Abstract
Toxoplasmosis is a globally prevalent zoonotic parasitic disease. Neonates with congenital infection can develop severe long-term sequelae, which can be mitigated or prevented through early diagnosis and therapeutic approaches. In this context, the main objective of this study was to describe the main treatments and evaluate the effectiveness of the current treatment protocols for gestational and congenital toxoplasmosis to prevent vertical transmission and to reduce clinical manifestations in neonates. This systematic review with a meta-analysis searched digital databases (PUBMED, SCOPUS, WEB OF SCIENCE, EMBASE, and COCHRANE) for observational cohort studies published between 1 January 2013 and 29 January 2025, evaluating treatment effectiveness in gestational and congenital toxoplasmosis. Risk ratios (RRs) were calculated using random effects models to assess infection risk and clinical manifestations in neonates. The study quality was assessed following the Joanna Briggs Institute protocol and fifty-six studies from 16 countries were included, comprising 11,090 pregnant women and 4138 children. Studies were predominantly from Brazil (38%), France, and Italy. Only 9% of the studies indicated knowledge of the serological status of the pregnant woman before the gestational stage. Of 10,148 women with confirmed toxoplasmosis, 8600 received treatment, with 18% of their children infected, compared to a 58% infection rate in untreated mothers’ children. Meta-analysis showed that treatment reduced infection risk (RR = 0.34 [0.21; 0.57]) and clinical manifestations (RR = 0.30 [0.17; 0.56]). While spiramycin or triple therapy showed similar effects, triple therapy demonstrated more consistent results (RR: 0.22 [0.15; 0.32]) compared to spiramycin alone (RR: 0.54 [0.06; 4.67]). In conclusion, treatment protocols for congenital or gestational toxoplasmosis have proven to be effective in reducing the risk of infection and clinical manifestations in neonates. Regarding the type of treatment, although they have similar responses, the use of triple therapy shows more consistent responses than isolated spiramycin. It can be also concluded that prevention and mitigation of congenital toxoplasmosis require standardized treatment protocols, improved diagnostic methods, and educational programs for women of childbearing age, as treatment initiation timing and protocol choice are crucial factors in determining outcomes.
1. Introduction
Toxoplasma gondii is one of the most successful parasitic pathogens worldwide, capable of infecting a remarkable range of avian and mammalian hosts, including humans [1]. This protozoan’s heteroxenous life cycle, with felids as definitive hosts, contributes to its widespread distribution and significant public health impact [2,3]. Humans can be infected by several routes, by eating undercooked meat of animals containing tissue cysts, by consuming food or water contaminated with cat feces, by contaminated environmental samples, by blood transfusion or organ transplantation, as well as transplacentally from mother to fetus [1]. While typically asymptomatic in immunocompetent hosts, T. gondii infection poses significant health challenges in two critical scenarios: immunocompromised individuals, where it can cause severe neurological complications, and pregnant women, where transplacental transmission puts the fetus at risk of congenital infection [3]. In addition to these implications, socioeconomic factors such as lower socioeconomic status and education of the population play a significant role in the prevalence of toxoplasmosis. Although socioeconomic factors influence toxoplasmosis prevalence, congenital infection can affect all demographic groups, making it a universal concern in maternal healthcare [4].
Considering this complex scenario, the therapeutic approach to toxoplasmosis is a significant challenge [5]. Conventional treatment is limited to the acute phase of the disease, with no effects on latent parasites; consequently, a cure is still not available. Furthermore, considerable toxic effects and long-term therapy contribute to high rates of treatment abandonment [6]. However, evidence from French studies suggests that preventive and therapeutic approaches during pregnancy can reduce the risk of symptoms and sequelae in children [7].
Regarding the type of treatment, anti-Toxoplasma chemotherapy consists of several medications that can be used individually or in combination. These include sulfadiazine (SDZ), pyrimethamine (PYR), sulfamethoxazole (SMT), trimethoprim (TMP), and spiramycin [8]. Currently, the gold standard treatment involves a combination of SDZ and PYR, with the option to use the combination of SMT and TMP, clindamycin, and spiramycin, all of which present a synergistic effect affecting the replication of the tachyzoite form of T. gondii [9,10]. New research on the impacts of toxoplasmosis highlights the need to increase institutional awareness of infection pathways and implement comprehensive, interdisciplinary actions to control transmission and optimize treatment [11].
Given the complex clinical context and varied therapeutic approaches, this systematic review with a meta-analysis aims to evaluate treatment protocols for gestational and congenital toxoplasmosis, focusing on their effectiveness in preventing vertical transmission and reducing clinical manifestations in newborns.
2. Methods
2.1. Search Strategy and Selection Criteria
This systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA-P) guidelines [12], and the method protocol used was previously published (Available online: https://www.protocols.io/view/protocol-of-a-systematic-review-with-metanalysis-f-e6nvwddp2lmk/v1 accessed on 10 February 2025) and registered on the PROSPERO platform (Available online: https://protocols.io/view/protocol-of-a-systematic-review-with-metanalysis-f-cymnxu5e.pdf accessed on 10 February 2025).
Searches were conducted in PUBMED, SCOPUS, WEB OF SCIENCE, EMBASE, and the COCHRANE Library, manually via Google Scholar, plus with reference lists of key articles, for studies published between 1 January 2013 and 29 January 2025. The search strategy combined three categories of terms (Treatment, Congenital toxoplasmosis, and Population) using Boolean operators ‘OR’ within categories and ‘AND’ between categories, as follows: (Treatment OR Drug OR Drugs OR Spiramycin OR Sulfadiazine OR “Folinic acid” OR Pyrimethamine) and (“Congenital toxoplasmosis”) and (Maternal OR Pregnant OR Children OR Child OR Newborn OR Baby OR Babies OR Pediatric OR Infant OR Neonate).
Studies were eligible with the following characteristics: (1) population: human, pregnant, and newborn; (2) intervention: treatment of toxoplasmosis; (3) control/comparator: children/mothers not treated for toxoplasmosis; (4) outcomes of interest: treatment protocols used and their effectiveness in reducing risk of vertical transmission and clinical manifestations in neonates; (5) languages: there was no language restriction; while no design restrictions were applied, only cohort studies (prospective or retrospective) met the inclusion criteria of reporting both T. gondii infection status and treatment data; (6) publication dates: within the specific time frame that has been set.
Excluded works included literature reviews, meta-analyses, letters to the editor, animal studies, studies unrelated to toxoplasmosis and treatment, studies that treat other comorbidities in addition to toxoplasmosis, full texts that were not accessible, and texts published before 2013. To avoid bias in the results, studies whose mothers had co-infection were excluded.
Regarding outcome assessment, vertical transmission was confirmed through serological tests (IgM and IgG), PCR in amniotic fluid, or placental examination. Clinical manifestations were documented via ophthalmological examination, neurological assessment, and imaging studies. Treatment effectiveness was evaluated by comparing infection rates and clinical manifestations between treated and untreated groups. These standardized outcome measures were used to ensure consistency in data extraction and analysis across included studies.
First, the titles and abstracts of the research studies were evaluated independently by two researchers. Duplicates and studies that did not meet the inclusion or exclusion criteria were excluded from the analyses. Title and abstract screening were conducted using Rayyan (https://www.rayyan.ai/), accessed on 10 February 2025, a software for organizing and managing systematic reviews independently, where each reviewer performed article selection blindly [13]. During the selection process, all data were cross-checked, and discrepancies were resolved through consensus and, if necessary, by the senior researcher. Finally, the full texts of eligible articles were read by the same researchers to decide on their definitive inclusion. For articles considered relevant to the review but not available in full for reading, authors were contacted to request the availability of the full article.
Data were collected using a standardized Excel spreadsheet (Microsoft Excel®, version 2016). The data extraction included the following: (1) bibliometrics (country where the study was conducted, title, journal, language, DOI, and publication year); (2) time of diagnosis; (3) initiation phase of therapy, therapeutic options; (4) dosage form of drugs; (5) dosage; (6) main clinical manifestations and sequels; (7) duration of treatment; (8) patient follow-up; (9) number of treated and untreated patients; (10) idiosyncrasies of the population or treatment.
After the selection phase, the data were extracted, and the quality assessment was performed using the Joanna Briggs Institute (JBI) critical appraisal checklist for cohort studies, which evaluates key methodological aspects including population selection, exposure measurement, identification of confounding factors, outcome assessment, and follow-up adequacy. Studies were rated on each criterion as ‘yes’, ‘no’, ‘unclear’, or ‘not applicable’ [14].
2.2. Data Synthesis and Analysis
The data were evaluated using the programming language “R” [15] through the supplements “meta” [16] and “metafor” [17]. The pooled effect estimates were computed from risk ratio (RR) differences between treated and untreated groups using random effects models due to expected clinical and methodological heterogeneity between studies. Statistical heterogeneity among studies was evaluated by Cochran’s Q test and I2 inconsistency test [18]. Data synthesis involved pooling RR for infection and clinical manifestations separately, with subgroup analyses performed by treatment type. Subgroups analysis was conducted through comparing the incidence of cases of congenital toxoplasmosis after different treatments in pregnant women. Forest plots were generated to present the pooled effect and the 95% confidence interval.
