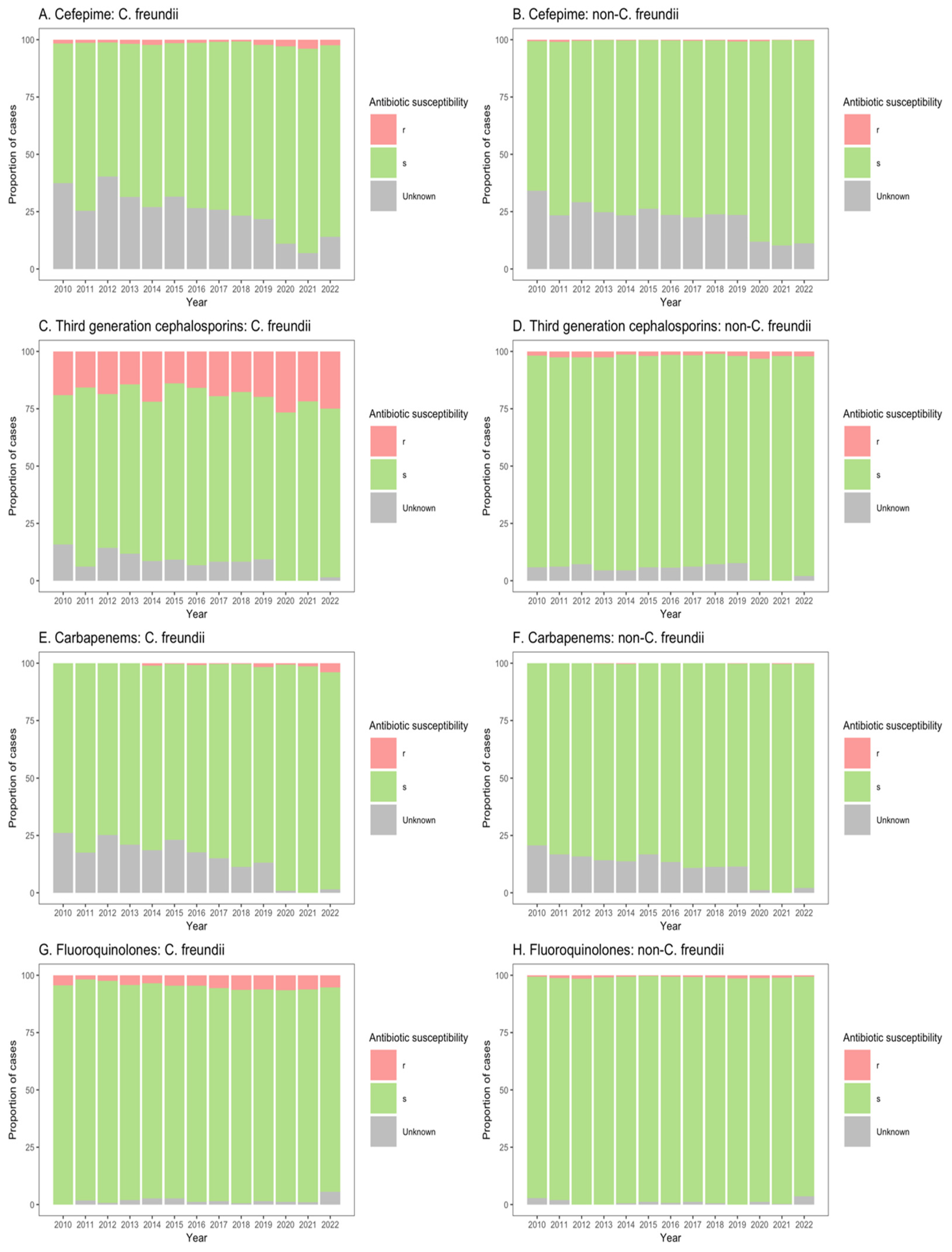
The susceptibilities of Citrobacter spp. isolates to clinically important antibiotics among patients from ICU, non-ICU inpatient wards, and outpatient departments are shown in Figure 3, Figure 4 and Figure 5. Among ICU patients, there was a considerable proportion of resistance to third-generation cephalosporins among C. freundii isolates (26.8–44.0%, Figure 3C), as compared with non-freundii isolates (1.7–6.9%, Figure 3D). C. freundii isolates also exhibited higher resistance rates to cefepime, carbapenems, and fluoroquinolones compared with non-freundii isolates among ICU patients (Figure 3).
