Abstract
Ticks and tick-borne pathogens pose a significant threat to human and animal health, yet the diversity and prevalence of tick-borne microorganisms in karst regions remains inadequately explored. In October 2023, a total of 274 Rhipicephalus microplus ticks were collected from livestock in Guizhou Province, which boasts the largest karst area in China. Pathogen identification was subsequently performed using PCR amplification, Sanger sequencing, and phylogenetic analysis. High microbial diversity was noted, with five bacterial species from the order Rickettsiales detected, including those from the genera Rickettsia (family Rickettsiaceae), Anaplasma, and Ehrlichia (family Anaplasmataceae). The overall prevalence of infection with at least one pathogen was remarkably high at 94.5%. The highest positive rate was observed for Candidatus Rickettsia jingxinensis at 90.9%. A novel Ehrlichia species, provisionally designated as Candidatus Ehrlichia carsus, was identified with a positive rate of 16.8%. In addition, Anaplasma marginale, Ehrlchia minasensis and Ehrlichia canis were detected in 15.3%, 4.7% and 1.5%, respectively. The co-infections involving two or three rickettsial species were observed in 34.3% ticks. These findings highlight the high diversity and prevalence of tick-borne rickettsial agents in the karst area, underscoring the need for enhanced surveillance and effective tick control to mitigate disease risks to both humans and livestock.
1. Introduction
Ticks are obligate blood-feeding arthropods and important vectors of a wide range of pathogens, including viruses, bacteria, and protozoa, which pose significant risks to both human and animal health [1,2,3,4]. Throughout their life cycle, from larva to adult, ticks depend on blood feeding for development, which enables them to acquire and transmit pathogens from hosts at every stage. Furthermore, many pathogens can be transmitted transovarially and transstadially, enabling ticks to function not only as vectors but also as reservoirs for these infectious agents [5]. The dual role further underscores the significance of ticks in pathogen transmission and complicates control efforts. Ticks pose a significant threat to the livestock industry. Their direct impact includes blood-sucking, leading to anemia and weight loss, while their indirect harm lies in transmitting pathogens, compromising animal husbandry, reducing productivity, and ultimately causing substantial economic losses [6].
The order Rickettsiales comprises a group of Gram-negative bacteria that includes three main lineages: Rickettsiaceae, Anaplasmataceae, and Candidatus Midichloriaceae [7,8]. With the continuous discovery of new pathogenic species and their associated diseases, the order Rickettsiales has garnered widespread attention [9]. For instance, Anaplasma capra, a zoonotic pathogen initially reported in China in 2015, can infect humans, goats, and sheep [10]. In 2022, Rickettsia aeschlimannii was first identified in patients in Xinjiang [11], and in 2024, Rickettsia solvaca was detected for the first time in patients in Inner Mongolia [12]. These findings highlight the ongoing emergence and spread of tick-borne rickettsial pathogens, emphasizing the need for continued surveillance and research to mitigate their impact on human and animal health.
Karst landscapes are widely distributed across the globe, found in regions such as the Balkan Peninsula, southwest China, Malaysia, and Indonesia. Karst areas of China account for approximately 15.6% of the global karst area [13]. Among these, Guizhou Province has the largest karst area, covering about 73% of the province’s land. The karst landscape of southwest China has vast primary forests and highly diverse ecosystems, making it recognized as one of the world’s biodiversity hotspots. The region’s unique ecological conditions and geographical features provide an ideal habitat for ticks [14]. However, research on ticks and tick-borne pathogens in karst regions remains limited, lacking a comprehensive understanding of the diversity and infection rates of tick-borne agents in this area.
Guizhou Province, characterized by its typical karst landscapes and well-developed animal husbandry, is a high-risk area for tick-borne diseases. Previous studies have identified various tick-borne pathogens in some areas of Guizhou, including Rickettsia monacensis, Candidatus Rickettsia jingxinensis (Ca. R. jingxinensis), Anaplasma capra, and Jingmen tick virus [15,16,17]. However, the unique ecological environment of the karst landscape may determine the presence of tick-borne agents. Therefore, the present study focuses on the karst regions of Guizhou. The objective is to elucidate the composition and distribution patterns of tick-borne pathogens in this region. Due to local environmental constraints and the limited number of sampling sites, parasitic ticks were collected from livestock in six counties. Despite the limited sample size, we conducted an epidemiological investigation of tick-borne pathogens, aiming to provide scientific evidence and data support for the prevention and control of tick-borne diseases in the region.
2. Materials and Methods
2.1. Sample Collection
In October 2023, both blood-feeding and host-questing ticks were collected from livestock at nine sampling sites within the karst region of Guizhou Province. The tick species were systematically identified by an entomologist (Yi Sun) through detailed morphological analysis, including to species, developmental stage, and sex.
2.2. DNA Extraction
Each tick was processed as an individual sample for DNA extraction. Ticks were placed into 1.5 mL centrifuge tubes with 2–3 steel beads and 200 µL RNase-free water. The tubes were then homogenized in a tissue grinder under the following conditions: 4 °C, 55 Hz for 300 s. Following homogenization, DNA was extracted according to the manufacturer’s protocol for the TaKaRa MiniBEST Viral RNA/DNA Extraction Kit Ver.5.0 (TaKaRa Bio Inc, Kusatsu, Japan). The DNA was then eluted in 200 µL RNase-free water for subsequent analysis.
2.3. PCR Assays and Sequencing
Nucleic acid samples extracted from ticks were used as templates for PCR amplification targeting specific gene fragments: the outer membrane protein A (ompA) gene, the 17 kDa gene, and the citrate synthase (gltA) gene for Rickettsia; the gltA gene, the heat shock protein (groEL) gene, and the major surface protein 4 (msp4) gene for Anaplasma; the 16S rRNA gene, gltA gene, and groEL gene for Ehrlichia; the 18S rRNA gene for Babesia and Theileria; the 16S rRNA and the 5S-23S rRNA gene for Borrelia (Table S1). Samples showing positive detection for all three gene fragments of Rickettsia, Anaplasma, and Ehrlichia were considered positive. All PCR-positive amplicons were subsequently sent to Beijing Tianyi Huiyuan Biotechnology Co., Ltd. for paired-end sequencing. The relevant sequences have been uploaded to GenBank (Table S2).