3. Results
3.1. Qualitative Results
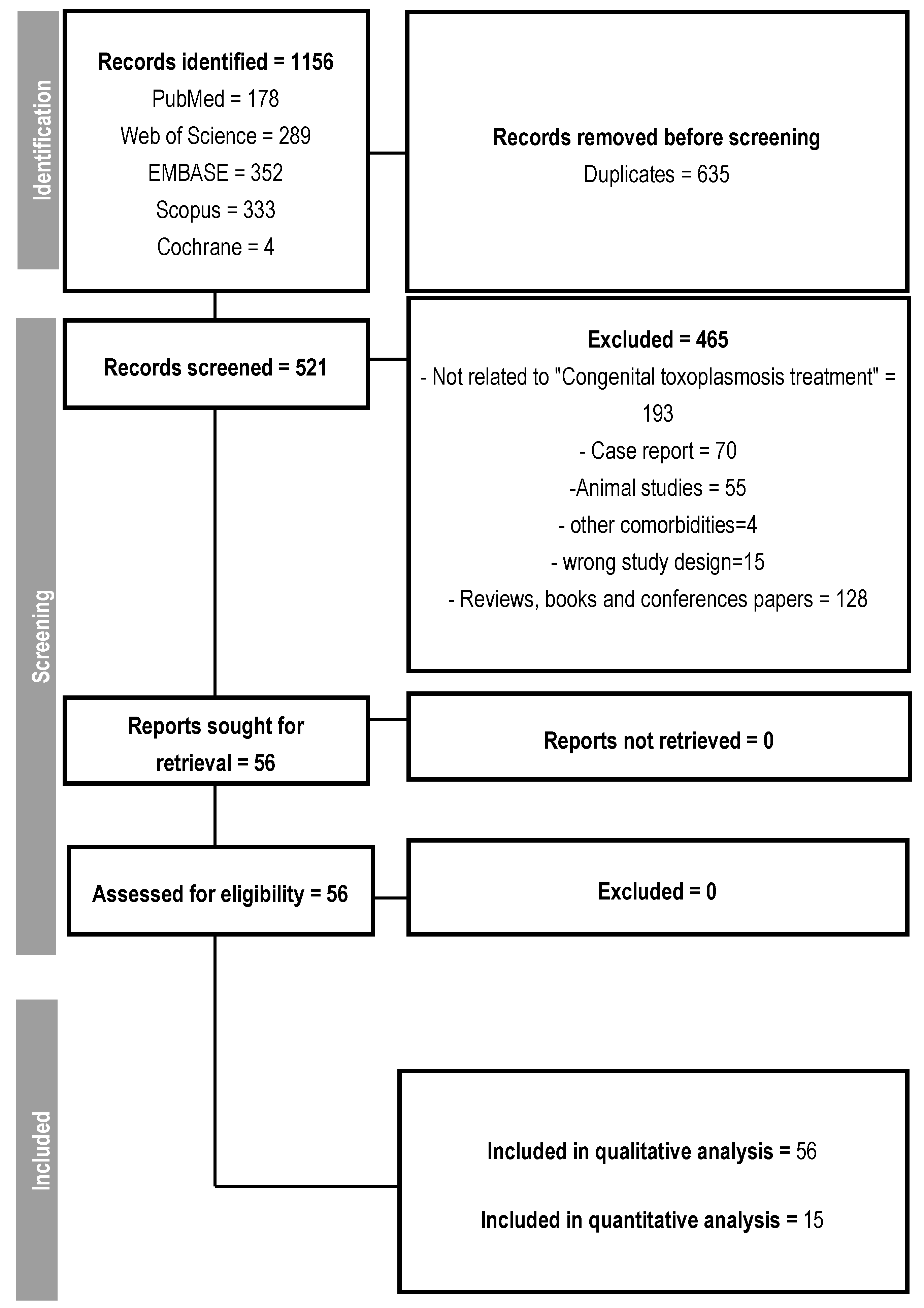
From the initial selection of 1156 studies, 635 were duplicates. Out of the remaining 521, 465 were excluded for pre-defined reasons, such as studies unrelated to the treatment of congenital toxoplasmosis, case reports, studies conducted on animals, reviews, and presentations at scientific conferences, resulting in the selection of 56 studies that addressed the treatment of congenital or gestational toxoplasmosis (Figure 1). No article was excluded due to its inaccessibility.
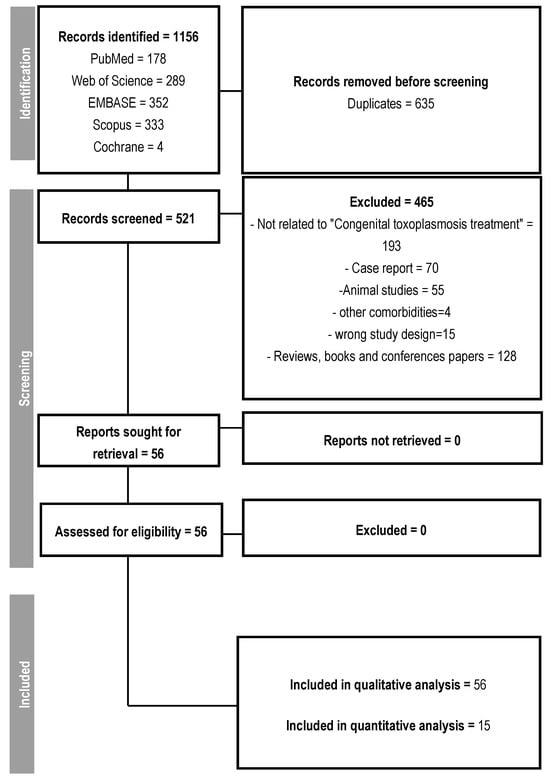
Figure 1.
Follow-up flowchart.
All 56 eligible studies contributed to the construction of data to elucidate the evidence, considering the following variables: time of diagnosis; initial phase of therapy, therapeutic options; pharmaceutical form of medications; dosage; main clinical manifestations and sequelae; treatment duration; patient follow-up; number of treated and untreated patients; population or treatment idiosyncrasies. The characteristics of all studies included in the qualitative analysis are shown in Supplementary Table S1. A total of 11,090 pregnant women and 4138 children were enrolled in these studies. Eligible studies were published between 2013 and 2025 in 16 countries. Out of the total number of studies analyzed, 20 were conducted in Brazil, followed by France and Italy, with 6 studies each. Moreover, 85% of the studies were conducted in Referral Centers.
Some studies have shown that the screening period for toxoplasmosis during pregnancy may vary from country to country, but indirect diagnostic methods for detecting IgM and IgG immunoglobulins were unanimous, albeit with variations in serological methodology [19,20,21,22]. In this regard, one of the mentioned diagnostic methods was the Sabin Feldman test, considered the gold standard for toxoplasmosis diagnosis. Some studies have demonstrated the use of other immunoglobulins such as IgA and IgE [23,24,25,26,27,28,29,30,31,32] to complement the diagnosis of Toxoplasma infection. Another methodology addressed in the studies was the avidity test, considered a useful tool for detecting the timing of infection [33]. Ultrasound imaging examinations in pregnant women were also present in all studies, while the PCR technique was present in 50% of the studies.
Regarding the knowledge of the pregnant woman’s serological status before the gestational stage, a relevant factor in defining the timing of infection if the infection was acquired during or early in pregnancy, which would facilitate the detection of the moment of seroconversion. However, this analysis was addressed in only 9% of the studies.
A total of 2652 IgG avidity tests were conducted, with 1595 of them detecting low avidity. Additionally, a total of 4284 PCR tests were performed, with 3724 on amniotic fluid and 560 on placenta or umbilical cord, resulting in 442 positive results for a total of 995 children diagnosed with congenital toxoplasmosis with follow-up after one year of age. Additionally, routine follow-up tests for pregnant women considered seronegative for toxoplasmosis during prenatal care are monthly in France and Italy [3,28,31,34,35,36]. Ultrasounds were conducted on pregnant women as a complementary examination to indirect diagnostic tests, and in children, fundoscopic eye examinations, transfontanelle ultrasounds performed at birth, and physical and neurological examinations were carried out in all studies conducted at reference centers.
According to Damar et al. [20], in Turkey, specifically in the city of Sanlúrfa, there is no established protocol considering that it is not a highly prevalent disease in the country. Similarly, in Japan, the frequency of prenatal screening exams, according to Hijikata et al. [37], is quarterly since it does not have a high incidence. In Vienna, Austria, the frequency of exams is bimonthly [22]. According to Carral [19], in Buenos Aires, Argentina, exams are repeated every trimester and during childbirth, and in Brazil, exams are repeated quarterly [38,39,40].
Out of a total of 10,148 pregnant women diagnosed with toxoplasmosis, 8600 received some form of treatment and had 1548 (18%) infected children, while 1586 untreated mothers had 922 children infected with T. gondii (58%).
In 71% of the studies, the triple therapy regimen (sulfadiazine, pyrimethamine, and folinic acid) associated or not with spiramycin is used as a treatment for gestational and congenital toxoplasmosis, with the following dosage regimens: pyrimethamine 25–50 mg/day, sulfadiazine 3 g/day, folinic acid 25–50 mg twice a week, and spiramycin 3 g/day. For neonates, the established regimen is pyrimethamine 3 mg/kg every 3 days, sulfadiazine 25 mg/kg every 8 hours, and folinic acid 50 mg every 7 days orally [41].
Only five studies cited the pharmaceutical form adopted for the treatment of neonates/children. The most common clinical manifestations in children of treated mothers were ophthalmological, while in children of untreated mothers, the predominant clinical manifestations were neurological. In total, 113 deaths were reported, of which 48% were spontaneous abortions, and 25% were terminations of pregnancy after amniocentesis results [26,42,43], with the remainder being represented by 15% stillbirths, 11% postnatal deaths, and 1% death during adolescence.
3.2. Quantitative Results
To perform the meta-analysis, only studies with groups larger than 10 individuals per group and that did not include only infected individuals were considered. This approach reduces the risk of bias associated with small sample sizes and studies in which both groups would have all individuals infected, which would not allow for infection risk analyses. As shown in Table 1, a total of 15 studies were included in the meta-analysis, some of which contained infection risk data, while others presented clinical manifestation data, leaving 10 for each analysis. However, the timing of maternal infection, a potential important confounder, could not be adequately analyzed as this information was not consistently reported across studies. Although the exact timing of infection was uncertain, studies reported mean treatment initiation at 24 ± 6 gestational weeks.

Table 1.
General characteristics of the studies included in the meta-analysis.
