Unveiling the Thermotolerance and Growth-Promoting Attributes of Endophytic Bacteria Derived from Oryza sativa: Implications for Sustainable Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Screening of Thermotolerant Endophyte Bacteria Isolates
2.2. Germination Bioassay for Evaluating Bacterial Inoculation Effects on Heat-Stressed Rice Seeds
2.3. 16S rRNA Gene Sequence Analyses
2.4. Evaluating Thermotolerant Endophytic Bacteria for Plant Growth-Promoting Traits
2.5. Soil Pot Experiment
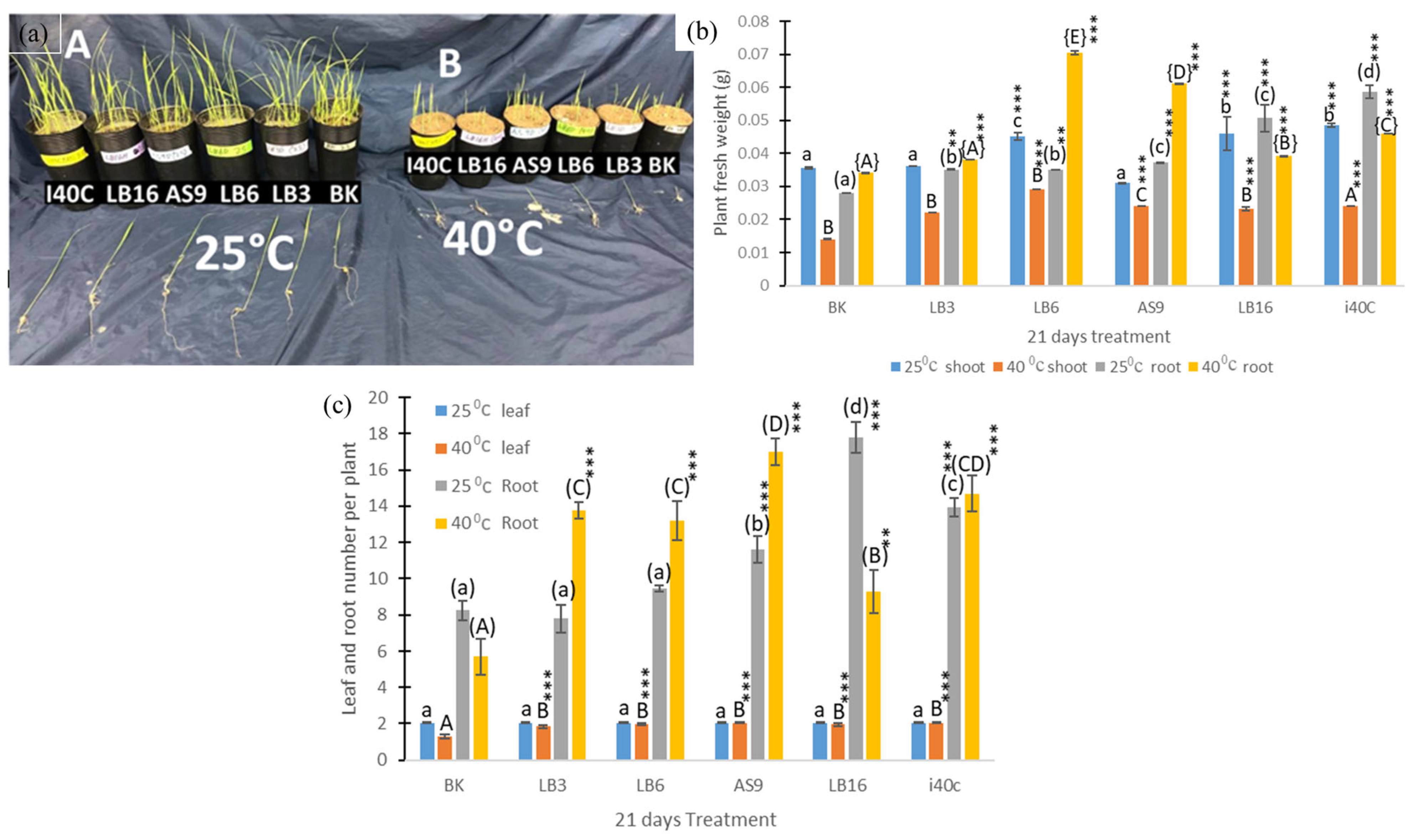
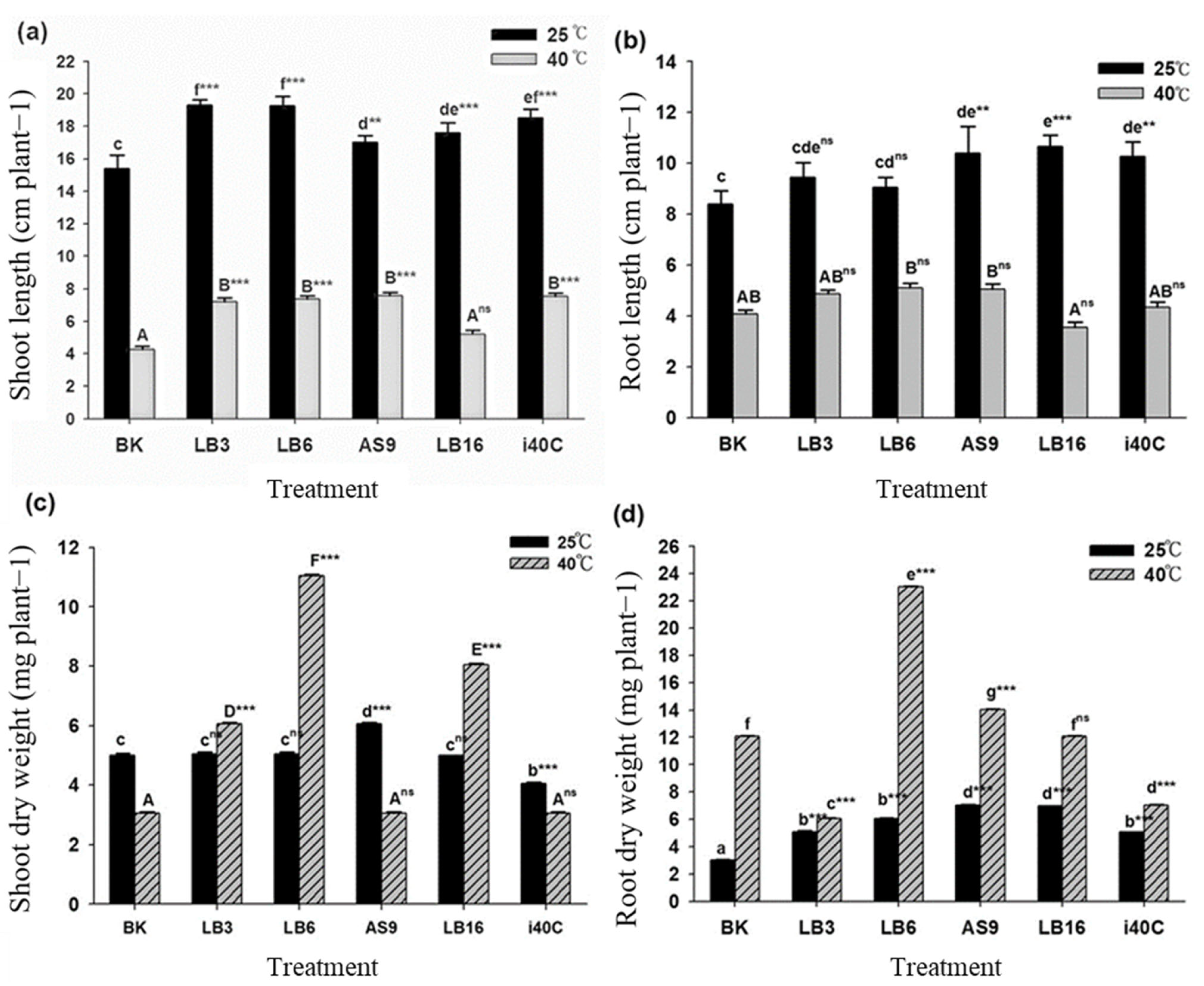
2.6. Measurement of Plant Growth Parameters
2.7. Analyzing Physiological Parameters in Response to Stress
2.8. Statistical Analysis
3. Results
3.1. Isolation of Thermotolerant Bacteria and Growth Temperature Test
3.2. Seed Germination Bioassay
3.3. Screening and Identification of Thermotolerant Endophytic Bacteria
3.4. Evaluating Nitrogen Fixation, Phosphate and Potassium Solubilization, IAA Production, and Siderophore Production
3.5. Plant Growth States
3.6. SPAD Chlorophyll Analysis
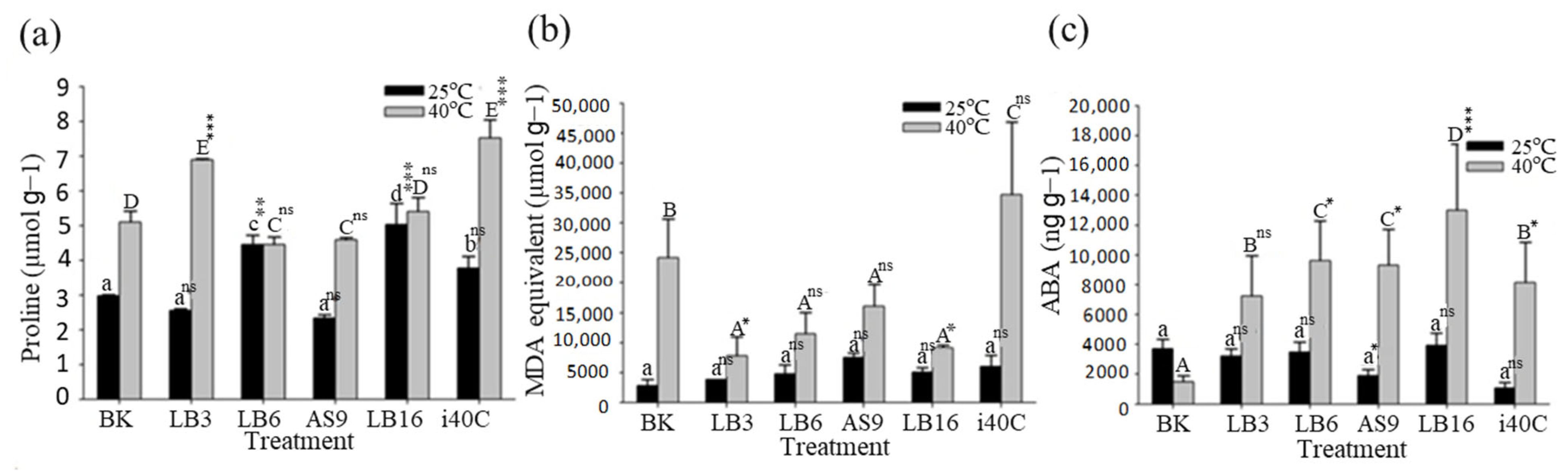
3.7. Osmoprotectant Proline Content
3.8. MDA Content
3.9. Endogenous Phytohormone Abscisic Acid (ABA)
3.10. Effect of Heat Stress and Inoculation on Measured Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathy, S.; Meena, S.; Babu, S.; Das, T.; Dhar, S. Phosphorus pools under integrated phosphorus management of upland direct-seeded rice (Oryza sativa) in North-Eastern Hill region of India. Indian J. Agron. 2021, 66, 495–497. [Google Scholar] [CrossRef]
- Awasthi, A.; Pattnayak, K.C.; Tandon, A.; Sarkar, A.; Chakraborty, M. Implications of climate change on surface temperature in North Indian states: Evidence from CMIP6 model ensembles. Front. Environ. Sci. 2023, 11, 1264757. [Google Scholar] [CrossRef]
- Anderson, R.; Bayer, P.E.; Edwards, D. Climate change and the need for agricultural adaptation. Curr. Opin. Plant Biol. 2020, 56, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Mayo, Z.A. Impact of temperature and rainfall on rice production in Punjab, Pakistan. Environ. Dev. Sustain. 2021, 23, 1706–1728. [Google Scholar] [CrossRef]