2.4. Phylogenetic Analysis
The obtained sequences were initially compared using the BLAST tool from NCBI (http://www.ncbi.nlm.nih.gov/BLAST, accessed on 15 January 2025). The reference sequences from different strains in GenBank were used for concatenated tree construction (Table S3). The sample sequences were then aligned with reference sequences using MAFFT (v7.487) software [18], followed by trimming of unreliable regions using Gblocks (v0.91b) [19]. The aligned sequences were subsequently concatenated. Phylogenetic trees based on individual gene fragments and concatenated genes were constructed using IQ-TREE (v2.1.4), with the optimal substitution models selected. Trees were generated using the maximum likelihood (ML) method. To assess the reliability of the phylogenetic tree, ultrafast bootstrap analysis with 1000 iterations was performed [20]. The resulting phylogenetic tree was annotated and visualized using the iTOL online tool (v7.1) (https://itol.embl.de/, accessed on 16 February 2025) [21]. Finally, genetic distances were calculated using Mega 11 [22].
2.5. Statistical Analyses
The count data are presented as n (%), with 95% confidence intervals (95% CI) calculated using the Wilson score method, which provides more accurate interval estimates, particularly for small sample sizes or proportions close to 0 or 1. Chi-square tests (χ2) were conducted to assess differences in positivity rates between groups [23]. When expected frequencies were low, Fisher’s exact test was considered as an alternative. For comparing positive rates of Rickettsia, Anaplasma, and Ehrlichia, paired sample analyses were performed, with Cochran’s Q test used to evaluate overall differences and McNemar’s test applied for pairwise comparisons. All statistical analyses were conducted using SPSS 22.0 software, applying two-sided tests with a significance level set at p < 0.05.
3. Results
3.1. Tick Sampling
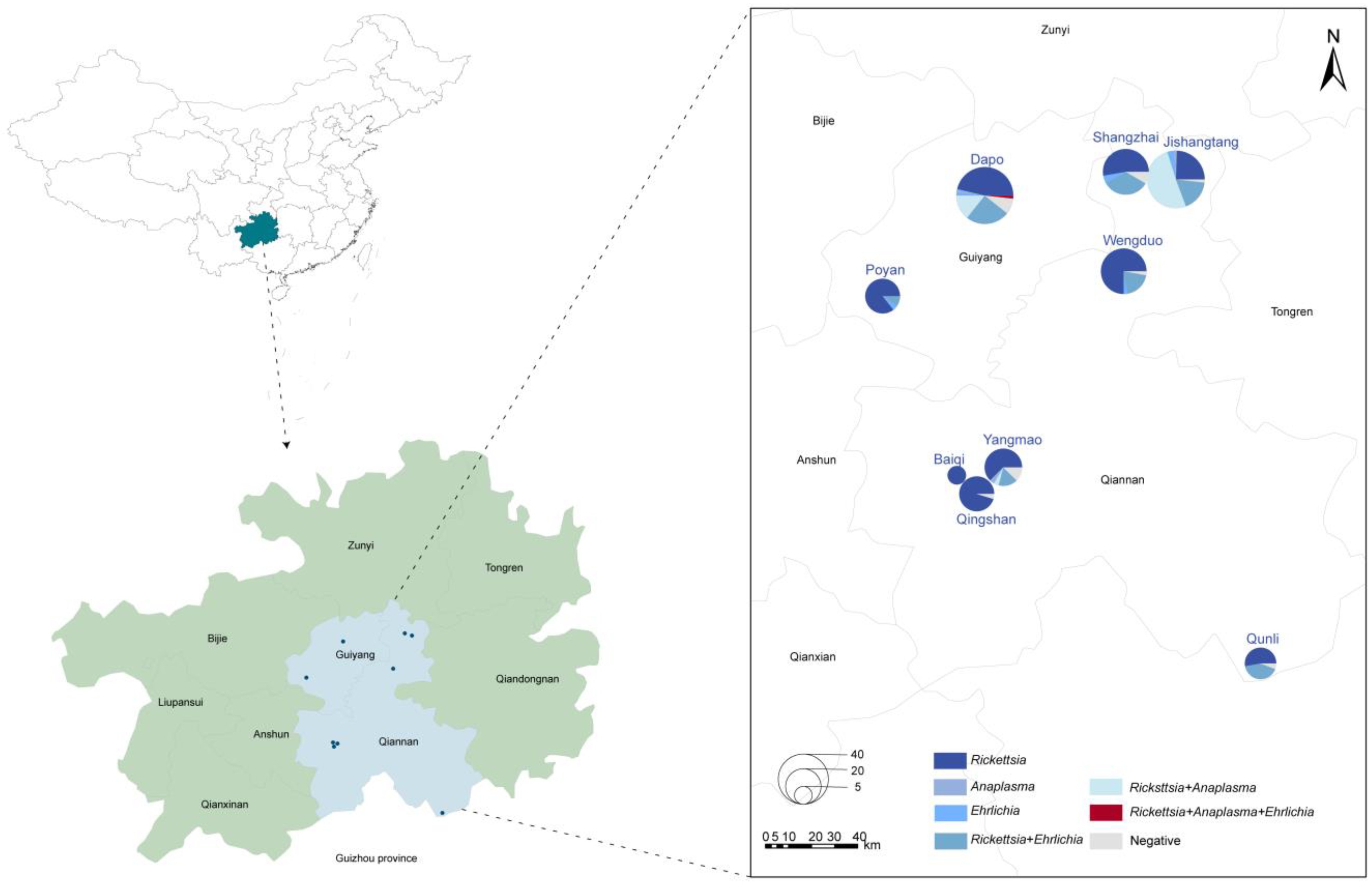
A total of 274 adult tick samples were collected from nine sampling sites in the karst region of Guizhou Province (Figure 1). Morphological identification confirmed that all the ticks were Rhipicephalus microplus (R. microplus), comprising 200 females and 74 males, with 183 blood-feeding ticks and 91 host-questing ticks. Except for one site, where goats served as tick hosts, the remaining sites hosted cattle.