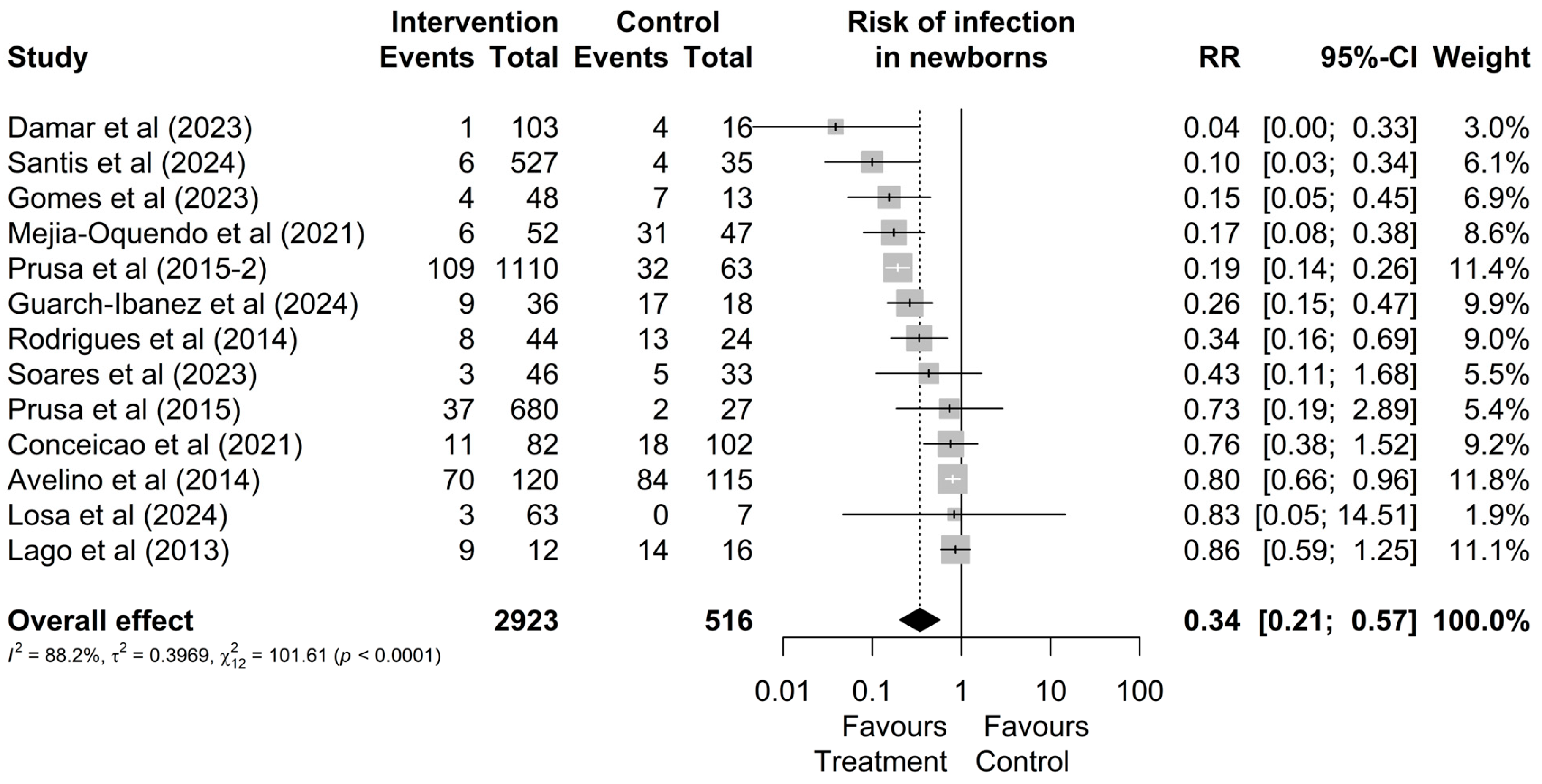
Regarding the risk of infection, out of the 2923 mothers treated during pregnancy and the 516 who remained untreated, it was observed that a total of 276 (9%) and 231 (45%) had infected newborns, respectively (Figure 2). Therefore, treatment reduces the possibility of vertical transmission (RR = 0.34 [0.21; 0.57]; Figure 2). Additionally, no outlier values were identified regarding the risk of infection.
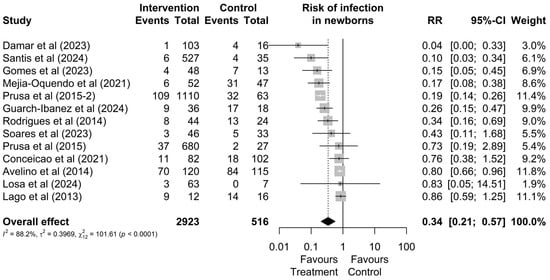
Figure 2.
Forest graph of the risk of infection in the fetus after gestational treatment for toxoplasmosis [20,22,24,30,38,44,45,46,47,48,49,50,51].
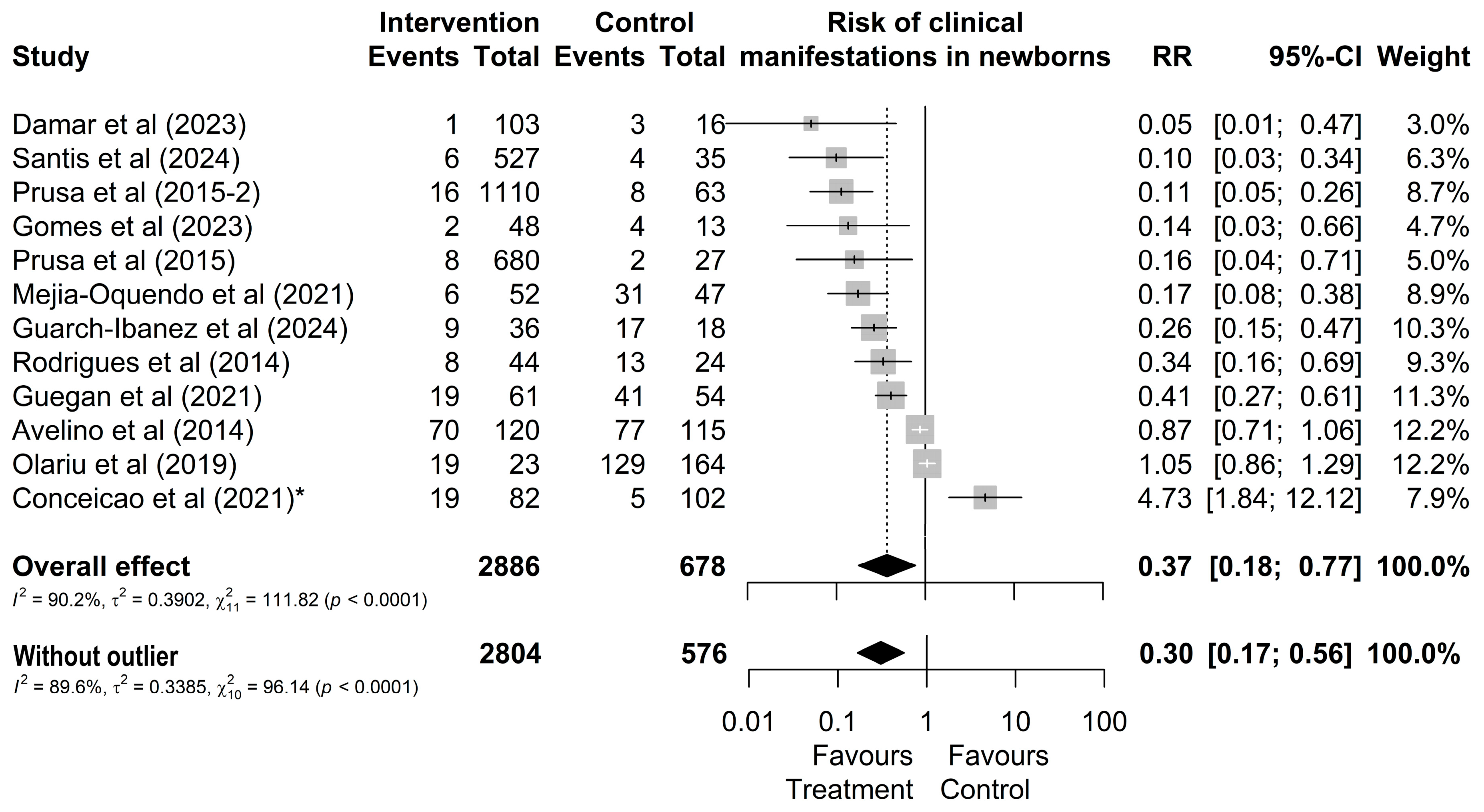
Regarding the clinical manifestations of newborns from treated or untreated mothers, it was observed that out of 2886 treated mothers, 183 (6%) had children who presented some type of clinical manifestation (Figure 3). In contrast, out of 678 untreated mothers, a total of 334 (49%) of their children presented one of the typical clinical manifestations (Figure 3). However, the study by Conceição et al. [24] was identified as an outlier and influential point without overlapping effects with other studies. By omitting this study, the result changes from RR = 0.37 [0.18; 0.77] (Figure 3) to RR = 0.30 [0.17; 0.56].
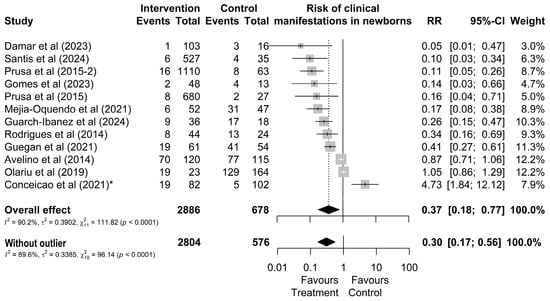
Figure 3.
Forest chart of the risk of clinical manifestations in children after gestational treatment for toxoplasmosis. (*) The outlier study [20,21,22,24,27,38,44,45,46,49,50,51].
