Clinical Manifestations of Non-Congenital CMV Infection in Infants and Immunocompetent Children: Review of Cases from the Past Decade
Abstract
1. Introduction
2. Literature Search
2.1. Search for Published Cases
2.2. Selected Papers
- -
- Eight because they reported the results of in vitro research;
- -
- Six because they focused on topics outside our area of interest (CMV effects on T reg activity, CMV-specific T cell immunity, vaccine response and differences in T effector response between immunocompetent children, CMV infection in a child with Down syndrome, radiological findings in pediatric CMV lung infection, and a study of CMV seroprevalence in the Russian adolescent population);
- -
- -
- -
- Therefore, 45 papers were included and are listed in Table 1. The main characteristics of the cases presented are shown.
3. Postnatal CMV Infection
3.1. Preterm Infants
3.2. Term Infants
3.3. Postnatal CMV Infection and Breast Milk
3.4. Postnatal CMV Treatment
4. Primary Infection in Childhood
4.1. Gastrointestinal Disease and Hepatitis
4.2. Respiratory Disease
4.3. Hematological Diseases
4.4. Brain Diseases
4.5. Vascular Damage
4.6. Adrenal Disease
4.7. Other Conditions
4.8. Therapy in Immunocompetent Children
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the Worldwide Seroprevalence of Cytomegalovirus: A Systematic Review and Meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A Systematic Literature Review of the Global Seroprevalence of Cytomegalovirus: Possible Implications for Treatment, Screening, and Vaccine Development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef]
- U.S. Centers for Disease Control and Prevention Clinical Overview of CMV and Congenital CMV. Available online: https://www.cdc.gov/cytomegalovirus/hcp/clinical-overview/index.html (accessed on 10 January 2025).
- Ho, M.; Suwansirikul, S.; Dowling, J.N.; Youngblood, L.A.; Armstrong, J.A. The Transplanted Kidney as a Source of Cytomegalovirus Infection. N. Engl. J. Med. 1975, 293, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in Hematopoietic Stem Cell Transplant Recipients. Hematol. Oncol. Clin. N. Am. 2011, 25, 151–169. [Google Scholar] [CrossRef]
- Razonable, R.R.; Hayden, R.T. Clinical Utility of Viral Load in Management of Cytomegalovirus Infection after Solid Organ Transplantation. Clin. Microbiol. Rev. 2013, 26, 703–727. [Google Scholar] [CrossRef]
- Kekre, N.; Tokessy, M.; Mallick, R.; McDiarmid, S.; Huebsch, L.; Bredeson, C.; Allan, D.; Tay, J.; Tinmouth, A.; Sheppard, D. Is Cytomegalovirus Testing of Blood Products Still Needed for Hematopoietic Stem Cell Transplant Recipients in the Era of Universal Leukoreduction? Biol. Blood Marrow Transplant. 2013, 19, 1719–1724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dioverti, M.V.; Razonable, R.R. Cytomegalovirus. Microbiol. Spectr. 2016, 4, 97–125. [Google Scholar] [CrossRef]
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries with Universal Screening: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2120736. [Google Scholar] [CrossRef]
- Paris, R.; Apter, D.; Boppana, S.; D’Aloia, M.; De Schrevel, N.; Delroisse, J.-M.; Grassano, L.; Guignard, A.; Panackal, A.A.; Roman, F.; et al. Incidence of Cytomegalovirus Primary and Secondary Infection in Adolescent Girls: Results From a Prospective Study. J. Infect. Dis. 2023, 228, 1491–1495. [Google Scholar] [CrossRef]
- Chung, M.L.; Sung, H.; Jung, E.; Lee, B.S.; Kim, K.S.; Kim, E.A.-R. Prevention of Human Milk-Acquired Cytomegalovirus Infection in Very-Low-Birth-Weight Infants. BMC Pediatr. 2023, 23, 244. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, S.; Xu, L.; Xu, X.; Huang, L.; Wang, Y.; Hao, C. Presence of Cytomegalovirus Infection Is Associated with an Unfavorable Outcome in Immunocompetent Infants With Pertussis. Front. Cell. Infect. Microbiol. 2022, 12, 800452. [Google Scholar] [CrossRef]
- Tomşa, N.-A.; Meliţ, L.E.; Bucur, G.; Văsieșiu, A.-M.; Mărginean, C.O. Cytomegalovirus, a “Friend” of SARS-CoV-2: A Case Report. Children 2024, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Y.; Chen, F.; Ruan, L.; Gu, L.; Wang, T.; Dong, H.; Wang, Y.; Hao, C.; Huang, L.; et al. Clinical Characteristics of Pediatric Patients Hospitalized with Community-Acquired Pneumonia and Cytomegalovirus DNA Detected in Bronchoalveolar Lavage Fluid. Front. Pediatr. 2024, 12, 1407174. [Google Scholar] [CrossRef]
