Industrial Microbial Technologies for Feed Protein Production from Non-Protein Nitrogen
Abstract
1. Introduction
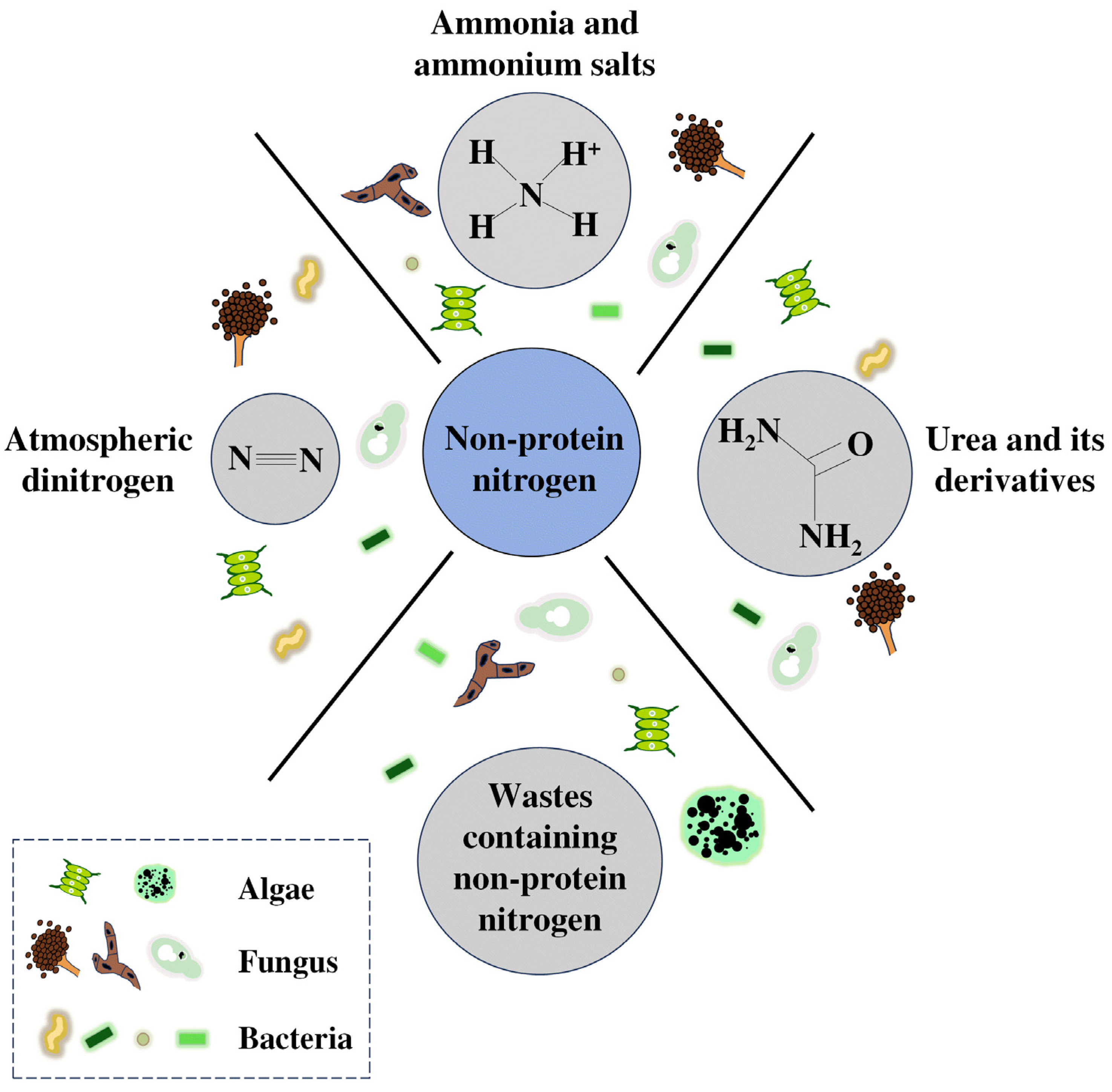
2. Assimilation of Non-Protein Nitrogen Sources by Microorganisms
2.1. Atmospheric Dinitrogen
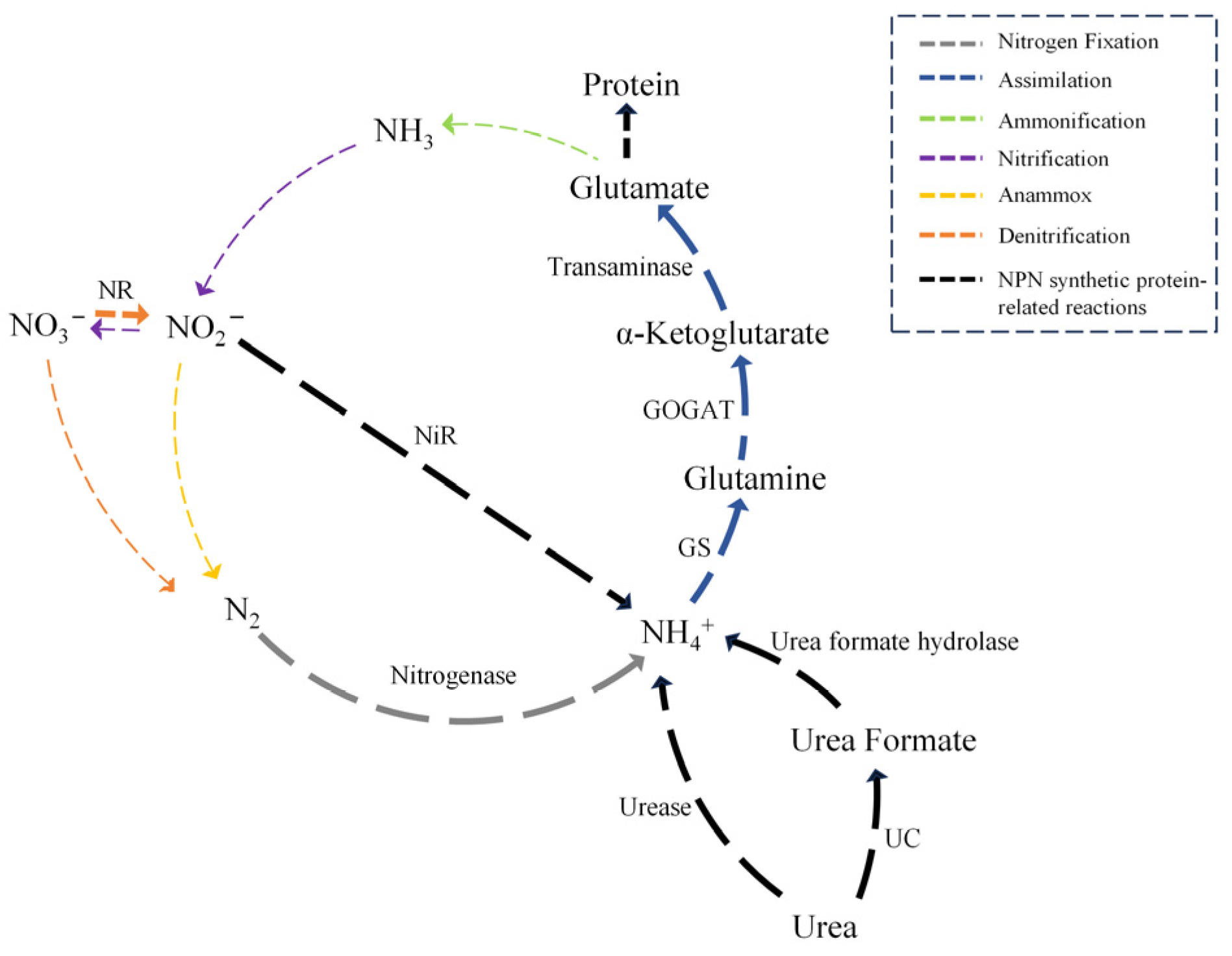
2.2. Urea and Its Derivatives
2.3. Ammonia and Ammonium Salts
2.4. Wastes Containing Non-Protein Nitrogen
3. Fundamental of Non-Protein Nitrogen Assimilation by Microorganisms
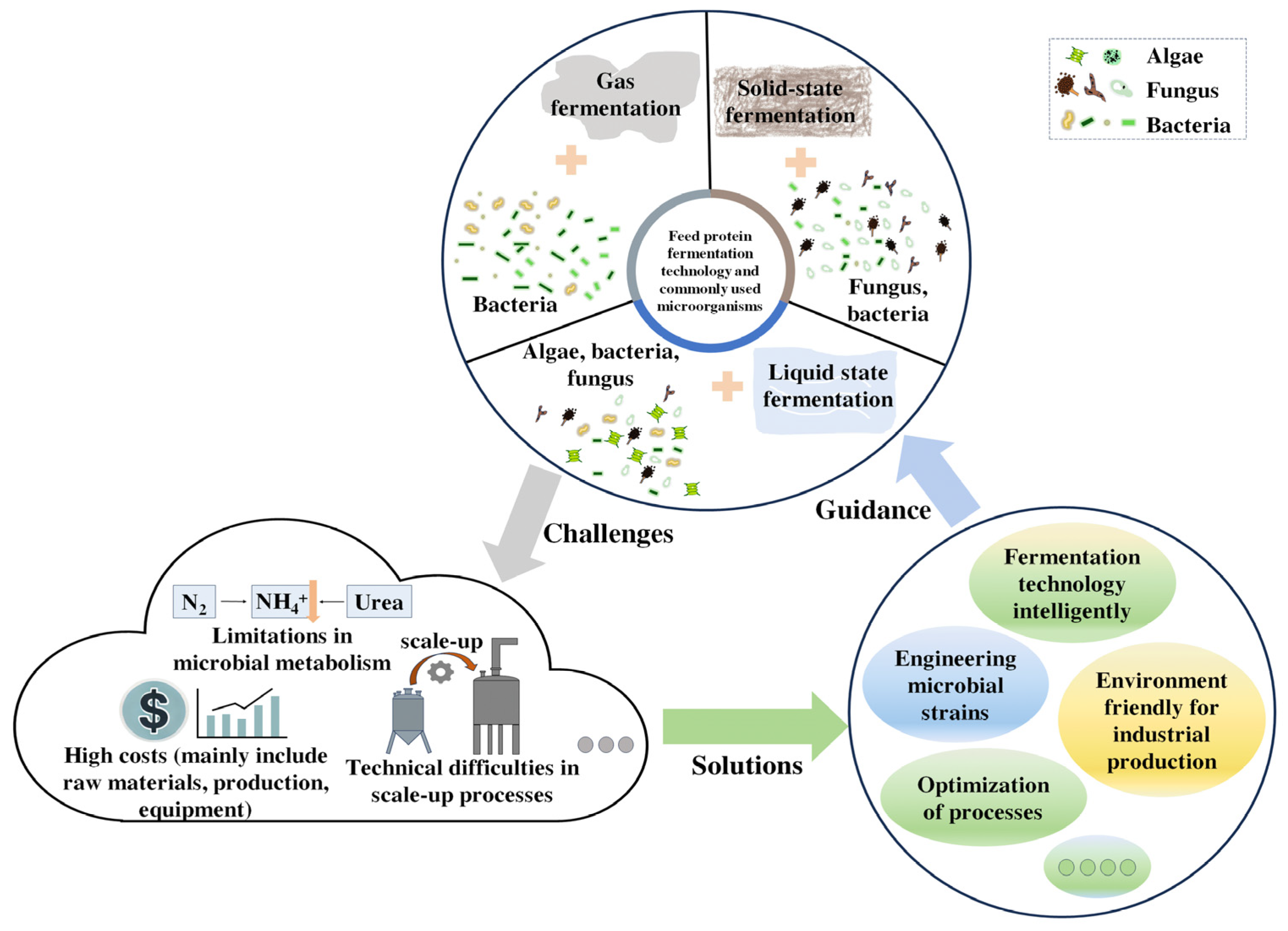
4. Industrial Fermentation Technology to Produce Feed Protein
4.1. Solid State Fermentation
4.2. Liquid State Fermentation
4.3. Gas Fermentation
5. Evolution and Screening of Industrial Strains Enhancing the Assimilation of Non-Protein Nitrogen
6. Conclusions and Further Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPN | Non-protein nitrogen |
| RDA | Recommended Dietary Allowance |
| UCYN-A | Candidatus Atelocyanobacter thalassa |
| KBM | KnipBio Meal |
| FDA | Food and Drug Administration |
| MAFF | Ministry of Agriculture, Forestry, and Fisheries |
| GFMP | Good Feed Manufacturing Practice |
| NR | Nitrate reductase |
| NiR | Nitrite reductase |
| GS | Glutamine synthetase |
| GOGAT | Glutamate synthetase |
| UC | Urea carboxylase |
| Cu-NiRs | Copper-type nitrite reductases |
| cd1NiRs | Cytochrome cd1-type nitrite reductases |
| ccNiRs | Multiheme nitrite reductase |
| SSF | Solid state fermentation |
| LSF | Liquid state fermentation |
| GF | Gas fermentation |
| ALE | Adaptive laboratory evolution |
| ARTP | Atmospheric pressure room temperature plasma |
| HTS | High-throughput screening |
References
- Lonnie, M.; Hooker, E.; Brunstrom, J.; Corfe, B.; Green, M.; Watson, A.; Williams, E.; Stevenson, E.; Penson, S.; Johnstone, A. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Watson, A.W.; Lonnie, M.; Peeters, W.M.; Oonincx, D.; Tsoutsoura, N.; Simon-Miquel, G.; Szepe, K.; Cochetel, N.; Pearson, A.G.; et al. Meeting the Global Protein Supply Requirements of a Growing and Ageing Population. Eur. J. Nutr. 2024, 63, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.; Panaite, S.-A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef]
- Liu, Y.; Aimutis, W.R.; Drake, M. Dairy, Plant, and Novel Proteins: Scientific and Technological Aspects. Foods 2024, 13, 1010. [Google Scholar] [CrossRef]
- Smil, V. Nitrogen and Food Production: Proteins for Human Diets. AMBIO A J. Hum. Environ. 2002, 31, 126–131. [Google Scholar] [CrossRef]
- Clark, M.; Tilman, D. Comparative Analysis of Environmental Impacts of Agricultural Production Systems, Agricultural Input Efficiency, and Food Choice. Environ. Res. Lett. 2017, 12, 064016. [Google Scholar] [CrossRef]
- Sodiq, A.; Baloch, A.A.B.; Khan, S.A.; Sezer, N.; Mahmoud, S.; Jama, M.; Abdelaal, A. Towards Modern Sustainable Cities: Review of Sustainability Principles and Trends. J. Clean. Prod. 2019, 227, 972–1001. [Google Scholar] [CrossRef]
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; Van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The Future Supply of Animal-Derived Protein for Human Consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Review: Feed Demand Landscape and Implications of Food-Not Feed Strategy for Food Security and Climate Change. Animal 2018, 12, 1744–1754. [Google Scholar] [CrossRef]
- Kim, S.W.; Less, J.F.; Wang, L.; Yan, T.; Kiron, V.; Kaushik, S.J.; Lei, X.G. Meeting Global Feed Protein Demand: Challenge, Opportunity, and Strategy. Annu. Rev. Anim. Biosci. 2019, 7, 221–243. [Google Scholar] [CrossRef]
- Parisi, G.; Tulli, F.; Fortina, R.; Marino, R.; Bani, P.; Dalle Zotte, A.; De Angelis, A.; Piccolo, G.; Pinotti, L.; Schiavone, A.; et al. Protein Hunger of the Feed Sector: The Alternatives Offered by the Plant World. Ital. J. Anim. Sci. 2020, 19, 1204–1225. [Google Scholar] [CrossRef]
- Santillan, E.; Yasumaru, F.; Vethathirri, R.S.; Thi, S.S.; Hoon, H.Y.; Sian, D.C.P.; Wuertz, S. Microbial Community-Based Protein from Soybean-Processing Wastewater as a Sustainable Alternative Fish Feed Ingredient. Sci. Rep. 2024, 14, 2620. [Google Scholar] [CrossRef]
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.-S.; Ciais, P.; Tubiello, F.N.; Smith, P.; Campbell, N.; Jain, A.K. Global Greenhouse Gas Emissions from Animal-based Foods are Twice those of Plant-based Foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef]
- Li, Y.P.; Ahmadi, F.; Kariman, K.; Lackner, M. Recent Advances and Challenges in Single Cell Protein (SCP) Technologies for Food and Feed Production. npj Sci. Food 2024, 8, 66. [Google Scholar] [CrossRef]
- Ayodele, T.; Tijani, A.; Liadi, M.; Alarape, K.; Clementson, C.; Hammed, A. Biomass-Based Microbial Protein Production: A Review of Processing and Properties. Front. Biosci.-Elite 2024, 16, 40. [Google Scholar] [CrossRef]
- Nadar, C.G.; Fletcher, A.; Moreira, B.R.D.A.; Hine, D.; Yadav, S. Waste to Protein: A Systematic Review of a Century of Advancement in Microbial Fermentation of Agro-industrial Byproducts. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13375. [Google Scholar] [CrossRef]
- Bojana, B.; Vucurovic, D.; Vasic, D.; Jevtic, M.R.; Dodic, S. Biotechnological Production of Sustainable Microbial Proteins from Agro-Industrial Residues and By-Products. Foods 2022, 12, 107. [Google Scholar] [CrossRef]
- Muniz, E.D.N.; Montenegro, R.T.D.Q.; Da Silva, D.N.; D’Almeida, A.P.; Gonçalves, L.R.B.; De Albuquerque, T.L. Advances in Biotechnological Strategies for Sustainable Production of Non-Animal Proteins: Challenges, Innovations, and Applications. Fermentation 2024, 10, 638. [Google Scholar] [CrossRef]
- Chama, N.T. Production of Single-Cell Protein from Different Substrates. Aust. J. Sci. Technol. 2019, 3, 148–153. [Google Scholar]
- Xie, R.; Wang, Y.; Chen, Q.; Guo, W.; Jiao, N.; Zheng, Q. Coupling Between Carbon and Nitrogen Metabolic Processes Mediated by Coastal Microbes in Synechococcus-Derived Organic Matter Addition Incubations. Front. Microbiol. 2020, 11, 1041. [Google Scholar] [CrossRef]
- Bibra, M.; Samanta, D.; Sharma, N.K.; Singh, G.; Johnson, G.R.; Sani, R.K. Food Waste to Bioethanol: Opportunities and Challenges. Fermentation 2022, 9, 8. [Google Scholar] [CrossRef]
- Jagannathan, P.; Muthukumaran, C.; Tamilarasan, K. A Sequential Pretreatment of Lignocelluloses in Bamboo Biomass to Fermentable Sugars by Acid/Enzymatic Hydrolysis. 3 Biotech 2017, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Alawad, I.; Ibrahim, H. Pretreatment of Agricultural Lignocellulosic Biomass for Fermentable Sugar: Opportunities, Challenges, and Future Trends. Biomass Convers. Biorefinery 2024, 14, 6155–6183. [Google Scholar] [CrossRef]
- Begum, W.; Saha, B.; Mandal, U. A Comprehensive Review on Production of Bio-Surfactants by Bio-Degradation of Waste Carbohydrate Feedstocks: An Approach towards Sustainable Development. RSC Adv. 2023, 13, 25599–25615. [Google Scholar] [CrossRef]
- Ahamefule, C.S.; Osilo, C.; Ahamefule, B.C.; Madueke, S.N.; Moneke, A.N. Simultaneous Production of Biofuel from Agricultural Wastes and Bioremediation of the Waste Substrates: A Review. Curr. Res. Microb. Sci. 2024, 7, 100305. [Google Scholar] [CrossRef]