3.3. Comparative Analysis Between Treatments
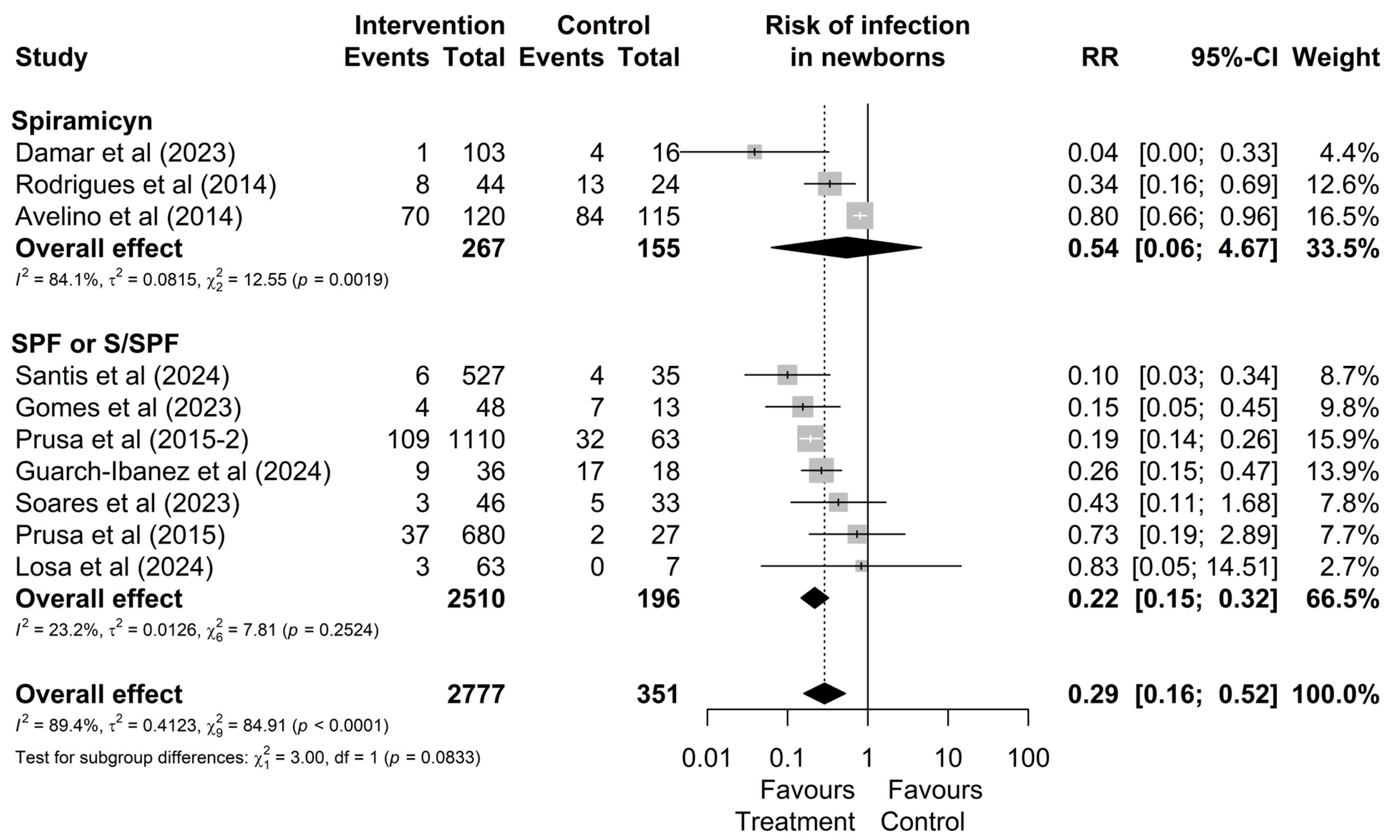
As shown in Figure 4, although no significant difference between treatments was observed in the overall analysis (p = 0.0833), the subgroup of studies involving mothers treated with the triple regimen demonstrated lower heterogeneity (I2 = 23.2%, χ2 p = 0.18) and statistically significant results (RR: 0.22 [0.15; 0.32]) compared to the group treated with spiramycin alone (I2 = 84.1%, χ2 p < 0.01), which showed non-significant results (RR: 0.54; [0.06; 4.67]). These findings suggest greater consistency in treatment effects for mothers who received the triple regimen.
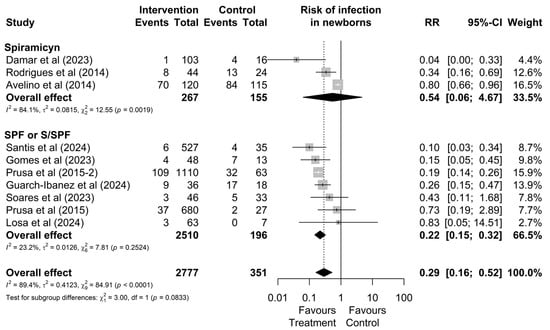
Figure 4.
Forest plot of the proportion of infected neonates divided into treatment drug subgroups. CI: confidence interval; studies with more than one drug subgroup; SPF: sulfadiazine + pyrimethamine + folinic acid; W/SPF: spiramycin alternated with SPF [20,22,30,38,44,45,46,48,50,51].
3.4. Risk of Bias
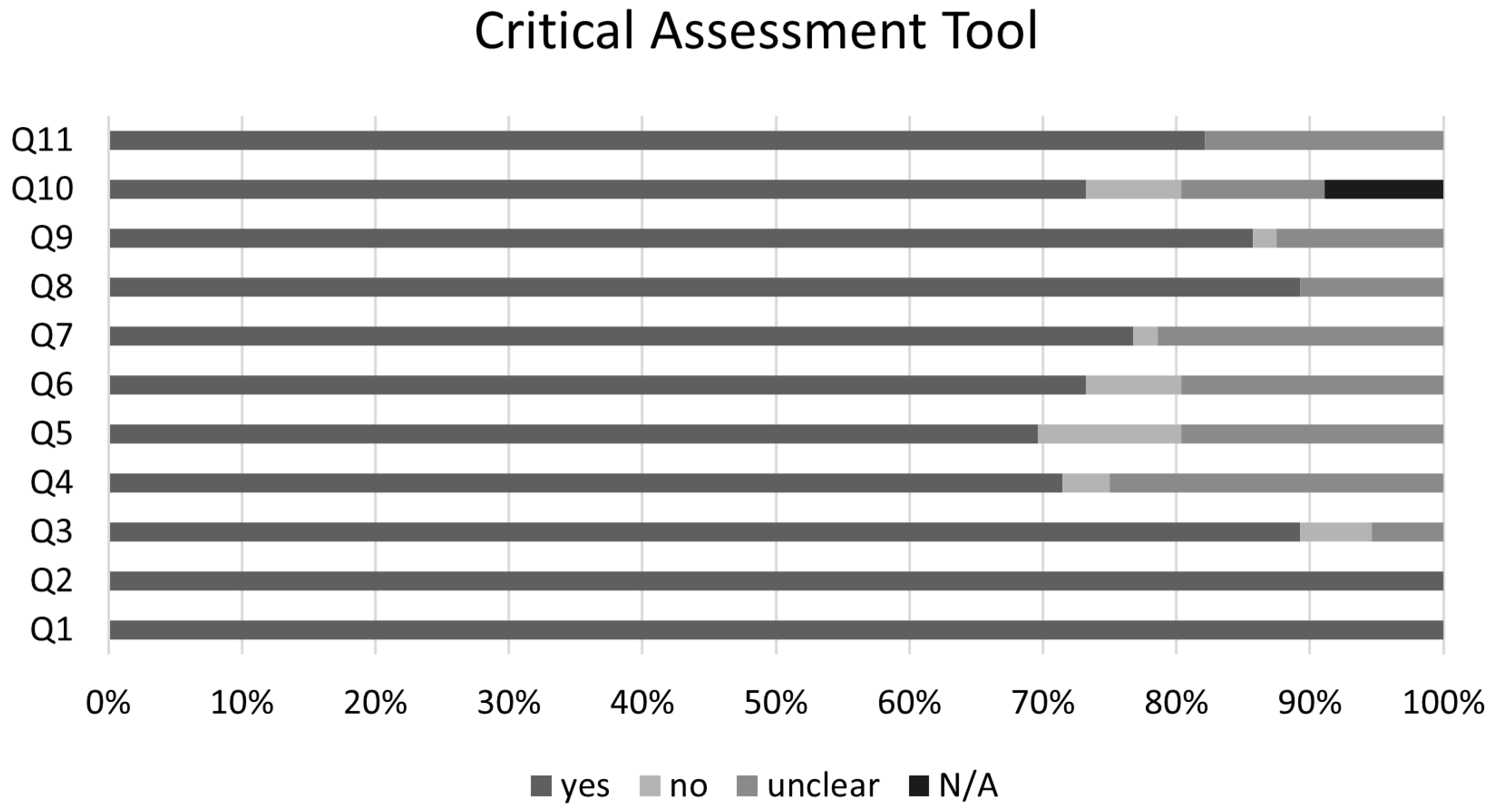
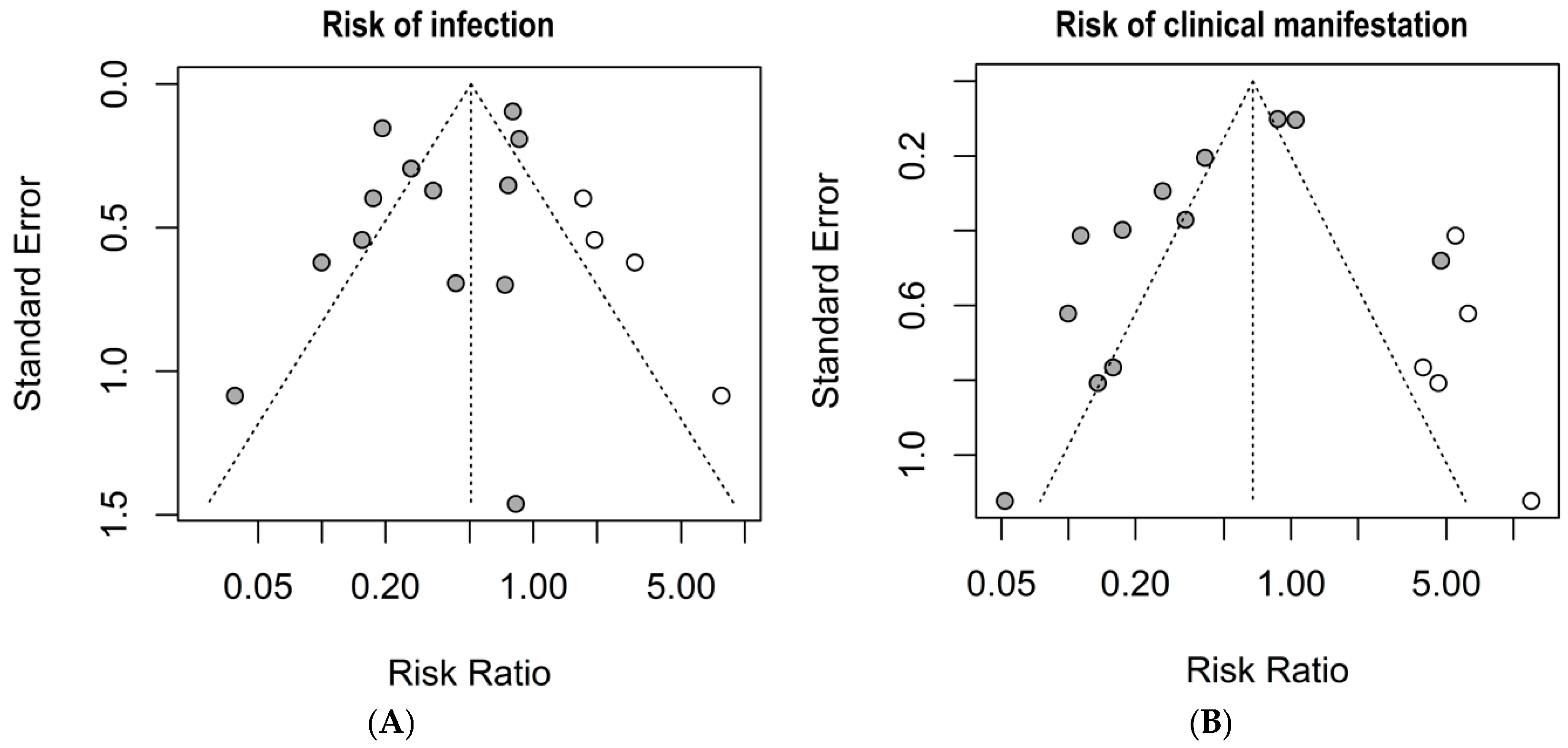
The graphical summary of the bias risk assessment using the Joanna Briggs Institute critical appraisal tool for all studies included in qualitative and quantitative analyses is shown in Figure 5. Overall, the analyzed studies demonstrated robust methodological quality, with 84% average adherence to the assessment instrument criteria. The risk of bias evaluation revealed that most studies (81%) presented a low risk, while only 11% showed moderate risk, and 8% were classified as high risk. This favorable distribution of bias risk levels substantiates the methodological rigor of the included studies, enhancing the reliability of the findings. No authors reported conflicts of interest. Regarding publication bias, the Egger’s test did not show an increased risk; however, funnel plots with the trim-and-fill method suggest the possible omission of three studies regarding the risk of infection and four studies regarding the risk of clinical manifestations (Figure 6).