- Munakata, Y.; Spina, R.; Slezack-Deschaumes, S.; Genestier, J.; Hehn, A.; Laurain-Mattar, D. Screening of endophytic bacteria of Leucojum aestivum ‘gravety giant’ as a potential source of alkaloids and as antagonist to some plant fungal pathogens. Microorganisms 2022, 10, 2089. [Google Scholar] [CrossRef]
- Ganie, S.A.; Bhat, J.A.; Devoto, A. The influence of endophytes on rice fitness under environmental stresses. Plant Mol. Biol. 2022, 109, 447–467. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world–bacterial life within plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Haldar, S.; Sengupta, S. An Overview of the Multifaceted Role of Plant Growth-Promoting Microorganisms and Endophytes in Sustainable Agriculture: Developments and Prospects. In Microbial Symbionts and Plant Health: Trends and Applications for Changing Climate; Springer: Singapore, 2023; pp. 179–208. [Google Scholar]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Zhang, Q.; White, J.F. Bioprospecting desert plants for endophytic and biostimulant microbes: A strategy for enhancing agricultural production in a Hotter, drier future. Biology 2021, 10, 961. [Google Scholar] [CrossRef]
- Liang, Z.; Lin, X.; Liao, Y.; Tang, T. Characteristics and diversity of endophytic bacteria in Panax notoginseng under high temperature analysed using full-length 16S rRNA sequencing. Arch. Microbiol. 2022, 204, 435. [Google Scholar] [CrossRef]
- Sharma, P.; Suman, A.; Aswini, K.; SaiPrasad, J.; Gond, S. Endophytic bacterial taxonomic and functional diversity in the seeds of wheat genotypes from different agroecologies. J. Plant Interact. 2023, 18, 2227652. [Google Scholar]
- Devi, R.; Kaur, T.; Negi, R.; Kour, D.; Chaubey, K.K.; Yadav, A.N. Indigenous plant growth-promoting rhizospheric and endophytic bacteria as liquid bioinoculants for growth of sweet pepper (Capsicum annuum L.). Biologia 2023, 78, 2623–2633. [Google Scholar]
- Zhang, M.; Yang, L.; Hao, R.; Bai, X.; Wang, Y.; Yu, X. Drought-tolerant plant growth-promoting rhizobacteria isolated from jujube (Ziziphus jujuba) and their potential to enhance drought tolerance. Plant Soil 2020, 452, 423–440. [Google Scholar]