- Gao, L.; Liu, X. Effects of Carbon Concentrations and Carbon to Nitrogen Ratios on Sporulation of Two Biological Control Fungi as Determined by Different Culture Methods. Mycopathologia 2010, 169, 475–481. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Hadiyarto, A.; Soetrisnanto, D.; Rosyidin, I.; Fitriana, A. Co-Digestion of Bagasse and Waterhyacinth for Biogas Production with Variation of C/N and Activated Sludge. J. Phys. Conf. Ser. 2019, 1295, 012050. [Google Scholar] [CrossRef]
- Panda, J.; Amrit, R.; Mishra, A.K.; Chakraborty, A.; Rustagi, S.; Nath, P.C.; Sarabandi, K.; Sarma, H.; Wagh, M.S.; Mohanta, Y.K. Sustainable Valorization of Fruit and Vegetable Waste for Bioactive Compounds: Advancing Functional Food and Wellness. Waste Biomass Valor 2025, 1–30. [Google Scholar] [CrossRef]
- Guida, S.; Van Peteghem, L.; Luqmani, B.; Sakarika, M.; McLeod, A.; McAdam, E.J.; Jefferson, B.; Rabaey, K.; Soares, A. Ammonia Recovery from Brines Originating from a Municipal Wastewater Ion Exchange Process and Valorization of Recovered Nitrogen into Microbial Protein. Chem. Eng. J. 2022, 427, 130896. [Google Scholar] [CrossRef]
- Olsen, P.M.; Horn, S.J.; Byrtusova, D.; Moen, L.F.; Shapaval, V.; Hansen, L.D. Assessment of Different Nitrogen Sources and Bioreactor Cultivation Strategies during Growth of Aurantiochytrium Limacinum on Spruce Sugars. Algal Res. 2025, 86, 103951. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Newbold, J.R. Effect of Ammonia Concentration on Rumen Microbial Protein Production In Vitro. Britisb J. Nutr. 2022, 127, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Ijaola, A.O.; Akamo, D.O.; George, T.T.; Sengul, A.; Adediji, M.Y.; Asmatulu, E. Algae as a Potential Source of Protein: A Review on Cultivation, Harvesting, Extraction, and Applications. Algal Res. 2024, 77, 103329. [Google Scholar] [CrossRef]
- Nandy, S.K.; Srivastava, R.K. A Review on Sustainable Yeast Biotechnological Processes and Applications. Microbiol. Res. 2018, 207, 83–90. [Google Scholar] [CrossRef]
- Koch, H.; Sessitsch, A. The Microbial-Driven Nitrogen Cycle and Its Relevance for Plant Nutrition. J. Exp. Bot. 2024, 75, 5547–5556. [Google Scholar] [CrossRef]
- Li, J.; Yuan, M.; Meng, N.; Li, H.; Sun, J.; Sun, B. Influence of Nitrogen Status on Fermentation Performances of Non- Saccharomyces Yeasts: A Review. Food Sci. Hum. Wellness 2024, 13, 556–567. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, Y.; Zhu, J.; Zhou, J.; Lin, H.; Fu, Y.; Zhou, Y. Transcriptomic Analysis of Nitrogen Metabolism Pathways in Klebsiella Aerogenes Under Nitrogen-Rich Conditions. Front. Microbiol. 2024, 15, 1323160. [Google Scholar] [CrossRef]
- Baumann, K.B.L.; Mazzoli, A.; Salazar, G.; Ruscheweyh, H.-J.; Müller, B.; Niederdorfer, R.; Sunagawa, S.; Lever, M.A.; Lehmann, M.F.; Bürgmann, H. Metagenomic and -Transcriptomic Analyses of Microbial Nitrogen Transformation Potential, and Gene Expression in Swiss Lake Sediments. ISME Commun. 2024, 4, ycae110. [Google Scholar] [CrossRef]
- Rojo, M.C.; Talia, P.M.; Lerena, M.C.; Ponsone, M.L.; Gonzalez, M.L.; Becerra, L.M.; Mercado, L.A.; Martín-Arranz, V.; Rodríguez-Gómez, F.; Arroyo-López, F.N.; et al. Evaluation of Different Nitrogen Sources on Growth and Fermentation Performance for Enhancing Ethanol Production by Wine Yeasts. Heliyon 2023, 9, e22608. [Google Scholar] [CrossRef]
- Tang, S.; Pan, W.; Zhou, J.; Ma, Q.; Yang, X.; Wanek, W.; Marsden, K.A.; Kuzyakov, Y.; Chadwick, D.R.; Wu, L.; et al. Soil Nitrogen and Phosphorus Regulate Decomposition of Organic Nitrogen Compounds in the Rothamsted Experiment. Soil Biol. Biochem. 2024, 196, 109502. [Google Scholar] [CrossRef]
- Hu, C.-C.; Liu, X.-Y.; Driscoll, A.W.; Kuang, Y.-W.; Brookshire, E.N.J.; Lü, X.-T.; Chen, C.-J.; Song, W.; Mao, R.; Liu, C.-Q.; et al. Global Distribution and Drivers of Relative Contributions among Soil Nitrogen Sources to Terrestrial Plants. Nat. Commun. 2024, 15, 6407. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhao, S.; Zheng, N.; Beckers, Y.; Wang, J. Urea Metabolism and Regulation by Rumen Bacterial Urease in Ruminants–A Review. Ann. Anim. Sci. 2018, 18, 303–318. [Google Scholar] [CrossRef]
- Dixon, R.; Kahn, D. Genetic Regulation of Biological Nitrogen Fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Massana, R. The Nitroplast: A Nitrogen-Fixing Organelle. Science 2024, 384, 160–161. [Google Scholar] [CrossRef]
- Guo, K.; Yang, J.; Yu, N.; Luo, L.; Wang, E. Biological Nitrogen Fixation in Cereal Crops: Progress, Strategies, and Perspectives. Plant Commun. 2023, 4, 100499. [Google Scholar] [CrossRef]
- Dai, H.; Wei, S.; Li, J.; Kong, W.; Wang, B.; Pei, J.; Wu, J. Fertilization Effects on Symbiotic and Free-Living Biological Nitrogen Fixations: Similar Effects but Different Mechanisms. Appl. Soil Ecol. 2024, 202, 105590. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Tian, C.; Fan, S.; Zheng, D.; Song, Y.; Gao, P.; Li, D. Effects of Nitrogen Supply on Hydrogen-Oxidizing Bacterial Enrichment to Produce Microbial Protein: Comparing Nitrogen Fixation and Ammonium Assimilation. Bioresour. Technol. 2024, 394, 130199. [Google Scholar] [CrossRef]
- Hu, X.; Vandamme, P.; Boon, N. Co-Cultivation Enhanced Microbial Protein Production Based on Autotrophic Nitrogen-Fixing Hydrogen-Oxidizing Bacteria. Chem. Eng. J. 2022, 429, 132535. [Google Scholar] [CrossRef]
- Hu, X.; Kerckhof, F.-M.; Ghesquière, J.; Bernaerts, K.; Boeckx, P.; Clauwaert, P.; Boon, N. Microbial Protein out of Thin Air: Fixation of Nitrogen Gas by an Autotrophic Hydrogen-Oxidizing Bacterial Enrichment. Environ. Sci. Technol. 2020, 54, 3609–3617. [Google Scholar] [CrossRef]
- Masson-Boivin, C. Symbiotic Nitrogen Fixation by Rhizobia—The Roots of a Success Story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Huang, K.; Wang, F.; Mei, Z. Molecular Mechanism and Agricultural Application of the NifA–NifL System for Nitrogen Fixation. Int. J. Mol. Sci. 2023, 24, 907. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.D.; Poole, P.S.; et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Westhead, O.; Barrio, J.; Bagger, A.; Murray, J.W.; Rossmeisl, J.; Titirici, M.-M.; Jervis, R.; Fantuzzi, A.; Ashley, A.; Stephens, I.E.L. Near Ambient N2 Fixation on Solid Electrodes versus Enzymes and Homogeneous Catalysts. Nat. Rev. Chem. 2023, 7, 184–201. [Google Scholar] [CrossRef]
- Riyaz, Z.; Khan, S.T. Nitrogen Fixation by Methanogenic Archaea, Literature Review and DNA Database-Based Analysis; Significance in Face of Climate Change. Arch. Microbiol. 2025, 207, 6. [Google Scholar] [CrossRef]
- Kartal, B.; Keltjens, J.T. Anammox Biochemistry: A Tale of Heme c Proteins. Trends Biochem. Sci. 2016, 41, 998–1011. [Google Scholar] [CrossRef]
- Ettwig, K.F.; Butler, M.K.; Le Paslier, D.; Pelletier, E.; Mangenot, S.; Kuypers, M.M.M.; Schreiber, F.; Dutilh, B.E.; Zedelius, J.; De Beer, D.; et al. Nitrite-Driven Anaerobic Methane Oxidation by Oxygenic Bacteria. Nature 2010, 464, 543–548. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial Reactive Oxygen and Nitrogen Species: Concepts and Controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Bennett, E.M.; Murray, J.W.; Isalan, M. Engineering Nitrogenases for Synthetic Nitrogen Fixation: From Pathway Engineering to Directed Evolution. BioDesign Res. 2023, 5, 0005. [Google Scholar] [CrossRef]