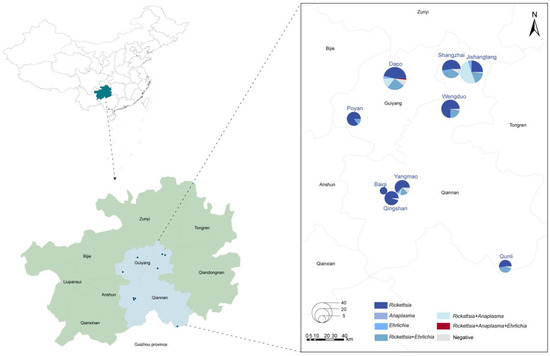
Figure 1.
Distribution of tick samples in Guizhou Province, China. Circle size corresponds to the number of ticks collected, while colors represent different pathogens detected in this study.
3.2. Phylogenic Analysis of Different Tick-Borne Pathogens
Molecular screening for additional pathogens, including Babesia, Theileria, and Borrelia yielded negative results. Our findings were exclusively focused on members of the order Rickettsiales, which included species from the spotted fever group of the family Rickettsiaceae, as well as two genera within the family Anaplasmataceae: Anaplasma and Ehrlichia.
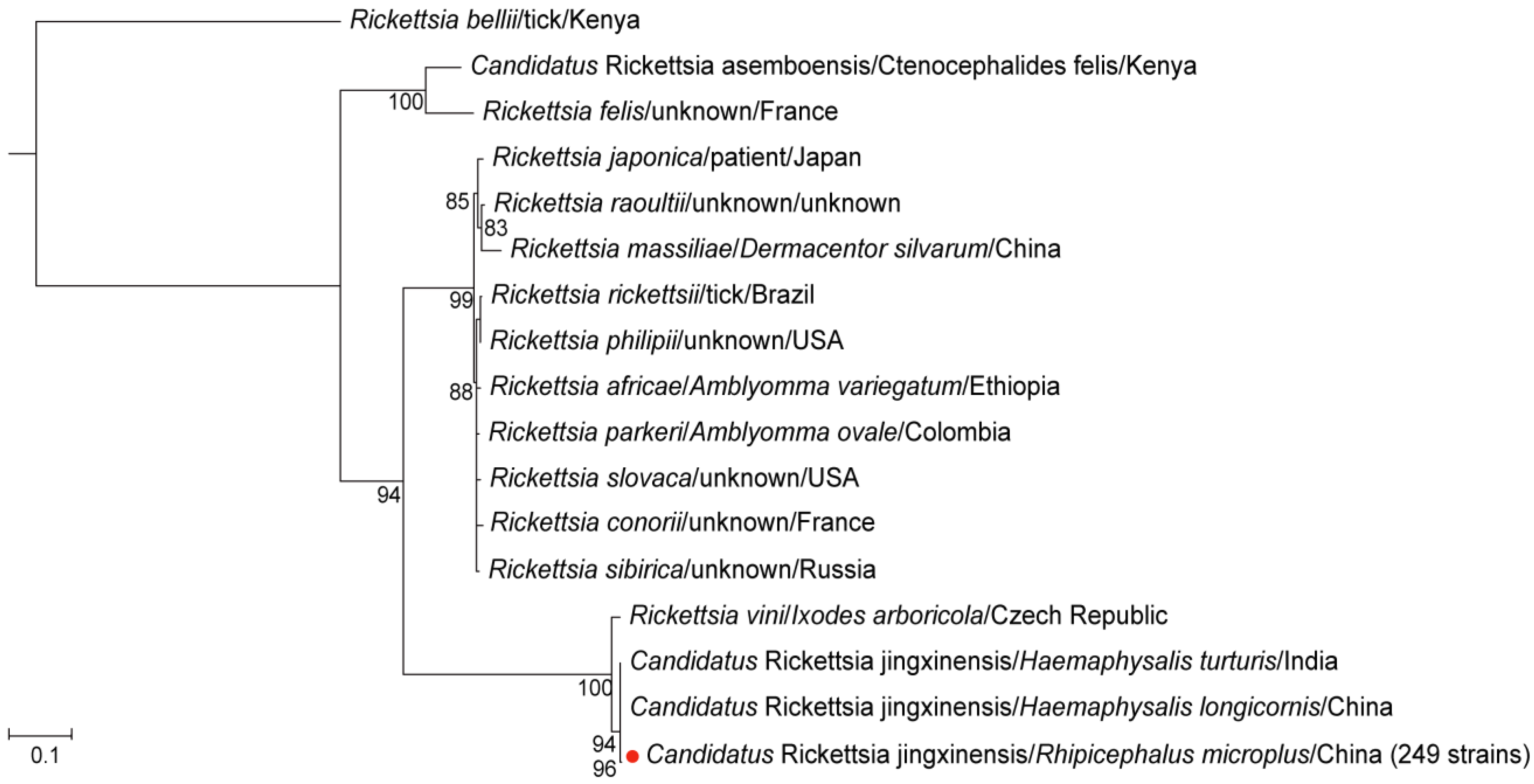
For Rickettsia, 249 samples tested positive for the ompA, gltA, and 17 kDa gene fragments, exhibiting identical sequences across all positive samples. The ompA gene sequence of these samples exhibited 100% identity with Candidatus Rickettsia jingxinensis identified in Haemaphysalis longicornis from Shaanxi Province, China (GenBank accession number: MH932061.1) [24] and Haemaphysalis spinigera from Idukki, Kerala, India (GenBank accession number: MN463682.1) (Figure S1A). The gltA gene sequence showed 100% identity with Ca. R. jingxinensis detected in Rhipicephalus microplus from China (GenBank accession number: MW114883.1) [25] (Figure S1B). The 17 kDa gene sequence also exhibited 100% identity with Candidatus Rickettsia jingxinensis detected in China (GenBank accession number: OR801788.1) (Figure S1C). Additionally, in the phylogenetic tree constructed from the concatenated three gene fragments, the detected Rickettsia clustered with Ca. R. jingxinensis detected in Shaanxi, China in 2019, and with the strain identified in India in 2020 (Figure 2). These findings collectively suggest that the Rickettsia identified in the positive samples is Ca. R. jingxinensis, yielding a positivity rate of 90.9% (95% CI: 86.9–93.7). No significant differences were observed in positivity rates of Ca. R. jingxinensis between genders or blood-feeding statuses.