- Checinska Sielaff, A.; Urbaniak, C.; Mohan, G.B.M.; Stepanov, V.G.; Tran, Q.; Wood, J.M.; Minich, J.; McDonald, D.; Mayer, T.; Knight, R. Characterization of the total and viable bacterial and fungal communities associated with the International Space Station surfaces. Microbiome 2019, 7, 1–21. [Google Scholar]
- Rodríguez-Rodríguez, R.M.; Guimarães, A.A.; de Castro, J.L.; Siqueira, J.O.; Carneiro, M.A.C.; de Souza Moreira, F.M. Rhizobia and endophytic bacteria isolated from rainforest fragments within an iron ore mining site of the Eastern Brazilian Amazon. Braz. J. Microbiol. 2021, 52, 1461–1474. [Google Scholar]
- Montes-Luz, B.; Conrado, A.C.; Ellingsen, J.K.; Monteiro, R.A.; de Souza, E.M.; Stacey, G. Acetylene Reduction Assay: A Measure of Nitrogenase Activity in Plants and Bacteria. Curr. Protoc. 2023, 3, e766. [Google Scholar]
- Berza, B.; Sekar, J.; Vaiyapuri, P.; Pagano, M.C.; Assefa, F. Evaluation of inorganic phosphate solubilizing efficiency and multiple plant growth promoting properties of endophytic bacteria isolated from root nodules Erythrina brucei. BMC Microbiol. 2022, 22, 276. [Google Scholar]
- Singh, P.; Kardile, H.B.; Rawal, S.; Kumar, M.; Dua, V.; Sharma, J.; Kumar, S. Beneficial Effects of Bacterial Endophytes Isolated From Potato Roots And Tubers on Nutrient Solubilization. Potato J. 2022, 49, 82–94. [Google Scholar]
- Fiodor, A.; Ajijah, N.; Dziewit, L.; Pranaw, K. Biopriming of seed with plant growth-promoting bacteria for improved germination and seedling growth. Front. Microbiol. 2023, 14, 1142966. [Google Scholar]
- Alemneh, A.A.; Cawthray, G.R.; Zhou, Y.; Ryder, M.H.; Denton, M.D. Ability to produce indole acetic acid is associated with improved phosphate solubilising activity of rhizobacteria. Arch. Microbiol. 2021, 203, 3825–3837. [Google Scholar]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [PubMed]
- Chowdappa, S.; Jagannath, S.; Konappa, N.; Udayashankar, A.C.; Jogaiah, S. Detection and characterization of antibacterial siderophores secreted by endophytic fungi from Cymbidium aloifolium. Biomolecules 2020, 10, 1412. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Singh, N.; Jindal, S.; Kaur, R.; Goyal, A.; Awasthi, R. Kjeldahl method. Adv. Tech. Anal. Chem. 2022, 1, 105. [Google Scholar]