- Han, Y.; Lv, M.; Liu, J.; He, S.; Shi, W.; Li, M.; Gao, Z. Agronomic Practices-Driven Response of Nitrogen-Related Microorganisms. Plant Soil 2025, 1–16. [Google Scholar] [CrossRef]
- Michel-Reydellet, N.; Kaminski, P.A. Azorhizobium caulinodans PII and GlnK Proteins Control Nitrogen Fixation and Ammonia Assimilation. J. Bacteriol. 1999, 181, 2655–2658. [Google Scholar] [CrossRef]
- Schnabel, T.; Sattely, E. Engineering Posttranslational Regulation of Glutamine Synthetase for Controllable Ammonia Production in the Plant Symbiont Azospirillum brasilense. Appl. Environ. Microbiol. 2021, 87, e00582-21. [Google Scholar] [CrossRef]
- Ito, Y.; Yoshidome, D.; Hidaka, M.; Araki, Y.; Ito, K.; Kosono, S.; Nishiyama, M. Improvement of the Nitrogenase Activity in Escherichia coli That Expresses the Nitrogen Fixation-Related Genes from Azotobacter vinelandii. Biochem. Biophys. Res. Commun. 2024, 728, 150345. [Google Scholar] [CrossRef]
- Tatemichi, Y.; Nakahara, T.; Ueda, M.; Kuroda, K. Construction of Recombinant Escherichia coli Producing Nitrogenase-Related Proteins from Azotobacter vinelandii. Biosci. Biotechnol. Biochem. 2021, 85, 2209–2216. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y.; Ma, Y.; Chen, Q.; Zhan, Y.; Lu, W.; Cai, L.; Hou, M.; Chen, S.; Yan, Y.; et al. Global Investigation of an Engineered Nitrogen-Fixing Escherichia coli Strain Reveals Regulatory Coupling between Host and Heterologous Nitrogen-Fixation Genes. Sci. Rep. 2018, 8, 10928. [Google Scholar] [CrossRef]
- Brown, K.A.; Harris, D.F.; Wilker, M.B.; Rasmussen, A.; Khadka, N.; Hamby, H.; Keable, S.; Dukovic, G.; Peters, J.W.; Seefeldt, L.C.; et al. Light-Driven Dinitrogen Reduction Catalyzed by a CdS:Nitrogenase MoFe Protein Biohybrid. Science 2016, 352, 448–450. [Google Scholar] [CrossRef]
- Dey, S.; Awata, T.; Mitsushita, J.; Zhang, D.; Kasai, T.; Matsuura, N.; Katayama, A. Promotion of Biological Nitrogen Fixation Activity of an Anaerobic Consortium Using Humin as an Extracellular Electron Mediator. Sci. Rep. 2021, 11, 6567. [Google Scholar] [CrossRef]
- Luo, Y.W.; Lou, Y.W.; Shi, D.; Kranz, S.A.; Hopkinson, B.M.; Hong, H.; Shen, R.; Zhang, F. Reduced Nitrogenase Efficiency Dominates Response of the Globally Important Nitrogen Fixer Trichodesmium to Ocean Acidification. Nat. Commun. 2019, 10, 1521. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on Materials & Methods to Produce Controlled Release Coated Urea Fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Foschi, F.G. Urea Cycle Disorders: A Case Report of a Successful Treatment with Liver Transplant and a Literature Review. World J. Gastroenterol. 2015, 21, 4063. [Google Scholar] [CrossRef]
- Mobley, H.L.; Hausinger, R.P. Microbial Ureases: Significance, Regulation, and Molecular Characterization. Microbiol. Rev. 1989, 53, 85–108. [Google Scholar] [CrossRef]
- Patra, A.K.; Aschenbach, J.R. Ureases in the Gastrointestinal Tracts of Ruminant and Monogastric Animals and Their Implication in Urea-N/Ammonia Metabolism: A Review. J. Adv. Res. 2018, 13, 39–50. [Google Scholar] [CrossRef]
- González-Martín, I.; Hernández-Hierro, J.M. Detection and Quantification of Additives (Urea, Biuret and Poultry Litter) in Alfalfas by Nir Spectroscopy with Fibre-Optic Probe. Talanta 2008, 76, 1130–1135. [Google Scholar] [CrossRef]
- Inácio, A.G.; Ítavo, C.C.B.F.; Dias, A.M.; Dos Santos Difante, G.; De Queiroz, J.F.; De Oliveira, L.C.S.; Dos Santos, G.T.; Ítavo, L.C.V. A New Feed Additive Composed of Urea and Soluble Carbohydrate Coated with Wax for Controlled Release in Ruminal Fluid. Sci. Rep. 2022, 12, 4487. [Google Scholar] [CrossRef]
- Esteves, E.A.; Martino, H.S.D.; Oliveira, F.C.E.; Bressan, J.; Costa, N.M.B. Chemical Composition of a Soybean Cultivar Lacking Lipoxygenases (LOX2 and LOX3). Food Chem. 2010, 122, 238–242. [Google Scholar] [CrossRef]
- Suriyapha, C.; Suntara, C.; Wanapat, M.; Cherdthong, A. Effects of Substituting Agro-Industrial by-Products for Soybean Meal on Beef Cattle Feed Utilization and Rumen Fermentation. Sci. Rep. 2022, 12, 21630. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, J.; Yuan, X.; Wang, D.; Luo, H.; Yang, R.; Wang, H. Urea Promotes Alkaline Anaerobic Fermentation of Waste Activated Sludge for Hydrogen Production. Bioresour. Technol. 2025, 418, 131900. [Google Scholar] [CrossRef]
- Tourna, M.; Stieglmeier, M.; Spang, A.; Könneke, M.; Schintlmeister, A.; Urich, T.; Engel, M.; Schloter, M.; Wagner, M.; Richter, A.; et al. Nitrososphaera viennensis, an Ammonia Oxidizing Archaeon from Soil. Proc. Natl. Acad. Sci. USA 2011, 108, 8420–8425. [Google Scholar] [CrossRef]
- Strope, P.K.; Nickerson, K.W.; Harris, S.D.; Moriyama, E.N. Molecular Evolution of Urea Amidolyase and Urea Carboxylase in Fungi. BMC Evol. Biol. 2011, 11, 80. [Google Scholar] [CrossRef]
- Konzock, O.; Zaghen, S.; Fu, J.; Kerkhoven, E.J. Urea Is a Drop-in Nitrogen Source Alternative to Ammonium Sulphate in Yarrowia lipolytica. iScience 2022, 25, 105703. [Google Scholar] [CrossRef]
- Brabender, M.; Hussain, M.S.; Rodriguez, G.; Blenner, M.A. Urea and Urine Are a Viable and Cost-Effective Nitrogen Source for Yarrowia lipolytica Biomass and Lipid Accumulation. Appl. Microbiol. Biotechnol. 2018, 102, 2313–2322. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, D.; Shi, Y.-C. Effects of Nitrogen Source on Ethanol Production in Very High Gravity Fermentation of Corn Starch. J. Taiwan Inst. Chem. Eng. 2017, 70, 229–235. [Google Scholar] [CrossRef]
- Chan-u-tit, P.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. High Level Ethanol Production by Nitrogen and Osmoprotectant Supplementation Under Very High Gravity Fermentation Conditions. Energies 2013, 6, 884–899. [Google Scholar] [CrossRef]
- Afrasiab, K.T.; Noppawan, D.; Imrana, N.S.; Nicom, L.; Sarote, S.; Wirat, V.; Pramuk, P. Utilization of Urea as a Nitrogen Source for Ethanol Production from Oil Palm Trunk Using Simultaneous Saccharification and Fermentation. Agric. Nat. Resour. 2021, 55, 448–455. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, W.; Zhang, G. Production of Single Cell Protein Using Waste Capsicum Powder Produced during Capsanthin Extraction. Lett. Appl. Microbiol. 2010, 50, 187–191. [Google Scholar] [CrossRef]
- Kumar, A.; Bera, S. Revisiting Nitrogen Utilization in Algae: A Review on the Process of Regulation and Assimilation. Bioresour. Technol. Rep. 2020, 12, 100584. [Google Scholar] [CrossRef]
- Su, Y. Revisiting Carbon, Nitrogen, and Phosphorus Metabolisms in Microalgae for Wastewater Treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef]
- Rosa, R.M.; Machado, M.; Vaz, M.G.M.V.; Lopes-Santos, R.; Nascimento, A.G.D.; Araújo, W.L.; Nunes-Nesi, A. Urea as a Source of Nitrogen and Carbon Leads to Increased Photosynthesis Rates in Chlamydomonas reinhardtii Under Mixotrophy. J. Biotechnol. 2023, 367, 20–30. [Google Scholar] [CrossRef]
- Ramanna, L.; Guldhe, A.; Rawat, I.; Bux, F. The Optimization of Biomass and Lipid Yields of Chlorella sorokiniana When Using Wastewater Supplemented with Different Nitrogen Sources. Bioresour. Technol. 2014, 168, 127–135. [Google Scholar] [CrossRef]
