Treatment Protocols for Gestational and Congenital Toxoplasmosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
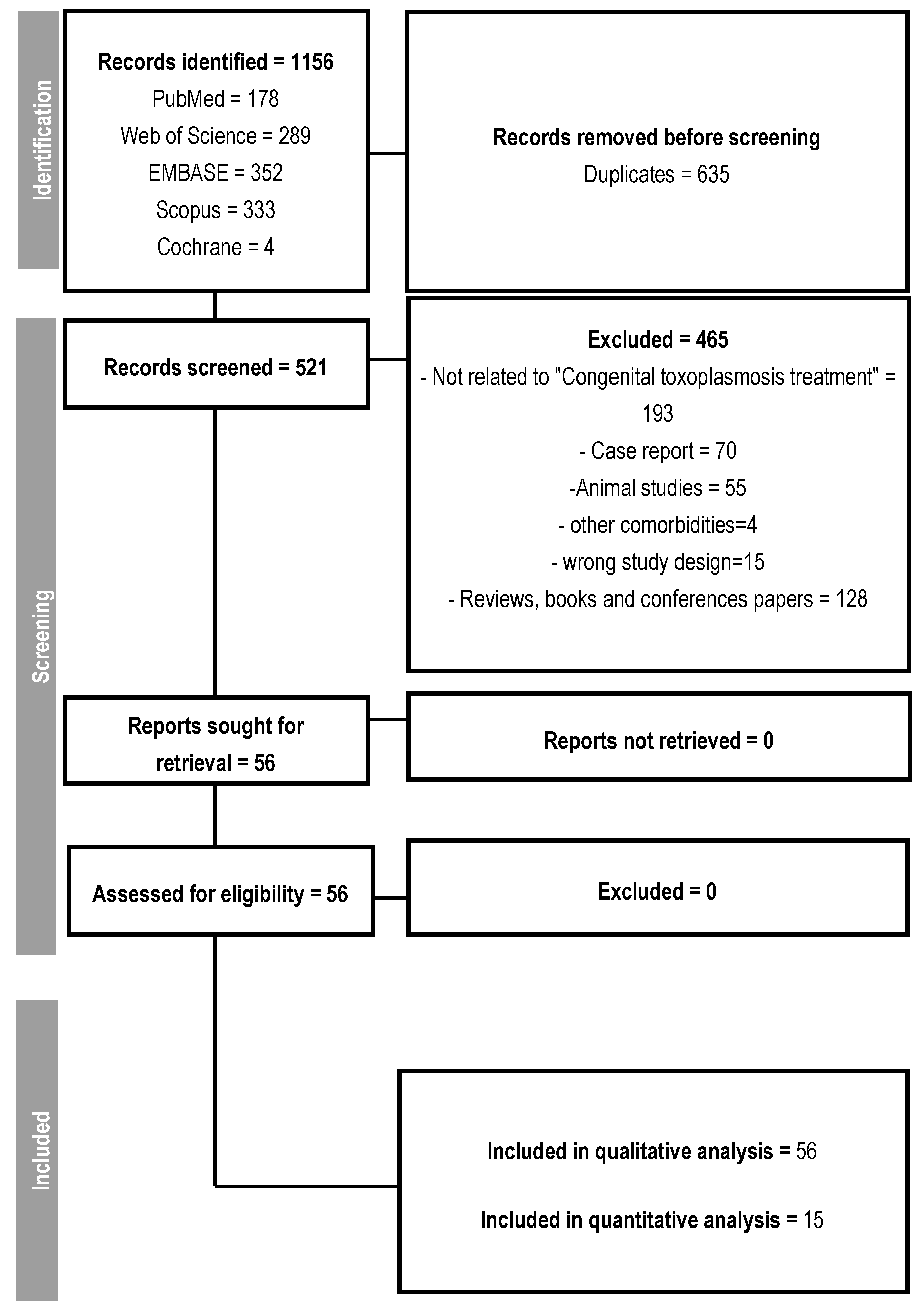
2.1. Search Strategy and Selection Criteria
2.2. Data Synthesis and Analysis
3. Results
3.1. Qualitative Results
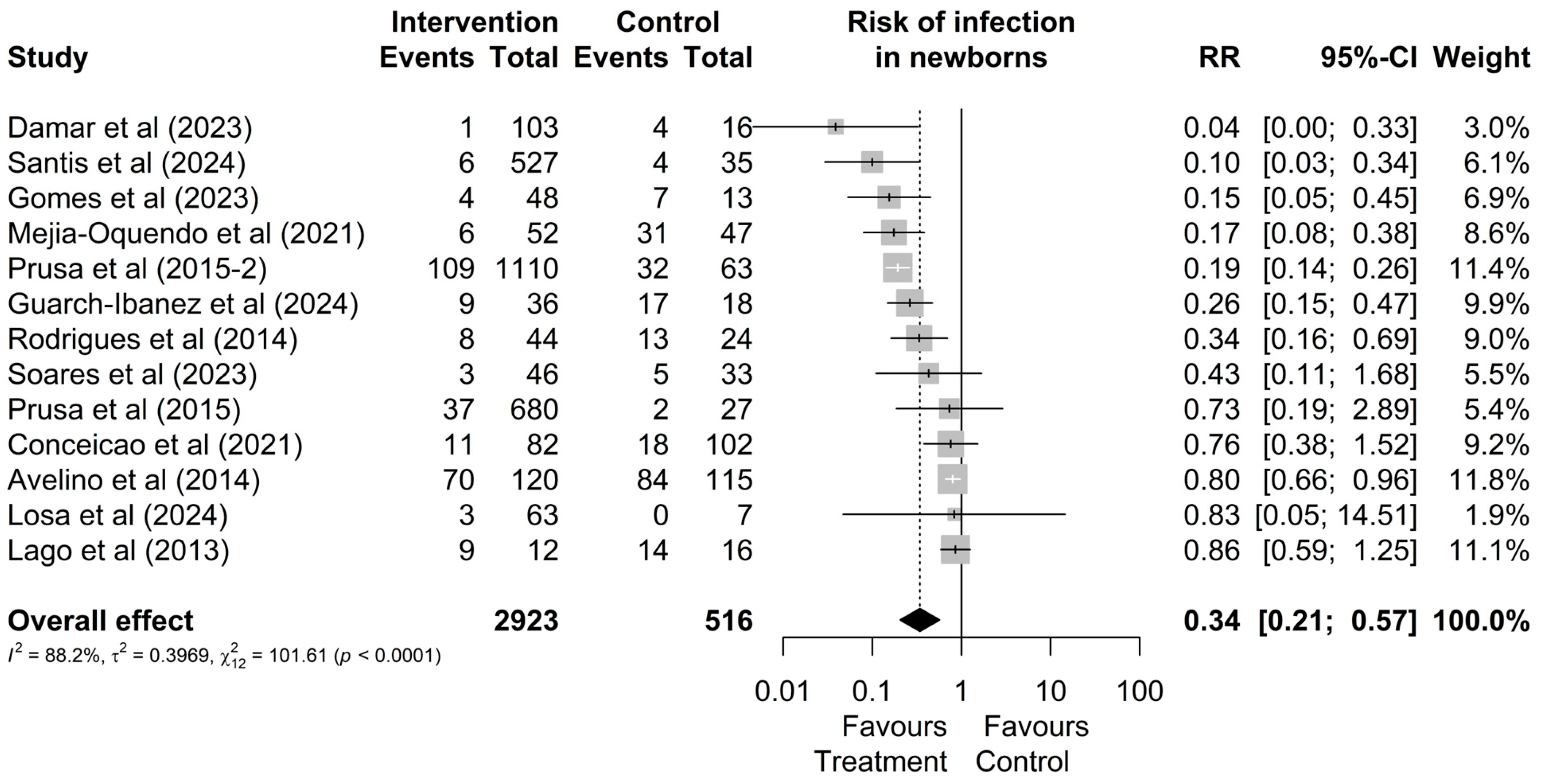
3.2. Quantitative Results
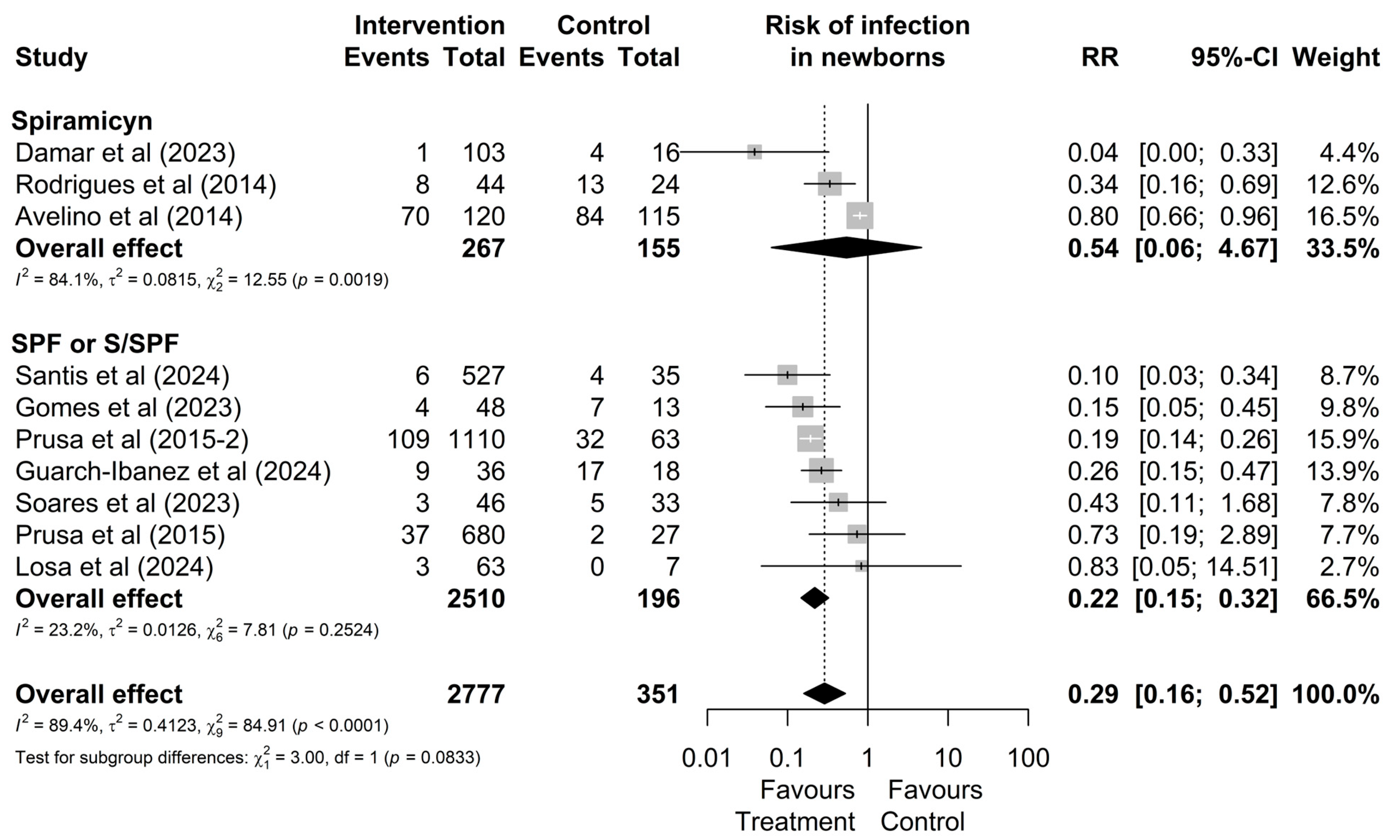
3.3. Comparative Analysis Between Treatments
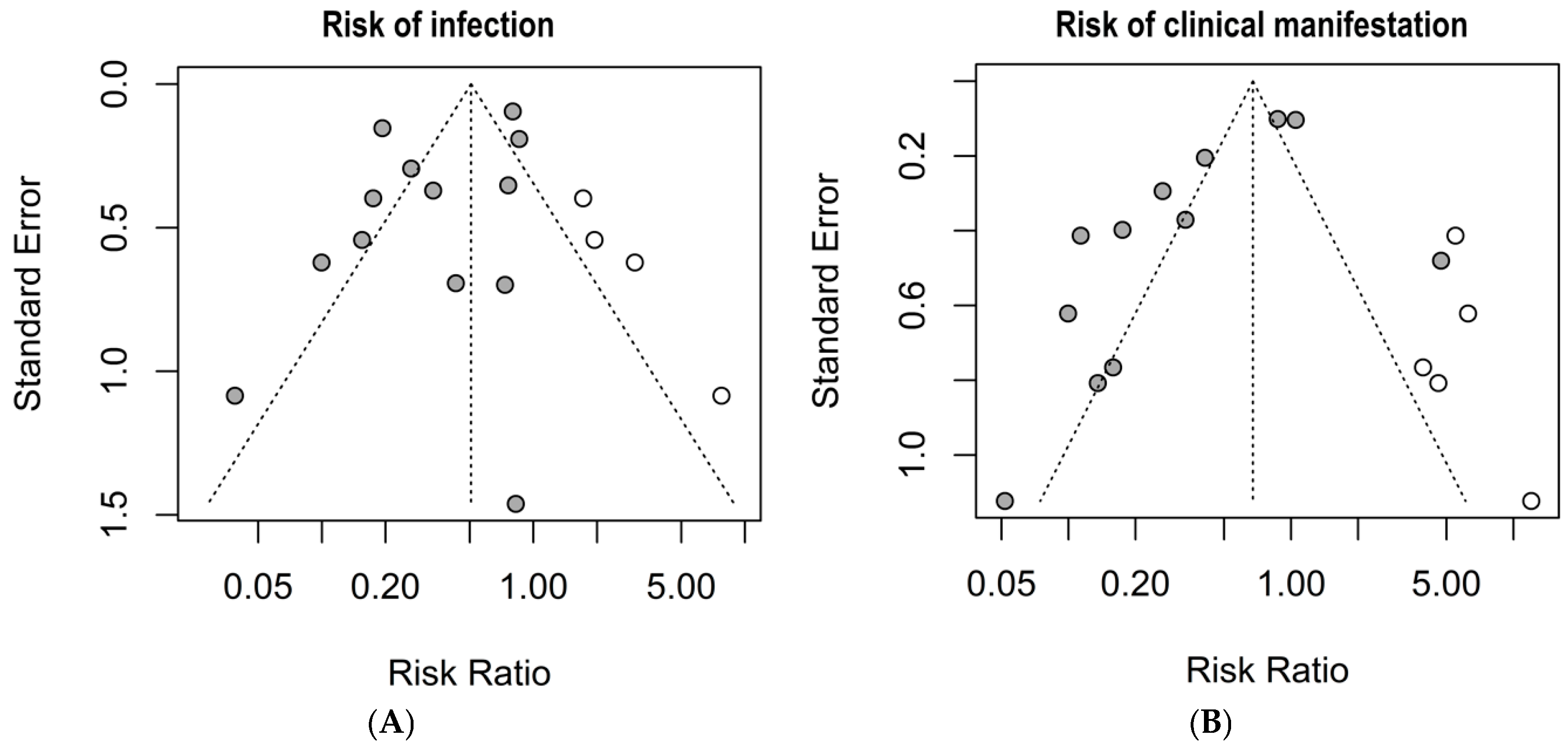
3.4. Risk of Bias
4. Discussion
- The occurrence of congenital toxoplasmosis remains a significant health problem in numerous countries. There is no global consensus on the traceability of toxoplasmosis during pregnancy. While some countries advocate monitoring all pregnant women, others do not recommend it, and the choice of treatment conduct is not yet well-established. The frequency of congenital toxoplasmosis is primarily associated with late, inaccurate, or nonexistent diagnosis during pregnancy, leading to delays or the absence of adequate treatment;
- This systematic review and meta-analysis of articles published from 2013 to 2025, selected based on inclusion and exclusion criteria, reveals an urgent need to establish standardization for therapeutic protocols. This is particularly crucial after the 16th or 18th week of pregnancy. Additionally, monthly monitoring of pregnant women with serological tests is recommended as a predictor to reduce the vertical transmission of Toxoplasma gondii. Ensuring proper healthcare access and promoting adequate treatment will improve the overall health of pregnant women and their children;
- Future studies on congenital toxoplasmosis that address procedures in the mother–child dyad should concentrate on new diagnostic tools, novel drugs with efficacy against potentially resistant genotypes and fewer side effects, as well as innovative strategies for health education aimed at women of childbearing age.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth 2019, 16, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.C.D.; Duarte, E.S.M. Atypical strains of Toxoplasma gondii and its impact on development of toxoplasmosis. Res. Soc. Dev. 2023, 12, 2. [Google Scholar] [CrossRef]
- Kamus, L.; Belec, S.; Lambrecht, L.; Abasse, S.L.; Olivier, S.; Combe, P.L.; Bonnave, P.-E.; Vauloup-Fellous, C.L.; Jaffe, C. Maternal and congenital toxoplasmosis in Mayotte: Prevalence, incidence and management. PLoS Negl. Trop. Dis. 2023, 17, e0011198. [Google Scholar] [CrossRef]
- Montoya, J.G.; Laessig, K.; Fazeli, M.S.; Siliman, G.; Yoon, S.S.; Drake-Shanahan, E.; Zhu, C.Y.; Akbary, A.; McLeod, R. A fresh look at the role of spiramycin in preventing a neglected disease: Meta-analyses of observational studies. Eur. J. Med. Res. 2021, 26, 143. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, T.; Zhai, S.Q.; Li, C.H. Recent progress on anti-Toxoplasma drugs discovery: Design, synthesis and screening. Eur. J. Med. Chem. 2019, 183, 111711. [Google Scholar] [CrossRef]
- Wallon, M.; Peyron, F. Effect of Antenatal Treatment on the Severity of Congenital Toxoplasmosis. Clin. Infect. Dis. 2015, 62, 811–812. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Devel. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef]
- Chorlton, S.D. Adjunctive bradyzoite-directed therapy for reducing complications of congenital toxoplasmosis. Med. Hypotheses 2019, 133, 109376. [Google Scholar] [CrossRef]
- Dos Santos, D.A.; Souza, H.F.S.; Silber, A.M.; Souza, T.D.A.C.B.D.; Ávila, A.R. Protein kinases on carbon metabolism: Potential targets for alternative chemotherapies against toxoplasmosis. Front. Cell. Infect. Microbiol. 2023, 13, 1175409. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs. The Joanna Briggs Institute Reviewers’. In Manual 2015: Methodology for JBI Scoping Reviews; Joanne Briggs Institute: Adelaide, Australia, 2015; pp. 1–24. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna R Foundation for Statistical Computing. 2019. Available online: https://www.r-project.org (accessed on 10 February 2025).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Carral, L.; Kaufer, F.; Olejnik, P.; Freuler, C.; Durlach, R. Prevention of congenital toxoplasmosis in a Buenos Aires hospital. Medicina 2013, 73, 238–242. [Google Scholar]
