Abstract
The Mycoplasmataceae are a family of bacteria that typically cause respiratory, arthritic, and genitourinary disease in humans. Mycoplasma spp. of animal origin are also the causative agents of porcine wheezing disease, chronic respiratory disease and arthritis in chickens and other conditions. These diseases have a significant impact on public health and the economic development of livestock breeding. Clinical prevention and treatment of mycoplasma infections is primarily dependent on the use of antibiotics. However, inappropriate and excessive use of antimicrobials has enabled resistance development that has become a significant clinical concern. Mycoplasma are also robust biofilm producers, and this process is a major factor for the persistence of these infections, especially in conjunction with common antibiotic resistance mechanisms, including target gene mutations and the action of efflux pumps. A mycoplasma biofilm refers to a structured and stable microbial community formed by Mycoplasma spp. adhering to biological or non-biological surfaces under suitable conditions and secreting extracellular polymers (EPS) such as polysaccharides. This process allows the microorganisms to adapt to their surrounding environment and survive during the growth process. These biofilms render bacteria more resistant to antimicrobials than planktonic bacteria, resulting in biofilm-associated infections that are more challenging to eradicate and more likely to recur. The current study reviews progress from the fields of biofilm formation, structure and identification, correlations between biofilms and drug resistance and virulence as well as methods of biofilm prevention and control. Our aim was to provide a reference basis for the subsequent in-depth understanding of the research of mycoplasma biofilms.
1. Biological Characteristics of Mycoplasma
Mycoplasma spp. are prokaryotes (class Mollicutes) that are characterized by the lack of a cell wall, a small genome (500–2000 kb), and a pleomorphic morphology (spherical, filamentous, or irregular) with diameters of 100–300 nm. Their reduced size and structural flexibility enable them to pass through 0.22 µm sterilizing-grade membrane filters, a feature that complicates containment in laboratory and clinical environments [1]. Mycoplasma have nutritional requirements that differ from most other bacteria due to their lifestyle that is tightly linked to the host. For instance, cultures require supplementation with 10–20% human or animal serum to provide essential cholesterol needed for their cell membranes. Additionally, the optimum pH for growth is 7.8–8.0 and they cannot survive at pH < 7.0. Most are facultative anaerobes and some strains grow better when 5% CO2 is added at the time of initial isolation. They form smooth, round, transparent colonies of uniform size and roundness on solid plates (fried egg morphology) (Figure 1) [2]. The absence of a cell wall precludes any effectiveness of β-lactam and glycopeptide antibiotics or fosfomycin [3].
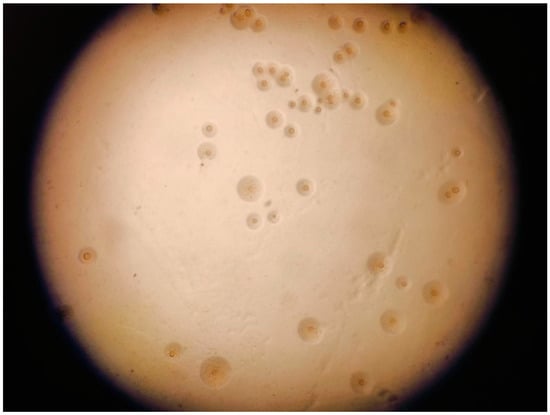
Figure 1.
Fried egg colonies of Mycoplasma gallisepticum.
Mycoplasma are widely found in nature in humans, animals, and plants, and there are a wide variety of extant species. The first mycoplasma was isolated by Nocard in 1898 and was classified as Mycoplasma mycoides in 1967 [4]. Subsequently, Mycoplasma spp. have been isolated from the mucosal surfaces of the respiratory and genital tracts in both humans and animals. These bacteria are highly prevalent, and this has serious implications for both human and animal health. They are important animal pathogens and can cause serious disease in cattle, pigs, and poultry and cause chronic infections in humans and animals, including pneumonia [5,6,7,8], chronic bronchitis [9], arthritis [10,11,12], mucositis [13,14], chorioamnionitis [15], and reproductive disorders [16,17,18,19]. Mycoplasma pneumoniae is a significant causative agent of respiratory disease in children, resulting in upper and lower respiratory tract infections. The clinical presentation is characterized by coughing, headache, wheezing, and other non-specific manifestations that are commonly observed in children [6,20]. The presence of Mycoplasma genitalium is associated with an increased risk of urethritis and proctitis in men and cervicitis and pelvic inflammatory disease in women. These conditions are linked to an elevated risk of preterm labor, miscarriage, and infertility [21]. Mycoplasma hyopneumoniae is the primary pathogen of Mycoplasma pneumonia of swine as well as porcine respiratory disease syndrome. M. hyopneumoniae can cause chronic respiratory symptoms such as coughing and wheezing in pigs and make the animals highly susceptible to secondary infection with other viral and bacterial respiratory pathogens [22,23]. Mycoplasma synoviae is the causative agent of respiratory tract disease in poultry that is manifested by airsacculitis and joint disease. The latter is characterized by inflammatory exudative bursitis or tarsal arthritis. Additionally, M. synoviae has been linked to decreased egg production and abnormal eggshell formation [24].