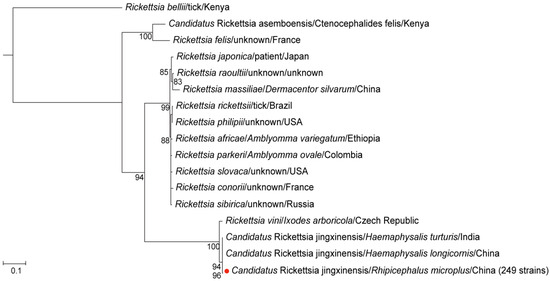
Figure 2.
The phylogenetic tree of Rickettsia was constructed based on concatenated ompA, gltA, and 17 kDa nucleotide sequences using the maximum likelihood (ML) method with 1000 bootstrap replicates. The red dots indicate the evolutionary positions of samples that tested positive for all three gene fragments.
For Anaplasma, a total of 42 samples tested positive for the gltA, groEL, and msp4 gene fragments. The gltA gene sequences from the 42 positive samples were identical and showed 100% identity with Anaplasma marginale (A. marginale) detected in R. microplus from Colombia (GenBank accession number: MT722102.1) [26] (Figure S2A). The groEL gene sequences from 32 of the positive samples displayed 100% identity and grouped with A. marginale detected in Bos taurus in Brazil (GenBank accession number: CP023730.1), while the remaining 10 sequences were identical and clustered with A. marginale from R. microplus in China (GenBank accession number: KX987395.1) [27] (Figure S2B). The msp4 gene sequences exhibited 99.8–100% identity and clustered phylogenetically with A. marginale strains from cattle in Malaysia (GenBank accession number: MT173810.1) and Brazil (GenBank accession number: MK570464.1) [28] (Figure S2C). Phylogenetic analysis further confirmed that the species identified in this study was A. marginale (Figure 3), with an overall positivity rate of 15.3% (95% CI: 11.5–20.1). The positivity rate was significantly higher in male ticks compared to female ticks (36.5% vs. 7.5%, p < 0.001) and notably lower in blood-feeding ticks compared to host-questing ticks (7.7% vs. 30.8%, p < 0.001).

Figure 3.
The phylogenetic tree of Anaplasma was constructed based on concatenated gltA, groEL, and msp4 nucleotide sequences using the maximum likelihood (ML) method with 1000 bootstrap replicates. The red dots indicate the evolutionary positions of samples that tested positive for all three gene fragments.
For Ehrlichia, a total of 63 samples tested positive for the 16S rRNA, gltA, and groEL gene fragments. Further phylogenetic analysis identified three distinct Ehrlichia species. In four positive samples, Ehrlichia canis (E. canis) was identified, with its 16S rRNA, gltA, and groEL gene sequences clustering with those of E. canis detected in R. microplus ticks from Hubei province of China (GenBank accession numbers: KX987326.1, MW428300.1, MW428319.1) [27,29], showing 100% sequence similarity, respectively (Figure S3A–C). In 13 positive samples, the gene fragments were identical across all three gene segments, sharing 100% sequence identity with Ehrlichia minasensis (E. minasensis) detected in Brazilian cattle (GenBank accession numbers: GCA_004181775.1, GCA_000825765.1) [30] and in R. microplus ticks from Hainan province of China (GenBank accession numbers: OR835907.1, OR555731.1, OR555740.1) [31] (Figure S3A–C). The remaining 46 positive samples exhibited 99.8–100% homology with each other for the 16S rRNA gene fragment and also shared 99.8–100% similarity with uncultured Ehrlichia sp. detected in R. microplus ticks from Hainan, China (GenBank accession numbers: OR835912.1, OR835909.1) [31] and Hyalomma anatolicum ticks from Pakistan (GenBank accession number: MN726921.1) [32], clustering together in the same branch of the phylogenetic tree (Figure S3A). The gltA gene sequences from these samples showed 100% homology with uncultured Ehrlichia sp. found in R. microplus ticks from Wuhan and Anhui provinces of China (GenBank accession numbers: KX987356.1, OQ185256.1) [27,33], grouping in the same clade (Figure S3B). Similarly, the groEL gene fragment exhibited 100% sequence identity with uncultured Ehrlichia sp. detected in Rhipicephalus annulatus ticks from Sri Lanka and R. microplus ticks from Anhui, China (GenBank accession numbers: MZ970589.1, OQ185235.1) [23,34], clustering in the same branch (Figure S3C). Based on these findings, we constructed a concatenated phylogenetic tree using all three gene fragments (Figure 4), which confirmed that, apart from E. canis and E. minasensis, the remaining 46 positive samples formed a distinct branch, suggesting the presence of a novel Ehrlichia species. Since this new member was found in a karst landscape region, we propose the provisional name Candidatus Ehrlichia carsus. The overall positivity rate for Ehrlichia detection in ticks was 23.0% (95% CI: 18.4–28.3). The positivity rate for Ehrlichia was significantly higher in female ticks compared to male ticks (27.5% vs. 10.8%, p < 0.004). Additionally, the positivity rate in blood-feeding ticks was significantly higher than that in host-questing ticks (29.0% vs. 11.0%, p < 0.001).

Figure 4.
The phylogenetic tree of Ehrlichia was constructed based on concatenated 16S rRNA, gltA, and groEL nucleotide sequences using the maximum likelihood (ML) method with 1000 bootstrap replicates. The red dots indicate the evolutionary positions of samples that tested positive for all three gene fragments.