- Damar Çakırca, T.; Can, İ.N.; Deniz, M.; Torun, A.; Akçabay, Ç.; Güzelçiçek, A. Toxoplasmosis: A Timeless Challenge for Pregnancy. Trop. Med. Infect. Dis. 2023, 8, 63. [Google Scholar] [CrossRef]
- Olariu, T.R.; Press, C.; Talucod, J.; Olson, K.; Montoya, J.G. Toxoplasmose congénitale aux États-Unis: Observations cliniques et sérologiques chez les nourrissons nés de mères traitées pendant la grossesse. Parasite 2019, 26, 13. [Google Scholar] [CrossRef]
- Prusa, A.-R.; Kasper, D.C.; Pollak, A.; Olischar, M.; Gleiss, A.; Hayde, M. Amniocentesis for the detection of congenital toxoplasmosis: Results from the nationwide Austrian prenatal screening program. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015, 21, 191.e1–191.e8. [Google Scholar] [CrossRef]
- Buonsenso, D.; Pata, D.; Colonna, A.T.; Iademarco, M.; de Santis, M.; Masini, L.; Conti, G.; Molle, F.; Baldascino, A.; Acampora, A.; et al. Spyramicine and Trimethoprim-Sulfamethoxazole Combination to Prevent Mother-To-Fetus Transmission of Toxoplasma gondii Infection in Pregnant Women: A 28-Years Single-center Experience. Pediatr. Infect. Dis. J. 2022, 41, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Conceição, A.R.; Belucik, D.N.; Missio, L.; Gustavo Brenner, L.; Henrique Monteiro, M.; Ribeiro, K.S.; Costa, D.F.; Valadão, M.C.D.S.; Commodaro, A.G.; de Oliveira Dias, J.R.; et al. Ocular Findings in Infants with Congenital Toxoplasmosis after a Toxoplasmosis Outbreak. Ophthalmology 2021, 128, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, T.E.; Gomes, A.O.; Coelho-dos-Reis, J.G.; Carneiro, A.C.A.V.; Machado, A.S.; Andrade, G.M.Q.; Vasconcelos-Santos, D.V.; Januário, J.N.; Peruhype-Magalhães, V.; Teixeira-Carvalho, A.; et al. Long-term impact of congenital toxoplasmosis on phenotypic and functional features of circulating leukocytes from infants one year after treatment onset. Clin. Immunol. 2021, 232, 108859. [Google Scholar] [CrossRef] [PubMed]
- Findal, G.; Helbig, A.; Haugen, G.; Jenum, P.A.; Stray-Pedersen, B. Management of suspected primary Toxoplasma gondii infection in pregnant women in Norway: Twenty years of experience of amniocentesis in a low-prevalence population. BMC Pregnancy Childbirth 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Guegan, H.; Stajner, T.; Bobic, B.; Press, C.; Olariu, R.T.; Olson, K.; Srbljanovic, J.; Montoya, J.G.; Djurković-Djaković, O.; Robert-Gangneux, F. Maternal Anti-Toxoplasma Treatment during Pregnancy Is Associated with Reduced Sensitivity of Diagnostic Tests for Congenital Infection in the Neonate. J. Clin. Microbiol. 2021, 59, e01368-20. [Google Scholar] [CrossRef]
- Piffer, S.; Lauriola, A.L.; Pradal, U.; Collini, L.; Dell’Anna, L.; Pavanello, L. Toxoplasma gondii infection during pregnancy: A ten-year observation in the province of Trento, Italy. Le Infez. Med. 2020, 28, 603–610. [Google Scholar]
- Olariu, T.R.; Remington, J.S.; McLeod, R.; Alam, A.; Montoya, J.G. Severe congenital toxoplasmosis in the United States: Clinical and serologic findings in untreated infants. Pediatr. Infect. Dis. J. 2011, 30, 1056–1061. [Google Scholar] [CrossRef]
- Soares, J.A.S.; Holzmann, A.P.F.; Alves, B.B.S.; Lima, C.F.Q.; Caldeira, A.P. Profile of pregnant women and children accompanied due to T. gondii exposure at a referred healthcare center: What has changed in 10 years? Rev. Bras. Saude Matern. Infant. 2023, 23, e20220225. [Google Scholar] [CrossRef]