- Podevin, M.; De Francisci, D.; Holdt, S.L.; Angelidaki, I. Effect of Nitrogen Source and Acclimatization on Specific Growth Rates of Microalgae Determined by a High-Throughput in Vivo Microplate Autofluorescence Method. J. Appl. Phycol. 2015, 27, 1415–1423. [Google Scholar] [CrossRef]
- Veaudor, T.; Cassier-Chauvat, C.; Chauvat, F. Genomics of Urea Transport and Catabolism in Cyanobacteria: Biotechnological Implications. Front. Microbiology. 2019, 10, 2052. [Google Scholar] [CrossRef]
- Hausinger, R.P. Metabolic Versatility of Prokaryotes for Urea Decomposition. J. Bacteriol. 2004, 186, 2520–2522. [Google Scholar] [CrossRef]
- Hailemariam, S.; Zhao, S.; He, Y.; Wang, J. Urea Transport and Hydrolysis in the Rumen: A Review. Anim. Nutr. 2021, 7, 989–996. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Zhang, L.; Ding, Z.; Shi, G. Understanding and Application of Bacillus Nitrogen Regulation: A Synthetic Biology Perspective. J. Adv. Res. 2023, 49, 1–14. [Google Scholar] [CrossRef]
- Shi, Y.; Niu, X.; Yang, H.; Chu, M.; Wang, N.; Bao, H.; Zhan, F.; Yang, R.; Lou, K. Optimization of the Fermentation Media and Growth Conditions of Bacillus velezensis BHZ-29 Using a Plackett–Burman Design Experiment Combined with Response Surface Methodology. Front. Microbiol. 2024, 15, 1355369. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Y.; Gong, A. Development of a Defined Medium for Corynebacterium glutamicum Using Urea as Nitrogen Source. 3 Biotech 2021, 11, 405. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Huang, W.; Kong, F.; Li, Y.; Xi, M.; Zheng, Z. Comparative Bioavailability of Ammonium, Nitrate, Nitrite and Urea to Typically Harmful Cyanobacterium Microcystis aeruginosa. Mar. Pollut. Bull. 2016, 110, 93–98. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Hu, C.; Liu, B. Long-Term Urea Fertilization Alters the Composition and Increases the Abundance of Soil Ureolytic Bacterial Communities in an Upland Soil. FEMS Microbiol. Ecol. 2019, 95, fiz044. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, W.; Chen, H.; Zhang, X.; He, C.; Rong, J.; Wang, Q. Improved Productivity of Neutral Lipids in Chlorella sp. A2 by Minimal Nitrogen Supply. Front. Microbiol. 2016, 7, 557. [Google Scholar] [CrossRef]
- Gómez Cardozo, J.R.; Beigbeder, J.-B.; Dantas, J.M.D.M.; Lavoie, J.-M. High-Gravity Fermentation for Bioethanol Production from Industrial Spent Black Cherry Brine Supplemented with Whey. Fermentation 2023, 9, 170. [Google Scholar] [CrossRef]
- Alharbi, R.M. Urea-N Influences Biomass Yield, Neutral Lipids Accumulation, and Unsaturated Fatty Acid Production in Photoautotrophically Grown Microalga Chlorella sp. Biocatal. Agric. Biotechnol. 2024, 56, 103056. [Google Scholar] [CrossRef]
- Sigurdarson, J.J.; Svane, S.; Karring, H. Development of a M9-based Urea Medium (M9U) for Sensitive and Real-time Monitoring of Ureolytic Activity of Bacteria and Cell-free Urease. MicrobiologyOpen 2020, 9, e976. [Google Scholar] [CrossRef]
- Salami, S.A.; Moran, C.A.; Warren, H.E.; Taylor-Pickard, J. A Meta-Analysis of the Effects of Slow-Release Urea Supplementation on the Performance of Beef Cattle. Animals 2020, 10, 657. [Google Scholar] [CrossRef]
- Salami, S.A.; Moran, C.A.; Warren, H.E.; Taylor-Pickard, J. Meta-Analysis and Sustainability of Feeding Slow-Release Urea in Dairy Production. PLoS ONE 2021, 16, e0246922. [Google Scholar] [CrossRef]
- Abdullah, E.Y.; Ali, H.T.; Ahmet, O.G. Decarbonization in Ammonia Production, New Technological Methods in Industrial Scale Ammonia Production and Critical Evaluations. Heliyon 2021, 7, e08257. [Google Scholar] [CrossRef]
- Bora, N.; Kumar Singh, A.; Pal, P.; Kumar Sahoo, U.; Seth, D.; Rathore, D.; Bhadra, S.; Sevda, S.; Venkatramanan, V.; Prasad, S.; et al. Green Ammonia Production: Process Technologies and Challenges. Fuel 2024, 369, 131808. [Google Scholar] [CrossRef]
- Brondi, M.; Eisa, M.; Bortoletto-Santos, R.; Drapanauskaite, D.; Reddington, T.; Williams, C.; Ribeiro, C.; Baltrusaitis, J. Recovering, Stabilizing, and Reusing Nitrogen and Carbon from Nutrient-Containing Liquid Waste as Ammonium Carbonate Fertilizer. Agriculture 2023, 13, 909. [Google Scholar] [CrossRef]
- Xu, D.; Zhong, B.; Wang, X.; Li, X.; Zhong, Y.; Yan, Z.; Yang, J.; Li, X.; Wang, Y.; Zhou, X. The Development Road of Ammonium Phosphate Fertilizer in China. Chin. J. Chem. Eng. 2022, 41, 170–175. [Google Scholar] [CrossRef]
- Wang, C.; Walsh, S.D.C.; Longden, T.; Palmer, G.; Lutalo, I.; Dargaville, R. Optimising Renewable Generation Configurations of Off-Grid Green Ammonia Production Systems Considering Haber-Bosch Flexibility. Energy Convers. Manag. 2023, 280, 116790. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Liu, M.; Wang, T.; Wang, L.; Xiao, H.; Li, J.; Duan, C.; Gao, L.; Liu, Y.; Yan, H.; Zhang, Y.; et al. Core Microbiota for Nutrient Digestion Remained and Ammonia Utilization Increased after Continuous Batch Culture of Rumen Microbiota In Vitro. Front. Microbiol. 2024, 15, 1331977. [Google Scholar] [CrossRef]
- Arandia-Gorostidi, N.; Jaffe, A.L.; Parada, A.E.; Kapili, B.J.; Casciotti, K.L.; Salcedo, R.S.R.; Baumas, C.M.J.; Dekas, A.E. Urea Assimilation and Oxidation Support Activity of Phylogenetically Diverse Microbial Communities of the Dark Ocean. ISME J. 2024, 18, wrae230. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Viana, T.; Ardö, Y.; Arneborg, N. Influence of Nitrogen Sources on Growth and Fermentation Performance of Different Wine Yeast Species during Alcoholic Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 10191–10207. [Google Scholar] [CrossRef]
- Papagianni, M.; Wayman, F.; Mattey, M. Fate and Role of Ammonium Ions during Fermentation of Citric Acid by Aspergillus niger. Appl. Environ. Microbiol. 2005, 71, 7178–7186. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Walter, B.; Wirtz, A.; Burkovski, A. Ammonium Toxicity in Bacteria. Curr. Microbiol. 2006, 52, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, F.; Liu, K.; Zhang, M.; Cheng, Y.; Wang, F.; Wang, S.; Han, R.; Xue, Z. Uncovering the Effects of Ammonium Sulfate on Neomycin B Biosynthesis in Streptomyces fradiae SF-2. Fermentation 2022, 8, 678. [Google Scholar] [CrossRef]
- Ardin, A.C.; Fujita, K.; Nagayama, K.; Takashima, Y.; Nomura, R.; Nakano, K.; Ooshima, T.; Matsumoto-Nakano, M. Identification and Functional Analysis of an Ammonium Transporter in Streptococcus mutans. PLoS ONE 2014, 9, e107569. [Google Scholar] [CrossRef]
- Lee, Y.J.; Moon, B.C.; Lee, D.K.; Ahn, J.H.; Gong, G.; Um, Y.; Lee, S.-M.; Kim, K.H.; Ko, J.K. Sustainable Production of Microbial Protein from Carbon Dioxide in the Integrated Bioelectrochemical System Using Recycled Nitrogen Sources. Water Res. 2025, 268, 122576. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the Productivity of Microalgae Cultivated in Wastewater toward Biofuel Production: A Critical Review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Metin, U.; Altınbaş, M. Evaluating Ammonia Toxicity and Growth Kinetics of Four Different Microalgae Species. Microorganisms 2024, 12, 1542. [Google Scholar] [CrossRef]