3.3. Prevalence of Different Tick-Borne Pathogens
Overall, in the 274 tick samples analyzed, the positive rate of Rickettsia was 90.9% (95% CI: 86.9–93.7), while Anaplasma and Ehrlichia were detected at rates of 15.3% (95% CI: 11.5–20.1) and 23.0% (95% CI: 18.4–28.3), respectively. Among these, 259 ticks (94.5%, 95% CI: 91.2–96.7) were found to be infected with at least one pathogen (Table 1). No significant differences in the positivity rates of tick-borne pathogens were observed between the different regions. Furthermore, the co-infection rate of two or more pathogens was 34.3% (95% CI: 28.9–40.1), primarily involving co-infections of Rickettsia with Anaplasma and Rickettsia with Ehrlichia (Table 2). These findings highlight the high prevalence of Rickettsia in tick populations and the frequent occurrence of co-infections, underscoring the complexity of tick-borne pathogen transmission.

Table 1.
The prevalence of tick-borne pathogens in ticks from karst area in Guizhou Province of southwest China.

Table 2.
Co-infection of different tick-borne pathogens in Guizhou Province.
4. Discussion
In this study, we screened R. microplus for pathogens and identified several members of the order Rickettsiales, including a novel Ehrlichia species that has not been previously reported in other karst regions. Notably, no Borrelia, Babesia, or Theileria species were detected. These findings highlight the critical need for ongoing monitoring of rickettsial infections in karst landscapes, as well as the importance of continuous surveillance of local arthropods, mammals, and humans to better understand and mitigate the risks posed by emerging tick-borne pathogens.
All tick samples collected in this study were R. microplus, whereas research in European karst regions has primarily focused on Ixodes ricinus. For example, Blažeková et al. [35] studied tick samples from the Slovak Karst National Park and identified several species, including Ixodes ricinus, Haemaphysalis concinna, Haemaphysalis inermis, Dermacentor reticulatus, and Dermacentor marginatus. They also detected pathogens such as Anaplasma spp., Bartonella spp., Rickettsia spp., Babesia spp., and Theileria spp. Similarly, studies in other karst regions of Slovakia have shown that Ixodes ricinus was the predominant tick species, harboring a diverse array of tick-borne pathogens, with the highest infection rate for Borrelia burgdorferi s.l., reaching 12.43% [36]. Additionally, Susnjar et al. [37] detected Borrelia spp. in Ixodes ricinus collected from the Coastal-Karst area in Slovenia. These findings differ from our study, particularly in terms of tick species and the pathogens they carry. This highlights the significant role of the ecological environment and host conditions in shaping tick communities and influencing their capacity for pathogen transmission in karst regions.
We observed differences in pathogen infection rates among Rickettsia, Anaplasma, and Ehrlichia in ticks. For Rickettsia, no significant variations in infection rates were found between sex and feeding status, suggesting a relatively uniform transmission potential across these groups. In contrast, Anaplasma exhibited distinct differences based on sex and feeding status, with non-blood-feeding ticks showing higher infection rates than blood-feeding ticks, and male ticks exhibiting higher infection rates than female ticks. Conversely, Ehrlichia displayed the opposite trend, with blood-feeding ticks having significantly higher infection rates than non-blood-feeding ticks, and female ticks showing higher infection rates than males. Previous studies have also found higher positivity rates for Borrelia in female ticks compared to males [38], likely due to differences in blood intake and feeding duration. Additionally, research has shown that Rhipicephalus sanguineus with excessive blood feeding exhibit a significantly higher Ehrlichia infection rate, potentially due to the increased vectorial capacity associated with higher blood intake [39]. Moreover, we detected a high rate of co-infection with multiple pathogens in ticks, offering important insights into the complex interactions among tick-borne pathogens. Co-infections may alter transmission patterns and contribute to divergent clinical manifestations in hosts, underscoring the necessity for enhanced vector surveillance programs. Such co-infection complexity poses diagnostic challenges and complicates therapeutic interventions in clinical settings. Of particular concern is Rickettsia, which was found to co-occur with both Anaplasma and Ehrlichia, due to its potential to cause severe health issues in humans and animals. The potential interactions between these pathogens within tick vectors may alter transmission efficiency, which could subsequently impact disease severity in infected hosts.
Additionally, we observed a high positivity rate of Ca. R. jingxinensis at 90.9%, which closely aligns with the 96.74% reported in the Qianxinan, Liupanshui, and Bijie regions of Guizhou Province [40], while the positivity rate is significantly higher than the 6.19% seen in the Qiandongnan region [14]. This notably elevated infection rate suggests that Ca. R. jingxinensis may exhibit strong adaptability and transmission capacity in Guizhou, potentially due to favorable ecological conditions, high vector abundance, or host availability. Further investigation is needed to elucidate the underlying factors contributing to this pattern. As a member of the spotted fever group Rickettsia, Ca. R. jingxinensis has a broad geographic distribution [25,41]. Clinical cases from South Korea have shown that Ca. R. jingxinensis can infect humans, causing symptoms such as fever, erythema, and eschar, highlighting a potential risk to human health [42]. Therefore, it is crucial to strengthen epidemiological surveillance of both human and animal populations in this region.
R. microplus is a primary vector for the transmission of A. marginale [43]. In this study, we detected A. marginale with a positivity rate of 15.3%, which exhibited high genetic similarity to strains found in Colombia, Brazil, China, and Malaysia. Previous studies have also reported the presence of A. marginale across various regions in China, including Hunan, Guangdong (central-southern), Chongqing, Sichuan (southwestern), and Liaoning (northeastern), further confirming the widespread distribution of this pathogen in China [44]. A. marginale is the most virulent species of Anaplasma affecting cattle, with infected animals commonly showing clinical signs such as fever, lethargy, dark urine, and jaundice. While infections with A. marginale can be fatal in adult cattle, many animals remain asymptomatic despite harboring the pathogen [45]. Given the high positivity rate of this pathogen in the region, its significant infectivity and pathogenicity, along with the critical role of livestock farming in the local economy, future efforts should prioritize strengthening surveillance and control measures for A. marginale and other related pathogens to reduce their negative impact on livestock production and economic stability.