2. Biofilms
A biofilm is defined as a structured community of microorganisms attached to a surface and embedded in its own secreted extracellular polymeric substance (EPS) matrix [25]. Its extracellular polymers form the material basis of the biofilm that includes as its primary components EPS, proteins, and extracellular DNA (eDNA) [26] that provide structural integrity to the biofilm (Table 1). Microbial communities typically account for 10–25% of the biofilm volume, and the remaining (75–90%) consists of EPS [27]. Adhesion–cohesion, scaffolding, mechanical stability, and protection are the most prominent features of the EPS component [28] and play a crucial role in protection from antibiotics and host immunity [29,30]. The EPS barrier effect makes bacteria less susceptible to antimicrobial agents and results in biofilm-forming Mycoplasma strains that have elevated levels of heat and desiccation resistance [31,32].

Table 1.
Primary components and functions of biofilms.
Planktonic mycoplasma adhere to biotic or abiotic surfaces such as mucosal membranes and implanted biomaterials. These biofilms represent a survival strategy under nutrient-limited conditions [38,39,40]. Numerous Mycoplasma species, including M. pneumoniae [41,42], are capable of forming biofilms in vivo and in vitro (Table 2).

Table 2.
Mycoplasma spp. that are known to form biofilms.
2.1. Formation and Structure of Biofilms
The formation of Mycoplasma spp. biofilms is a dynamic and complex process, and like other bacteria, the initial stages are characterized by adhesion to host tissues or inanimate surfaces (such as glass). This is accomplished via its variable surface antigen (Vsa) proteins present on the surface [41]. The length and structure of Vsa proteins affect their adhesion ability. Short Vsa proteins facilitate biofilm formation, while long Vsa proteins may inhibit adhesion by steric hindrance and allow a planktonic lifestyle [52]. After initial adhesion, mycoplasma cells begin to secrete extracellular polymers (polysaccharides, lipids, DNA, or proteins) to form an EPS extracellular matrix, which surrounds the cells together and allows surface adhesion [34]. This aggregation forms microcolonies that are the basis of the biofilm structure. Over time, microcolonies develop into mature biofilms, characterized by complex three-dimensional structures that include honeycomb- and tower-like structures. These features are connected via the extracellular matrix to form channels that facilitate the transport of nutrients and the removal of waste [27]. As the biofilm ages, the nutrients within the biofilm are insufficient to support the internal community, and a large amount of waste and toxins accumulate. Some Mycoplasma spp. also express nucleases and glycosidases to degrade EPS, resulting in transformation into a free state for secondary infection [31]. Quorum sensing (QS) plays an important role in the process of biofilm development. QS is a microbial communication mechanism that coordinates the group behavior between microbial cells by producing and detecting signal molecules called autoinducers (AIs), influencing virulence gene expression, toxin production, and biofilm formation [53,54]. Although the genome of mycoplasma is relatively small and lacks a complex gene-regulation system, some mycoplasmas may still have a quorum sensing mechanism. For example, studies have shown that the biofilm formation of Mycoplasma genitalium is associated with the quorum sensing metabolic pathway, in which the protein translocase subunit is involved, and the biofilm formation contributes to the antibiotic tolerance of this mycoplasma [55].
The mechanisms of biofilm formation vary depending on species and environmental conditions. For example, particular strains of M. bovis form robust biofilms in vitro, while others do not, and this has been linked to selective adhesin expression [52]. In the case of M. bovis, biofilm formation ability is correlated with molecular type identified via amplified fragment length polymorphism (AFLP) and random amplified polymorphic DNA (RAPD). In particular, subgroup B are strong biofilm formers, while subgroup A are poor biofilm formers [31]. Additionally, M. bovis biofilm formation is significantly enhanced when co-cultured with the Gram-positive Trueperella pyogenes, and the morphological characteristics of these biofilms resemble those formed during in vivo infections [56]. The polymicrobial interactions between these two pathogens induce biofilm formation that increases resistance to antimicrobial agents and thereby exacerbates the progression of chronic pneumonia.
Multiple experiments have also demonstrated that biofilm structures are diverse, and this phenotypic heterogeneity is most likely the result of differences in gene expression [57]. For example, M. pneumoniae can form volcano-like biofilm structures [41] (Figure 2), while M. synoviae biofilms exhibit mushroom- and tower-like structures [46]. M. pulmonis biofilms formed on tracheal epithelia in vivo are also tower-like structures [49].
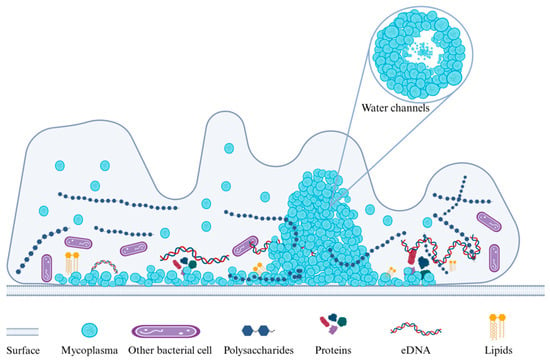
Figure 2.