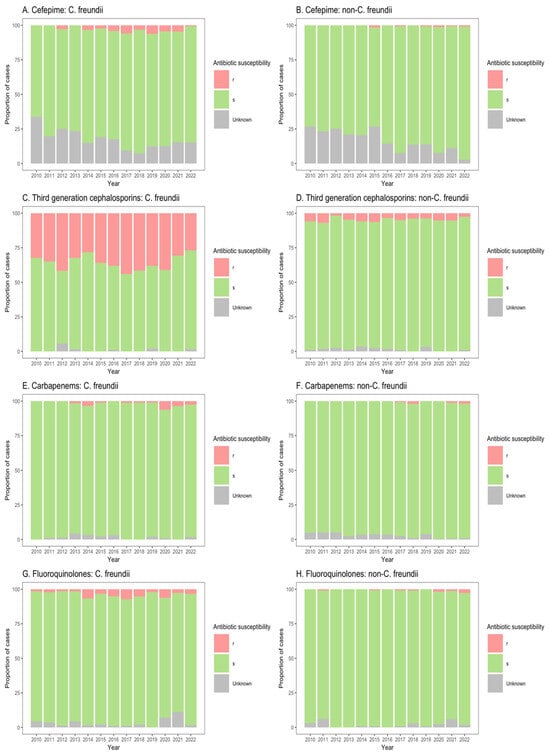
Figure 3.
Susceptibility of Citrobacter freundii and non-freundii isolates to cefepime (A,B), third-generation cephalosporins (C,D), carbapenems (E,F), and fluoroquinolones (G,H) among patients admitted to intensive care units from 55 ANRESIS acute care hospitals in Switzerland, 2010–2022. Isolates were considered as ‘s’ (susceptible or susceptible, increased exposure), r’ (resistant), or ‘Unknown’.
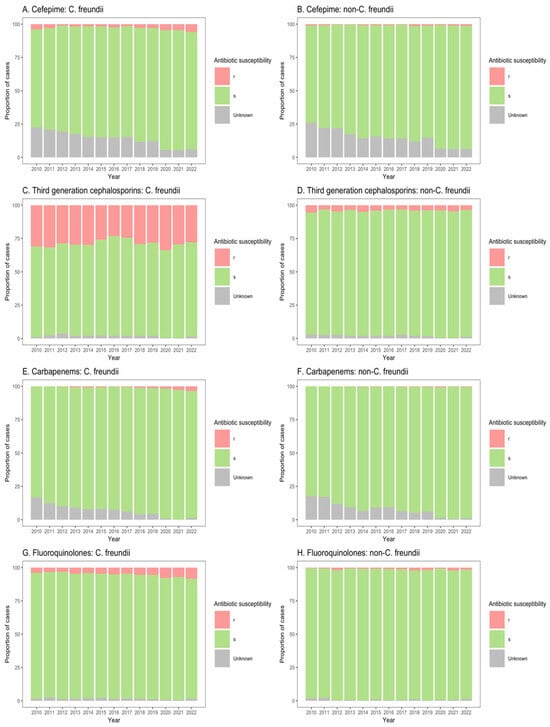