We also detected three Ehrlichia species: a novel strain provisionally named Ca. E. carsus, E. minasensis, and E. canis. The novel Ca. E. carsus detected in this study formed a distinct evolutionary branch in the combined phylogenetic analysis, suggesting it may represent an uncharacterized Ehrlichia species. Its 16S gene fragment showed 100% homology with an uncultured Ehrlichia species detected on Hainan Island. While the pathogenicity and host range of this species remain undetermined, this discovery adds new evidence of the diversity of pathogens carried by ticks in the karst regions of Guizhou. The positivity rate for E. minasensis was 4.7%, which is higher than the 1.68% reported in tick samples from Yingshan County, Hubei [46]. Phylogenetic analysis revealed that the 16S gene fragment of E. minasensis closely resembles that of E. minasensis detected on Hainan Island, China. However, the groEL and gltA gene fragments, along with the combined phylogenetic analysis, showed a strong phylogenetic relationship with E. minasensis from Mato Grosso, Brazil [47,48]. E. canis primarily infects canines but can also affect humans, typically causing mild or asymptomatic clinical manifestations [49]. Therefore, future research should be performed using whole-genome sequencing and functional experiments to further elucidate the taxonomic classification and biological characteristics of this strain. Notably, both Hainan Island, China, and Mato Grosso, Brazil, are situated in typical karst landscapes [50,51], and this unique ecological environment may provide an ideal habitat for ticks and their pathogens, potentially influencing pathogen transmission. Consequently, future studies should investigate the role of karst ecosystems in shaping tick communities and pathogen transmission, providing valuable insights for tick-borne disease prevention and control.
As an exploratory epidemiological study, this research was conducted at specific sites across six counties in Guizhou Province over a relatively short sampling period. Consequently, the findings may not fully reflect the overall prevalence of tick-borne agents in the karst region of Guizhou. Nevertheless, given the high risk of these diseases in the area, this study provides valuable baseline data for disease prevention and control efforts while laying a crucial foundation for future large-scale research.
5. Conclusions
In summary, the prevalence of tick-borne pathogens and the co-infection rate in R. microplus ticks from the karst region of Guizhou were relatively high. Therefore, it is crucial to raise awareness among local farmers about effective tick management and disease prevention strategies. Additionally, strengthening monitoring and control efforts targeting ticks and their associated pathogens will be essential not only for protecting livestock health but also for reducing the risk of human infections and safeguarding public health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13040765/s1, Table S1: PCR primers used in this study; Table S2: Sequences of Rickettsia, Anaplasma, and Ehrlichia deposited in GenBank; Table S3: Reference sequences utilized for combined phylogenetic analysis; Figure S1: Phylogenetic trees of Rickettsia were constructed using the maximum likelihood (ML) method with 1000 bootstrap replicates. (A) ompA gene; (B) gltA gene; (C) 17 kDa gene; Figure S2: Phylogenetic trees of Anaplasma were constructed using the maximum likelihood (ML) method with 1000 bootstrap replicates. (A) gltA gene; (B) groEL gene; (C) msp4 gene; Figure S3: Phylogenetic trees of Ehrlichia were constructed using the maximum likelihood (ML) method with 1000 bootstrap replicates. (A) 16S rRNA gene; (B) gltA gene; (C) groEL gene.
Author Contributions
Y.-T.L.: Writing—original draft, Investigation; Y.-F.W.: Data curation; Writing—original draft; M.-Z.Z.: Investigation; D.-Y.Z.: Data curation; Y.S.: Investigation; C.-W.G.: Investigation; L.Z.: Investigation; X.-M.C.: Investigation; W.-C.C.: Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2023YFC2305901), the Foundation of State Key Laboratory of Pathogen and Biosecurity of China (Grant No. SKLPBS2226), and the National Natural Science Foundation of China (Grant No. 82160633).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dantas-Torres, F.; Chomel, B.B.; Otranto, D. Ticks and tick-borne diseases: A one health perspective. Trends Parasitol. 2012, 28, 437–446. [Google Scholar]
- Jia, N.; Wang, J.; Shi, W.; Du, L.; Sun, Y.; Zhan, W.; Jiang, J.F.; Wang, Q.; Zhang, B.; Ji, P.; et al. Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell 2020, 182, 1328–1340.e13. [Google Scholar] [PubMed]
- Pace, E.J.; O’Reilly, M. Tickborne diseases: Diagnosis and management. Am. Fam. Physician 2020, 101, 530–540. [Google Scholar] [PubMed]
- Fang, L.Q.; Liu, K.; Li, X.L.; Liang, S.; Yang, Y.; Yao, H.W.; Sun, R.X.; Sun, Y.; Chen, W.J.; Zuo, S.Q.; et al. Emerging tick-borne infections in mainland China: An increasing public health threat. Lancet Infect. Dis. 2015, 15, 1467–1479. [Google Scholar] [PubMed]
- Johnson, N. Ticks: Biology, Ecology, and Diseases, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 25–40, 65–72. [Google Scholar]