- Hameed, A.; Chen, Y.-P.; Shen, F.-T.; Lin, S.-Y.; Huang, H.-I.; Lin, Y.-W.; Young, C.-C. Evaluation of a subtropical maize-rice rotation system maintained under long-term fertilizer inputs for sustainable intensification of agriculture. Appl. Soil Ecol. 2023, 184, 104772. [Google Scholar]
- Tiwari, S.; Lata, C.; Chauhan, P.S.; Nautiyal, C.S.J.P.P. Biochemistry. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar]
- Lin, S.-Y.; Hameed, A.; Tsai, C.-F.; Young, C.-C. Description of Flavobacterium agricola sp. nov., an auxin producing bacterium isolated from paddy field. Antonie Van Leeuwenhoek 2023, 116, 1345–1357. [Google Scholar]
- Zhang, H.; Tu, Y.; Kang, J.; Song, W.; Zheng, L. Blue light dosage affects photosynthesis, chlorophyll, and antioxidant properties of Mesembryanthemum crystallinum. Photosynthetica 2021, 59, 547–556. [Google Scholar]
- Alexander, A.; Singh, V.K.; Mishra, A. Halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front. Microbiol. 2020, 11, 568289. [Google Scholar]
- Williams, P.; De Mallorca, M.S. Abscisic acid and gibberellin-like substances in roots and root nodules of Glycine max. Plant Soil 1982, 65, 19–26. [Google Scholar]
- Castro-Cegrí, A.; Sierra, S.; Hidalgo-Santiago, L.; Esteban-Muñoz, A.; Jamilena, M.; Garrido, D.; Palma, F. Postharvest Treatment with Abscisic Acid Alleviates Chilling Injury in Zucchini Fruit by Regulating Phenolic Metabolism and Non-Enzymatic Antioxidant System. Antioxidants 2023, 12, 211. [Google Scholar] [CrossRef]
- Cummins, W.; Sondheimer, E.J.P. Activity of the asymmetric isomers of abscisic acid in a rapid bioassay. Planta 1973, 111, 365–369. [Google Scholar] [PubMed]
- Yang, J.; Zhou, Q.; Shen, K.; Song, N.; Ni, L. Controlling nanodomain morphology of epoxy thermosets templated by poly (caprolactone)-block-poly (dimethylsiloxane)-block-poly (caprolactone) ABA triblock copolymer. RSC Adv. 2018, 8, 3705–3715. [Google Scholar] [PubMed]
- Arachchige, S.M.; Razzaq, A.; Dai, H.-Y.; Wang, J. Confronting Heat Stress in Crops Amid Global Warming: Impacts, Defense Mechanisms, and Strategies for Enhancing Thermotolerance. Crop Breed. Genet. Genom. 2024, 6, 20240011. [Google Scholar]