The volcano-like biofilm structure of M. pneumoniae is composed of densely arranged cells closely connected via EPS. Sparse water channels are present in the biofilm interior that promote the exchange of nutrients and waste and support the growth and maintenance of the biofilm.
Surface materials can significantly affect bacterial adhesion such that biofilm morphologies are dependent on surface material characteristics [58,59]. For example, M. gallisepticum biofilms exhibit lichen-like and ice-arabesque structures on glass coverslips and present cupola- or igloo-shaped structures on plastics [50]. EPS also can determine structures, and M. genitalium biofilms contain high levels of N-acetylglucosamine, indicating that EPS formation can be species-specific and may affect the final biofilm structure [29]. Polysaccharides are key components of the ECM and play the primary supporting role in the membrane structure. Mycoplasma–polysaccharide interactions are also influenced by type and genetic strain differences that also affect structure [42]. In addition, the presence of eDNA can promote the stability of biofilm, and biofilms formed in vitro involve large cell variants that contribute to eDNA release and enable M. hyopneumoniae to form biofilms on non-biological surfaces [26,47]. Biofilm formation and structures are influenced by a variety of factors, and understanding these processes is essential for interpreting the complexity and diversity of mycoplasma biofilms and developing clinical treatment methods.
2.2. Methods for the Identification of the Biofilm
2.2.1. Staining Methods
Crystal Violet is a commonly used biofilm staining agent that can bind to and stain cells in the biofilm, thereby quantifying the relative amount of biomembrane formation through light absorption (OD value) or observing the structural characteristics of the biomembrane through microscopic imaging [60]. Although Crystal Violet can stain both living and dead bacteria, these can be distinguished by the MTT metabolic assay.
The Congo red staining method has the characteristics of rapid reaction, simple operation, and stable staining, which is another method for detecting biofilms in vitro. Its principle is that Congo red can combine with extracellular polymers to form dry red–brown crystals. This method can identify the presence or absence of biofilms, as well as quantify the polysaccharides and cellulose in biofilms [61]. However, Kang et al. [46] found that when staining the biofilms formed by M. synoviae, the sensitivity of Congo red staining is not as high as that of crystal violet staining.
2.2.2. Imaging Assays
Biofilm can be microscopically imaged, and this provides a qualitative and quantitative detection method. Most commonly, scanning electron microscopy (SEM), focused ion beam (FIB), and confocal laser scanning microscopy (CLSM) are used for the direct observation of the structure and morphology of biofilms. The SEM provides a detailed view of the biofilm and its extracellular polymers and enables detection of towers and channels. The principal constraints associated with SEM are sample dehydration to ensure their suitability for vacuum operations, and this cumbersome process may potentially compromise the structural integrity of the matrix [62]. The FIB microscope is an effective tool for examining the internal structure of biofilms and eliminates the need for time-consuming pretreatment procedures. Its principle is to remove the surface layer or cross-section of the sample by grinding or cutting to observe the internal structural characteristics of the biofilm [63]. High-resolution CLSM is a widely employed analytical tool for the detection and visualization of the composition of biofilm matrices. Furthermore, it can provide a detailed quantitative characterization of the microstructure within the biofilm, including the biomass and spatial distribution of the microbial population [27,64], and can be used to quantify fluorescence signals [65]. The combination of CLSM and SEM enables the visualization and quantification of the two-dimensional and three-dimensional complex structures of mycoplasma biofilms.
2.2.3. Genetic Testing
Biofilms are subject to regulation by several genes, and the application of genome-wide analysis has enabled the development of a more precise assay for the screening of biofilm-associated genes. At present, this method has been employed to identify pivotal genes associated with the regulation of biofilm formation in M. gallisepticum that include manB (gene encoding phosphomannomutase), oppA and oppD (components of the oligopeptide permeases complex), pdh (pyruvate dehydrogenase), eno (enolase), relA ((p)ppGpp synthase/phosphodiesterase), msbA (ABC transporter), deoA (thymidine phosphorylase), gapA (adhesin), and rpoS (stationary phase sigma factors) [66]. The genes oppA and oppD are components of the ATP-dependent oligopeptide transporter complex protein family members that play different roles in bacterial transport over the plasma membrane [66]. DeoA is a key enzyme necessary for the salvage pathway for deoxyribonucleotide synthesis and to use deoxyribonucleosides as a carbon and energy source. In particular, microcolonies of Mycoplasma hominis that have entered an energy-deficient state due to aging or other factors will preferentially choose deoxyribonucleotides as an energy source that assists in survival under these environmental stresses, including antimicrobial treatment [67].