- Desta, B. Review on the impact of ticks on livestock health and productivity. J. Biol. Agric. Healthc. 2016, 6, 1–7. [Google Scholar]
- Salje, J. Cells within cells: Rickettsiales and the obligate intracellular bacterial lifestyle. Nat. Rev. Microbiol. 2021, 19, 375–390. [Google Scholar]
- Giannotti, D.; Boscaro, V.; Husnik, F.; Vannini, C.; Keeling, P.J. The “other” Rickettsiales: An overview of the family “Candidatus Midichloriaceae”. Appl. Environ. Microbiol. 2022, 88, e0243221. [Google Scholar]
- Kang, Y.J.; Diao, X.N.; Zhao, G.Y.; Chen, M.H.; Xiong, Y.; Shi, M.; Fu, W.M.; Guo, Y.J.; Pan, B.; Chen, X.P.; et al. Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evol. Biol. 2014, 14, 167. [Google Scholar]
- Li, H.; Zheng, Y.C.; Ma, L.; Jia, N.; Jiang, B.G.; Jiang, R.R.; Huo, Q.B.; Wang, Y.W.; Liu, H.B.; Chu, Y.L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar]
- Yang, M.; Jia, Y.; Dong, Z.; Zhang, Y.; Xie, S.; Liu, Q.; Wang, Y. Rickettsia aeschlimannii infection in a woman from Xingjiang, northwestern China. Vector-Borne Zoonotic Dis. 2022, 22, 55–57. [Google Scholar]
- Duan, L.; Zhang, L.; Hou, X.; Bao, Z.; Zeng, Y.; He, L.; Liu, Z.; Zhou, H.; Hao, Q.; Dong, A. Surveillance of tick-borne bacterial infection in ticks and forestry populations in Inner Mongolia, China. Front. Public Health 2024, 12, 1302133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Chen, W.H.; Li, D.; Shu, H.L.; Liang, J.D.; Zhao, J.H.; Tian, W.Y.; Han, Y.F. Four new araneogenous species and a new genus in Hypocreales (Clavicipitaceae, Cordycipitaceae) from the karst region of China. MycoKeys 2025, 112, 335–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Meng, C.; Zhang, B.; Wang, X.; Tian, J.; Tang, G.; Wang, W.; Li, N.; Li, M.; Xu, X.; et al. Prevalence of spotted fever group Rickettsia and Candidatus Lariskella in multiple tick species from Guizhou Province, China. Biomolecules 2022, 12, 1701. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, K.; Zhao, S.; Yan, Y.; Wang, H.; Jing, J.; Jian, F.; Wang, R.; Zhang, L.; Ning, C. Detection and phylogenetic characterization of Anaplasma capra: An. emerging pathogen in sheep and goats in China. Front. Cell. Infect. Microbiol. 2018, 8, 283. [Google Scholar] [CrossRef]
- Guo, J.J.; Lin, X.D.; Chen, Y.M.; Hao, Z.Y.; Wang, Z.X.; Yu, Z.M.; Lu, M.; Li, K.; Qin, X.C.; Wang, W.; et al. Diversity and circulation of Jingmen tick virus in ticks and mammals. Virus Evol. 2020, 6, veaa051. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. Mega11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, E.K.; Liapunova, N.A.; Tuul, D.; Otgonsuren, G.; Nomin, D.; Erdenebat, N.; Abmed, D.; Danchinova, G.A.; Sato, K.; Kawabata, H.; et al. Co-infections with multiple pathogens in natural populations of Ixodes persulcatus ticks in Mongolia. Parasit. Vectors 2022, 15, 236. [Google Scholar] [CrossRef]
- Guo, W.P.; Wang, Y.H.; Lu, Q.; Xu, G.; Luo, Y.; Ni, X.; Zhou, E.M. Molecular detection of spotted fever group rickettsiae in hard ticks, northern China. Transbound. Emerg. Dis. 2019, 66, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, W.B.; Pan, Y.S.; Jiang, B.G.; Du, C.H.; Que, T.C.; Zhan, L.; Wu, J.H.; Yu, M.H.; Cui, X.M.; et al. Detection of novel spotted fever group Rickettsiae (Rickettsiales: Rickettsiaceae) in Ticks (Acari: Ixodidae) in southwestern China. J. Med. Entomol. 2021, 58, 1363–1369. [Google Scholar] [CrossRef]
- Cotes-Perdomo, A.P.; Oviedo, A.; Castro, L.R. Molecular detection of pathogens in ticks associated with domestic animals from the Colombian Caribbean region. Exp. Appl. Acarol. 2020, 82, 137–150. [Google Scholar] [CrossRef]
- Lu, M.; Tian, J.H.; Yu, B.; Guo, W.P.; Holmes, E.C.; Zhang, Y.Z. Extensive diversity of Rickettsiales bacteria in ticks from Wuhan, China. Ticks Tick-Borne Dis. 2017, 8, 574–580. [Google Scholar] [CrossRef] [PubMed]
- de Souza Ramos, I.A.; Herrera, H.M.; Fernandes, S.J.; do Amaral, R.B.; Zanatto, D.C.S.; da Silva, T.M.V.; Horta, B.L.S.; Campos, J.B.V.; Alves, J.V.A.; de Macedo, G.C.; et al. Genetic diversity of Anaplasma marginale in beef cattle in the Brazilian Pantanal. Ticks Tick-Borne Dis. 2019, 10, 805–814. [Google Scholar] [CrossRef]
- Lu, M.; Tian, J.; Pan, X.; Qin, X.; Wang, W.; Chen, J.; Guo, W.; Li, K. Identification of Rickettsia spp., Anaplasma spp., and an Ehrlichia canis-like agent in Rhipicephalus microplus from southwest and south-central China. Ticks Tick-Borne Dis. 2022, 13, 101884. [Google Scholar] [CrossRef]
- Aguiar, D.M.; Araujo, J.P.J.; Nakazato, L.; Bard, E.; Cabezas-Cruz, A. Complete genome sequence of an Ehrlichia minasensis strain isolated from cattle. Microbiol. Resour. Announc. 2019, 8, e00161-19. [Google Scholar] [CrossRef]
- Intirach, J.; Lv, X.; Sutthanont, N.; Cai, B.; Champakaew, D.; Chen, T.; Han, Q.; Lv, Z. Molecular and next-generation sequencing analysis of tick-borne pathogens of Rhipicephalus ticks (Acari: Ixodidae) in cattle and dogs. Acta Trop. 2024, 252, 107138. [Google Scholar] [CrossRef]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabbar, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasit. Vectors 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liao, J.; Chen, Q.; Ding, J.; Chang, H.; Lyu, Y.; Yu, L.; Wen, B.; Sun, Y.; Qin, T. Diversity of Rickettsiales bacteria in five species of ticks collected from Jinzhai County, Anhui Province, China in 2021–2022. Front. Microbiol. 2023, 14, 1141217. [Google Scholar] [CrossRef] [PubMed]