- Scherholz, M.L.; Curtis, W.R. Achieving pH Control in Microalgal Cultures through Fed-Batch Addition of Stoichiometrically-Balanced Growth Media. BMC Biotechnol. 2013, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.D.; Simionov, I.-A.; Petrea, S.M.; Georgescu, P.-L.; Ifrim, G.A.; Iticescu, C. Efficiency of Microalgae Employment in Nutrient Removal (Nitrogen and Phosphorous) from Municipal Wastewater. Water 2025, 17, 260. [Google Scholar] [CrossRef]
- Kundu, P.; Dutta, N.; Bhattacharya, S. Application of Microalgae in Wastewater Treatment with Special Reference to Emerging Contaminants: A Step towards Sustainability. Front. Anal. Sci. 2024, 4, 1513153. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of Nitrogen Utilization by Soil Microorganisms–A Review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, D.; Huang, L.; Zeng, Y.; Liu, J.; Xiang, H.; Zheng, Y. The Role of Glutamine Synthetase in Regulating Ammonium Assimilation and Iron-Only Nitrogenase Expression in a Photosynthetic Diazotroph. Microbiol. Spectr. 2023, 11, e04953-22. [Google Scholar] [CrossRef]
- Kawade, K.; Tabeta, H.; Ferjani, A.; Hirai, M.Y. The Roles of Functional Amino Acids in Plant Growth and Development. Plant Cell Physiol. 2023, 64, 1482–1493. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient Recovery from Wastewater Streams by Microalgae: Status and Prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, Y.; Yu, G.; Wang, Q.; He, N.; Chen, Z.; He, H.; Zhu, X.; Li, P.; Zhang, F.; et al. Changing Patterns of Global Nitrogen Deposition Driven by Socio-Economic Development. Nat. Commun. 2025, 16, 46. [Google Scholar] [CrossRef]
- Liu, T.; Duan, H.; Lücker, S.; Zheng, M.; Daims, H.; Yuan, Z.; Guo, J. Sustainable Wastewater Management through Nitrogen-Cycling Microorganisms. Nat. Water 2024, 2, 936–952. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, L.; Hu, J.; Huang, W.; Lou, L. Microbiome Data Analysis via Machine Learning Models: Exploring Vital Players to Optimize Kitchen Waste Composting System. Bioresour. Technol. 2023, 388, 129731. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, V.; Cheng, L.; Hussain, A.; Ormeci, B. Nitrogen Removal from Wastewater: A Comprehensive Review of Biological Nitrogen Removal Processes, Critical Operation Parameters and Bioreactor Design. J. Environ. Chem. Eng. 2022, 10, 107387. [Google Scholar] [CrossRef]
- Selvam, A.; Ilamathi, P.M.K.; Udayakumar, M.; Murugesan, K.; Banu, J.R.; Khanna, Y.; Wong, J. Food Waste Properties. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 11–41. ISBN 978-0-12-819148-4. [Google Scholar]
- Manu, M.K.; Li, D.; Liwen, L.; Jun, Z.; Varjani, S.; Wong, J.W.C. A Review on Nitrogen Dynamics and Mitigation Strategies of Food Waste Digestate Composting. Bioresour. Technol. 2021, 334, 125032. [Google Scholar] [CrossRef] [PubMed]
- Kwan, T.H.; Hu, Y.; Lin, C.S.K. Valorisation of Food Waste via Fungal Hydrolysis and Lactic Acid Fermentation with Lactobacillus casei Shirota. Bioresour. Technol. 2016, 217, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Alrbai, M.; Al-Dahidi, S.; Shboul, B.; Abusorra, M.; Hayajneh, H. Techno-Economic Feasibility Study of Ammonia Recovery from Sewage Sludge Digestate in Wastewater Treatment Plants. Clean. Environ. Syst. 2024, 15, 100235. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic Granular Sludge Technology: Mechanisms of Granulation and Biotechnological Applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef]
- Mohammadkhani, G.; Mahboubi, A.; Plöhn, M.; Funk, C.; Ylitervo, P. Total Ammonia Removal from Anaerobic Digestion Effluents of Municipal Sewage Sludge Using Nordic Microalgae. Algal Res. 2024, 84, 103802. [Google Scholar] [CrossRef]
- Sancho, I.; Licon, E.; Valderrama, C.; De Arespacochaga, N.; López-Palau, S.; Cortina, J.L. Recovery of Ammonia from Domestic Wastewater Effluents as Liquid Fertilizers by Integration of Natural Zeolites and Hollow Fibre Membrane Contactors. Sci. Total Environ. 2017, 584–585, 244–251. [Google Scholar] [CrossRef]
- Yu, Y.; Lei, Z.; Yuan, T.; Jiang, Y.; Chen, N.; Feng, C.; Shimizu, K.; Zhang, Z. Simultaneous Phosphorus and Nitrogen Recovery from Anaerobically Digested Sludge Using a Hybrid System Coupling Hydrothermal Pretreatment with MAP Precipitation. Bioresour. Technol. 2017, 243, 634–640. [Google Scholar] [CrossRef]
- Qi, B.; Jiang, X.; Wang, H.; Li, J.; Zhao, Q.; Li, R.; Wang, W. Resource Recovery from Liquid Digestate of Swine Wastewater by an Ultrafiltration Membrane Bioreactor (UF-MBR) and Reverse Osmosis (RO) Process. Environ. Technol. Innov. 2021, 24, 101830. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Du, X.; Gao, T.; Cheng, Z.; Fu, W.; Wang, S. Ammonia Inhibition in Anaerobic Digestion of Organic Waste: A Review. Int. J. Environ. Sci. Technol. 2024, 22, 3927–3942. [Google Scholar] [CrossRef]
- Mangwe, M.C.; Mason, W.A.; Reed, C.B.; Spaans, O.K.; Pacheco, D.; Bryant, R.H. A Systematic Review and Meta-Analysis of Cow-Level Factors Affecting Milk Urea Nitrogen and Urinary Nitrogen Output Under Pasture-Based Diets. J. Dairy Sci. 2025, 108, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Vivekanand, V.; Mohanakrishna, G.; Pattnaik, B.; Muddapur, U.M.; Aminabhavi, T.M. Production of Bioactive Phenolic Compounds from Agricultural By-Products towards Bioeconomic Perspectives. J. Clean. Prod. 2023, 414, 137460. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Calabrese, G.; Di Bella, G.; Cicero, N.; Dugo, G. Production of Single Cell Protein (SCP) from Food and Agricultural Waste by Using Saccharomyces cerevisiae. Nat. Prod. Res. 2018, 32, 648–653. [Google Scholar] [CrossRef]
- Yi, Y.; Li, J.; Zhou, P.; Jia, F.; Chen, Y.; Li, D. Production of Single Cell Protein Rich in Potassium by Nectaromyces Rattus Using Biogas Slurry and Molasses. J. Environ. Manag. 2024, 350, 119627. [Google Scholar] [CrossRef]
- Yan, J.; Han, B.; Gui, X.; Wang, G.; Xu, L.; Yan, Y.; Madzak, C.; Pan, D.; Wang, Y.; Zha, G.; et al. Engineering Yarrowia lipolytica to Simultaneously Produce Lipase and Single Cell Protein from Agroindustrial Wastes for Feed. Sci. Rep. 2018, 8, 758. [Google Scholar] [CrossRef]
- Spalvins, K.; Zihare, L.; Blumberga, D. Single Cell Protein Production from Waste Biomass: Comparison of Various Industrial by-Products. Energy Procedia 2018, 147, 409–418. [Google Scholar] [CrossRef]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single Cell Protein Production through Multi Food-Waste Substrate Fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Putri, D.; Ulhidayati, A.; Musthofa, I.A.; Wardani, A.K. Single Cell Protein Production of Chlorella sp. Using Food Processing Waste as a Cultivation Medium. IOP Conf. Ser. Earth Environ. Sci. 2018, 131, 012052. [Google Scholar] [CrossRef]
- Van Peteghem, L.; Matassa, S.; Rabaey, K.; Sakarika, M. Microbial Protein from Recovered Nitrogen: Nutritional Quality, Safety, and Feasibility Assessment. Sci. Total Environ. 2023, 892, 164525. [Google Scholar] [CrossRef]
- Voutilainen, E.; Pihlajaniemi, V.; Parviainen, T. Economic Comparison of Food Protein Production with Single-Cell Organisms from Lignocellulose Side-Streams. Bioresour. Technol. Rep. 2021, 14, 100683. [Google Scholar] [CrossRef]