Transposon-enabled sequencing is a novel high-throughput sequencing technology that enables the identification of genes essential for bacterial growth in diverse environments and has been utilized in biofilm characterization. This technique has been applied to identify the adhesion gene VlhA and the M. gallisepticum mutant encoding the adhesion-related factor enolase, as well as the genes involved in EPS synthesis (manB, ABC transporter permease, and ABC transporter ATP-binding protein). This technique utilizes an M. gallisepticum transposon Mini-Tn4001SpGm mutagenesis system [68].
3. Correlation Between Biofilm and Resistance of Mycoplasma
Mycoplasma spp. drug resistance mechanisms include target gene mutations and the expression of efflux pumps. For instance, resistance to fluoroquinolones and aminoglycosides in Tibetan yak M. bovis isolates included a single-site base mutation, while doubly mutated isolates led to the emergence of highly resistant strains [69]. In another study, 23/36 strains of M. anserisalpingitidis showed a nearly 50% reduction in MIC values after exposure to the efflux pump inhibitor carbon cyanide m-chlorophenylhydrazone (CCCP), thus linking efflux pumps with significant drug resistance [70]. In these contexts, biofilm formation also reduces sensitivity to antibiotics, and drug resistance is significantly enhanced when compared to free-living isolates [29]. At full maturity, antibiotics are typically ineffective against mycoplasma biofilms. For example, M. hyopneumoniae can persist in biofilms despite exposure to enrofloxacin, tylosin, and florfenicol even at 10× MIC of the cognate planktonic forms [61]. M. synoviae sensitivity to enrofloxacin, doxycycline, tylosin, and tiamulin decreased following biofilm formation, and the minimal biofilm inhibitory concentration (MBIC) of tylosin increased 4.24-fold compared to the MIC of tylosin [46]. In addition, significantly low negative correlations have been observed between the MBIC of enrofloxacin and biomass and M. gallisepticum biofilms that were found to be more resistant to tetracycline and gentamicin than planktonic M. gallisepticum [47]. These strains also displayed a greater capacity for biofilm formation, resulting in reduced antibiotic sensitivity [66]. M. pneumoniae biofilms formed in vitro at 48 and 72 h exhibited complete resistance to erythromycin concentrations of up to 512 µg mL−1 [71]. The MBIC of U. urealyticum to tetracycline and levofloxacin after the formation of a biofilm was 4-8-fold or higher than the MIC of its planktonic counterparts, and its quinolone-resistant strains produce more biofilms than the sensitive strains [72,73].
The resistance characteristics of Mycoplasma spp. are therefore linked to biofilm formation ability, although exceptions to this have been reported. For example, M. bovis biofilms demonstrated no significant resistance to the effects of hygromycin, enrofloxacin, and dafloxacin [31], and no correlation was found between biofilm formation capacity and antibiotic susceptibility in M. anserisalpingitidis [32]. In U. urealyticum, biofilm formation did not alter its sensitivity to azithromycin and erythromycin [74]. To improve the comparability of anti-biofilm studies, it will be necessary to establish a standardized method to accurately define the biofilm sensitivity endpoint parameters [75]. Therefore, the relationship between Mycoplasma spp. biofilms and drug resistance requires further investigation that takes into account the specific Mycoplasma species, culture conditions, and biofilm-forming ability.
4. Correlation Between Biofilm and Virulence
Mycoplasmas are the smallest self-replicating prokaryotes, and their restricted genome size necessitates acquisition of host nutrients. Membrane lipoproteins and proteins are recognized virulence factors in this organism. Invasiveness is mediated by surface adhesins and accessory proteins; capsular polysaccharides and invasion enzymes and biofilm formation all contribute to host survival, environmental adaptation, and immune evasion [76]. Biofilms are therefore a virulence factor in these bacteria and are key components of their pathogenesis [77]. Mycoplasma biofilms and planktonic bacteria also differ in immunoreactivity. For instance, six immunoreactive proteins linked to M. bovis biofilm formation have been identified, and the HSP-70 family member DnaK displayed the highest level of immunoreactivity and was linked to immune system evasion [74]. In M. hyopneumoniae, a significant positive correlation between biofilm formation ability and virulence was identified, and attenuated strains were also low biofilm formers [78]. The P1 adhesin precursor protein in the strongly biofilm-forming M. gallisepticum NX-01 has also been identified [66]. The expression of variable membrane surface lipoproteins (Vsps) has also been highly and positively correlated with M. bovis biofilm formation. Strains that expressed VspF were poor biofilm formers, while VspO or VspB expressers were strong biofilm formers [31]. This suggested that different strains possess disparate abilities to form biofilms that are most likely associated with variations in the expression of virulence-related genes, including the molecular type. However, the highly pathogenic strain Mycoplasma mycoides subsp. mycoides SC was unable to produce biofilms in an air/liquid interface model, whereas its cognate strain Mycoplasma mycoides subsp. mycoides LC was able to form biofilms [31,79]; both the highly virulent strain M. gallisepticum S6 and the weakly virulent strain M. gallisepticum 6/85 were able to form dense biofilms, indicating that the ability to form biofilms does not necessarily correlate with pathogenicity [47].