- Parisi, S.G.; Basso, M.; Del Vecchio, C.; Andreis, S.; Franchin, E.; Bello, F.D.; Pagni, S.; Biasolo, M.A.; Manganelli, R.; Barzon, L.; et al. Virological Testing of Cerebrospinal Fluid in Children Aged Less than 14 Years with a Suspected Central Nervous System Infection: A Retrospective Study on 304 Consecutive Children from January 2012 to May 2015. Eur. J. Paediatr. Neurol. 2016, 20, 588–596. [Google Scholar] [CrossRef]
- Tezer, H.; Kanik Yüksek, S.; Gülhan, B.; Özkaya Parlakay, A.N.; Tuna Kirsaçlioğlu, C. Cytomegalovirus Hepatitis in 49 Pediatric Patients with Normal Immunity. Turk. J. Med. Sci. 2016, 46, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Çelikel, E.; Tezer, H.; Kanik-Yuksek, S.; Gülhan, B.; Ozkaya-Parlakay, A.; Yaralı, N. Evaluation of 98 Immunocompetent Children with Cytomegalovirus Infection: Importance of Neurodevelopmental Follow-Up. Eur. J. Pediatr. 2015, 174, 1101–1107. [Google Scholar] [CrossRef]
- Reckziegel, M.; Weber-Osel, C.; Egerer, R.; Gruhn, B.; Kubek, F.; Walther, M.; Wilhelm, S.; Zell, R.; Krumbholz, A. Viruses and Atypical Bacteria in the Respiratory Tract of Immunocompromised and Immunocompetent Patients with Airway Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1581–1592. [Google Scholar] [CrossRef]
- Uemura, S.; Mori, T.; Nino, N.; Sakakibara, N.; Takafuji, S.; Myojin, S.; Takami, Y.; Morioka, I.; Nishimura, N.; Kugo, M.; et al. An Infant with Refractory Cytomegalovirus-induced Thrombocytopenia. Clin. Case Rep. 2020, 8, 75–78. [Google Scholar] [CrossRef]
- Bedell, M.; Price, T.; Wang, Q.; Rea, B. Cytomegalovirus (CMV) Appendicitis in an Immunocompetent Child Masked by Prominent Lymphoid Hyperplasia. Fetal Pediatr. Pathol. 2024, 44, 82–84. [Google Scholar] [CrossRef]
- Aboelsoud, K.; Yu, Z.; Doolittle, R. Postnatally Acquired Cytomegalovirus Masquerading as Bronchopulmonary Dysplasia in a Premature Infant in the Neonatal Intensive Care Unit. Am. J. Med. Sci. 2024, 367, S223–S224. [Google Scholar]
- Salemi, T.F.; McLean, V.R.; Jnah, A.J. Congenital and Postnatal Cytomegalovirus: Case Series and State of the Science for Neonatal Providers. Neonatal Netw. 2024, 43, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Correia de Azevedo, A.; Nobre, S.; Fonseca, P. Multisystemic Disease in a Child and Successful Recovery with Antiviral Treatment. Cureus 2024, 16, e53893. [Google Scholar] [CrossRef]
- Eid, M.; Ghanem, A.; Saloum, E. Cytomegalovirus Viremia in an 11-Year-Old Child with Sickle Cell Disease Manifested Only with Fever: A Case Report. Ann. Med. Surg. 2023, 85, 3666–3669. [Google Scholar] [CrossRef]
- Ferrua, C.; Lemoine, A.; Mosca, A.; Lopes, A.-A. Clinical Manifestation of Cytomegalovirus-Associated Protein-Losing Enteropathy in Children. Nutrients 2023, 15, 2844. [Google Scholar] [CrossRef] [PubMed]
- Semwal, P.; Bolia, R.; Rajvanshi, N.; Phulware, R.H.; Bhat, N.K. Gastric Outlet Obstruction Due to Cytomegalovirus Infection in an Immunocompetent Child. Indian J. Gastroenterol. 2023, 42, 577–579. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Hsu, K.-H.; Huang, C.-G.; Chiang, M.-C.; Chu, S.-M.; Chen, C.-L.; Hsu, J.-F.; Chueh, H.-Y. Clinical Characteristics of Infants with Symptomatic Congenital and Postnatal Cytomegalovirus Infection—An 11-Year Multicenter Cohort Study in Taiwan. Children 2023, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Cascardo, C.; Kazyak, K.; Bohr, M.; Amin, M.; Gebara, S.; Freij, B.J. S3355 Postnatal Cytomegalovirus Infection as a Potential Trigger for Very Early Onset Crohn’s Disease in an Immunocompetent Infant. Am. J. Gastroenterol. 2022, 117, e2125–e2126. [Google Scholar] [CrossRef]
- Minihan, L.; Lee Oei, J.; Bajuk, B.; Palasanthiran, P. Postnatal Cytomegalovirus Infection: Is It Important? A 10-Year Retrospective Case-Control Study of Characteristics and Outcomes in Very Preterm and Very Low Birth Weight Infants. Pediatr. Infect. Dis. J. 2022, 41, 579–586. [Google Scholar] [CrossRef]
- Wagoner, M.; Saliba, C.; Melkonian, V.; Miyata, M.; Blewett, C.; Greenspon, J. A Rare Case of Cytomegalovirus-Induced Hepatitis Presenting in a Pediatric Patient as a Hepatic Mass. J. Pediatr. Surg. Case Rep. 2022, 79, 102212. [Google Scholar] [CrossRef]