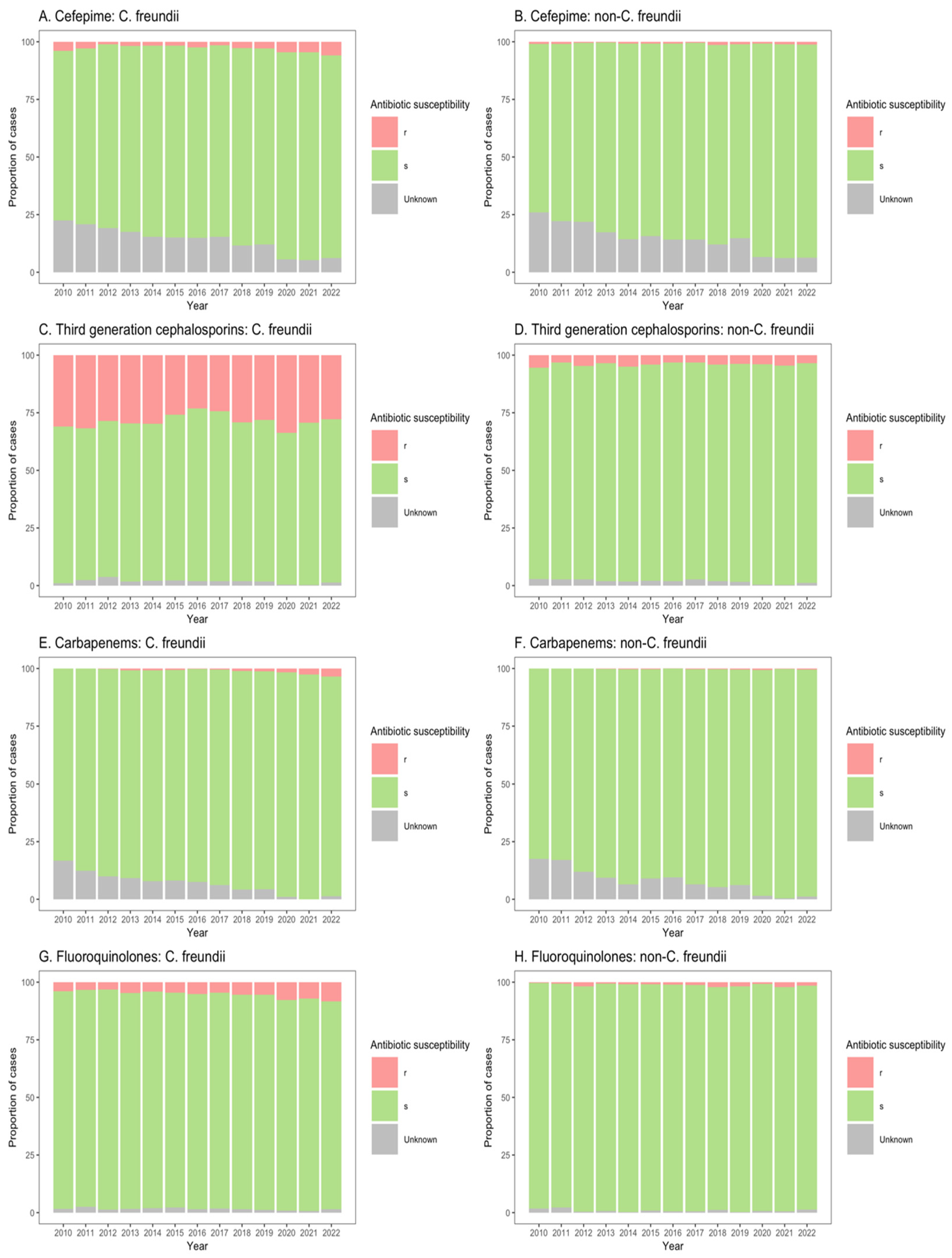
Figure 4.
Susceptibility of Citrobacter freundii and non-freundii isolates to cefepime (A,B), third-generation cephalosporins (C,D), carbapenems (E,F), and fluoroquinolones (G,H) among patients in non-ICU inpatient wards across 55 ANRESIS acute care hospitals in Switzerland, 2010–2022. Isolates were considered as ‘s’ (susceptible or susceptible, increased exposure), r’ (resistant), or ‘Unknown’.
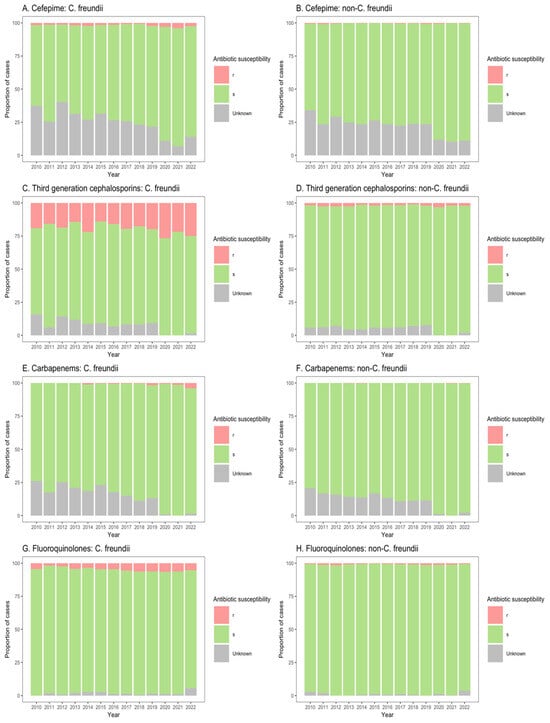
Figure 5.