Alterations in the survival patterns of Mycoplasma spp. during the formation of biofilms may result in variations in the expression levels of virulence genes. M. pneumoniae can produce the CARDS toxin that results in host cell vacuolization and activation of the NLRP3 inflammasome [80,81]. However, biofilm growth and CARDS toxin expression in M. pneumoniae S1 decrease over time [82]. CARDS toxin and the activities of enzymes associated with the production of H2S and H2O2 were highest during the initial stages of biofilm formation of M. pneumoniae and subsequently declined over time [71]. Together, these studies indicated that biofilm formation ability is closely linked to pathogenicity and the ability to establish persistent infections in vivo [83].
5. Strategies for Biofilm Control
Biofilms are the leading cause of many persistent and chronic bacterial infections [84]. The emergence of microbial biofilms that adhere to implantable devices or mucosal tissues is a significant global health concern. Biofilms typically increase antibiotic MIC values and may approach levels where the administered drug then reaches toxic levels. A thorough understanding of the mechanism of mycoplasma biofilm formation and the relationship between biofilms and drug resistance and virulence demonstrates that timely prevention, control, and removal of biofilms is a key to resolving mycoplasma biofilm infections. However, antibiotics that target the mycoplasma biofilm have not yet been developed, so finding new strategies to counteract the biofilm is essential for the prevention and treatment of mycoplasma biofilm-associated infections.
5.1. Physical Methods
When biofilm formation proceeds beyond the adhesion to microcolony and mature biofilm formation, the cells become more recalcitrant to treatment strategies. Therefore, if possible, tackling the developmental process already at the initial adhesion step is most successful to prevent biofilm formation. Disinfection and instrument sterilization and physical methods are commonly used to control biofilms that include ultrasound, ultraviolet light, spray washing, and exposure to elevated temperatures. Chemical reagents such as ethanol and alkaline detergents are also effective against Mycoplasma spp. [85]. Biofilm-associated infections associated with adherence to non-biological surfaces can be treated using ultrasound, vortex agitation, and other techniques to isolate adherent bacteria or release bacteria from biofilms [86]. For instance, SMARTF magnetic fluid, which contains iron oxide nano/microparticles, can mechanically remove bacterial cells attached inside flow cells or catheters, and its biofilm removal efficiency is much higher than that of the standard scraping and vortexing methods [87]. Bacterial biofilms exist in drinking water systems [88], and mycoplasma can be transmitted to humans and animals via water sources and can pose health risks. Ultrasonic and low-frequency electromagnetic pulse technologies are commonly used to eliminate biofilm in water by inhibiting bacterial adhesion and colonization. Altering the physicochemical properties of adsorption surfaces to inhibit microbial adhesion can also be used and includes enhancing smoothness, wettability, and hydrophilicity or applying surface coatings. The development of new materials with anti-adhesion properties in medical devices can help prevent infections of implanted materials [89].
5.2. Biological Formulations
Novel anti-biofilm agents, including plant-derived natural compounds, antimicrobial peptides, and biofilm matrix-degrading enzymes, can play significant roles in preventing bacterial adhesion and suppressing genes associated with biofilm formation. Antimicrobial peptides synthesized from derivatives of the amphipathic, cationic antimicrobial peptide magainin 2 exhibit antibacterial activity, membrane-disrupting properties, and anti-M. pneumoniae effects [90]. Sinefungin, a 5′-aminoalkyl analog of S-adenosyl-L-homocysteine, has been demonstrated to inhibit biofilm formation of M. pneumoniae and Streptococcus pneumoniae in vitro. It functions as a pan-inhibitor against S-adenosylmethionine-dependent methyltransferases and thus holds promise as an inhibitor of mycoplasma biofilms [91,92]. Natural compounds such as phenolics, essential oils, terpenoids, plant lectins, alkaloids, peptides, and polyacetylenes have also demonstrated significant anti-biofilm properties [93]. Small molecule inhibitors are compounds that can interact with biomacromolecules such as proteins and nucleic acids, altering their biological activity [94]. The novel narrow-spectrum SM4 and SM9 were screened for their inhibitory effects on the growth of M. gallisepticum and were able to demonstrate their antibacterial activity by reducing the invasion of M. gallisepticum to host cells and changing the cell membrane integrity of M. gallisepticum. Low molecular weight and membrane-active antibacterial properties allow efficacy against M. gallisepticum and M. synoviae biofilm in vitro [95,96]. SMs showed minimal toxicity on chicken cells and can be used in combination with probiotics, representing a prospective drug to control M. gallisepticum biofilms [97]. Additionally, nanoparticles can serve as drug delivery carriers and are capable of penetrating the EPS barrier to target antibiotic delivery, thereby enhancing bioavailability [98]. Zinc oxide nanoparticles (ZnO NPs) show broad antibacterial activity and anti-biofilm activity against pathogenic bacteria and have a sustainable application prospect [99,100]. Aivlosin in combination with ZnO NPs in the treatment is effective in treating M. gallisepticum infections in vivo by reducing the level of pro-inflammatory cytokines [101]. Phytochemical-synthesized ZnO NPs, such as Corydalis yanhusuo-ZnO NPs and Albizia chinensis-ZnO NPs, significantly reduce the pathological damage caused by M. pneumoniae [102,103]. These demonstrate that the ZnO NPs hold great potential in the field of anti-mycoplasma biofilm. In summary, these findings contribute to the development of formulations aimed at eradicating mycoplasma biofilms.