- Valentini, P.; Buonsenso, D.; Barone, G.; Serranti, D.; Calzedda, R.; Ceccarelli, M.; Speziale, D.; Ricci, R.; Masini, L. Spiramycin/cotrimoxazole versus pyrimethamine/sulfonamide and spiramycin alone for the treatment of toxoplasmosis in pregnancy. J. Perinatol. 2015, 35, 90–94. [Google Scholar] [CrossRef]
- Yamada, H.; Tanimura, K.; Deguchi, M.; Tairaku, S.; Morizane, M.; Uchida, A.; Ebina, Y.; Nishikawa, A. A cohort study of maternal screening for congenital Toxoplasma gondii infection: 12 years’ experience. J. Infect. Chemother. 2019, 25, 427–430. [Google Scholar] [CrossRef]
- Trotta, M.; Trotta, A.; Spataro, E.; Giache, S.; Borchi, B.; Zammarchi, L.; Campolmi, I.; Galli, L.; Pasquini, L. Primary toxoplasmosis acquired during early pregnancy: Is it currently overestimated? Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Fricker-Hidalgo, H.; Cimon, B.; Chemla, C.; Darde, M.L.; Delhaes, L.; L’Ollivier, C.; Godineau, N.; Houze, S.; Paris, L.; Quinio, D.; et al. Toxoplasma seroconversion with negative or transient immunoglobulin M in pregnant women: Myth or reality? A French multicenter retrospective study. J. Clin. Microbiol. 2013, 51, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, A.; Chincoli, A.; de Gennaro, A.C.; Calvario, A.; Amendolara, M.; Del Gaudio, G.; Laforgia, N.; Carbonara, S. Congenital toxoplasmosis and proposal of a new classification for the likelihood of primary maternal infection: Analysis of 375 cases in Southeast Italy. J. Matern. Neonatal Med. 2020, 33, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Wallon, M.; Peyron, F.; Cornu, C.; Vinault, S.; Abrahamowicz, M.; Kopp, C.; Binquet, C. Congenital toxoplasma infection: Monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin. Infect. Dis. 2013, 56, 1223–1231. [Google Scholar] [CrossRef]
- Hijikata, M.; Morioka, I.; Okahashi, A.; Nagano, N.; Kawakami, K.; Komatsu, A.; Kawana, K.; Ohyama, S.; Fujioka, K.; Tanimura, K.; et al. A prospective cohort study of newborns born to mothers with serum Toxoplasma gondii immunoglobulin M positivity during pregnancy. J. Infect. Chemother. 2022, 28, 486–491. [Google Scholar] [CrossRef]
- Gomes-Ferrari-Strang, A.G.; Ferrar, R.G.; Falavigna-Guilherme, A.L. Gestational toxoplasmosis treatment changes the child’s prognosis: A cohort study in southern Brazil. PLoS Negl. Trop. Dis. 2023, 17, e0011544. [Google Scholar] [CrossRef]
- Righi, N.C.; Hermes, L.; Piccini, A.D.; Branco, J.C.; Skupien, J.A.; Weinmann, A.R.M.; Valadao, M.C.D.; Schuch, N.J. Epidemiological profile of gestational and congenital toxoplasmosis cases arising out of the population outbreak. Sci. Med. 2021, 31, e40108. [Google Scholar] [CrossRef]
- Tibúrcio, J.D.; Vasconcelos-Santos, D.V.; Vasconcelos, G.C.; Carellos, E.V.M.; Romanelli, R.M.d.C.; Januario, J.N.; Andrade, G.M.Q. Psychometric properties of CVFQ7-BR-toxo to evaluate vision-related quality of life in children with congenital toxoplasmosis in Brazil. Arq. Bras. Oftalmol. 2022, 85, 46–58. [Google Scholar] [CrossRef]
- Bartholo, B.B.G.R.; Monteiro, D.L.M.; Rodrigues, N.C.P.; Trajano, A.J.B.; de Jesus, N.R.; Cardoso, F.F.O.; de Souza, F.M.; Werner, H.; Araujo Júnior, E. Treatment of Acute Toxoplasmosis in Pregnancy: Influence in the Mother-to-Child Transmission. J. Obstet. Gynaecol. Can. 2020, 42, 1505–1510. [Google Scholar] [CrossRef]
- Avci, M.E.; Arslan, F.; Çiftçi, S.; Ekiz, A.; Tüten, A.; Yildirim, G.; Madazli, R. Role of spiramycin in prevention of fetal toxoplasmosis. J. Matern. Neonatal Med. 2016, 29, 2073–2076. [Google Scholar] [CrossRef]
- Mandelbrot, L.; Kieffer, F.; Sitta, R.; Laurichesse-Delmas, H.; Winer, N.; Mesnard, L.; Berrebi, A.; Le Bouar, G.; Bory, J.-P.P.; Cordier, A.-G.G.; et al. Prenatal therapy with pyrimethamine plus sulfadiazine vs spiramycin to reduce placental transmission of toxoplasmosis: A multicenter, randomized trial. Am. J. Obstet. Gynecol. 2018, 219, e1–e386. [Google Scholar] [CrossRef]