- Van Peteghem, L.; Matassa, S.; Sakarika, M. Fueling the Protein Transition: Can Waste-Derived Ethanol Enable Efficient and High-Quality Microbial Protein Production? Bioresour. Technol. 2025, 418, 131990. [Google Scholar] [CrossRef]
- Peterson, E.C.; Siao, R.; Chua, G.G.; Busran, C.T.; Pavlovic, R.; Thong, A.; Hermansen, C.; Sofeo, N.; Kanagasundaram, Y.; Weingarten, M.; et al. Single Cell Protein and Oil Production from Solid Cocoa Fatty Acid Distillates Co-Fed Ethanol. Bioresour. Technol. 2023, 387, 129630. [Google Scholar] [CrossRef]
- Campos-Valdez, A.; Kirchmayr, M.R.; Barrera-Martínez, I.; Casas-Godoy, L. Sustainable Production of Single-Cell Oil and Protein from Wastepaper Hydrolysate: Identification and Optimization of a Rhodotorula Mucilaginosa Strain as a Promising Yeast. FEMS Yeast Res. 2023, 23, foad044. [Google Scholar] [CrossRef] [PubMed]
- Devanthi, P.V.P.; Pratama, F.; Pramanda, I.T.; Bani, M.D.; Kadar, A.D.; Kho, K. Exploring the Potential of Aspergillus oryzae for Sustainable Mycoprotein Production Using Okara and Soy Whey as Cost-Effective Substrates. J. Fungi 2024, 10, 555. [Google Scholar] [CrossRef]
- Santin, A.; Russo, M.T.; Ferrante, M.I.; Balzano, S.; Orefice, I.; Sardo, A. Highly Valuable Polyunsaturated Fatty Acids from Microalgae: Strategies to Improve Their Yields and Their Potential Exploitation in Aquaculture. Molecules 2021, 26, 7697. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Batista Meneses, D.; Montes De Oca-Vásquez, G.; Vega-Baudrit, J.R.; Rojas-Álvarez, M.; Corrales-Castillo, J.; Murillo-Araya, L.C. Pretreatment Methods of Lignocellulosic Wastes into Value-Added Products: Recent Advances and Possibilities. Biomass Convers. Biorefinery 2022, 12, 547–564. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Zhang, M. Hemicellulose and Unlocking Potential for Sustainable Applications in Biomedical, Packaging, and Material Sciences: A Narrative Review. Int. J. Biol. Macromol. 2024, 280, 135657. [Google Scholar] [CrossRef]
- Kavya; Vashisht, M.; Jain, B.; Shrivastava, S. Transforming Waste into Wealth: A Review on Microbial Conversion of Organic Municipal Wastes to Value-Added Products. Discov. Environ. 2024, 2, 112. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, X.; Hua, Y.; Guo, M.; Sun, X.; Huang, Y.; Dong, L.; Yu, S. Enhancing Nitrogen Removal in Combined Sewage Overflows by Using Bio-Fluidized Bed with Ceramic Waste Powder Carriers: Effects and Mechanisms. Environ. Sci. Pollut. Res. 2024, 31, 65252–65263. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial Wastewater Treatment: Current Trends, Bottlenecks, and Best Practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhou, T.; Bai, J.; Zhang, Y.; Li, J.; Zhou, C.; Zhou, B. Nitrogen-Containing Wastewater Fuel Cells for Total Nitrogen Removal and Energy Recovery Based on Cl•/ClO• Oxidation of Ammonia Nitrogen. Water Res. 2023, 235, 119914. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Rasool, K.; Hussain, S.; Shahzad, A.; Miran, W.; Mahmoud, K.A.; Ali, N.; Almomani, F. Comprehensive Insights into Sustainable Conversion of Agricultural and Food Waste into Microbial Protein for Animal Feed Production. Rev. Environ. Sci. Biotechnol. 2023, 22, 527–562. [Google Scholar] [CrossRef]
- Shen, M.; Song, B.; Zhou, C.; Almatrafi, E.; Hu, T.; Zeng, G.; Zhang, Y. Recent Advances in Impacts of Microplastics on Nitrogen Cycling in the Environment: A Review. Sci. Total Environ. 2022, 815, 152740. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, A.; Jiao, J.; Shen, W.; Yang, L.; Zhou, C.; Tan, Z. Effects of Non-Protein Nitrogen Sources on In Vitro Rumen Fermentation Characteristics and Microbial Diversity. Front. Anim. Sci. 2022, 3, 891898. [Google Scholar] [CrossRef]
- Xu, X.; Hui, D.; King, A.W.; Song, X.; Thornton, P.E.; Zhang, L. Convergence of Microbial Assimilations of Soil Carbon, Nitrogen, Phosphorus and Sulfur in Terrestrial Ecosystems. Sci. Rep. 2015, 5, 17445. [Google Scholar] [CrossRef]
- Einsle, O. Catalysis and Structure of Nitrogenases. Curr. Opin. Struct. Biol. 2023, 83, 102719. [Google Scholar] [CrossRef]
- Campbell, W.H. NITRATE REDUCTASE STRUCTURE, FUNCTION AND REGULATION: Bridging the Gap between Biochemistry and Physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef]
- Neis, E.; Dejong, C.; Rensen, S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Yelamanchi, S.D.; Jayaram, S.; Thomas, J.K.; Gundimeda, S.; Khan, A.A.; Singhal, A.; Keshava Prasad, T.S.; Pandey, A.; Somani, B.L.; Gowda, H. A Pathway Map of Glutamate Metabolism. J. Cell Commun. Signal. 2016, 10, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Glutamate: A Truly Functional Amino Acid. Amino Acids 2013, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-P.; Su, Y.; Jiang, S.; Liang, W.; Lou, Z.; Frugier, F.; Xu, P.; Murray, J.D. Applying Conventional and Cell-Type-Specific CRISPR/Cas9 Genome Editing in Legume Plants. aBIOTECH 2024, 1–15. [Google Scholar] [CrossRef]
- Shang, Y.; Shi, H.; Liu, M.; Lan, P.; Li, D.; Liu, X.; Wang, M.; Zhang, Z.; Chen, S. Using Synthetic Biology to Express Nitrogenase Biosynthesis Pathway in Rice and to Overcome Barriers of Nitrogenase Instability in Plant Cytosol. Trends Biotechnol. 2025, 2615, S0167779924003627. [Google Scholar] [CrossRef]
- Joshi, R.; Sharma, V.; Kuila, A. Fermentation Technology: Current Status and Future Prospects. In Principles and Applications of Fermentation Technology; Kuila, A., Sharma, V., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 1–13. ISBN 978-1-119-46026-8. [Google Scholar]
- Yang, X.; Yuan, L.; Zeeshan, M.; Yang, C.; Gao, W.; Zhang, G.; Wang, C. Optimization of Fermentation Conditions to Increase the Production of Antifungal Metabolites from Streptomyces sp. KN37. Microb. Cell Factories 2025, 24, 26. [Google Scholar] [CrossRef]
- Pereira, A.A.; Yaverino-Gutierrez, M.A.; Monteiro, M.C.; Souza, B.A.; Bachheti, R.K.; Chandel, A.K. Precision Fermentation in the Realm of Microbial Protein Production: State-of-the-Art and Future Insights. Food Res. Int. 2025, 200, 115527. [Google Scholar] [CrossRef]
- Soccol, C.R.; Costa, E.S.F.D.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.D.S. Recent Developments and Innovations in Solid State Fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Pandey, A. Solid-State Fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Mienda, B.S.; Idi, A.; Umar, A. Microbiological Features of Solid State Fermentation and Its Applications. An Overview. Res. Biotechnol. 2011, 2, 21–26. [Google Scholar]
- Betchem, G.; Monto, A.R.; Lu, F.; Billong, L.F.; Ma, H. Prospects and Application of Solid-State Fermentation in Animal Feed Production-a Review. Ann. Anim. Sci. 2024, 33, 1123–1137. [Google Scholar] [CrossRef]
- Li, B.; Zhao, C.; Sun, Q.; Chen, K.; Zhao, X.; Xu, L.; Yang, Z.; Peng, H. Effects of Ammonification–Steam Explosion Pretreatment on the Production of True Protein from Rice Straw during Solid-State Fermentation. Sustainability 2023, 15, 5964. [Google Scholar] [CrossRef]
- Marius, K.S.; Mahamadi, N.; Ibrahim, K.; Iliassou, M.; Sonagnon, H.S.K.; Yerobessor, D.; Wahauwouele, H.C.; Essodolom, T.; Alfred, S.T. Production of Single Cell Protein (SCP) and Essentials Amino Acids from Candida utilis FMJ12 by Solid State Fermentation Using Mango Waste Supplemented with Nitrogen Sources. Afr. J. Biotechnol. 2018, 17, 716–723. [Google Scholar] [CrossRef]