- 60th Annual Meeting of the European Society for Paediatric Endocrinology (ESPE). Horm. Res. Paediatr. 2022, 95, 1–616. [CrossRef]
- Wan Natrah, W.; Lili, Y.; Maryam, M. Child with Cytomegalovirus Associated Acute Demyelinating Encephalomyelitis: A Case Report. Bangladesh J. Med. Sci. 2022, 21, 749–753. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Aguiar, G.B.; Kamer, C.; Farias, L.; Matsuda, J. Acute Disseminated Encephalomyelitis Related to a Cytomegalovirus Infection in an Immunocompetent Patient. Cureus 2021, 13, 12795. [Google Scholar] [CrossRef]
- Bayrak, N.A.; Polat, E.; Erdogan, F. Cytomegalovirus Colitis in Immunocompetent and Immunocompromised Children: A 2-Center Study. Pediatr. Infect. Dis. J. 2021, 40, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Howard-Jones, A.R.; Cristerna-Tarrasa, G.H.; Khan, R.; Stormon, M.; Arbuckle, S.; Britton, P.N. Severe Postnatal Cytomegalovirus Enterocolitis in Immunocompetent Term Infants Requiring Total Parenteral Nutrition. JPGN Rep. 2021, 2, e110. [Google Scholar] [CrossRef]
- Choudhary, A.; Mehta, A. Cytomegalovirus Infection Presenting as Severe Sepsis, Hepatitis, and Autoimmune Hemolytic Anemia in an Immunocompetent Child in Pediatric Intensive Care Unit. Pediatr. Infect. Dis. J. 2021, 40, e453–e454. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cheng, Y.; Peng, Q.; Chen, K. Clinical Features, Treatment Courses, and Distribution of Cytomegalovirus Genotypes among Thrombocytopenia Patients Aged Younger than 12 Months. Am. J. Perinatol. 2021, 38, 1403–1411. [Google Scholar] [CrossRef]
- Patel, R.M.; Shenvi, N.; Knezevic, A.; Hinkes, M.; Bugg, G.W.; Stowell, S.R.; Roback, J.D.; Easley, K.A.; Josephson, C. Observational Study of Cytomegalovirus from Breast Milk and Necrotising Enterocolitis. Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 259–265. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Ye, Z.; Zheng, C.; Jiang, Z.; Huang, Y. Cytomegalovirus Enteritis with Intractable Diarrhea in Infants from a Tertiary Care Center in China. Scand. J. Gastroenterol. 2020, 55, 55–61. [Google Scholar] [CrossRef]
- Tuna Kirsaclioglu, C.; Hizal, G.; Karakus, E.; Sayli, T.R. An Unusual Presentation of Cytomegalovirus Infection: Generalized Edema. Med. Princ. Pr. 2020, 29, 94–96. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, L. Cytomegalovirus Encephalitis in Immunocompetent Infants: A 15-Year Retrospective Study at a Single Center. Int. J. Infect. Dis. 2019, 82, 106–110. [Google Scholar] [CrossRef]
- ASCIA 2019 Conference Abstracts: Poster 1. Intern. Med. J. 2019, 49, 5–33. [CrossRef]
- Loureiro, B.; Batalha, S.; Rocha, E.; Maia, R.; Kjöllerström, P. Refractory Immune Hemolytic Anemia in an Immunocompetent Infant with Cytomegalovirus Infection. Pediatr. Blood Cancer 2019, 66, e27791. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Kawano, Y.; Kawada, J.; Ito, Y.; Hara, S. A Case of Refractory Cytomegalovirus-Related Thrombocytopenia That Achieved Complete Remission without Antiviral Therapy. J. Infect. Chemother. 2018, 24, 995–997. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.J.; Kim, Y.; Choi, E.M.; Shim, Y.J.; Kim, H.S.; Suh, J.K.; Kim, J.Y.; Lee, K.S.; Park, S.Y.; Lee, J.M.; et al. Clinical Characteristics and Treatment Courses for Cytomegalovirus-Associated Thrombocytopenia in Immunocompetent Children after Neonatal Period. Blood Res. 2018, 53, 110. [Google Scholar] [CrossRef]
- Isleyen, F.; Tekin, M.; Konca, C. Thrombocytopenia in Infants: CMV-Related ITP or CMV-Related Thrombocytopenia? Indian. J. Hematol. Blood Transfus. 2018, 34, 774–775. [Google Scholar] [CrossRef]
- Khalifeh, H.; Mourad, Y.; Chamoun, C. Infantile Cytomegalovirus-Associated Severe Warm Autoimmune Hemolytic Anemia: A Case Report. Children 2017, 4, 94. [Google Scholar] [CrossRef]
- Silwedel, C.; Frieauff, E.; Thomas, W.; Liese, J.G.; Speer, C.P. Secondary Haemophagocytic Lymphohistiocytosis Triggered by Postnatally Acquired Cytomegalovirus Infection in a Late Preterm Infant. Infection 2017, 45, 355–359. [Google Scholar] [CrossRef]