- Avelino, M.M.; Amaral, W.N.; Rodrigues, I.M.; Rassi, A.R.; Gomes, M.B.; Costa, T.L.; Castro, A.M. Congenital toxoplasmosis and prenatal care state programs. BMC Infect. Dis. 2014, 14, 33. [Google Scholar] [CrossRef]
- De Santis, M.; Tartaglia, S.; Apicella, M.; Visconti, D.; Noia, G.; Valentini, P.; Lanzone, A.; Santangelo, R.; Masini, L. The prevention of congenital toxoplasmosis using a combination of Spiramycin and Cotrimoxazole: The long-time experience of a tertiary referral centre. Trop. Med. Int. Health 2024, 29, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Guarch-Ibáñez, B.; Carreras-Abad, C.; Frick, M.A.; Blázquez-Gamero, D.; Baquero-Artigao, F.; Fuentes, I. REIV-TOXO Project: Results from a Spanish cohort of congenital toxoplasmosis (2015–2022). The beneficial effects of prenatal treatment on clinical outcomes of infected newborns. PLoS Neglected Trop. Dis. 2024, 18, e0012619. [Google Scholar] [CrossRef]
- Lago, E.G.; Oliveira, A.P.; Bender, A.L. Presence and duration of anti-Toxoplasma gondii immunoglobulin M in infants with congenital toxoplasmosis. J. Pediatr. 2014, 90, 363–369. [Google Scholar] [CrossRef]
- Losa, A.; Carvalho, I.; Sousa, B.; Ashworth, J.; Guedes, A.; Carreira, L.; Pinho, L.; Godinho, C. Congenital Toxoplasmosis Diagnosis: Challenges and Management Outcomes. Cureus 2024, 16, e52971. [Google Scholar] [CrossRef]
- Mejia-Oquendo, M.; Marulanda-Ibarra, E.; Gomez-Marin, J.E. Evaluation of the impact of the first evidence-based guidelines for congenital toxoplasmosis in Armenia (Quindío) Colombia: An observational retrospective analysis. Lancet Reg. Health—Am. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Prusa, A.-R.; Kasper, D.C.; Pollak, A.; Gleiss, A.; Waldhoer, T.; Hayde, M. The Austrian toxoplasmosis register, 1992–2008. Clin. Infect. Dis. 2015, 60, e4–e10. [Google Scholar] [CrossRef]
- Rodrigues, I.M.; Costa, T.L.; Avelar, J.B.; Amaral, W.N.; Castro, A.M.; Avelino, M.M. Assessment of laboratory methods used in the diagnosis of congenital toxoplasmosis after maternal treatment with spiramycin in pregnancy. BMC Infect. Dis. 2014, 14, 349. [Google Scholar] [CrossRef]
- Felín, M.S.; Wang, K.; Moreira, A.; Grose, A.; Leahy, K.; Zhou, Y.; Clouser, F.A.; Siddiqui, M.; Leong, N.; Goodall, P.; et al. Building Programs to Eradicate Toxoplasmosis Part I: Introduction and Overview. Curr. Pediatr. Rep. 2022, 10, 57–92. [Google Scholar] [CrossRef]
- Ferguson, D.J. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int. J. Parasitol. 2004, 34, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Lago, E.G.; Endres, M.M.; Scheeren, M.F.D.C.; Fiori, H.H. Ocular Outcome of Brazilian Patients with Congenital Toxoplasmosis. Pediatr. Infect. Dis. J. 2021, 40, e21–e27. [Google Scholar] [CrossRef] [PubMed]
- Wallon, M.; Garweg, J.G.; Abrahamowicz, M.; Cornu, C.; Vinault, S.; Quantin, C.; Bonithon-Kopp, C.; Picot, S.; Peyron, F.; Binquet, C. Ophthalmic outcomes of congenital toxoplasmosis followed until adolescence. Pediatrics 2014, 133, e601–e608. [Google Scholar] [CrossRef]
- McLeod, R.; Lykins, J.; Gwendolyn Noble, A.; Rabiah, P.; Swisher, C.N.; Heydemann, P.T.; McLone, D.; Frim, D.; Withers, S.; Clouser, F.; et al. Management of Congenital Toxoplasmosis. Curr. Pediatr. Rep. 2014, 2, 166–194. [Google Scholar] [CrossRef]
- World Health Organization. Promoting Safety of Medicines for Children; WHO Press: Geneva, Switzerland, 2007; 59p, ISBN 978-92-4-156343-7. [Google Scholar]
- Koçak, Ö.; Kan, Ö.; Kocak, O.; Kan, O. Results of the Toxoplasmosis Screening in 9311 Pregnant Women in a Tertiary Center in Turkey. Flora 2020, 25, 332–338. [Google Scholar] [CrossRef]
- Kalem, A.K.; Hasanoǧlu, I.; Ayhan, M.; Kayaaslan, B.; Eser, F.; Oǧuz, Y.; Avşar, F.Y.; Güner, R. Toxoplasmosis in pregnancy: Test, treatment and outcome. Eur. Res. J. 2022, 8, 296–303. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar]