- Dasch, G.A.; Eremeeva, M.E.; Zambrano, M.L.; Premaratna, R.; Kularatne, S.A.M.; Jayanthe Rajapakse, R.P.V. Molecular characterization of Rickettsial agents in ticks (Acari: Ixodidae) from Sri Lanka. Am. J. Trop. Med. Hyg. 2022, 106, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Blazekova, V.; Stanko, M.; Sprong, H.; Kohl, R.; Zubrikova, D.; Vargova, L.; Bona, M.; Miklisova, D.; Vichova, B. Ixodiphagus hookeri (Hymenoptera: Encyrtidae) and tick-borne pathogens in ticks with sympatric occurrence (and different activities) in the Slovak Karst National Park (Slovakia), Central Europe. Pathogens 2024, 13, 385. [Google Scholar] [CrossRef]
- Bona, M.; Blaňárová, L.; Stanko, M.; Mošanský, L.; Čepčeková, E.; Víchová, B. Impact of climate factors on the seasonal activity of ticks and temporal dynamics of tick-borne pathogens in an area with a large tick species diversity in Slovakia, Central Europe. Biologia 2021, 77, 1619–1631. [Google Scholar] [CrossRef]
- Susnjar, J.; Cerar Kisek, T.; Strasek Smrdel, K.; Ruzic-Sabljic, E.; Adam, K.; Ivovic, V. Detection, identification and genotyping of Borrelia spp. in ticks of Coastal-Karst and Littoral-Inner Carniola regions in Slovenia. Folia Parasitol. 2023, 70, 7. [Google Scholar] [CrossRef]
- Strnad, M.; Honig, V.; Ruzek, D.; Grubhoffer, L.; Rego, R.O.M. Europe-wide meta-analysis of Borrelia burgdorferi Sensu Lato prevalence in questing Ixodes ricinus ticks. Appl. Environ. Microbiol. 2017, 83, e00609-17. [Google Scholar] [CrossRef]
- Shu, C.; Intirach, J.; Zhou, Y.; Gao, S.; Lv, X.; Jiao, H.; Hu, Y.; Lv, Z. Microbial community characteristics and pathogens detection in Rhipicephalus sanguineus and Haemaphysalis hystricis from Hainan Island, China. Front. Microbiol. 2024, 15, 1450219. [Google Scholar] [CrossRef]
- Lu, M.; Meng, C.; Gao, X.; Sun, Y.; Zhang, J.; Tang, G.; Li, Y.; Li, M.; Zhou, G.; Wang, W.; et al. Diversity of Rickettsiales in Rhipicephalus microplus ticks collected in domestic ruminants in Guizhou Province, China. Pathogens 2022, 11, 1108. [Google Scholar] [CrossRef]
- Gual-Gonzalez, L.; Torres, M.E.; Self, S.C.W.; Cantillo-Barraza, O.; Nolan, M.S. Spotted fever group Rickettsia spp. molecular and serological evidence among Colombian vectors and animal hosts: A historical review. Insects 2024, 15, 170. [Google Scholar] [CrossRef]
- Seo, J.Y.; Park, J.S.; Lee, H.I.; Ju, J.W. Molecular identification of spotted fever group Rickettsiae in ticks in the Republic of Korea. Pathogens 2024, 13, 575. [Google Scholar] [CrossRef]
- Byaruhanga, C.; Collins, N.E.; Knobel, D.L.; Khumalo, Z.T.H.; Chaisi, M.E.; Oosthuizen, M.C. Molecular detection and phylogenetic analysis of Anaplasma marginale and Anaplasma centrale amongst transhumant cattle in north-eastern Uganda. Ticks Tick-Borne Dis. 2018, 9, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, R.; Liu, Z.; Niu, Q.; Guan, G.; Liu, G.; Luo, J.; Yin, H. Insight into the genetic diversity of Anaplasma marginale in cattle from ten provinces of China. Parasit. Vectors 2017, 10, 565. [Google Scholar] [CrossRef]
- Al-Hosary, A.; Raileanu, C.; Tauchmann, O.; Fischer, S.; Nijhof, A.M.; Silaghi, C. Epidemiology and genotyping of Anaplasma marginale and co-infection with piroplasms and other Anaplasmataceae in cattle and buffaloes from Egypt. Parasit. Vectors 2020, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Pan, K.; Teng, Z.; Wang, H.; Zhang, X.; Ren, H.; Yi, L.; He, J.; Cai, K.; Qin, T. Molecular detection reveals diverse tick-borne bacterial and protozoan pathogens in two tick species from Yingshan County of Hubei Province, China in 2021–2022. Front Microbiol 2023, 14, 1298037. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.C.; Zweygarth, E.; Ribeiro, M.F.; da Silveira, J.A.; de la Fuente, J.; Grubhoffer, L.; Valdes, J.J.; Passos, L.M. New species of Ehrlichia isolated from Rhipicephalus (Boophilus) microplus shows an ortholog of the E. canis major immunogenic glycoprotein gp36 with a new sequence of tandem repeats. Parasit. Vectors 2012, 5, 291. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Zweygarth, E.; Broniszweska, M.; Passos, L.M.; Ribeiro, M.F.; Manrique, M.; Tobes, R.; de la Fuente, J. Complete genome sequence of Ehrlichia mineirensis, a novel organism closely related to Ehrlichia canis with a new host association. Genome Announc. 2015, 3, e01450-14. [Google Scholar] [CrossRef]
- Sgroi, G.; D’Alessio, N.; Veneziano, V.; Rofrano, G.; Fusco, G.; Carbonara, M.; Dantas-Torres, F.; Otranto, D.; Iatta, R. Ehrlichia canis in human and tick, Italy, 2023. Emerg. Infect. Dis. 2024, 30, 2651–2654. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y. The distribution, protection and utilization of karst caves in Hainan province, China. In Proceedings of the 16th International Congress of Speleology, Guilin, China, 21–28 July 2013. [Google Scholar]
- Galvão, P.; Halihan, T.; Hirata, R. Evaluating karst geotechnical risk in the urbanized area of Sete Lagoas, Minas Gerais, Brazil. Hydrogeol. J. 2015, 23, 1499–1513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).