Susceptibility of Citrobacter freundii and non-freundii isolates to cefepime (A,B), third-generation cephalosporins (C,D), carbapenems (E,F), and fluoroquinolones (G,H) among outpatient departments across 55 ANRESIS acute care hospitals in Switzerland, 2010–2022. Isolates were considered as ‘s’ (susceptible or susceptible, increased exposure), r’ (resistant), or ‘Unknown’.
Similarly, for patients in both non-ICU inpatient wards and outpatient departments, there was a considerable proportion of resistance to third-generation cephalosporins among C. freundii isolates (23.2–33.8% of isolates from non-ICU inpatients (Figure 4C); 13.9–26.6% of isolates from outpatients (Figure 5C)), as compared with non-freundii isolates (3.2–5.4% of isolates from non-ICU inpatients (Figure 4D); 1.1–3.1% of isolates from outpatient departments (Figure 5D)). C. freundii isolates also demonstrated greater proportions of resistance to cefepime and fluoroquinolones, as compared with non-freundii isolates, among patients in non-ICU inpatient wards and outpatient departments (Figure 4 and Figure 5). The proportion of isolates demonstrating carbapenem resistance remained low across all settings: 1.0–6.3% for C. freundii and 0.8–1.9% for non-freundii isolates from ICU patients (Figure 3E,F); 0.1–3.5% for C. freundii and 0.1–0.7% for non-freundii isolates from non-ICU inpatients (Figure 4E,F); 0.3–3.9% for C. freundii and 0.1–0.4% for non-freundii isolates from outpatients (Figure 5E,F).
4. Discussion
We used surveillance data from 55 Swiss acute care hospitals participating in ANRESIS to describe the clinical and epidemiological features of Citrobacter spp. over a period of 13 years. Our findings indicate an increase in the annual incidence of Citrobacter spp. BSIs. We found a higher incidence among male versus female patients, in those aged ≥65 years, as compared with younger patients, and those living in the German-speaking region of Switzerland, as compared with the Latin-speaking region. We also demonstrated an increase in the proportion of Citrobacter spp. among positive urinary tract samples, particularly from 2017 onwards among male patients and in those ≥65 years. These findings support our assertion that, although Citrobacter spp. have long been considered to be pathogens of low virulence, they are, in fact, emerging pathogens of clinical importance in Switzerland.
There are few studies on the burden and trends of Citrobacter spp. for comparative analysis. For example, Citrobacter spp. are not included in the European Antimicrobial Resistance Surveillance Network (EARS-Net) or WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS) reporting. Nevertheless, a 10-year period surveillance study conducted in Hungary from 2008 to 2017 revealed an increase in urinary tract infections (UTIs) caused by Citrobacter spp. among inpatients and outpatients [10]. The study found that the most affected age groups were those under 10 years and over 60 years of age [10]. In the Czech Republic, from 2011 to 2019, Hrbacek et al. also reported an increase in the prevalence of Citrobacter spp. from positive urine samples [11]. A recent systematic review documented an increasing number of Citrobacter infections or colonization since 2010, particularly in Asian countries [2]. Further, several European surveillance studies have reported increasing rates of Citrobacter spp. isolates carrying extended-spectrum beta-lacatamase and carbapenemase genes [1,3,4]. We found a considerable proportion of C. freundii isolates demonstrating resistance to third-generation cephalosporins among patients in ICU, most likely based on AmpC β-lactamases [12,13]. In contrast, the proportion of isolates with carbapenem resistance remained very low in our study.