- Brito, R.M.d.M.; Bessa, G.d.L.; Bastilho, A.L.; Dantas-Torres, F.; de Andrade-Neto, V.F.; Bueno, L.L.; Fujiwara, R.T.; Magalhães, L.M.D. Genetic diversity of Toxoplasma gondii in South America: Occurrence, immunity, and fate of infection. Parasites Vectors 2023, 16, 461. [Google Scholar]
- Andrade, F.M.; Santana, E.F.M.; Júnior, E.A.; Andrade, S.G.A.; Bortoletti Filho, J.; Amed, A.M.; Moron, A.F. Polymerase chain reaction analysis of amniotic fluid for diagnosis of fetal toxoplasmosis. Clin. Exp. Obstet. Gynecol. 2019, 46, 593–595. [Google Scholar] [CrossRef]
- Boudaouara, Y.; Aoun, K.; Maatoug, R.; Souissi, O.; Bouratbine, A.; Ben Abdallah, R. Congenital toxoplasmosis in Tunisia: Prenatal and neonatal diagnosis and postnatal follow-up of 35 cases. Am. J. Trop. Med. Hyg. 2018, 98, 1722–1726. [Google Scholar] [CrossRef]
- Bouhlel, S.; Ben Abdallah, R.; Aoun, K.; Maatoug, R.; Souissi, O.; Bouratbine, A. Management of Toxoplasmic Seroconversion in the Third Trimester of Pregnancy in Tunisia. Bull. De La Soc. De Pathol. Exot. (1990) 2018, 111, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Capobiango, J.D.; Breganó, R.M.; Navarro, I.T.; Neto, C.P.R.; Casella, A.M.B.; Mori, F.M.R.L.; Pagliari, S.; Inoue, I.T.; Reiche, E.M.V. Congenital toxoplasmosis in a reference center of Paraná, Southern Brazil. Braz. J. Infect. Dis. 2014, 18, 364–3671. [Google Scholar] [CrossRef] [PubMed]
- Carellos, E.V.M.; de Andrade, J.Q.; Romanelli, R.M.C.; Tibúrcio, J.D.; Januário, J.N.; Vasconcelos-Santos, D.V.; Figueiredo, R.M.; de Andrade, G.M.Q. High Frequency of Bone Marrow Depression During Congenital Toxoplasmosis Therapy in a Cohort of Children Identified By Neonatal Screening in Minas Gerais, Brazil. Pediatr. Infect. Dis. J. 2017, 36, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Caro-Garzón, J.D.; Gómez-Henck, C.; Jaramillo-Giraldo, T.; Cifuentes-Botero, J.M.; Gómez-Marín, J.E. Evaluation of the avidity test for the follow up on children treated for congenital toxoplasmosis during the first year of life. Iatreia 2021, 34, 25–32. [Google Scholar] [CrossRef]
- De Paula, H.L.; de Lucca, L.; Vendrame, S.A.; Wess, L.C.; Stein, C.d.S.; Moresco, R.N.; Beck, S.T.; Gonçalves, T.d.L. Delta-aminolevulinate dehydratase enzyme activity and the oxidative profile of pregnant women being treated for acute toxoplasmosis. Microb. Pathog. 2022, 164, 105455. [Google Scholar] [CrossRef]
- Diesel, A.A.; Zachia, S.d.A.; Müller, A.L.L.; Perez, A.V.; Uberti, F.A.d.F.; Magalhães, J.A.d.A. Follow-up of Toxoplasmosis during Pregnancy: Ten-Year Experience in a University Hospital in Southern Brazil. Rev. Bras. Ginecol. Obstet. 2019, 41, 539–547. [Google Scholar] [CrossRef]
- Donadono, V.; Saccone, G.; Maruotti, G.M.; Berghella, V.; Migliorini, S.; Esposito, G.; Sirico, A.; Tagliaferri, S.; Ward, A.; Mazzarelli, L.L. Incidence of toxoplasmosis in pregnancy in Campania: A population-based study on screening, treatment, and outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 316–321. [Google Scholar] [CrossRef]
- Donadono, V.; Saccone, G.; Sarno, L.; Esposito, G.; Mazzarelli, L.L.; Sirico, A.; Guida, M.; Martinelli, P.; Zullo, F.; Maruotti, G.M. Association between lymphadenopathy after toxoplasmosis seroconversion in pregnancy and risk of congenital infection. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 45–51. [Google Scholar] [CrossRef]
- Evangelista, F.F.; Mantelo, F.M.; de Lima, K.K.; Marchioro, A.A.; Beletini, L.F.; de Souza, A.H.; Santana, P.L.; Riedo, C.d.O.; Higa, L.T.; Guilherme, A.L.F. Prospective evalution of pregnant women with suspected acute toxoplasmosis treated in a reference prenatal care clinic at a university teaching hospital in Southern Brazil. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, 46. [Google Scholar] [CrossRef]