- Maxwell, O.I.; Chinwuba, U.B.; Onyebuchukwu, M.G. Protein Enrichment of Potato Peels Using Saccharomyces Cerevisiae via Solid-State Fermentation Process. Adv. Chem. Eng. Sci. 2019, 09, 99–108. [Google Scholar] [CrossRef]
- Kong, S.; Wang, S.; He, Y.; Wang, N.; Wang, Z.; Weng, L.; Liu, D.; Zhao, X.; Chen, J.; Xu, J.; et al. Three-Stage Solid-State Fermentation Technology for Distillers’ Grain Feed Protein Based on Different Microorganisms Considering Oxygen Requirements. Fermentation 2024, 10, 550. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Wang, N.; Wang, S.; Zeng, S.; Xu, Z.; Liu, D.; Zhao, X.; Liu, F.; Xu, J.; et al. Efficient Conversion of Corn Straw to Feed Protein through Solid-State Fermentation Using a Thermophilic Microbial Consortium. Waste Manag. 2025, 194, 298–308. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Wang, Z.; Xu, Z.; Zhuang, W.; Liu, D.; Lv, Y.; Wang, S.; Xu, J.; Ying, H. Solid-State Fermentation of Corn Straw Using Synthetic Microbiome to Produce Fermented Feed: The Feed Quality and Conversion Mechanism. Sci. Total Environ. 2024, 920, 171034. [Google Scholar] [CrossRef]
- Chen, J.; Cai, Y.; Wang, Z.; Xu, Z.; Li, J.; Ma, X.; Zhuang, W.; Liu, D.; Wang, S.; Song, A.; et al. Construction of a Synthetic Microbial Community Based on Multiomics Linkage Technology and Analysis of the Mechanism of Lignocellulose Degradation. Bioresour. Technol. 2023, 389, 129799. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Wang, Z.; Shen, C.; Liu, D.; Shen, X.; Weng, L.; He, Y.; Wang, S.; Wang, J.; et al. Pretreatment of Luzhou Distiller’s Grains for Feed Protein Production Using Crude Enzymes Produced by a Synthetic Microbial Consortium. Bioresour. Technol. 2023, 390, 129852. [Google Scholar] [CrossRef]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in Solid State Fermentation Technology: Design, Applications and Engineering Aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef]
- Durand, A. Bioreactor Designs for Solid State Fermentation. Biochem. Eng. J. 2003, 13, 113–125. [Google Scholar] [CrossRef]
- Mattedi, A.; Sabbi, E.; Farda, B.; Djebaili, R.; Mitra, D.; Ercole, C.; Cacchio, P.; Del Gallo, M.; Pellegrini, M. Solid-State Fermentation: Applications and Future Perspectives for Biostimulant and Biopesticides Production. Microorganisms 2023, 11, 1408. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Luan, G.; Ma, Y.; Lou, W.; Chen, R.; Feng, D.; Zhang, S.; Sun, J.; Lu, X. Engineered Hypermutation Adapts Cyanobacterial Photosynthesis to Combined High Light and High Temperature Stress. Nat. Commun. 2023, 14, 1238. [Google Scholar] [CrossRef] [PubMed]
- Artola, A.; Font, X.; Moral-Vico, J.; Sánchez, A. The Role of Solid-State Fermentation to Transform Existing Waste Treatment Plants Based on Composting and Anaerobic Digestion into Modern Organic Waste-Based Biorefineries, in the Framework of Circular Bioeconomy. Front. Chem. Eng. 2024, 6, 1463785. [Google Scholar] [CrossRef]
- Bürck, M.; Lemes, A.C.; Egea, M.B.; Braga, A.R.C. Exploring the Potential and Challenges of Fermentation in Creating Foods: A Spotlight on Microalgae. Fermentation 2024, 10, 649. [Google Scholar] [CrossRef]
- Aloo, S.O.; Park, S.; Oyinloye, T.M.; Lee, Y.-W.; Cho, Y.E.; Park, S.J.; Oh, D.-H. Effects of Liquid State vs. Solid State Lactic Fermentation on Drying, Nutritional Composition, Phytochemical Profile, and In Vitro Neuro-Related Bioactivities of Cheungsam Industrial Hempseed (Korean Breed). Food Biosci. 2025, 63, 105708. [Google Scholar] [CrossRef]
- Tian, Y.; Li, J.; Meng, J.; Li, J. High-Yield Production of Single-Cell Protein from Starch Processing Wastewater Using Co-Cultivation of Yeasts. Bioresour. Technol. 2023, 370, 128527. [Google Scholar] [CrossRef]
- Zhang, B.; Ren, D.; Liu, Q.; Liu, X.; Bao, J. Coproduction of Single Cell Protein and Lipid from Lignocellulose Derived Carbohydrates and Inorganic Ammonia Salt with Soluble Ammonia Recycling. Bioresour. Technol. 2023, 384, 129345. [Google Scholar] [CrossRef]
- Clement, P.N.; Nwokoro, O. Production of Single Cell Protein from Hydrolyzed Pineapple (Ananas comosus) Peel Using Fungi. Bio-Research 2019, 15, 961–973. [Google Scholar] [CrossRef]
- Cerrone, F.; O’Connor, K.E. Cultivation of Filamentous Fungi in Airlift Bioreactors: Advantages and Disadvantages. Appl. Microbiol. Biotechnol. 2025, 109, 41. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Chatzikonstantinou, A.V.; Xiros, C.; Katapodis, P.; Stamatis, H. Mycoprotein Production by Submerged Fermentation of the Edible Mushroom Pleurotus ostreatus in a Batch Stirred Tank Bioreactor Using Agro-Industrial Hydrolysate. Foods 2023, 12, 2295. [Google Scholar] [CrossRef] [PubMed]
- Niyigaba, T.; Küçükgöz, K.; Kołożyn-Krajewska, D.; Królikowski, T.; Trząskowska, M. Advances in Fermentation Technology: A Focus on Health and Safety. Appl. Sci. 2025, 15, 3001. [Google Scholar] [CrossRef]
- Marcellin, E.; Angenent, L.T.; Nielsen, L.K.; Molitor, B. Recycling Carbon for Sustainable Protein Production Using Gas Fermentation. Curr. Opin. Biotechnol. 2022, 76, 102723. [Google Scholar] [CrossRef]
- Woern, C.; Grossmann, L. Microbial Gas Fermentation Technology for Sustainable Food Protein Production. Biotechnol. Adv. 2023, 69, 108240. [Google Scholar] [CrossRef]
- Raziq, A. Single Cell Protein (SCP) Production and Potential Substrates: A Comprehensive Review. Pure Appl. Biol. 2020, 9, 3. [Google Scholar] [CrossRef]
- Vlaeminck, E.; Uitterhaegen, E.; Quataert, K.; Delmulle, T.; Kontovas, S.-S.; Misailidis, N.; Ferreira, R.G.; Petrides, D.; De Winter, K.; Soetaert, W.K. Single-Cell Protein Production from Industrial Off-Gas through Acetate: Techno-Economic Analysis for a Coupled Fermentation Approach. Fermentation 2023, 9, 771. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Xu, J.; Ma, S.; Liang, X.; Wei, Z.; Li, D.; Xue, M. C1 Gas Protein: A Potential Protein Substitute for Advancing Aquaculture Sustainability. Rev. Aquac. 2023, 15, 1179–1197. [Google Scholar] [CrossRef]
- Xu, J.; Wang, J.; Ma, C.; Wei, Z.; Zhai, Y.; Tian, N.; Zhu, Z.; Xue, M.; Li, D. Embracing a Low-Carbon Future by the Production and Marketing of C1 Gas Protein. Biotechnol. Adv. 2023, 63, 108096. [Google Scholar] [CrossRef]
- Jain, S.; Heffernan, J.; Joshi, J.; Watts, T.; Marcellin, E.; Greening, C. Microbial Conversion of Waste Gases into Single-Cell Protein. Microbiol. Aust. 2023, 44, 27–30. [Google Scholar] [CrossRef]
- Li, S.; Zuo, X.; Carpenter, M.D.; Verduzco, R.; Ajo-Franklin, C.M. Microbial Bioelectronic Sensors for Environmental Monitoring. Nat. Rev. Bioeng. 2024, 3, 30–49. [Google Scholar] [CrossRef]
- Kholif, A.E.; Anele, A.; Anele, U.Y. Microbial Feed Additives in Ruminant Feeding. AIMS Microbiol. 2024, 10, 542–571. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Guo, C.; Xu, S.; Chen, J.; Zhou, J. High-Throughput Screening Technology in Industrial Biotechnology. Trends Biotechnol. 2020, 38, 888–906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Gao, C.; Song, W.; Wei, W.; Wu, J.; Liu, L.; Chen, X. Engineering Status of Protein for Improving Microbial Cell Factories. Biotechnol. Adv. 2024, 70, 108282. [Google Scholar] [CrossRef]