The main strength of this study is that it is based on a large national database of Citrobacter spp. from 55 acute care hospitals, which account for 72% of annual patient days across Switzerland. This study, therefore, offers an important contribution to otherwise sparse epidemiological data on Citrobacter spp. burden, temporal trends, and resistance patterns. Further, we restricted our analyses to account for an increase in the number of acute care hospitals contributing data to ANRESIS throughout the study period. Our analyses were also stratified by linguistic regions of Switzerland (German- versus Latin-speaking), reflecting the country’s linguistic and cultural heterogeneity. Our results from urinary tract samples highlight the need for further studies to explain the increase in the proportion of Citrobacter spp. among urinary tract samples, particularly among male patients and in those ≥65 years from 2017 onwards.
Nevertheless, there are a number of limitations. The ANRESIS database does not include clinical information or genotypic data. We were also unable to distinguish infection from colonization for non-invasive specimens, thereby affecting our assessment of the Citrobacter spp. infection burden beyond BSIs. Finally, we were not able to distinguish community-acquired from healthcare-associated infections, limiting our understanding of the role of nosocomial acquisition in the observed trends. However, the increase in Citrobacter spp. BSIs and the proportion of carbapenem resistance among ICU patients observed during the early COVID-19 pandemic could be an indirect indicator of related infection control challenges.
In conclusion, our study offers valuable insights into the epidemiology of Citrobacter spp. in Switzerland. The findings indicate that Citrobacter spp. are emerging as clinically important pathogens. Further epidemiological and molecular studies are needed to elucidate the mechanisms underlying these trends.
Author Contributions
Conceptualization, P.F., R.G., M.G., A.K. and S.H.; methodology, P.F., R.G., M.G., A.K. and S.H.; software, P.F. and R.G.; validation, P.F., R.G. and S.H.; formal analysis, P.F., R.G. and S.H.; investigation, P.F., R.G., M.G., A.K. and S.H.; data curation, P.F., R.G., M.G., and A.K.; writing—original draft preparation, P.F., R.G., M.G., A.K. and S.H.; writing—review and editing, P.F., R.G., M.G., N.B., A.K. and S.H.; supervision, R.G., A.K. and S.H.; project administration, A.K. and S.H. All authors have read and agreed to the published version of the manuscript.
Funding
P.F. acknowledges support from the Federal Commission for Scholarships for Foreign Students for the Swiss Government Excellence Scholarship (ESKAS No. 2022.0465) for the academic years 2022–2025. ANRESIS is funded by the Federal Office of Public Health and the University of Bern, Switzerland.
Institutional Review Board Statement
As the analysis was performed on de-identified public health surveillance data, neither ethical review nor individual informed consent was required according to the Swiss law for research on humans (Article 33, Paragraph 2, Human Research Act).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are provided within the manuscript. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank all laboratories participating in the ANRESIS network: ADMED Microbiology, La Chaux-de-Fonds; Bacteriology Laboratory, Geneva University Hospitals, Geneva; Cantonal Hospital Bruderholz; Central Institute, Hôpitaux Valaisans (ICHV), Sitten; Central Laboratory, Cantonal Hospital Graubünden, Chur; Central Laboratory, Microbiology Section, Cantonal Hospital Baden; Centre for Laboratory Medicine, Cantonal Hospital Luzern; Centre for Laboratory Medicine, Cantonal Hospital Schaffhausen; Centre for Laboratory Medicine Risch, Schaan; Centre for Laboratory Medicine St. Gallen; Clinical Microbiology, University Hospital, Basel; Hôpital du Jura, Laboratoire, Delémont; Microbiology Laboratory, Dianalabs, Geneva; Institute for Infectious Diseases, University Bern; Institute for Laboratory Medicine, Cantonal Hospital Aarau; Institute for Medical Microbiology, University Hospital