- Ludwig, A.; Fernandes, F.D.; Guerra, R.R.; Braünig, P.; Ramos, L.S.; Pacheco, L.S.; Sangioni, L.A.; Vogel, F.S.F. Molecular detection of Toxoplasma gondii in placentas of women who received therapy during gestation in a toxoplasmosis outbreak. Infect. Genet. Evol. 2022, 97, 105145. [Google Scholar] [CrossRef]
- Mueller, R.A.S.; Frota, A.C.C.; Barreto, D.D.M.; Vivacqua, D.P.F.; Loria, G.B.; Lebreiro, G.P.; Martins, M.G.; Potsch, M.V.; Maia, P.D.; Coutinho, R.L.M.; et al. Congenital Toxoplasmosis: Missed Opportunities for Diagnosis and Prevention. J. Trop. Pediatr. 2021, 67, fmaa069. [Google Scholar] [CrossRef] [PubMed]
- Pengsaa, K.; Hattasingh, W. Congenital toxoplasmosis: An uncommon disease in Thailand. Paediatr. Int. Child Health 2015, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Prasil, P.; Sleha, R.; Kacerovsky, M.; Bostik, P. Comparison of adverse reactions of spiramycin versus pyrimethamine/sulfadiazine treatment of toxoplasmosis in pregnancy: Is spiramycin really the drug of choice for unproven infection of the fetus? J. Matern.-Fetal Neonatal Med. 2023, 36, 2215377. [Google Scholar] [CrossRef] [PubMed]
- Saghrouni, F.; Khammari, I.; Ben Abdeljelil, J.; Yaacoub, A.; Meksi, S.G.; Ach, H.; Garma, L.; Fathallah, A.; Ben Saïd, M. La toxoplasmose congénitale: à propos de 21 cas. J. Pédiatr. Puéricult. 2013, 26, 83–89. [Google Scholar] [CrossRef]
- Teil, J.; Dupont, D.; Charpiat, B.; Corvaisier, S.; Vial, T.; Leboucher, G.; Wallon, M.; Peyron, F. Treatment of Congenital Toxoplasmosis: Safety of the Sulfadoxine-Pyrimethamine Combination in Children Based on a Method of Causality Assessment. Pediatr. Infect. Dis. J. 2016, 35, 634–638. [Google Scholar] [CrossRef]
- Villar, B.B.D.L.F.; Neves, E.d.S.; Louro, V.C.; Lessa, J.F.; Rocha, D.N.; Gomes, L.H.F.; Junior, S.C.G.; Pereira, J.P.; Moreira, M.E.L.; Guida, L.d.C. Toxoplasmosis in pregnancy: A clinical, diagnostic, and epidemiological study in a referral hospital in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2020, 24, 517–523. [Google Scholar] [CrossRef]
- Yamamoto, L.; Targa, L.S.; Sumita, L.M.; Shimokawa, P.T.; Rodrigues, J.C.; Kanunfre, K.A.; Okay, T.S. Association of Parasite Load Levels in Amniotic Fluid With Clinical Outcome in Congenital Toxoplasmosis. Obstet. Gynecol. 2017, 130, 335–345. [Google Scholar] [CrossRef]
- Zuluaga, L.M.; Hernández, J.C.; Castaño, C.F.; Donado, J.H. Effect of antenatal spiramycin treatment on the frequency of retinochoroiditis due to congenital toxoplasmosis in a Colombian cohort. Biomedica 2017, 37 (Suppl. S1), 86–91. [Google Scholar] [CrossRef]


| Study | Country | Population (*) | Mother Treatment (Diagnosis Trimester/Week Initiated Protocol) | Newborn Treatment (*) | Conclusions |
|---|---|---|---|---|---|
| Avelino et al., 2014 [44] | Brazil | 120 treated PW, 115 untreated PW, 162 treated NB, 0 untreated NB | S (2T,25W) | SPF | Treatment of pregnant women with spiramycin reduces the possibility of transmission of infection to the fetus. However, a lack of proper treatment is associated with the onset of the neural–optical form of congenital infection. Primary preventive measures should be increased for all pregnant women during the prenatal period, and secondary prophylaxis through surveillance of seroconversion in seronegative pregnant woman should be introduced to reduce the severity of congenital infection in the environment. |
| Conceição et al., 2021 [24] | Brazil | 82 treated PW, 102 untreated PW, 29 treated NB, 0 untreated NB | Undefined (3T,29W) | SPF-C | High prevalence rates of clinical manifestations were observed in infants with congenital toxoplasmosis after a waterborne toxoplasmosis outbreak, the largest yet described. Cerebral calcifications were higher in infants with ocular abnormalities, and maternal infection during the third pregnancy trimester was associated with a higher rate of congenital toxoplasmosis independent of maternal treatment. |