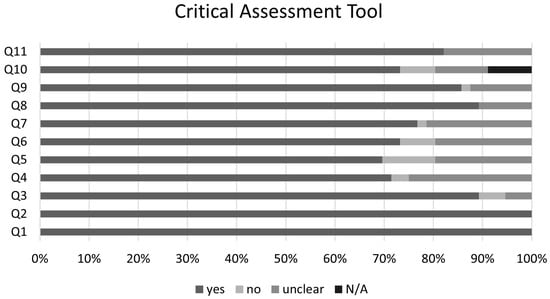
Figure 5.
Joanna Briggs Institute Critical Assessment Tool. Q1. Were the two groups similar and recruited from the same population? Q2. Were the exposures measured similarly to assign people to both exposed and unexposed groups? Q3. Was the exposure measured in a valid and reliable way? Q4. Were confounding factors identified? Q5. Were strategies to deal with confounding factors stated? Q6. Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? Q7. Were the outcomes measured in a valid and reliable way? Q8. Was the follow up time reported and sufficient to be long enough for outcomes to occur? Q9. Was follow up complete, and if not, were the reasons to loss to follow up described and explored? Q10.Were strategies to address incomplete follow up utilized? Q11. Was appropriate statistical analysis used? N/A—not applicable.
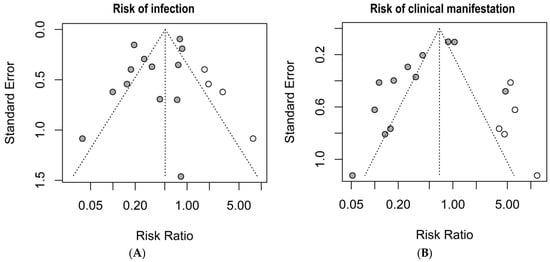
Figure 6.
Publication bias risk by the trim-and-fill method and funnel plots. (A) risk of infection; (B) risk of clinical manifestations. Grey circles: Published studies included in the meta-analysis. White circles: Hypothetical missing studies identified through trim-and-fill analysis. These represent potentially unpublished studies that, if included, would balance the funnel plot and produce a more symmetrical distribution around the estimated true effect size.
4. Discussion
The most effective approach to preventing congenital toxoplasmosis requires comprehensive management from diagnosis through treatment of the mother–child pair. Our meta-analysis demonstrated that maternal treatment significantly reduces both vertical transmission risk (RR = 0.34 [0.21; 0.57) and clinical manifestations in newborns (RR = 0.30 [0.17; 0.56]). While both spiramycin alone and triple therapy (sulfadiazine, pyrimethamine, and folinic acid) showed similar efficacy, triple therapy demonstrated more consistent results with lower heterogeneity between studies. In this systematic review, only 9% of studies reported prior knowledge of women’s serological status during the preconception period, highlighting a critical gap in preventive care. These findings emphasize the urgent need to implement protocols promoting early serological screening in women of childbearing age, particularly in countries with high toxoplasmosis incidence, as determining the timing of infection is crucial for optimal therapeutic management [36].
The implementation of monthly serological monitoring represents a critical strategy in preventing congenital toxoplasmosis. This is particularly evident when examining data from certain regions of high-incidence countries like Italy [28] and Brazil [38], where reported rates are 0.12% and 0.15%, respectively. While economic constraints in low- and middle-income countries often challenge the feasibility of monthly testing during prenatal care, the scientific evidence strongly supports that regular monitoring is essential for early detection and appropriate intervention [32]. This is primarily because the effectiveness of antiparasitic treatment significantly decreases once the parasite establishes intracellular infection beyond the initial parasitemia phase.
The critical importance of comprehensive diagnostic approaches is further emphasized by discordant immunoglobulin levels as reported by Fricker-Hidalgo et al. [34], demonstrating that interpretation of current tests extends beyond standard parameters. Misinterpretation or relativization of results outside recommended levels may result in irreversible fetal harm. Beyond standard IgG and IgM measurements, complementary testing for anti-Toxoplasma IgA and IgE isotypes [27,29], PCR techniques [36], and proper timing of diagnostic procedures are crucial for accurate diagnosis. The PCR test timing is particularly critical, as it should be performed between the 16th and 30th weeks of gestation and not exceed 4–6 weeks from the estimated infection date to avoid false-negative results. However, the accuracy of the PCR method remains unclear, as the occurrence of true false positive and false negative rates still need to be determined, even considering that diagnosis of congenital toxoplasmosis using a combination of IgG avidity in maternal blood and multiplex nested PCR in amniotic fluid and neonatal blood is helpful to detect a high-risk pregnancy, as well as to diagnose T. gondii infection [32].
The number of infected cells and intracellular parasite concentration significantly influence infection pathophysiology, emphasizing the importance of early and accurate diagnosis for optimal prognosis [50]. Variations in PCR diagnostic outcomes may result from multiple factors, including reduced test sensitivity, suboptimal timing of specimen collection relative to infection onset [29,34,50], or treatment initiation effects [27]. These technical challenges underscore why a comprehensive diagnostic approach is essential, incorporating multiple methodologies to ensure accurate detection and monitoring.
While this multi-method diagnostic approach represents the ideal standard of care, its implementation faces significant challenges, particularly in resource-limited settings. Healthcare systems in low- and middle-income countries must balance the substantial costs of comprehensive testing against the potential consequences of delayed or missed diagnoses. This economic consideration becomes particularly relevant given that regular monitoring remains fundamental for successful treatment outcomes, as the parasite’s intracellular behavior after initial parasitemia significantly reduces antiparasitic drug efficacy.
The complexity is further compounded by the specificity of acquired immunity to primary T. gondii genotype contact. Exposure to different genotypes requires development of new immunological memory, highlighting the importance of continued serological monitoring throughout pregnancy to identify recent infections. This biological characteristic, combined with the time-sensitive nature of effective intervention, reinforces why sustained monitoring, despite its associated costs, remains a cornerstone of effective prevention strategies.
When T. gondii infection is confirmed during pregnancy, treatment protocols should follow a clear progression based on gestational timing and diagnostic findings [52]. Initial treatment with spiramycin is indicated until the 16th week of gestation. After this period, the therapeutic approach should be guided by amniocentesis results and ultrasonographic findings. Detection of parasitic DNA through PCR or identification of morphological changes via ultrasound suggests fetal infection, necessitating a transition to triple therapy with sulfadiazine, pyrimethamine, and folinic acid. This protocol modification considers the parasite’s complex immunological interactions, as acquired immunity is genotype-specific [53]. The development of new immunological responses is required when encountering different T. gondii genotypes, making sustained monitoring essential throughout pregnancy for detecting potential new or reactivated infections. This biological characteristic underscores the importance of a flexible therapeutic approach that can be adjusted based on ongoing diagnostic findings and the specific nature of the infection.
Multiple factors influence the detection of fetal infection, including infection timing, PCR sample collection timing, and treatment initiation, all of which can affect test sensitivity [42]. While imaging studies provide valuable diagnostic information, they cannot definitively rule out fetal infection, particularly retinal involvement. Our meta-analysis demonstrates that maternal treatment reduces both transmission risk (42%) and clinical manifestations (40%), with subgroup analyses indicating more consistent outcomes for combination therapy (SPF or S/SPF) compared to spiramycin alone, aligning with findings from Montoya et al. [4]. These results support transitioning from spiramycin monotherapy to combination treatment after the initial gestational period, as spiramycin alone does not effectively treat fetal infection.
The timing of the immunological response presents additional diagnostic challenges in neonatal cases. The interval between placental and fetal infection allows anti-Toxoplasma maternal IgG antibody transfer, which can suppress fetal antibody production. This dynamic may result in negative anti-Toxoplasma IgM antibodies at birth despite infection, even with highly sensitive testing methods. Conversely, when maternal infection occurs near delivery, neonates may develop positive serology within weeks after birth, though subsequent confirmatory testing may become negative. These variable serological patterns emphasize the importance of comprehensive follow-up, as demonstrated by Lago et al. [54], who found that initiating treatment within two months of life serves as a protective factor against late-onset retinochoroiditis, which may manifest later in life [55].
Neonatal treatment during the first year of life aims to control infection until the child develops a sufficient immune response to inhibit parasite proliferation. McLeod et al. [56] emphasizes that treatment requires individually compounded formulations based on the child’s weight, aligning with World Health Organization guidelines for patient safety and pharmacotherapeutic principles [56,57]. The implementation of standardized syrup formulations is particularly crucial as these medications are typically only available in adult tablet form, helping optimize dosing while minimizing handling errors and inappropriate administration. However, as Trotta et al. [33] note, even with appropriate postnatal treatment, serious complications may occur, especially in cases of first-trimester infection.