Zürich; Institute for Microbiology, Université de Lausanne; Laboratoire et analyses médicales, Etablissements Hospitaliers du Nord Vaudois, eHnv; Laboratory for Infectious Diseases, University Children’s Hospital Zürich; Labor Bioanalytica AG, Luzern; Labor Langenthal, Oberaargau Hospital, Langenthal; Labor MCL AG, Niederwald; Laboratory Medicine EOLAB, Department of Microbiology, Bellinzona; Labormedizin, Kantonsspital Winterthur; Labor Rothen, Basel; Labor Solothurner Spitäler, Solothurn; Labor Spital, Einsiedeln; Laborteam, Goldach; Labpoint, Avenches; Microbiology Laboratory Hôpital Fribourgeois, Fribourg; Microbiology Laboratory, Hospital Thurgau; Microbiology Laboratory, Unilabs, Coppet; Promed SA, Laboratoire medical, Marly; Viollier AG, Basel.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANRESIS | Swiss National Centre for Antibiotic Resistance |
| BSI | Bloodstream infection |
| C. freundii | Citrobacter freundii |
| CI | Confidence interval |
| C. koseri | Citrobacter koseri |
| ICU | Intensive care unit |
| IRR | Incidence rate ratio |
References
- Arana, D.M.; Ortega, A.; González-Barberá, E.; Lara, N.; Bautista, V.; Gómez-Ruíz, D.; Sáez, D.; Fernández-Romero, S.; Aracil, B.; Pérez-Vázquez, M.; et al. Carbapenem-Resistant Citrobacter spp. Isolated in Spain from 2013 to 2015 Produced a Variety of Carbapenemases Including VIM-1, OXA-48, KPC-2, NDM-1 and VIM-2. J. Antimicrob. Chemother. 2017, 72, 3283–3287. [Google Scholar] [CrossRef] [PubMed]
- Fonton, P.; Hassoun-Kheir, N.; Harbarth, S. Epidemiology of Citrobacter spp. Infections among Hospitalized Patients: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2024, 24, 662. [Google Scholar]
- Yao, Y.; Falgenhauer, L.; Falgenhauer, J.; Hauri, A.M.; Heinmüller, P.; Domann, E.; Chakraborty, T.; Imirzalioglu, C. Carbapenem-Resistant Citrobacter spp. as an Emerging Concern in the Hospital-Setting: Results From a Genome-Based Regional Surveillance Study. Front. Cell. Infect. Microbiol. 2021, 11, 744431. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, K.; Sarvikivi, E.; Arifulla, D.; Pietikäinen, R.; Forsblom-Helander, B.; Tarkka, E.; Anttila, V.-J.; Grönroos, J.O.; Rintala, E.; Kauranen, J.; et al. Three Clusters of Carbapenemase-Producing Citrobacter Freundii in Finland, 2016–2020. J. Antimicrob. Chemother. 2021, 76, 2697–2701. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Sun, Q.; Huang, Z.-X.; Wang, H.-Y.; Zhang, R.; Chen, G. Colistin Resistance Gene Mcr-1 in Gut Flora of Children. Int. J. Antimicrob. Agents 2017, 50, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Swiss Centre for Antibiotic Resistance (ANRESIS). Available online: https://www.anresis.ch/ (accessed on 1 August 2024).
- Eucast. Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 9 February 2025).
- CLSI Standards & Guidelines. Shop for CLSI Standards. Available online: https://clsi.org/standards/ (accessed on 9 February 2025).
- OFSP (Office Fédéral de la Santé Publique). Chiffres-Clés des Hôpitaux Suisses. Available online: https://www.bag.admin.ch/bag/fr/home/zahlen-und-statistiken/zahlen-fakten-zu-spitaelern/kennzahlen-der-schweizer-spitaeler.html (accessed on 2 August 2024).
- Gajdács, M.; Urbán, E. Resistance Trends and Epidemiology of Citrobacter-Enterobacter-Serratia in Urinary Tract Infections of Inpatients and Outpatients (RECESUTI): A 10-Year Survey. Medicina 2019, 55, 285. [Google Scholar] [CrossRef] [PubMed]
- Hrbacek, J.; Cermak, P.; Zachoval, R. Current Antibiotic Resistance Patterns of Rare Uropathogens: Survey from Central European Urology Department 2011–2019. BMC Urol. 2021, 21, 61. [Google Scholar]
- Barlow, M.; Hall, B.G. Origin and Evolution of the AmpC β-Lactamases of Citrobacter Freundii. Antimicrob. Agents Chemother. 2002, 46, 1190. [Google Scholar] [PubMed]
- Tariq, F.N.; Shafiq, M.; Khawar, N.; Habib, G.; Gul, H.; Hayat, A.; Rehman, M.U.; Moussa, I.M.; Mahmoud, E.A.; Elansary, H.O. The Functional Repertoire of AmpR in the AmpC β-Lactamase High Expression and Decreasing β-Lactam and Aminoglycosides Resistance in ESBL Citrobacter Freundii. Heliyon 2023, 9, e19486. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).