| Damar et al., 2023 [20] | Turkey | 103 treated PW, 16 untreated PW, 3 treated NB, 2 untreated NB | S (2T,15W) | C-SX | In conclusion, although Toxoplasma seroprevalence was found to be high in our region, there was a paucity in diagnosis, follow-up, and treatment. Our findings support that prenatal spiramycin prophylaxis is effective in preventing the transmission of parasites from mother to child. |
| De Santis et al., 2024 [45] | Italy | 537 treated PW, 35 untreated PW, 34 treated NB, 0 untreated NB | S/SPF-Cl/S | Undefined | The study discusses the efficacy of available treatments to reduce the risk of vertical transmission of toxoplasmosis during pregnancy, highlighting the controversy over their effectiveness. Although a large randomized clinical trial would be ideal to validate or modify current clinical practices, randomization against placebo is considered unethical. The authors’ experience indicates that maternal treatment with spiramycin and cotrimoxazole, even with negative amniocentesis, can significantly reduce the rate of transmission of congenital toxoplasmosis without causing harm to the mother or fetus. |
| Gomes-Ferrari-Strang et al., 2023 [38] | Brazil | 48 treated PW, 13 untreated PW, 61 treated NB, 0 untreated NB | S-S/SPF (2T,26W) | SPF | Based on the follow-up of women with acute T. gondii infection and their children, through a multidisciplinary team, the availability of anti-T. gondii serology and pre- and post-natal treatments reduced the risk of toxoplasmosis transmission. |
| Guarch-Ibáñez et al., 2024 [46] | Spain | 36 treated PW, 18 untreated PW, 0 treated NB, 0 untreated NB | S-SPF-S/SPF | S/SPF | Since cases detected by prenatal screening and treated with SPI and/or PSA presented fewer complications at birth and during follow-up, it is recommended to implement universal screening in Spain and in countries with similar epidemiological data. Long-term follow-up of the REIV-TOXO cohort will provide more information on late complications and the effects of pre- and postnatal treatments. |
| Guegan et al., 2021 [27] | Frace/Serbia/USA | 61 treated PW, 54 untreated PW, 0 treated NB, 0 untreated NB | S-SPF-S/SPF (3T,28W) | Undefined | The sensitivity of PCR for detecting Toxoplasma in blood was also reduced by maternal treatment from 39.1% to 23.2%. These results highlight that anti-Toxoplasma therapy during pregnancy may set back biological evidence of neonatal infection at birth and underline the need for a careful serological follow-up of infants with normal workup. |
| Lago et al., 2014 [47] | Brazil | 12 treated PW, 16 untreated PW, 59 treated NB, 6 untreated NB | Undefined | Undefined | Even with high sensitivity methods, children with congenital toxoplasmosis can have a negative anti-Toxoplasma IgM result at birth. It is important not to interrupt the monitoring of infants with suspected congenital toxoplasmosis simply because they present a negative anti-Toxoplasma IgM result. |
| Losa et al., 2024 [48] | Portugal | 63 treated PW, 7 untreated PW, 0 treated NB, 0 untreated NB | S-SPF-S/SPF | S/SPF | The lower incidence observed in the study, compared to Europe, may be related to the reduction in the prevalence of toxoplasmosis, the effectiveness of primary infection prevention measures, and a well-structured prenatal screening program, which allows early initiation of treatment to prevent vertical transmission. |
| Mejia-Oquendo et al., 2021 [49] | Colombia | 52 treated PW, 47 untreated PW, 0 treated NB, 0 untreated NB | S-SX/P (2T,22W) | Undefined | The study showed that an early detection program for gestational toxoplasmosis implemented at a public health center in Armenia, Quindío, correctly followed evidence-based guidelines. Diagnostic tests were requested in a timely manner, with adequate follow-up of seronegative pregnant women and timely initiation of treatment. Before the implementation of the guidelines, some mothers were not treated, and their children had more ocular and neurological sequelae, something that decreased after the adoption of the recommendations. However, the frequency of infection did not decrease compared to previous studies, and there were failures in the reporting of some IgA results. |