The lack of global consensus on toxoplasmosis screening during pregnancy presents an ongoing challenge [58]. While some countries mandate comprehensive maternal monitoring, others do not recommend routine screening [32,59]. Our systematic review provides evidence supporting standardized protocols that include therapeutic regimen modification after the 16th–18th weeks of pregnancy, coupled with monthly serological monitoring. This approach is particularly important given that congenital toxoplasmosis is primarily associated with delayed maternal diagnosis and subsequent treatment initiation [39]. The evidence from our analysis supports improving surveillance of women of childbearing age, pregnant women, and infected children, as such measures have demonstrated reduced incidence of congenital toxoplasmosis [38]. Additionally, our findings reinforce the importance of immediate postnatal treatment initiation and standardized medication preparation protocols [44].
Our meta-analysis presents important limitations that should be considered. The high heterogeneity observed could not be fully explained by our subgroup analyses. Important clinical factors, such as the gestational age at infection, were not consistently reported across studies, limiting our ability to control for this potential confounder. Additionally, the included studies varied in design and diagnostic criteria, contributing to the observed heterogeneity. Consequently, the aggregated results should be interpreted with caution, especially since only observational studies were included. While our findings strongly support monthly monitoring and comprehensive diagnostic approaches, we acknowledge the economic challenges faced by healthcare systems, particularly in low- and middle-income countries. Treatment approaches did not account for potential differences in gestational infection by different genotypes, which could influence treatment resistance and the immune response. Indeed, there are several studies showing that certain genotypes display differential sensitivity to common treatments that are preconized nowadays [60,61]. For instance, type I strains, known for their high virulence in mice, may exhibit resistance to some drugs due to differences in their metabolic pathways or drug target sites; type II and III strains, which are more prevalent in human infections, may show varying degrees of susceptibility based on their enzyme activity related to folate metabolism and drug detoxification; and atypical strains found in specific geographic regions may have unique genetic mutations that affect drug efficacy [60,61]. Understanding these genotype-specific responses is crucial for optimizing treatment strategies and developing more effective therapies against T. gondii infections. Future research should focus on developing and validating cost-effective monitoring strategies that maintain clinical efficacy while considering resource constraints in different healthcare settings. Thus, future studies on congenital toxoplasmosis need to concentrate on new diagnostic tools, novel drugs with efficacy against potentially resistant genotypes and fewer side effects, as well as innovative strategies for health education aimed at women of childbearing age.
Overall, the qualitative analysis of this systematic review focused on the main aspects defined as useful to clinical practice and that may serve as guidelines for future research. First, prior knowledge of the serological status of women of childbearing age, and the detection of seroconversion in non-reactive pregnant women or those reinfected by T. gondii, should be encouraged monthly. This contributes to more assertive and prompter decision-making regarding treatment and better prognosis for infections caused by this disease. Additionally, we suggest the implementation of adequate formulas specifically prepared for neonates and children to minimize improper manipulation of these medications through home preparations. This allows for the optimization of medication doses, reduces the risk of using these medications in inadequate doses, and promotes the rational use of these medications, contributing to the goal of minimizing clinical manifestations in these children.
In conclusion:
- The occurrence of congenital toxoplasmosis remains a significant health problem in numerous countries. There is no global consensus on the traceability of toxoplasmosis during pregnancy. While some countries advocate monitoring all pregnant women, others do not recommend it, and the choice of treatment conduct is not yet well-established. The frequency of congenital toxoplasmosis is primarily associated with late, inaccurate, or nonexistent diagnosis during pregnancy, leading to delays or the absence of adequate treatment;
- This systematic review and meta-analysis of articles published from 2013 to 2025, selected based on inclusion and exclusion criteria, reveals an urgent need to establish standardization for therapeutic protocols. This is particularly crucial after the 16th or 18th week of pregnancy. Additionally, monthly monitoring of pregnant women with serological tests is recommended as a predictor to reduce the vertical transmission of Toxoplasma gondii. Ensuring proper healthcare access and promoting adequate treatment will improve the overall health of pregnant women and their children;
- Future studies on congenital toxoplasmosis that address procedures in the mother–child dyad should concentrate on new diagnostic tools, novel drugs with efficacy against potentially resistant genotypes and fewer side effects, as well as innovative strategies for health education aimed at women of childbearing age.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13040723/s1, Table S1: Cohort selected studies among 1089 initially screened meeting inclusion but not exclusion criteria, with a focus on the treatment of congenital toxoplasmosis. Refs. [45,46,47,48,49,51,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81] can be found in Supplementary Materials.
Author Contributions
S.K.R. participated in the study design, data collection and analysis, wrote the original manuscript, and approval its final version; I.M.M. participated in the data selection, statistical analysis, and review of this manuscript and approved its final version; A.C.R.C. participated in the data analysis and review of this manuscript and approved its final version; A.C.A.M.P. participated in the data collection and review and approval of the final version of this manuscript; T.W.P.M. participated in the idealization, planning, review, and approval of the final version; J.R.M. participated in the study design, data collection and analysis, and review and approval of the final version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Brazilian research agencies (Fundação de Amparo à Pesquisa do Estado de Minas Gerais-FAPEMIG, Grant # RED-00198-23 and #APQ-01313-14; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) Grant # 312201/2021-4 and # INCT-Toxo 406572/2022-4; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES, Grant # AUXPE-02450/09-7).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. Ecohealth 2019, 16, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.C.D.; Duarte, E.S.M. Atypical strains of Toxoplasma gondii and its impact on development of toxoplasmosis. Res. Soc. Dev. 2023, 12, 2. [Google Scholar] [CrossRef]
- Kamus, L.; Belec, S.; Lambrecht, L.; Abasse, S.L.; Olivier, S.; Combe, P.L.; Bonnave, P.-E.; Vauloup-Fellous, C.L.; Jaffe, C. Maternal and congenital toxoplasmosis in Mayotte: Prevalence, incidence and management. PLoS Negl. Trop. Dis. 2023, 17, e0011198. [Google Scholar] [CrossRef]
- Montoya, J.G.; Laessig, K.; Fazeli, M.S.; Siliman, G.; Yoon, S.S.; Drake-Shanahan, E.; Zhu, C.Y.; Akbary, A.; McLeod, R. A fresh look at the role of spiramycin in preventing a neglected disease: Meta-analyses of observational studies. Eur. J. Med. Res. 2021, 26, 143. [Google Scholar] [CrossRef]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, T.; Zhai, S.Q.; Li, C.H. Recent progress on anti-Toxoplasma drugs discovery: Design, synthesis and screening. Eur. J. Med. Chem. 2019, 183, 111711. [Google Scholar] [CrossRef]
- Wallon, M.; Peyron, F. Effect of Antenatal Treatment on the Severity of Congenital Toxoplasmosis. Clin. Infect. Dis. 2015, 62, 811–812. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Devel. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef]
- Chorlton, S.D. Adjunctive bradyzoite-directed therapy for reducing complications of congenital toxoplasmosis. Med. Hypotheses 2019, 133, 109376. [Google Scholar] [CrossRef]
- Dos Santos, D.A.; Souza, H.F.S.; Silber, A.M.; Souza, T.D.A.C.B.D.; Ávila, A.R. Protein kinases on carbon metabolism: Potential targets for alternative chemotherapies against toxoplasmosis. Front. Cell. Infect. Microbiol. 2023, 13, 1175409. [Google Scholar] [CrossRef]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs. The Joanna Briggs Institute Reviewers’. In Manual 2015: Methodology for JBI Scoping Reviews; Joanne Briggs Institute: Adelaide, Australia, 2015; pp. 1–24. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna R Foundation for Statistical Computing. 2019. Available online: https://www.r-project.org (accessed on 10 February 2025).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Spiegelhalter, D.J. A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Ser. A Stat. Soc. 2009, 172, 137–159. [Google Scholar] [CrossRef]