- Al-Eyadhy, A.A.; Hasan, G.; Bassrawi, R.; Al-Jelaify, M.; Temsah, M.-H.; Alhaboob, A.; Al-Sohime, F.; Alabdulhafid, M. Cytomegalovirus Associated Severe Pneumonia, Multi-Organ Failure and Ganciclovir Associated Arrhythmia in Immunocompetent Child. J. Infect. Chemother. 2017, 23, 844–847. [Google Scholar] [CrossRef]
- Min, C.-Y.; Song, J.Y.; Jeong, S.J. Characteristics and Prognosis of Hepatic Cytomegalovirus Infection in Children: 10 Years of Experience at a University Hospital in Korea. Korean J. Pediatr. 2017, 60, 261. [Google Scholar] [CrossRef]
- Tsunoda, T.; Inui, A.; Iwasawa, K.; Oikawa, M.; Sogo, T.; Komatsu, H.; Ito, Y.; Fujisawa, T. Acute Liver Dysfunction Not Resulting from Hepatitis Virus in Immunocompetent Children. Pediatr. Int. 2017, 59, 551–556. [Google Scholar] [CrossRef]
- Álvarez-Hernández, L.; Cuevas-Castillejos, J.E.; Cuevas-Castillejos, H.; Aboitiz-Rivera, C.M.; Blachman-Braun, R. Different Clinical Manifestations in Two Siblings with Cytomegalovirus Infection. Rev. Médica Del. Hosp. Gen. México 2017, 80, 174–177. [Google Scholar] [CrossRef]
- Goelz, R.; Hamprecht, K.; Klingel, K.; Poets, C.F. Intestinal Manifestations of Postnatal and Congenital Cytomegalovirus Infection in Term and Preterm Infants. J. Clin. Virol. 2016, 83, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sue, P.K.; Salazar-Austin, N.M.; McDonald, O.G.; Rishi, A.; Cornish, T.C.; Arav-Boger, R. Cytomegalovirus Enterocolitis in Immunocompetent Young Children: A Report of Two Cases and Review of the Literature. Pediatr. Infect. Dis. J. 2016, 35, 573–576. [Google Scholar] [CrossRef]
- Kao, W.-T.; Lin, W.-C.; Tseng, Y.-H.; Chen, T.-H. Transient Cerebral Arteriopathy in a Child Associated with Cytomegalovirus Infection. Child. Neurol. Open 2015, 2, 2329048X15602025. [Google Scholar] [CrossRef] [PubMed]
- Vigué, M.-G.; Tuaillon, E.; Makinson, A.; Bullen, G.M.; Foulongne, V.; Segondy, M.; Perre, P.V.; Jeziorski, E. Lymphoma-like Syndrome: 4 Case Reports About Atypical Presentation of Primary Cytomegalovirus Infection in Immunocompetent Children. Medicine 2015, 94, e855. [Google Scholar] [CrossRef]
- Raja, J.; De Quesada, G. Heterophile Antibody Positive, Acute Cytomegaloviral Infection in an Immunocompetent Pre-Teen: An Atypical Presentation of an Atypical Infection. J. Infect. Public Health 2015, 8, 101–103. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Lima, M.; Impellizzeri, P.; Romeo, C.; Gitto, E. Colonic Stenosis Post-Necrotizing Enterocolitis in Term Newborn with Acquired Cytomegalovirus Infection. Chirurgia 2015, 110, 175–178. [Google Scholar]
- Cinel, G.; Pekcan, S.; Özçelik, U.; Alp, A.; Yalçın, E.; Doğru Ersöz, D.; Kiper, N. Cytomegalovirus Infection in Immunocompetent Wheezy Infants: The Diagnostic Value of CMV PCR in Bronchoalveolar Lavage Fluid. J. Clin. Pharm. Ther. 2014, 39, 399–403. [Google Scholar] [CrossRef]
- Louazon, T.; Collardeau, S.; Lachaux, A. Colite à cytomégalovirus chez un enfant immunocompétent. Arch. Pédiatrie 2014, 21, 1016–1019. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Buoncompagni, A.; Minoia, F.; Coccia, M.C.; Martini, A.; Picco, P. Cytomegalovirus-Related Necrotising Vasculitis Mimicking Henoch-Schönlein Syndrome. Clin. Exp. Rheumatol. 2014, 32, S73–S75. [Google Scholar]
- Novakova, V.; Hamprecht, K.; Müller, A.M.; Arellano-Galindo, J.; Ehlen, M.; Horneff, G. Severe Postnatal CMV Colitis with an Extensive Colonic Stenosis in a 2-Month-Old Male Immunocompetent Term Infant Infected via Breast Milk. J. Clin. Virol. 2014, 59, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, M.T.; Rotolo, N.; Di Dio, G.; Franzonello, C.; Gennaro, A.; Lionetti, E.; Leonardi, S. Cytomegalovirus Colitis and Cow’s Milk Allergy in an Immunocompetent Infant: Is a Causal or Casual Relationship? Dig. Liver Dis. 2014, 46, e110–e111. [Google Scholar] [CrossRef]
- Taktak, A.; Acar, B.; Gür, G.; Tiryaki, T.; Karakuş, E.; Çaycı, F.Ş.; Uncu, N.; Çakar, N. Cytomegalovirus-Related Hemorrhagic Cystitis in an Immunocompetent Child. Ren. Fail. 2014, 36, 1148–1150. [Google Scholar] [CrossRef]