| Olariu et al., 2019 [21] | USA and Romania | 23 treated PW, 164 untreated PW, 0 treated NB, 0 untreated NB | Undefined | Undefined | These findings provide further evidence that anti-parasitic treatment if administered during pregnancy can contribute to better clinical outcomes, even in countries where systematic screening and treatment have not been routinely implemented. |
| Prusa et al., 2015 [22] | Austria | 660 treated PW, 27 untreated PW, 35 treated NB, 4 untreated NB | S-SPF-S/SPF-SZ (3T,28W) | S/SPF | Amniocentesis is indicated in women with acute maternal infection and facilitated targeted therapies in pregnant women and their offspring. In women with late T. gondii infection, negative amniotic fluid PCR made treatment of infants unnecessary. Serological and clinical follow-up of infants is important to confirm the infection status of the infant. Recommendations, based on our 17-year experience, to improve the current diagnostic strategies and to reduce unnecessary amniocentesis, are given. |
| Prusa et al., 2015-2 [50] | Austria | 1110 treated PW, 63 untreated PW, 141 treated NB, 0 untreated NB | S/SPF (3T,30W) | S/SPF | Results from the Austrian Toxoplasmosis Register show the efficiency of the prenatal screening program. Our results are of clinical relevance for infants, healthcare systems, and policy makers to consider preventive T. gondii screening as a potential tool to reduce the incidence of congenital toxoplasmosis. |
| Rodrigues et al., 2014 [51] | Brazil | 44 treated PW, 24 untreated PW, 46 treated NB, 0 untreated NB | S (1T,13W) | SPF | The higher proportion of infants without clinical symptoms in group 1 (70.4%) suggests that maternal treatment with spiramycin delays fetal infection, reducing the clinical sequelae of the disease in newborns. Given the low sensitivity of the tests used, when there is suspicion of congenital transmission, several serological and parasitological tests are required in order to confirm or exclude congenital toxoplasmosis in newborns. |
| Soares et al., 2023 [30] | Brazil | 46 treated PW, 33 untreated PW, 79 treated NB, 0 untreated NB | S-SPF-S/SPF (3T,28W) | SPF | A positive advance was observed regarding the care provided for the mother–child binomial affected by T. gondii, with a reduction in negative outcomes for the child. However, there are still challenges concerning the diagnosis and proper management of the disease. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, S.K.; Mariano, I.M.; Cunha, A.C.R.; Pajuaba, A.C.A.M.; Mineo, T.W.P.; Mineo, J.R. Treatment Protocols for Gestational and Congenital Toxoplasmosis: A Systematic Review and Meta-Analysis. Microorganisms 2025, 13, 723. https://doi.org/10.3390/microorganisms13040723
Ribeiro SK, Mariano IM, Cunha ACR, Pajuaba ACAM, Mineo TWP, Mineo JR. Treatment Protocols for Gestational and Congenital Toxoplasmosis: A Systematic Review and Meta-Analysis. Microorganisms. 2025; 13(4):723. https://doi.org/10.3390/microorganisms13040723
Chicago/Turabian StyleRibeiro, Sissi Kelly, Igor Moraes Mariano, Ana Clara Ribeiro Cunha, Ana Cláudia Arantes Marquez Pajuaba, Tiago Wilson Patriarca Mineo, and José Roberto Mineo. 2025. "Treatment Protocols for Gestational and Congenital Toxoplasmosis: A Systematic Review and Meta-Analysis" Microorganisms 13, no. 4: 723. https://doi.org/10.3390/microorganisms13040723
APA StyleRibeiro, S. K., Mariano, I. M., Cunha, A. C. R., Pajuaba, A. C. A. M., Mineo, T. W. P., & Mineo, J. R. (2025). Treatment Protocols for Gestational and Congenital Toxoplasmosis: A Systematic Review and Meta-Analysis. Microorganisms, 13(4), 723. https://doi.org/10.3390/microorganisms13040723