- Mishra, S.; Spaccarotella, K.; Gido, J.; Samanta, I.; Chowdhary, G. Effects of heat stress on plant-nutrient relations: An update on nutrient uptake, transport, and assimilation. Int. J. Mol. Sci. 2023, 24, 15670. [Google Scholar] [CrossRef]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef]
- Islam, M.N.; Masud, A.A.C.; Alam, M.M.; Islam, M.N.; Rahman, M.L.; Hasanuzzaman, M. Osmolyte-induced water deficit stress mitigation during panicle initiation stage in transplanted rice (Oryza sativa L.). Horizon 2022, 9, 9–20. [Google Scholar]
- Zulfiqar, F.; Ashraf, M. Proline alleviates abiotic stress induced oxidative stress in plants. J. Plant Growth Regul. 2023, 42, 4629–4651. [Google Scholar]
- Shaffique, S.; Khan, M.A.; Wani, S.H.; Pande, A.; Imran, M.; Kang, S.-M.; Rahim, W.; Khan, S.A.; Bhatta, D.; Kwon, E.-H. A review on the role of endophytes and plant growth promoting rhizobacteria in mitigating heat stress in plants. Microorganisms 2022, 10, 1286. [Google Scholar] [CrossRef]
- Anas, M.; Khalid, A.; Saleem, M.H.; Ali Khan, K.; Ahmed Khattak, W.; Fahad, S. Symbiotic Synergy: Unveiling Plant-Microbe Interactions in Stress Adaptation. J. Crop Health 2025, 77, 1–21. [Google Scholar]
- Lastochkina, O.; Garshina, D.; Allagulova, C.; Fedorova, K.; Koryakov, I.; Vladimirova, A. Application of endophytic Bacillus subtilis and salicylic acid to improve wheat growth and tolerance under combined drought and Fusarium root rot stresses. Agronomy 2020, 10, 1343. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Z.; Xie, J.; Hesselberg-Thomsen, V.; Tan, T.; Zheng, D.; Strube, M.L.; Dragoš, A.; Shen, Q.; Zhang, R. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022, 16, 774–787. [Google Scholar] [PubMed]
- Carreiras, J.; Cruz-Silva, A.; Fonseca, B.; Carvalho, R.C.; Cunha, J.P.; Proença Pereira, J.; Paiva-Silva, C.; Santos, S.A.; Janeiro Sequeira, R.; Mateos-Naranjo, E. Improving Grapevine Heat Stress Resilience with Marine Plant Growth-Promoting Rhizobacteria Consortia. Microorganisms 2023, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Notununu, I.; Moleleki, L.; Roopnarain, A.; Adeleke, R. Effects of plant growth-promoting rhizobacteria on the molecular responses of maize under drought and heat stresses: A review. Pedosphere 2022, 32, 90–106. [Google Scholar]