- Cao, K.; Cui, Y.; Sun, F.; Zhang, H.; Fan, J.; Ge, B.; Cao, Y.; Wang, X.; Zhu, X.; Wei, Z.; et al. Metabolic Engineering and Synthetic Biology Strategies for Producing High-Value Natural Pigments in Microalgae. Biotechnol. Adv. 2023, 68, 108236. [Google Scholar] [CrossRef]
- Onn, S.M.; Koh, G.J.; Yap, W.H.; Teoh, M.-L.; Low, C.-F.; Goh, B.-H. Recent Advances in Genetic Engineering of Microalgae: Bioengineering Strategies, Regulatory Challenges and Future Perspectives. J. Appl. Phycol. 2024, 37, 247–264. [Google Scholar] [CrossRef]
- Grossmann, M.; Kießling, F.; Singer, J.; Schoeman, H.; Schröder, M.-B.; Von Wallbrunn, C. Genetically Modified Wine Yeasts and Risk Assessment Studies Covering Different Steps within the Wine Making Process. Ann. Microbiol. 2011, 61, 103–115. [Google Scholar] [CrossRef]
- Yang, P.; Condrich, A.; Lu, L.; Scranton, S.; Hebner, C.; Sheykhhasan, M.; Ali, M.A. Genetic Engineering in Bacteria, Fungi, and Oomycetes, Taking Advantage of CRISPR. DNA 2024, 4, 427–454. [Google Scholar] [CrossRef]
- Zimmermann, A.; Prieto-Vivas, J.E.; Voordeckers, K.; Bi, C.; Verstrepen, K.J. Mutagenesis Techniques for Evolutionary Engineering of Microbes-Exploiting CRISPR-Cas, Oligonucleotides, Recombinases, and Polymerases. Trends Microbiol. 2024, 32, 884–901. [Google Scholar] [CrossRef]
- Bleisch, R.; Freitag, L.; Ihadjadene, Y.; Sprenger, U.; Steingröwer, J.; Walther, T.; Krujatz, F. Strain Development in Microalgal Biotechnology-Random Mutagenesis Techniques. Life 2022, 12, 961. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, X.; Rang, J.; Xia, L. Application and Research Progress of ARTP Mutagenesis in Actinomycetes Breeding. Gene 2024, 929, 148837. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wei, D.; Xing, X. Rapid Screening of High-Protein Auxenochlorella Pyrenoidosa Mutant by an Integrated System of Atmospheric and Room Temperature Plasma Mutagenesis and High-Throughput Microbial Microdroplet Culture. Algal Res. 2024, 80, 103509. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, J.; Wei, H.; Liu, Q.; Xu, W.; Bao, Y. Optimizing Mycelial Protein Yield in Pleurotus Djamor via ARTP Mutagenesis and Hybridization Strategies. J. Biotechnol. 2024, 386, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, B.; Zhang, X.; Men, P.; Gu, M.; Zhou, Y.; Hu, W.; Wang, Z.; Wang, M.; Huang, X.; et al. Improving the Production of Micafungin Precursor FR901379 in Coleophoma Empetri Using Heavy-Ion Irradiation and Its Mechanism Analysis. Mycology 2024, 1–15. [Google Scholar] [CrossRef]
- Vasina, M.; Velecky, J.; Planas, I.J.; Marques, S.M.; Skarupova, J.; Damborsky, J.; Bednar, D.; Mazurenko, S.; Prokop, Z. Tools for Computational Design and High-Throughput Screening of Therapeutic Enzymes. Adv. Drug Deliv. Rev. 2022, 183, 114143. [Google Scholar] [CrossRef]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive Laboratory Evolution Principles and Applications in Industrial Biotechnology. Biotechnol. Adv. 2022, 54, 107795. [Google Scholar] [CrossRef]
- Barrick, J.E.; Lenski, R.E. Genome Dynamics during Experimental Evolution. Nat. Rev. Genet. 2013, 14, 827–839. [Google Scholar] [CrossRef]
- Loewe, L.; Hill, W.G. The Population Genetics of Mutations: Good, Bad and Indifferent. Philos. Trans. R. Soc. B 2010, 365, 1153–1167. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Ji, X.-J.; Chen, S.-L.; Guo, D.-S.; Huang, H. Adaptive Evolution of Schizochytrium sp. by Continuous High Oxygen Stimulations to Enhance Docosahexaenoic Acid Synthesis. Bioresour. Technol. 2016, 211, 374–381. [Google Scholar] [CrossRef]
- Konstantinidis, D.; Pereira, F.; Geissen, E.; Grkovska, K.; Kafkia, E.; Jouhten, P.; Kim, Y.; Devendran, S.; Zimmermann, M.; Patil, K.R. Adaptive Laboratory Evolution of Microbial Co-cultures for Improved Metabolite Secretion. Mol. Syst. Biol. 2021, 17, e10189. [Google Scholar] [CrossRef]
- Blasche, S.; Kim, Y.; Mars, R.A.T.; Machado, D.; Maansson, M.; Kafkia, E.; Milanese, A.; Zeller, G.; Teusink, B.; Nielsen, J.; et al. Metabolic Cooperation and Spatiotemporal Niche Partitioning in a Kefir Microbial Community. Nat. Microbiol. 2021, 6, 196–208. [Google Scholar] [CrossRef]
- Ding, X.; Yang, W.; Du, X.; Chen, N.; Xu, Q.; Wei, M.; Zhang, C. High-Level and -Yield Production of L-Leucine in Engineered Escherichia coli by Multistep Metabolic Engineering. Metab. Eng. 2023, 78, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.; Lee, J.H.; Yoo, M.; Hwang, S.; Sung, B.H.; Cho, S.; Palsson, B.; Kim, S.C.; Cho, B.-K. Adaptive Laboratory Evolution of a Genome-Reduced Escherichia coli. Nat. Commun. 2019, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- LaCroix, R.A.; Sandberg, T.E.; O’Brien, E.J.; Utrilla, J.; Ebrahim, A.; Guzman, G.I.; Szubin, R.; Palsson, B.O.; Feist, A.M. Use of Adaptive Laboratory Evolution To Discover Key Mutations Enabling Rapid Growth of Escherichia coli K-12 MG1655 on Glucose Minimal Medium. Appl. Environ. Microbiol. 2015, 81, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Hu, G.; Li, X.; Gao, C.; Song, W.; Wei, W.; Wu, J.; Liu, L. A Synthetic Methylotroph Achieves Accelerated Cell Growth by Alleviating Transcription-Replication Conflicts. Nat. Commun. 2025, 16, 31. [Google Scholar] [CrossRef]
| NPN Type | Waste Sources | Concentration | Reference |
|---|---|---|---|
| Ammonia and ammonium salts | Food waste (average) | 0.76 g/kg | [132] |
| Anaerobic fermentation digestate food waste | 1.1~9.6 kg N/t | [133] | |
| Hydrolysates of food waste | 1081 mg/L | [134] | |
| Digestate of Wastewater treatment plant | 4040.74 mg/L | [135] | |
| Municipal wastewater | 100 mg/L | [136] | |
| Anaerobic digestion effluents of municipal sewage sludge | 1540 mg/L | [137] | |
| Wastewater treatment plant treated water | 35 mg/L | [138] | |
| Anaerobically digested sludge from wastewater treatment plant | 318.80 mg/L | [139] | |
| Liquid digestate from swine farm | 532 mg/L | [140] | |
| Chicken manure | 2937 mg/L | [141] | |
| Urea | Cattle urine | 50~438.3 g/kg | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Cai, Y.; Wang, F.; He, Y.; Yang, Y.; Guo, Z.; Liu, M.; Ren, H.; Wang, S.; Liu, D.; et al. Industrial Microbial Technologies for Feed Protein Production from Non-Protein Nitrogen. Microorganisms 2025, 13, 742. https://doi.org/10.3390/microorganisms13040742
Ye Y, Cai Y, Wang F, He Y, Yang Y, Guo Z, Liu M, Ren H, Wang S, Liu D, et al. Industrial Microbial Technologies for Feed Protein Production from Non-Protein Nitrogen. Microorganisms. 2025; 13(4):742. https://doi.org/10.3390/microorganisms13040742
Chicago/Turabian StyleYe, Yuxin, Yafan Cai, Fei Wang, Yi He, Yuxuan Yang, Zhengxiang Guo, Mengyu Liu, Huimin Ren, Shilei Wang, Dong Liu, and et al. 2025. "Industrial Microbial Technologies for Feed Protein Production from Non-Protein Nitrogen" Microorganisms 13, no. 4: 742. https://doi.org/10.3390/microorganisms13040742
APA StyleYe, Y., Cai, Y., Wang, F., He, Y., Yang, Y., Guo, Z., Liu, M., Ren, H., Wang, S., Liu, D., Xu, J., & Wang, Z. (2025). Industrial Microbial Technologies for Feed Protein Production from Non-Protein Nitrogen. Microorganisms, 13(4), 742. https://doi.org/10.3390/microorganisms13040742