- Carral, L.; Kaufer, F.; Olejnik, P.; Freuler, C.; Durlach, R. Prevention of congenital toxoplasmosis in a Buenos Aires hospital. Medicina 2013, 73, 238–242. [Google Scholar]
- Damar Çakırca, T.; Can, İ.N.; Deniz, M.; Torun, A.; Akçabay, Ç.; Güzelçiçek, A. Toxoplasmosis: A Timeless Challenge for Pregnancy. Trop. Med. Infect. Dis. 2023, 8, 63. [Google Scholar] [CrossRef]
- Olariu, T.R.; Press, C.; Talucod, J.; Olson, K.; Montoya, J.G. Toxoplasmose congénitale aux États-Unis: Observations cliniques et sérologiques chez les nourrissons nés de mères traitées pendant la grossesse. Parasite 2019, 26, 13. [Google Scholar] [CrossRef]
- Prusa, A.-R.; Kasper, D.C.; Pollak, A.; Olischar, M.; Gleiss, A.; Hayde, M. Amniocentesis for the detection of congenital toxoplasmosis: Results from the nationwide Austrian prenatal screening program. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015, 21, 191.e1–191.e8. [Google Scholar] [CrossRef]
- Buonsenso, D.; Pata, D.; Colonna, A.T.; Iademarco, M.; de Santis, M.; Masini, L.; Conti, G.; Molle, F.; Baldascino, A.; Acampora, A.; et al. Spyramicine and Trimethoprim-Sulfamethoxazole Combination to Prevent Mother-To-Fetus Transmission of Toxoplasma gondii Infection in Pregnant Women: A 28-Years Single-center Experience. Pediatr. Infect. Dis. J. 2022, 41, E223–E227. [Google Scholar] [CrossRef] [PubMed]
- Conceição, A.R.; Belucik, D.N.; Missio, L.; Gustavo Brenner, L.; Henrique Monteiro, M.; Ribeiro, K.S.; Costa, D.F.; Valadão, M.C.D.S.; Commodaro, A.G.; de Oliveira Dias, J.R.; et al. Ocular Findings in Infants with Congenital Toxoplasmosis after a Toxoplasmosis Outbreak. Ophthalmology 2021, 128, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, T.E.; Gomes, A.O.; Coelho-dos-Reis, J.G.; Carneiro, A.C.A.V.; Machado, A.S.; Andrade, G.M.Q.; Vasconcelos-Santos, D.V.; Januário, J.N.; Peruhype-Magalhães, V.; Teixeira-Carvalho, A.; et al. Long-term impact of congenital toxoplasmosis on phenotypic and functional features of circulating leukocytes from infants one year after treatment onset. Clin. Immunol. 2021, 232, 108859. [Google Scholar] [CrossRef] [PubMed]
- Findal, G.; Helbig, A.; Haugen, G.; Jenum, P.A.; Stray-Pedersen, B. Management of suspected primary Toxoplasma gondii infection in pregnant women in Norway: Twenty years of experience of amniocentesis in a low-prevalence population. BMC Pregnancy Childbirth 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Guegan, H.; Stajner, T.; Bobic, B.; Press, C.; Olariu, R.T.; Olson, K.; Srbljanovic, J.; Montoya, J.G.; Djurković-Djaković, O.; Robert-Gangneux, F. Maternal Anti-Toxoplasma Treatment during Pregnancy Is Associated with Reduced Sensitivity of Diagnostic Tests for Congenital Infection in the Neonate. J. Clin. Microbiol. 2021, 59, e01368-20. [Google Scholar] [CrossRef]
- Piffer, S.; Lauriola, A.L.; Pradal, U.; Collini, L.; Dell’Anna, L.; Pavanello, L. Toxoplasma gondii infection during pregnancy: A ten-year observation in the province of Trento, Italy. Le Infez. Med. 2020, 28, 603–610. [Google Scholar]
- Olariu, T.R.; Remington, J.S.; McLeod, R.; Alam, A.; Montoya, J.G. Severe congenital toxoplasmosis in the United States: Clinical and serologic findings in untreated infants. Pediatr. Infect. Dis. J. 2011, 30, 1056–1061. [Google Scholar] [CrossRef]
- Soares, J.A.S.; Holzmann, A.P.F.; Alves, B.B.S.; Lima, C.F.Q.; Caldeira, A.P. Profile of pregnant women and children accompanied due to T. gondii exposure at a referred healthcare center: What has changed in 10 years? Rev. Bras. Saude Matern. Infant. 2023, 23, e20220225. [Google Scholar] [CrossRef]
- Valentini, P.; Buonsenso, D.; Barone, G.; Serranti, D.; Calzedda, R.; Ceccarelli, M.; Speziale, D.; Ricci, R.; Masini, L. Spiramycin/cotrimoxazole versus pyrimethamine/sulfonamide and spiramycin alone for the treatment of toxoplasmosis in pregnancy. J. Perinatol. 2015, 35, 90–94. [Google Scholar] [CrossRef]
- Yamada, H.; Tanimura, K.; Deguchi, M.; Tairaku, S.; Morizane, M.; Uchida, A.; Ebina, Y.; Nishikawa, A. A cohort study of maternal screening for congenital Toxoplasma gondii infection: 12 years’ experience. J. Infect. Chemother. 2019, 25, 427–430. [Google Scholar] [CrossRef]
- Trotta, M.; Trotta, A.; Spataro, E.; Giache, S.; Borchi, B.; Zammarchi, L.; Campolmi, I.; Galli, L.; Pasquini, L. Primary toxoplasmosis acquired during early pregnancy: Is it currently overestimated? Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Fricker-Hidalgo, H.; Cimon, B.; Chemla, C.; Darde, M.L.; Delhaes, L.; L’Ollivier, C.; Godineau, N.; Houze, S.; Paris, L.; Quinio, D.; et al. Toxoplasma seroconversion with negative or transient immunoglobulin M in pregnant women: Myth or reality? A French multicenter retrospective study. J. Clin. Microbiol. 2013, 51, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Vimercati, A.; Chincoli, A.; de Gennaro, A.C.; Calvario, A.; Amendolara, M.; Del Gaudio, G.; Laforgia, N.; Carbonara, S. Congenital toxoplasmosis and proposal of a new classification for the likelihood of primary maternal infection: Analysis of 375 cases in Southeast Italy. J. Matern. Neonatal Med. 2020, 33, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Wallon, M.; Peyron, F.; Cornu, C.; Vinault, S.; Abrahamowicz, M.; Kopp, C.; Binquet, C. Congenital toxoplasma infection: Monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin. Infect. Dis. 2013, 56, 1223–1231. [Google Scholar] [CrossRef]
- Hijikata, M.; Morioka, I.; Okahashi, A.; Nagano, N.; Kawakami, K.; Komatsu, A.; Kawana, K.; Ohyama, S.; Fujioka, K.; Tanimura, K.; et al. A prospective cohort study of newborns born to mothers with serum Toxoplasma gondii immunoglobulin M positivity during pregnancy. J. Infect. Chemother. 2022, 28, 486–491. [Google Scholar] [CrossRef]
- Gomes-Ferrari-Strang, A.G.; Ferrar, R.G.; Falavigna-Guilherme, A.L. Gestational toxoplasmosis treatment changes the child’s prognosis: A cohort study in southern Brazil. PLoS Negl. Trop. Dis. 2023, 17, e0011544. [Google Scholar] [CrossRef]
- Righi, N.C.; Hermes, L.; Piccini, A.D.; Branco, J.C.; Skupien, J.A.; Weinmann, A.R.M.; Valadao, M.C.D.; Schuch, N.J. Epidemiological profile of gestational and congenital toxoplasmosis cases arising out of the population outbreak. Sci. Med. 2021, 31, e40108. [Google Scholar] [CrossRef]
- Tibúrcio, J.D.; Vasconcelos-Santos, D.V.; Vasconcelos, G.C.; Carellos, E.V.M.; Romanelli, R.M.d.C.; Januario, J.N.; Andrade, G.M.Q. Psychometric properties of CVFQ7-BR-toxo to evaluate vision-related quality of life in children with congenital toxoplasmosis in Brazil. Arq. Bras. Oftalmol. 2022, 85, 46–58. [Google Scholar] [CrossRef]
- Bartholo, B.B.G.R.; Monteiro, D.L.M.; Rodrigues, N.C.P.; Trajano, A.J.B.; de Jesus, N.R.; Cardoso, F.F.O.; de Souza, F.M.; Werner, H.; Araujo Júnior, E. Treatment of Acute Toxoplasmosis in Pregnancy: Influence in the Mother-to-Child Transmission. J. Obstet. Gynaecol. Can. 2020, 42, 1505–1510. [Google Scholar] [CrossRef]
- Avci, M.E.; Arslan, F.; Çiftçi, S.; Ekiz, A.; Tüten, A.; Yildirim, G.; Madazli, R. Role of spiramycin in prevention of fetal toxoplasmosis. J. Matern. Neonatal Med. 2016, 29, 2073–2076. [Google Scholar] [CrossRef]
- Mandelbrot, L.; Kieffer, F.; Sitta, R.; Laurichesse-Delmas, H.; Winer, N.; Mesnard, L.; Berrebi, A.; Le Bouar, G.; Bory, J.-P.P.; Cordier, A.-G.G.; et al. Prenatal therapy with pyrimethamine plus sulfadiazine vs spiramycin to reduce placental transmission of toxoplasmosis: A multicenter, randomized trial. Am. J. Obstet. Gynecol. 2018, 219, e1–e386. [Google Scholar] [CrossRef]
- Avelino, M.M.; Amaral, W.N.; Rodrigues, I.M.; Rassi, A.R.; Gomes, M.B.; Costa, T.L.; Castro, A.M. Congenital toxoplasmosis and prenatal care state programs. BMC Infect. Dis. 2014, 14, 33. [Google Scholar] [CrossRef]
- De Santis, M.; Tartaglia, S.; Apicella, M.; Visconti, D.; Noia, G.; Valentini, P.; Lanzone, A.; Santangelo, R.; Masini, L. The prevention of congenital toxoplasmosis using a combination of Spiramycin and Cotrimoxazole: The long-time experience of a tertiary referral centre. Trop. Med. Int. Health 2024, 29, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Guarch-Ibáñez, B.; Carreras-Abad, C.; Frick, M.A.; Blázquez-Gamero, D.; Baquero-Artigao, F.; Fuentes, I. REIV-TOXO Project: Results from a Spanish cohort of congenital toxoplasmosis (2015–2022). The beneficial effects of prenatal treatment on clinical outcomes of infected newborns. PLoS Neglected Trop. Dis. 2024, 18, e0012619. [Google Scholar] [CrossRef]
- Lago, E.G.; Oliveira, A.P.; Bender, A.L. Presence and duration of anti-Toxoplasma gondii immunoglobulin M in infants with congenital toxoplasmosis. J. Pediatr. 2014, 90, 363–369. [Google Scholar] [CrossRef]
- Losa, A.; Carvalho, I.; Sousa, B.; Ashworth, J.; Guedes, A.; Carreira, L.; Pinho, L.; Godinho, C. Congenital Toxoplasmosis Diagnosis: Challenges and Management Outcomes. Cureus 2024, 16, e52971. [Google Scholar] [CrossRef]