- Kadambari, S.; Whittaker, E.; Lyall, H. Postnatally Acquired Cytomegalovirus Infection in Extremely Premature Infants: How Best to Manage? Arch. Dis. Child. Fetal Neonatal Ed. 2020, 105, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Martins-Celini, F.P.; Yamamoto, A.Y.; Passos, D.M.; Do Nascimento, S.D.; Lima, E.V.; Di Giovanni, C.M.; Quadrado, E.R.S.; Barta, R.; Aragon, D.C.; Do Prado, S.I.; et al. Incidence, Risk Factors, and Morbidity of Acquired Postnatal Cytomegalovirus Infection Among Preterm Infants Fed Maternal Milk in a Highly Seropositive Population. Clin. Infect. Dis. 2016, 63, 929–936. [Google Scholar] [CrossRef]
- Hamele, M.; Flanagan, R.; Loomis, C.A.; Stevens, T.; Fairchok, M.P. Severe Morbidity and Mortality with Breast Milk Associated Cytomegalovirus Infection. Pediatr. Infect. Dis. J. 2010, 29, 84–86. [Google Scholar] [CrossRef]
- McCracken, G.H. Congenital Cytomegalic Inclusion Disease: A Longitudinal Study of 20 Patients. Am. J. Dis. Child. 1969, 117, 522. [Google Scholar] [CrossRef]
- Ballard, R.A. Acquired Cytomegalovirus Infection in Preterm Infants. Arch. Pediatr. Adolesc. Med. 1979, 133, 482. [Google Scholar] [CrossRef]
- Hamprecht, K.; Maschmann, J.; Vochem, M.; Dietz, K.; Speer, C.P.; Jahn, G. Epidemiology of Transmission of Cytomegalovirus from Mother to Preterm Infant by Breastfeeding. Lancet 2001, 357, 513–518. [Google Scholar] [CrossRef]
- Stark, A.; Cantrell, S.; Greenberg, R.G.; Permar, S.R.; Weimer, K.E.D. Long-Term Outcomes after Postnatal Cytomegalovirus Infection in Low Birthweight Preterm Infants: A Systematic Review. Pediatr. Infect. Dis. J. 2021, 40, 571–581. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Chatzakis, C.; Lilleri, D.; Blazquez-Gamero, D.; Alarcon, A.; Bourgon, N.; Foulon, I.; Fourgeaud, J.; Gonce, A.; Jones, C.E.; et al. Consensus Recommendation for Prenatal, Neonatal and Postnatal Management of Congenital Cytomegalovirus Infection from the European Congenital Infection Initiative (ECCI). Lancet Reg. Health Eur. 2024, 40, 100892, Erratum in Lancet Reg. Health Eur. 2024, 42, 100974. https://doi.org/10.1016/j.lanepe.2024.100974. [Google Scholar] [CrossRef]
- Nijman, J.; van Loon, A.M.; Krediet, T.G.; Verboon-Maciolek, M.A. Maternal and Neonatal Anti-Cytomegalovirus IgG Level and Risk of Postnatal Cytomegalovirus Transmission in Preterm Infants. J. Med. Virol. 2013, 85, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Mussi-Pinhata, M.M.; Pinto, P.C.G.; Yamamoto, A.Y.; Berencsi, K.; de Souza, C.B.S.; Andrea, M.; Duarte, G.; Jorge, S.M. Placental Transfer of Naturally Acquired, Maternal Cytomegalovirus Antibodies in Term and Preterm Neonates. J. Med. Virol. 2003, 69, 232–239. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Tang, J.; Xu, C.; Chen, L.; Xu, B.; Dai, Y.; Hu, Y.; Zhou, Y.-H. Minimal Adverse Outcomes of Postnatal Cytomegalovirus Infection in Term or Moderate and Late Preterm Infants. Front. Pediatr. 2023, 11, 1048282. [Google Scholar] [CrossRef]
- Stagno, S.; Reynolds, D.W.; Pass, R.F.; Alford, C.A. Breast Milk and the Risk of Cytomegalovirus Infection. N. Engl. J. Med. 1980, 302, 1073–1076. [Google Scholar] [CrossRef]
- Pass, R.F.; Anderson, B. Mother-to-Child Transmission of Cytomegalovirus and Prevention of Congenital Infection. J. Pediatr. Infect. Dis. Soc. 2014, 3, S2–S6. [Google Scholar] [CrossRef]
- d’Angelo, P.; Zelini, P.; Zavaglio, F.; Piccini, S.; Cirasola, D.; Arossa, A.; Spinillo, A.; Lilleri, D.; Baldanti, F. Correlates of Postnatal Human Cytomegalovirus Transmission in Term Babies in the First Year. J. Med. Virol. 2023, 95, e29105. [Google Scholar] [CrossRef] [PubMed]
- Hamprecht, K.; Goelz, R. Postnatal Cytomegalovirus Infection Through Human Milk in Preterm Infants. Clin. Perinatol. 2017, 44, 121–130. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Dollard, S.C.; Josephson, C.D.; Schmid, D.S.; Bialek, S.R. Breast Milk–Acquired Cytomegalovirus Infection and Disease in VLBW and Premature Infants. Pediatrics 2013, 131, e1937–e1945. [Google Scholar] [CrossRef]
- Maschmann, J.; Müller, D.; Lazar, K.; Goelz, R.; Hamprecht, K. New Short-Term Heat Inactivation Method of Cytomegalovirus (CMV) in Breast Milk: Impact on CMV Inactivation, CMV Antibodies and Enzyme Activities. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F604–F608. [Google Scholar] [CrossRef]