- Sharma, M.M.M.; Sharma, P.; Kapoor, D.; Beniwal, P.; Mehta, S. Phytomicrobiome community: An agrarian perspective towards resilient agriculture. In Plant Performance Under Environmental Stress: Hormones, Biostimulants and Sustainable Plant Growth Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 493–534. [Google Scholar]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Jalota, K.; Agarwal, C.; Pal, P.; Jindal, S. Biochemical Responses of Plants to Individual and Combined Abiotic Stresses. In Plant Secondary Metabolites and Abiotic Stress; Wikly: Hoboken, NJ, USA, 2024; pp. 1–34. [Google Scholar]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Fakhar, A.; Rafique, M.; Chen, Y. Plant growth-promoting rhizobacteria eliminate the effect of drought stress in plants: A review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar]
- Xiong, R.; Chen, Y. Molecular mechanisms and nutrient regulation of crop root responses to drought stress: Interactions with rhizosphere microorganisms. In Sustainable Agriculture Under Drought Stress; Elsevier: Amsterdam, The Netherlands, 2025; pp. 499–509. [Google Scholar]
- Khan, M.S.; Zulfiqar, I. Microbial mitigation of drought stress in plants: Adaptations to climate change. In Abiotic Stress in Plants—Adaptations to Climate Change; IntechOpen: London, UK, 2023. [Google Scholar]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R. Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Hung, S.-H.; Huang, E.; Huang, C.-C. Roles of plant growth-promoting rhizobacteria (PGPR) in stimulating salinity stress defense in plants: A review. Int. J. Mol. Sci. 2021, 22, 3154. [Google Scholar] [CrossRef]


| Strain | N Fixation (n Mole Ethylene h−1) | P Solubilization | K Solubilization | IAA Production (mg mL−1) | Production of Siderophore | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 30 °C | 40 °C | 45 °C | 30 °C | 40 °C | 45 °C | 30 °C | 40 °C | 45 °C | 30 °C | 40 °C | 45 °C | |
| DF18 | 0.06 ± 0.02 a | − | + | ++ | − | − | − | 7.75 ± 0.1 b | 3.22 ± 2.71 a | 14.26 ± 0.32 e | ++ | ++ | ++ |
| DF36 | 0.02 ± 0.01 a | - | + | ++ | + | + | + | 4.65 ± 0.29 a | 3.35 ± 0.06 a | 1.74 ± 0.33 a | ++ | ++ | ++ |
| LB16 | 0.24 ± 0.02 a | ++ | + | − | + | − | − | 15.24 ± 0.32 d | 13.71 ± 0.2 a | 8.73 ± 0.33 d | + | ++ | + |