- Mejia-Oquendo, M.; Marulanda-Ibarra, E.; Gomez-Marin, J.E. Evaluation of the impact of the first evidence-based guidelines for congenital toxoplasmosis in Armenia (Quindío) Colombia: An observational retrospective analysis. Lancet Reg. Health—Am. 2021, 1, 100010. [Google Scholar] [CrossRef]
- Prusa, A.-R.; Kasper, D.C.; Pollak, A.; Gleiss, A.; Waldhoer, T.; Hayde, M. The Austrian toxoplasmosis register, 1992–2008. Clin. Infect. Dis. 2015, 60, e4–e10. [Google Scholar] [CrossRef]
- Rodrigues, I.M.; Costa, T.L.; Avelar, J.B.; Amaral, W.N.; Castro, A.M.; Avelino, M.M. Assessment of laboratory methods used in the diagnosis of congenital toxoplasmosis after maternal treatment with spiramycin in pregnancy. BMC Infect. Dis. 2014, 14, 349. [Google Scholar] [CrossRef]
- Felín, M.S.; Wang, K.; Moreira, A.; Grose, A.; Leahy, K.; Zhou, Y.; Clouser, F.A.; Siddiqui, M.; Leong, N.; Goodall, P.; et al. Building Programs to Eradicate Toxoplasmosis Part I: Introduction and Overview. Curr. Pediatr. Rep. 2022, 10, 57–92. [Google Scholar] [CrossRef]
- Ferguson, D.J. Use of molecular and ultrastructural markers to evaluate stage conversion of Toxoplasma gondii in both the intermediate and definitive host. Int. J. Parasitol. 2004, 34, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Lago, E.G.; Endres, M.M.; Scheeren, M.F.D.C.; Fiori, H.H. Ocular Outcome of Brazilian Patients with Congenital Toxoplasmosis. Pediatr. Infect. Dis. J. 2021, 40, e21–e27. [Google Scholar] [CrossRef] [PubMed]
- Wallon, M.; Garweg, J.G.; Abrahamowicz, M.; Cornu, C.; Vinault, S.; Quantin, C.; Bonithon-Kopp, C.; Picot, S.; Peyron, F.; Binquet, C. Ophthalmic outcomes of congenital toxoplasmosis followed until adolescence. Pediatrics 2014, 133, e601–e608. [Google Scholar] [CrossRef]
- McLeod, R.; Lykins, J.; Gwendolyn Noble, A.; Rabiah, P.; Swisher, C.N.; Heydemann, P.T.; McLone, D.; Frim, D.; Withers, S.; Clouser, F.; et al. Management of Congenital Toxoplasmosis. Curr. Pediatr. Rep. 2014, 2, 166–194. [Google Scholar] [CrossRef]
- World Health Organization. Promoting Safety of Medicines for Children; WHO Press: Geneva, Switzerland, 2007; 59p, ISBN 978-92-4-156343-7. [Google Scholar]
- Koçak, Ö.; Kan, Ö.; Kocak, O.; Kan, O. Results of the Toxoplasmosis Screening in 9311 Pregnant Women in a Tertiary Center in Turkey. Flora 2020, 25, 332–338. [Google Scholar] [CrossRef]
- Kalem, A.K.; Hasanoǧlu, I.; Ayhan, M.; Kayaaslan, B.; Eser, F.; Oǧuz, Y.; Avşar, F.Y.; Güner, R. Toxoplasmosis in pregnancy: Test, treatment and outcome. Eur. Res. J. 2022, 8, 296–303. [Google Scholar] [CrossRef]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar]
- Brito, R.M.d.M.; Bessa, G.d.L.; Bastilho, A.L.; Dantas-Torres, F.; de Andrade-Neto, V.F.; Bueno, L.L.; Fujiwara, R.T.; Magalhães, L.M.D. Genetic diversity of Toxoplasma gondii in South America: Occurrence, immunity, and fate of infection. Parasites Vectors 2023, 16, 461. [Google Scholar]
- Andrade, F.M.; Santana, E.F.M.; Júnior, E.A.; Andrade, S.G.A.; Bortoletti Filho, J.; Amed, A.M.; Moron, A.F. Polymerase chain reaction analysis of amniotic fluid for diagnosis of fetal toxoplasmosis. Clin. Exp. Obstet. Gynecol. 2019, 46, 593–595. [Google Scholar] [CrossRef]
- Boudaouara, Y.; Aoun, K.; Maatoug, R.; Souissi, O.; Bouratbine, A.; Ben Abdallah, R. Congenital toxoplasmosis in Tunisia: Prenatal and neonatal diagnosis and postnatal follow-up of 35 cases. Am. J. Trop. Med. Hyg. 2018, 98, 1722–1726. [Google Scholar] [CrossRef]
- Bouhlel, S.; Ben Abdallah, R.; Aoun, K.; Maatoug, R.; Souissi, O.; Bouratbine, A. Management of Toxoplasmic Seroconversion in the Third Trimester of Pregnancy in Tunisia. Bull. De La Soc. De Pathol. Exot. (1990) 2018, 111, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Capobiango, J.D.; Breganó, R.M.; Navarro, I.T.; Neto, C.P.R.; Casella, A.M.B.; Mori, F.M.R.L.; Pagliari, S.; Inoue, I.T.; Reiche, E.M.V. Congenital toxoplasmosis in a reference center of Paraná, Southern Brazil. Braz. J. Infect. Dis. 2014, 18, 364–3671. [Google Scholar] [CrossRef] [PubMed]
- Carellos, E.V.M.; de Andrade, J.Q.; Romanelli, R.M.C.; Tibúrcio, J.D.; Januário, J.N.; Vasconcelos-Santos, D.V.; Figueiredo, R.M.; de Andrade, G.M.Q. High Frequency of Bone Marrow Depression During Congenital Toxoplasmosis Therapy in a Cohort of Children Identified By Neonatal Screening in Minas Gerais, Brazil. Pediatr. Infect. Dis. J. 2017, 36, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Caro-Garzón, J.D.; Gómez-Henck, C.; Jaramillo-Giraldo, T.; Cifuentes-Botero, J.M.; Gómez-Marín, J.E. Evaluation of the avidity test for the follow up on children treated for congenital toxoplasmosis during the first year of life. Iatreia 2021, 34, 25–32. [Google Scholar] [CrossRef]
- De Paula, H.L.; de Lucca, L.; Vendrame, S.A.; Wess, L.C.; Stein, C.d.S.; Moresco, R.N.; Beck, S.T.; Gonçalves, T.d.L. Delta-aminolevulinate dehydratase enzyme activity and the oxidative profile of pregnant women being treated for acute toxoplasmosis. Microb. Pathog. 2022, 164, 105455. [Google Scholar] [CrossRef]
- Diesel, A.A.; Zachia, S.d.A.; Müller, A.L.L.; Perez, A.V.; Uberti, F.A.d.F.; Magalhães, J.A.d.A. Follow-up of Toxoplasmosis during Pregnancy: Ten-Year Experience in a University Hospital in Southern Brazil. Rev. Bras. Ginecol. Obstet. 2019, 41, 539–547. [Google Scholar] [CrossRef]
- Donadono, V.; Saccone, G.; Maruotti, G.M.; Berghella, V.; Migliorini, S.; Esposito, G.; Sirico, A.; Tagliaferri, S.; Ward, A.; Mazzarelli, L.L. Incidence of toxoplasmosis in pregnancy in Campania: A population-based study on screening, treatment, and outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 240, 316–321. [Google Scholar] [CrossRef]
- Donadono, V.; Saccone, G.; Sarno, L.; Esposito, G.; Mazzarelli, L.L.; Sirico, A.; Guida, M.; Martinelli, P.; Zullo, F.; Maruotti, G.M. Association between lymphadenopathy after toxoplasmosis seroconversion in pregnancy and risk of congenital infection. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 45–51. [Google Scholar] [CrossRef]
- Evangelista, F.F.; Mantelo, F.M.; de Lima, K.K.; Marchioro, A.A.; Beletini, L.F.; de Souza, A.H.; Santana, P.L.; Riedo, C.d.O.; Higa, L.T.; Guilherme, A.L.F. Prospective evalution of pregnant women with suspected acute toxoplasmosis treated in a reference prenatal care clinic at a university teaching hospital in Southern Brazil. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, 46. [Google Scholar] [CrossRef]
- Ludwig, A.; Fernandes, F.D.; Guerra, R.R.; Braünig, P.; Ramos, L.S.; Pacheco, L.S.; Sangioni, L.A.; Vogel, F.S.F. Molecular detection of Toxoplasma gondii in placentas of women who received therapy during gestation in a toxoplasmosis outbreak. Infect. Genet. Evol. 2022, 97, 105145. [Google Scholar] [CrossRef]
- Mueller, R.A.S.; Frota, A.C.C.; Barreto, D.D.M.; Vivacqua, D.P.F.; Loria, G.B.; Lebreiro, G.P.; Martins, M.G.; Potsch, M.V.; Maia, P.D.; Coutinho, R.L.M.; et al. Congenital Toxoplasmosis: Missed Opportunities for Diagnosis and Prevention. J. Trop. Pediatr. 2021, 67, fmaa069. [Google Scholar] [CrossRef] [PubMed]
- Pengsaa, K.; Hattasingh, W. Congenital toxoplasmosis: An uncommon disease in Thailand. Paediatr. Int. Child Health 2015, 35, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Prasil, P.; Sleha, R.; Kacerovsky, M.; Bostik, P. Comparison of adverse reactions of spiramycin versus pyrimethamine/sulfadiazine treatment of toxoplasmosis in pregnancy: Is spiramycin really the drug of choice for unproven infection of the fetus? J. Matern.-Fetal Neonatal Med. 2023, 36, 2215377. [Google Scholar] [CrossRef] [PubMed]
- Saghrouni, F.; Khammari, I.; Ben Abdeljelil, J.; Yaacoub, A.; Meksi, S.G.; Ach, H.; Garma, L.; Fathallah, A.; Ben Saïd, M. La toxoplasmose congénitale: à propos de 21 cas. J. Pédiatr. Puéricult. 2013, 26, 83–89. [Google Scholar] [CrossRef]
- Teil, J.; Dupont, D.; Charpiat, B.; Corvaisier, S.; Vial, T.; Leboucher, G.; Wallon, M.; Peyron, F. Treatment of Congenital Toxoplasmosis: Safety of the Sulfadoxine-Pyrimethamine Combination in Children Based on a Method of Causality Assessment. Pediatr. Infect. Dis. J. 2016, 35, 634–638. [Google Scholar] [CrossRef]
- Villar, B.B.D.L.F.; Neves, E.d.S.; Louro, V.C.; Lessa, J.F.; Rocha, D.N.; Gomes, L.H.F.; Junior, S.C.G.; Pereira, J.P.; Moreira, M.E.L.; Guida, L.d.C. Toxoplasmosis in pregnancy: A clinical, diagnostic, and epidemiological study in a referral hospital in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2020, 24, 517–523. [Google Scholar] [CrossRef]
- Yamamoto, L.; Targa, L.S.; Sumita, L.M.; Shimokawa, P.T.; Rodrigues, J.C.; Kanunfre, K.A.; Okay, T.S. Association of Parasite Load Levels in Amniotic Fluid With Clinical Outcome in Congenital Toxoplasmosis. Obstet. Gynecol. 2017, 130, 335–345. [Google Scholar] [CrossRef]
- Zuluaga, L.M.; Hernández, J.C.; Castaño, C.F.; Donado, J.H. Effect of antenatal spiramycin treatment on the frequency of retinochoroiditis due to congenital toxoplasmosis in a Colombian cohort. Biomedica 2017, 37 (Suppl. S1), 86–91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).