- Aceti, A.; Cavallarin, L.; Martini, S.; Giribaldi, M.; Vitali, F.; Ambretti, S.; Zambrini, V.; Corvaglia, L. Effect of Alternative Pasteurization Techniques on Human Milk’s Bioactive Proteins. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Resch, B. How to Provide Breast Milk for the Preterm Infant and Avoid Symptomatic Cytomegalovirus Infection with Possible Long-Term Sequelae. Life 2022, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Bardanzellu, F.; Fanos, V.; Reali, A. Human Breast Milk-Acquired Cytomegalovirus Infection: Certainties, Doubts and Perspectives. Curr. Pediatr. Rev. 2019, 15, 30–41. [Google Scholar] [CrossRef]
- Schattner, A. The Wide Spectrum of Presentations of Cytomegalovirus Infection in Immunocompetent Hosts: An Exhaustive Narrative Review. Pathogens 2024, 13, 667. [Google Scholar] [CrossRef]
- Yang, T.; Lian, H.; Liao, J.; Zeng, Y.; Li, J.; Lin, C.; Lin, M. Epidemiological Characteristics and Meteorological Factors of Acute Respiratory Infections (ARIs) in Hospitalized Children in Eastern Guangdong, China. Sci. Rep. 2024, 14, 25518. [Google Scholar] [CrossRef]
- Bussel, J.B.; Kuter, D.J.; George, J.N.; McMillan, R.; Aledort, L.M.; Conklin, G.T.; Lichtin, A.E.; Lyons, R.M.; Nieva, J.; Wasser, J.S.; et al. AMG 531, a Thrombopoiesis-Stimulating Protein, for Chronic ITP. N. Engl. J. Med. 2006, 355, 1672–1681. [Google Scholar] [CrossRef]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of Terminology, Definitions and Outcome Criteria in Immune Thrombocytopenic Purpura of Adults and Children: Report from an International Working Group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Rocca, M.A. Acute Disseminated Encephalomyelitis. In White Matter Diseases; Springer International Publishing: Cham, Switzerland, 2020; pp. 109–125. ISBN 978-3-030-38620-7. [Google Scholar]
- Kennedy, P.G.E. Viral Encephalitis: Causes, Differential Diagnosis, and Management. J. Neurol. Neurosurg. Psychiatry 2004, 75, i10–i15. [Google Scholar] [CrossRef] [PubMed]
- Kahl, M.; Siegel-Axel, D.; Stenglein, S.G.; Jahn; Sinzger, C. Efficient Lytic Infection of Human Arterial Endothelial Cells by Human Cytomegalovirus Strains. J. Virol. 2000, 74, 7628–7635. [Google Scholar] [CrossRef]
- Rodríguez-Pla, A.; Stone, J.H. Vasculitis and Systemic Infections. Curr. Opin. Rheumatol. 2006, 18, 39–47. [Google Scholar] [CrossRef]
- Pulakhandam, U.; Dincsoy, H.P. Cytomegaloviral Adrenalitis and Adrenal Insufficiency in AIDS. Am. J. Clin. Pathol. 1990, 93, 651–656. [Google Scholar] [CrossRef]
- Nassoro, D.D.; Mkhoi, M.L.; Sabi, I.; Meremo, A.J.; Lawala, P.S.; Mwakyula, I.H. Adrenal Insufficiency: A Forgotten Diagnosis in HIV/AIDS Patients in Developing Countries. Int. J. Endocrinol. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Hurt, C.; Tammaro, D. Diagnostic Evaluation of Mononucleosis-Like Illnesses. Am. J. Med. 2007, 120, e1–e911. [Google Scholar] [CrossRef]
- Horwitz, C.A.; Henle, W.; Henle, G. Diagnostic Aspects of the Cytomegalovirus Mononucleosis Syndrome in Previously Healthy Persons. Postgrad. Med. 1979, 66, 153–158. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Red Book: 2021–2024 Report of the Committee on Infectious Diseases. Cytomegalovirus Infection, 32nd ed.; Kimberlin, D.W., Banerjee, R., Barnett, E.D., Lynfield, R., Sawyer, M.H., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- Plosa, E.J.; Esbenshade, J.C.; Fuller, M.P.; Weitkamp, J.-H. Cytomegalovirus Infection. Pediatr. Rev. 2012, 33, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Avila-Agüero, M.L.; Paris, M.M.; Alfaro, W.; Avila-Agüero, C.R.; Faingezicht, I. Ganciclovir Therapy in Cytomegalovirus (CMV) Infection in Immunocompetent Pediatric Patients. Int. J. Infect. Dis. 2003, 7, 278–281. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.A.; Neely, J.; Farhoudi, N. ITP in Children: Pathophysiology and Current Treatment Approaches. J. Pediatr. Hematol. Oncol. 2013, 35, 1–13. [Google Scholar] [CrossRef]
- Shrestha, R.; Rondelli, D.; Sherpa, M.T. Cytomegalovirus: A Possible Cause of Persistent Refractory Immune Thrombocytopenic Purpura. J. Adv. Intern. Med. 2014, 3, 42–45. [Google Scholar] [CrossRef]
- DiMaggio, D.; Anderson, A.; Bussel, J.B. Cytomegalovirus Can Make Immune Thrombocytopenic Purpura Refractory. Br. J. Haematol. 2009, 146, 104–112. [Google Scholar] [CrossRef]