| LB3 | 0.03 ± 0.02 a | - | + | + | + | + | + | 7.79 ± 0.24 b | 6.63 ± 32 b | 3.90 ± 0.29 b | ++ | ++ | ++ |
| AS10 | 0.38 ± 0.04 a | - | − | − | − | − | − | 11.10 ± 0.33 c | 0.86 ± 0.3 a | 5.13 ± 0.30 c | + | + | + |
| i40C | 0.01 ± 0.006 a | - | − | − | − | − | − | 7.34 ± 0.33 b | 12.71 ± 0.33 c | 33.93 ± 0.33 g | + | ++ | ++ |
| AS9 | 0.02 ± 0.01 a | - | − | − | − | − | − | 54.04 ± 0.33 f | 14.40 ± 0.33 c | 36.07 ± 0.33 h | + | + | + |
| LB6 | 0.13 ± 0.0.07 a | + | + | + | − | − | − | 28.71 ± 0.13 e | 40.18 ± 0.33 d | 23.63 ± 0.33 f | + | ++ | ++ |
| Source of Variation | Heat Stress | Inoculation | Heat Stress × Inoculation |
|---|---|---|---|
| Shoot length | 1986 *** | 19 *** | 2 * |
| Root length | 370 *** | 2 ns | 3 ** |
| Shoot dry weight | 800 *** | 777 *** | 297 *** |
| Root dry weight | 1681 *** | 961 *** | 625 *** |
| Chlorophyll SPAD | 172 *** | 8 *** | 0.9 ns |
| Proline | 139 *** | 12 *** | 15 *** |
| MDA content | 23 *** | 3 * | 2 * |
| Abscisic acid | 19 *** | 1.8 ns | 1.9 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlamini, W.N.; Lai, W.-A.; Chen, W.-C.; Shen, F.-T. Unveiling the Thermotolerance and Growth-Promoting Attributes of Endophytic Bacteria Derived from Oryza sativa: Implications for Sustainable Agriculture. Microorganisms 2025, 13, 766. https://doi.org/10.3390/microorganisms13040766
Dlamini WN, Lai W-A, Chen W-C, Shen F-T. Unveiling the Thermotolerance and Growth-Promoting Attributes of Endophytic Bacteria Derived from Oryza sativa: Implications for Sustainable Agriculture. Microorganisms. 2025; 13(4):766. https://doi.org/10.3390/microorganisms13040766
Chicago/Turabian StyleDlamini, Wonder Nathi, Wei-An Lai, Wen-Ching Chen, and Fo-Ting Shen. 2025. "Unveiling the Thermotolerance and Growth-Promoting Attributes of Endophytic Bacteria Derived from Oryza sativa: Implications for Sustainable Agriculture" Microorganisms 13, no. 4: 766. https://doi.org/10.3390/microorganisms13040766
APA StyleDlamini, W. N., Lai, W.-A., Chen, W.-C., & Shen, F.-T. (2025). Unveiling the Thermotolerance and Growth-Promoting Attributes of Endophytic Bacteria Derived from Oryza sativa: Implications for Sustainable Agriculture. Microorganisms, 13(4), 766. https://doi.org/10.3390/microorganisms13040766