5.3. Natural Medicines
Organic compounds extracted from natural products, such as phenolics, essential oils, terpenoids, plant lectins, alkaloids, peptides, and polyacetylenes, have also demonstrated significant anti-biofilm properties [93]. For instance, magnolol (5,5′-diallyl-2,2′-dihydroxybiphenyl) is a natural compound with anti-inflammatory, antibacterial, antioxidant, and anticancer properties that significantly inhibits the growth of M. synoviae, M. gallisepticum, and M. hyopneumoniae in vitro and inhibits M. synoviae biofilm formation by destroying cell membrane integrity and inhibiting protegenin A production. Energy-related metabolic pathways, including the citrate cycle, glycolysis/gluconeogenesis, and pyruvate metabolism, were significantly disturbed [104,105]. Magnolol has an MIC of 15.63 µg/mL against M. synoviae, demonstrating good antibacterial activity, and also has inhibitory effects on other Mycoplasma spp. strains. At a dose of 1 mg/kg, magnolol exhibited low cytotoxicity to chicken embryos and significantly reduced the pathogenicity of M. synoviae, indicating that it is relatively safe for host cells at the effective dose [105]. Moreover, studies have demonstrated that the volatile components of propolis have significant biological activities, including antioxidant, anticancer, and antiviral properties, with the main compounds characterized as monoterpenes and sesquiterpenes, and exhibit good biocompatibility with normal cells [106,107,108,109,110]. These compounds also have antibacterial potential against Gram-positive and negative bacteria [111,112]. Geopropolis volatile oil (VO) 403 was extracted from Melipona bicolor schencki. The primary constituents identified were α-pinene, β-pinene, limonene, and terpinen-4-ol. The MIC of VO 403 was 424 ± 0 μg mL-1 against the mycoplasma strains. And it showed 15.25% eradication activity and 13.20% inhibition of M. pneumoniae biofilm formation after 24 h at 2× its MIC [113]. A combination of natural medicines and antibiotics has also displayed inhibitory effects on biofilm formation and exhibited synergistic or additive effects [114,115]. As natural active medicines are abundant resources, natural medicines possess characteristics such as high safety, low toxicity, and antibacterial effects. They undoubtedly have promising prospects for long-term development in the prevention and treatment of Mycoplasma spp. biofilm-associated infections.
5.4. Gene Engineering
Transposons have been widely used in Mycoplasma spp. to specifically target and modify genes and have been used successfully to construct biofilm formation defect mutant strains [68,116,117,118]. It is necessary to evaluate their immunogenicity and protective effects, which are expected to be used for the development of live attenuated vaccines. A previous study reported that a transposon mutant library was also used to identify two adhesin genes, gapA and crmA, required for adhesion and biofilm formation [66,68,119]. The application of genetically modified microorganisms shows great potential in combating persistent bacterial infections and biofilms. A weakly virulent M. pneumoniae strain that was constructed and modified through oligonucleotide recombination and Cas9-mediated negative selection was able to express proteins targeting the peptidoglycan of pathogenic bacteria, thereby secreting enzymes that resist biofilms and kill bacteria [117,120,121]. The CRISPR/Cas9-HDR method has also been used to target and knock out genes involved in quorum sensing and adhesion to reduce biofilm formation. This provides a feasible strategy for combating biofilms [122]. Together, these studies provide new perspectives for treating clinically relevant bacterial biofilm infections and demonstrate the potential of novel microbial therapies in the future prevention and treatment of biofilm infections.
6. Opportunities and Challenges
Bacterial biofilms are linked to 65% of microbial infections, and 80% of chronic infections are related to bacterial biofilms [123]. These infections are the primary result of biofilms formed on indwelling medical devices and mucosal or soft tissues. For example, the vaginal pathogens M. genitalium and M. homini can form biofilms. Some species can also combine with other bacterial vaginal pathogens to produce polymicrobial biofilms that allow host persistence [124]. The use of antimicrobial agents to combat biofilms can also result in resistance, and the biofilms cannot be completely eradicated. This is also a primary reason for the high recurrence rate of female reproductive tract infections [125]. The inconsistency between in vivo and in vitro drug sensitivity leads to poor efficacy of antimicrobial therapy, indicating that classical antibiotic susceptibility tests need to consider the impact of biofilms on the tests. It is therefore necessary to develop a standardized method for antibiotic susceptibility testing that specifically targets biofilms. This will improve the consistency between tests and clinical practice [75].