- Levy, A.S.; Bussel, J. Immune Thrombocytopenic Purpura: Investigation of the Role of Cytomegalovirus Infection. Br. J. Haematol. 2004, 126, 622–623. [Google Scholar] [CrossRef]
- Kistangari, G.; McCrae, K.R. Immune Thrombocytopenia. Hematol. Oncol. Clin. N. Am. 2013, 27, 495–520. [Google Scholar] [CrossRef]
- Xiao, Y.; Lin, W.; Liu, Q.; Jin, R.; Fei, H. Direct Infection of Colony Forming Unit-Megakaryocyte by Human Cytomegalovirus Contributes the Pathogenesis of Idiopathic Thrombocytopenic Purpura. J. Huazhong Univ. Sci. Technol. 2006, 26, 555–557. [Google Scholar] [CrossRef]

| Author, Year | Clinical Manifestation (n Cases) | Age (Median Age) | Type of Infection (n Cases) | Antiviral Therapy (n Cases) | Other Therapy (n Cases) |
|---|---|---|---|---|---|
| Bedell M, 2024 [20] | Appendicitis (1) | 7 years old | Primary | No | No |
| Aboelsoud K, 2024 [21] | Pneumonitis, thrombocytopenia (1) | Born at 25 weeks of gestational age | Postnatal | No | Corticosteroids |
| Salemi T, 2024 [22] | Sepsis-like syndrome (1) | 39 days old | Postnatal | Ganciclovir | No |
| Rodrigues A, 2024 [23] | Disseminate infection and multi-organ involvement (1) | 7 years old | Primary | Ganciclovir and valganciclovir | No |
| Eid M, 2023 [24] | Prolonged fever (1) | 11 years old | Primary | Ganciclovir | No |
| Ferrua C, 2023 [25] | Protein-losing enteropathy (21) | 1–133.7 months old (median 29.7 months) | Primary | Antiviral (5) (drug not specified) | Immunoglobulin (2) Corticosteroids (2) |
| Semwal P, 2023 [26] | Gastritis and gastric outlet obstruction (1) | 4 years old | Primary | Ganciclovir and valganciclovir | No |
| Chen YN, 2023 [27] | 251 cases:
| <90th day of life (140 cases) >90th day of life (111 cases) | Postnatal | Ganciclovir (32) | No |
| Cascardo C, 2022 [28] | Colitis (1) | 140 days old | Postnatal | Valganciclovir | Corticosteroids |
| Minihan L, 2022 [29] | Total preterm (48) (<32 weeks and/or <1500 g)
| 47–82 days old | Postnatal | Ganciclovir (6) Ganciclovir and valganciclovir (5) | No |
| Wagoner M, 2022 [30] | Hepatitis and hemophagocytic lymphohistiocytosis (1) | 18 months old | Primary | No | Corticosteroids |
| Fuchs S, 2022 [31] | Adrenal injury (1) | 10 weeks old | Primary | Ganciclovir | Corticosteroids |
| Wan Natrah WY, 2022 [32] | Acute disseminated encephalomyelitis (1) | 11 years old | Primary | No | Corticosteroids |
| Da Silva RC, 2021 [33] | Acute disseminated encephalomyelitis (1) | 17 years old | Primary | Acyclovir | Corticosteroids |
| Bayrak N, 2021 [34] | Colitis (8) | 2–23 months old (9.8 months) | Primary | Ganciclovir (4) Ganciclovir + valganciclovir (2) | No |
| Howard-Jones AR, 2021 [35] | Enterocolitis, hepatitis, and thrombocytopenia (1) | 10 weeks old | Postnatal | Ganciclovir | No |
| Choudhary A, 2021 [36] | Sepsis, progressive hepatitis, and autoimmune hemolytic anemia (1) | 11 months old | Primary | Ganciclovir | Immunoglobulin |
| Hu H, 2021 [37] | Thrombocytopenia (8) | <12 months old | Postnatal | No | Corticosteroids (8) Immunoglobulin (6) |
| Patel RM, 2020 [38] | Total CMV + (33)
| NA (mean GA: 27.9 weeks) | Postnatal | Not specified | Not specified |
| Wang Y, 2020 [39] | Colitis (8) | 2–6.3 months old (2.5 months) | Postnatal | Ganciclovir (6) | No |
| Tuna Kirsaclioglu C, 2020 [40] | Protein-losing gastropathy (1) | 5 years old | Primary | No | No |
| Guo Y, 2019 [41] | Encephalitis (18) | 37–790 days old (5.1 months) | Primary | Ganciclovir (11) | No |
| Goh J, 2019 [42] | Enterocolitis (1) | 4 months old | Postnatal | Ganciclovir | No |
| Loureiro B, 2019 [43] | Autoimmune hemolytic anemia (1) | 6 months old | Postnatal | No | Corticosteroids Immunoglobulin |
| Nishio Y, 2018 [44] | Thrombocytopenia (1) | 20 months old | Primary | No | Immunoglobulin |
| Jin MJ, 2018 [45] | Thrombocytopenia (29)
| >6 months old | Primary | Ganciclovir or Ganciclovir + valganciclovir (8) | Immunoglobulin (13) Corticosteroids (4) Eight patients underwent combined therapy |
| Isleyen F, 2018 [46] | CMV-related secondary immune thrombocytopenia (3) | 2.5–11 months old | Primary | No | Immunoglobulin (3) |
| Khalifeh HK, 2017 [47] | Autoimmune hemolytic anemia (1) | 6 months old | Primary | No | Immunoglobulin Corticosteroids |
| Silwedel C, 2017 [48] | Hemophagocytic lymphohistiocytosis (1) | 9 weeks old (born late preterm) | Postnatal | Ganciclovir | No |