The initial step of biofilm formation does not necessarily require surface adhesion. Bacteria can attach to surfaces in the form of biofilms and can also form biofilm aggregates in a free-floating state [126,127]. This discovery has significant implications for human health and public hygiene. For instance, the biofilm tower structure of M. pneumoniae is derived from pre-existing aggregates in vitro, and this contributes to both the spread and persistence of the biofilm lifecycle [128]. Therefore, it is necessary to further verify whether Mycoplasma spp. can form non-surface adhesion biofilm aggregates in vivo or in vitro. However, the lack of reliable in vivo imaging technology for biofilm detection is a major obstacle to the study of these biofilms [129]. Therefore, the development of in vivo biofilm detection and tracing methods will promote the study of the interaction between biofilm and tissues and organs. Moreover, Mycoplasma spp. lack the classical repertoire of virulence genes common to pathogenic species, and the lack of targeted and efficient genetic tools for genome manipulation hinders the study of the functional characteristics of virulence genes related to pathogenicity [130,131]. This is also one of the important challenges in combating these biofilms. These studies will contribute to an understanding of the relationships between biofilm and clinical pathogenicity.
7. Conclusions
Mycoplasma biofilms are currently under study in the fields of human and veterinary medicine. Mycoplasmas can cause a multitude of diseases in humans and animals. Furthermore, there is a discernible upward trend in the diversity, resistance, and virulence of these organisms. The emergence of multidrug-resistant mycoplasmas and their secondary bacterial infectious diseases has constituted a frequent clinical challenge. The ineffectiveness of antimicrobials and vaccines has resulted in significant economic losses for the global farming industry. The formation of biofilms is one of the important reasons for these phenomena, underscoring the urgent need for the development of novel strategies to eradicate mycoplasma biofilms. Furthermore, the mechanisms of mycoplasma biofilm formation, drug resistance, and virulence factor expression are the current focus of research to better prevent and treat related diseases caused via these biofilms.
Author Contributions
J.L. and B.D.: investigation, writing—original draft, writing—review and editing, visualization. W.L.: writing—review and editing. J.Q., Y.L., X.W., M.L. and H.Y.: reviewing and editing. N.Z.: writing—review and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported financially by the National Natural Science Foundation of China (No. 32202866) and Guangdong University Science and Technology Service for Rural Revitalization (2021ZDZX4015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this systematic review are from previously reported studies and datasets and have been cited.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Young, L.; Sung, J.; Stacey, G.; Masters, J.R. Detection of Mycoplasma in cell cultures. Nat. Protoc. 2010, 5, 929–934. [Google Scholar] [CrossRef]
- Harwick, H.J.; Kalmanson, G.M.; Guze, L.B. Human Diseases Associated with Mycoplasmas—With an Appendix on Simple Culture Techniques. Calif. Med. 1972, 116, 1–7. [Google Scholar]
- Gautier-Bouchardon, A.V. Antimicrobial Resistance in Mycoplasma spp. Microbiol. Spectr. 2018, 6, 10.1128. [Google Scholar] [CrossRef] [PubMed]
- Shepard, M.C.; Calvy, G.L. The Role of Mycoplasma (Pleuropneumonia-like Organisms) in Human Disease. N. Engl. J. Med. 1965, 272, 848–851. [Google Scholar] [CrossRef]
- Goodnight, W.H.; Soper, D.E. Pneumonia in pregnancy. Crit. Care Med. 2005, 33, S390–S397. [Google Scholar] [CrossRef]
- Waites, K.B.; Talkington, D.F. Mycoplasma pneumoniae and its role as a human pathogen. Clin. Microbiol. Rev. 2004, 17, 697–728. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-Acquired pneumonia requiring hospitalization among U.S. children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef]
- Caswell, J.L.; Archambault, M. Mycoplasma bovis pneumonia in cattle. Anim. Health Res. Rev. 2007, 8, 161–186. [Google Scholar] [CrossRef]
- Buscho, R.O.; Saxtan, D.; Shultz, P.S.; Finch, E.; Mufson, M.A. Infections with viruses and Mycoplasma pneumoniae during exacerbations of chronic bronchitis. J. Infect. Dis. 1978, 137, 377–383. [Google Scholar] [CrossRef]
- Phuah, C.-L.; Javid, B.; Aliyu, S.H.; Lever, A.M.L. A case of Mycoplasma hominis septic arthritis postpartum. J. Infect. 2007, 55, e135–e137. [Google Scholar] [CrossRef]