| Al-Eyadhy AA, 2017 [49] | Pneumonia, multi-organ failure (1) | 12 years old | Primary | Ganciclovir | No |
| Min CY, 2017 [50] | Hepatitis (123) | 14 days–11.3 months old (8.5 months) | Primary | No | No |
| Tsunoda T, 2017 [51] | Hepatitis (13) | 1–12 months old (7 months) | Primary | Not specified | Not specified |
| Alvarez-Hernadez L, 2017 [52] | Mononucleosis-like syndrome (1) | 5 years old | Primary | Ganciclovir | No |
| Goelz R, 2016 [53] | Abdominal distension, bloody diarrhea, NEC with perforation, and volvulus with intestinal necrosis (15) * Diarrhea (2) ** | NA *: preterm 23–25 weeks **: term | Postnatal | Ganciclovir (5) | No |
| Sue P, 2016 [54] | Invasive enterocolitis (21) | 21 days old–14 months old (2 months) | Postnatal | Ganciclovir (11) Ganciclovir and valganciclovir (1) Valganciclovir (1) No (8) | No |
| Kao W, 2015 [55] | Transient cerebral arteriopathy (1) | 2.5 years old | Primary | Ganciclovir | Immunoglobulin Corticosteroids |
| Viguè MG, 2015 [56] | Lymphoma-like syndrome (4) | 33–82 months old | Primary | No | Corticosteroids (1) |
| Raja J, 2015 [57] | Mononucleosis-like syndrome (1) | 12 years old | Primary | No | No |
| Marseglia L, 2014 [58] | Colonic stenosis post NEC (1) | Newborn | Postnatal | Ganciclovir | No |
| Cinel G, 2014 [59] | Pneumonitis (25) | 4–30 months old (11.3 months) | Primary | Ganciclovir (14) | Corticosteroids (1) |
| Louazon T, 2014 [60] | Colitis (1) | 10 weeks old | Postnatal | No | No |
| D’Alessandro M, 2014 [61] | Necrotizing vasculitis mimicking Henoch–Schönlein syndrome (1) | 3 years old | Primary | No | Corticosteroids |
| Novakova V, 2014 [62] | Severe colitis (1) | 2 months old | Postnatal | Ganciclovir | Corticosteroids Immunoglobulin |
| Garozzo MT, 2014 [63] | Colitis (1) | 8 weeks old | Postnatal | Ganciclovir | No |
| Taktak A, 2014 [64] | Hemorrhagic renal cystitis (1) | 3 years old | Primary | No | No |
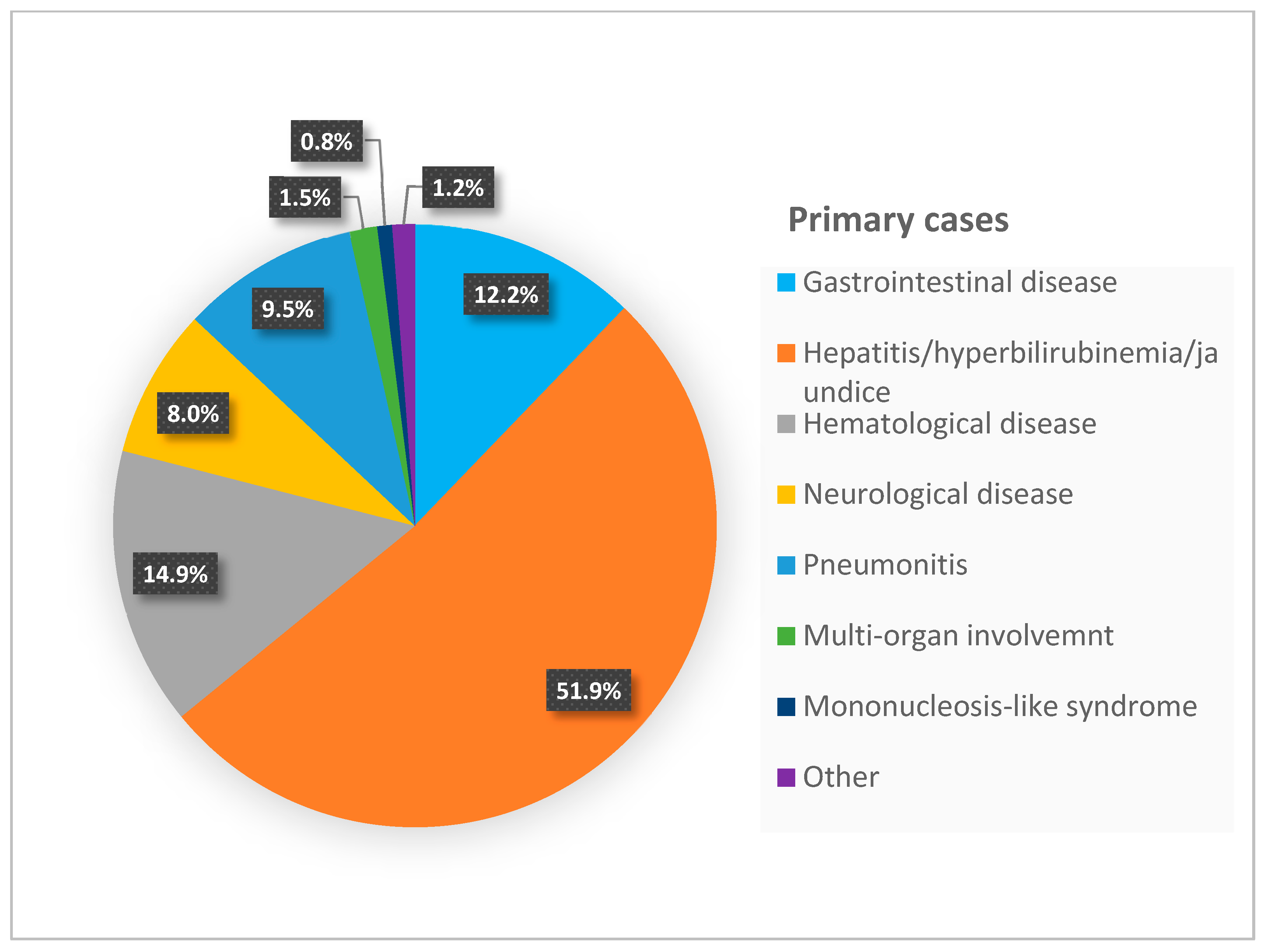
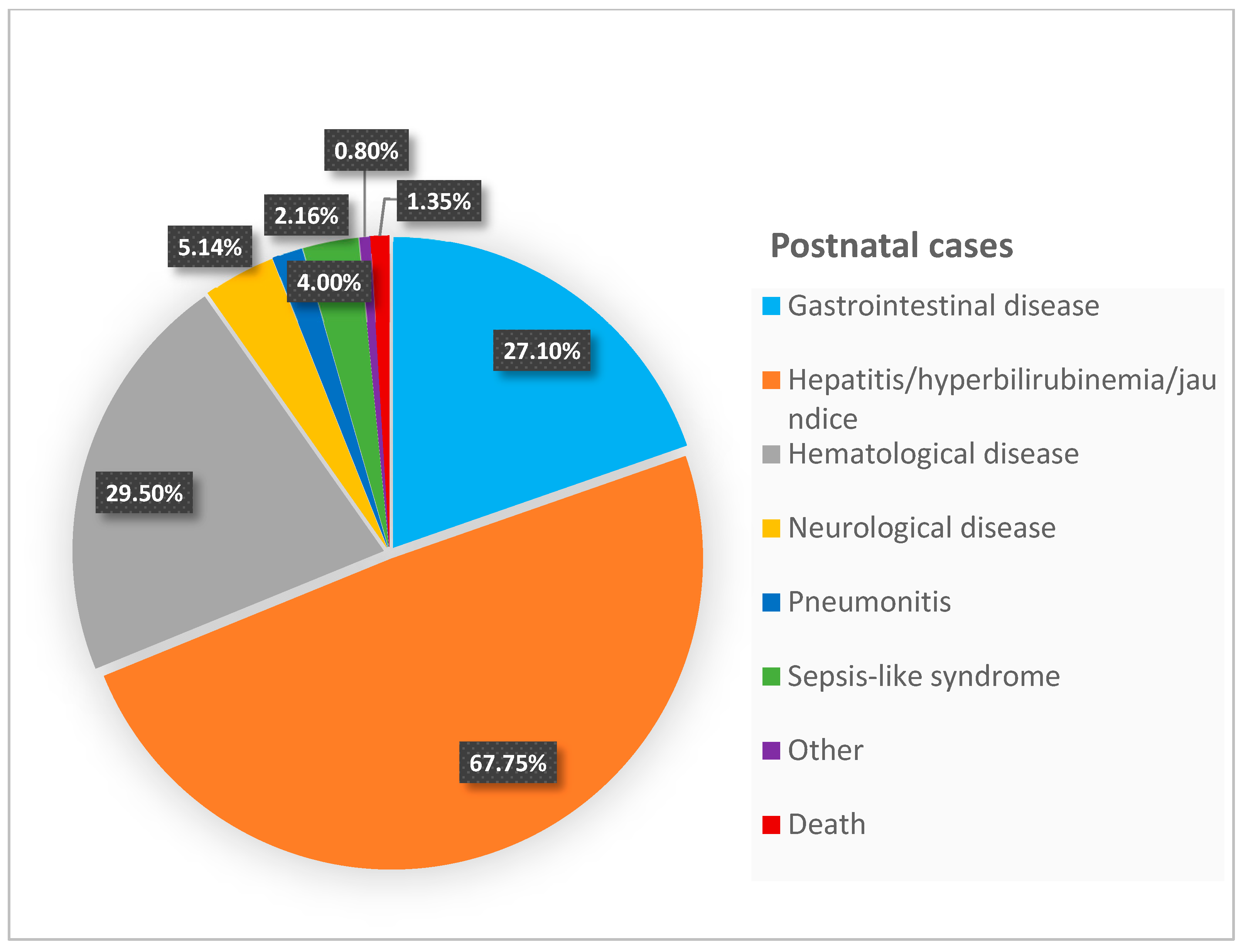
| Manifestations in 369 Postnatal Cases * Number (%) | Manifestations in 262 Primary Cases Number (%) | |
|---|---|---|
| Gastrointestinal disease | 100 (27.1%) | 32 (12.2%) |
| Hepatitis/hyperbilirubinemia/jaundice | 250 (67.75%) | 136 (51.9%) |
| Hematological disease | 109 (29.5%) | 39 (14.9%) |
| Pneumonitis | 8 (2.16%) | 25 (9.5%) |
| Neurological disease | 19 (5.14%) | 21 (8%) |
| Multi-organ involvement | ----- | 4 (1.5%) |
| Mononucleosis-like syndrome | ----- | 2 (0.8%) |
| Sepsis-like syndrome | 15 (4%) | --- |
| Other | 3 (0.8%) | 3 (1.2%) |
| Death | 5 (1.35%) | --- |
| Exclusion of other infectious diseases | EBV, HSV, Hepatitis, Influenza, bacterial infection, other related to pathology |
| Serological evaluation | IgG and IgM, IgG avidity index |
| Quantitative PCR on biological fluids | Urine, saliva, blood Bronchoalveolar lavage (in pulmonary infection) CSF (in neurological desease) |
| CMV search in biopsy samples related to pathology (i.e., gastrointestinal tract, bone marrow…) | “Owl eye” inclusion bodies on histological examination Positive immunohistochemistry for CMV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzialla, C.; Salomè, S.; Mondì, V. Clinical Manifestations of Non-Congenital CMV Infection in Infants and Immunocompetent Children: Review of Cases from the Past Decade. Microorganisms 2025, 13, 772. https://doi.org/10.3390/microorganisms13040772
Tzialla C, Salomè S, Mondì V. Clinical Manifestations of Non-Congenital CMV Infection in Infants and Immunocompetent Children: Review of Cases from the Past Decade. Microorganisms. 2025; 13(4):772. https://doi.org/10.3390/microorganisms13040772
Chicago/Turabian StyleTzialla, Chryssoula, Serena Salomè, and Vito Mondì. 2025. "Clinical Manifestations of Non-Congenital CMV Infection in Infants and Immunocompetent Children: Review of Cases from the Past Decade" Microorganisms 13, no. 4: 772. https://doi.org/10.3390/microorganisms13040772
APA StyleTzialla, C., Salomè, S., & Mondì, V. (2025). Clinical Manifestations of Non-Congenital CMV Infection in Infants and Immunocompetent Children: Review of Cases from the Past Decade. Microorganisms, 13(4), 772. https://doi.org/10.3390/microorganisms13040772