- Johnson, S.; Sidebottom, D.; Bruckner, F.; Collins, D. Identification of Mycoplasma fermentans in synovial fluid samples from arthritis patients with inflammatory disease. J. Clin. Microbiol. 2000, 38, 90–93. [Google Scholar] [CrossRef]
- Morrow, C.J.; Bradbury, J.M.; Gentle, M.J.; Thorp, B.H. The development of lameness and bone deformity in the broiler following experimental infection with Mycoplasma gallisepticum or Mycoplasma synoviae. Avian Pathol. 1997, 26, 169–187. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, B. Mycoplasma-Induced Rash and Mucositis. N. Engl. J. Med. 2023, 389, 1601. [Google Scholar] [CrossRef]
- Li, T.; Lee, N. Mycoplasma pneumoniae—Associated Mucositis. N. Engl. J. Med. 2018, 379, 1262. [Google Scholar] [CrossRef]
- Shurin, P.A.; Alpert, S.; Rosner, B.; Driscoll, S.G.; Lee, Y.-H.; McCormack, W.M.; Santamarina, B.A.G.; Kass, E.H. Chorioamnionitis and Colonization of the Newborn Infant with Genital Mycoplasmas. N. Engl. J. Med. 1975, 293, 5–8. [Google Scholar] [CrossRef]
- Lis, R.; Rowhani-Rahbar, A.; Manhart, L.E. Mycoplasma genitalium infection and female reproductive tract disease: A meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 61, 418–426. [Google Scholar] [CrossRef]
- Mavedzenge, S.N.; Weiss, H.A. Association of Mycoplasma genitalium and HIV infection: A systematic review and meta-analysis. AIDS 2009, 23, 611–620. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, H.L.; Xu, K.R.; Wang, S.Y.; Fan, L.Q.; Zhu, W.B. Mycoplasma and ureaplasma infection and male infertility: A systematic review and meta-analysis. Andrology 2015, 3, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Feberwee, A.; de Wit, J.J.; Landman, W.J.M. Induction of eggshell apex abnormalities by Mycoplasma synoviae: Field and experimental studies. Avian Pathol. 2009, 38, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Medjo, B.; Atanaskovic-Markovic, M.; Radic, S.; Nikolic, D.; Lukac, M.; Djukic, S. Mycoplasma pneumoniae as a causative agent of community-acquired pneumonia in children: Clinical features and laboratory diagnosis. Ital. J. Pediatr. 2014, 40, 104. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.E.; Bradshaw, C.S.; Manhart, L.E. Update in Epidemiology and Management of Mycoplasma genitalium Infections. Infect. Dis. Clin. N. Am. 2023, 37, 311–333. [Google Scholar] [CrossRef]
- Maes, D.; Segales, J.; Meyns, T.; Sibila, M.; Pieters, M.; Haesebrouck, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008, 126, 297–309. [Google Scholar] [CrossRef]
- Pieters, M.G.; Maes, D. Mycoplasmosis. In Diseases of Swine; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 863–883. [Google Scholar]
- Feberwee, A.; de Wit, S.; Dijkman, R. Clinical expression, epidemiology, and monitoring of Mycoplasma gallisepticum and Mycoplasma synoviae: An update. Avian Pathol. 2022, 51, 2–18. [Google Scholar] [CrossRef]
- Filloux, A.; Vallet, I. Biofilm: Set-up and organization of a bacterial community. Med. Sci. M/S 2003, 19, 77–83. [Google Scholar]
- Raymond, B.B.A.; Jenkins, C.; Turnbull, L.; Whitchurch, C.B.; Djordjevic, S.P. Extracellular DNA release from the genome-reduced pathogen Mycoplasma hyopneumoniae is essential for biofilm formation on abiotic surfaces. Sci. Rep. 2018, 8, 10373. [Google Scholar] [CrossRef]
- Awadh, A.A.; Kelly, A.F.; Forster-Wilkins, G.; Wertheim, D.; Giddens, R.; Gould, S.W.; Fielder, M.D. Visualisation and biovolume quantification in the characterisation of biofilm formation in Mycoplasma fermentans. Sci. Rep. 2021, 11, 11259. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Daubenspeck, J.M.; Totten, A.H.; Needham, J.; Feng, M.; Balish, M.F.; Atkinson, T.P.; Dybvig, K. Mycoplasma genitalium Biofilms Contain Poly-GlcNAc and Contribute to Antibiotic Resistance. Front. Microbiol. 2020, 11, 585524. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.L.; Dybvig, K. Biofilms protect Mycoplasma pulmonis cells from lytic effects of complement and gramicidin. Infect. Immun. 2007, 75, 3696–3699. [Google Scholar] [CrossRef]
- Mcauliffe, L.; Ellis, R.J.; Miles, K.; Aylinget, R.D.; Nicholas, R.A.J. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology 2006, 152 Pt 4, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Bekő, K.; Nagy, E.Z.; Grózner, D.; Kreizinger, Z.; Gyuranecz, M. Biofilm formation and its impact on environmental survival and antibiotic resistance of Mycoplasma anserisalpingitidis strains. Acta Vet. Hung. 2022; epub ahead of print. [Google Scholar]
- Zhu, X.; Dordet-Frisoni, E.; Gillard, L.; Ba, A.; Hygonenq, M.-C.; Sagné, E.; Nouvel, L.X.; Maillard, R.; Assié, S.; Guo, A.; et al. Extracellular DNA: A Nutritional Trigger of Mycoplasma bovis Cytotoxicity. Front. Microbiol. 2019, 10, 2753. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.M.; Daubenspeck, J.M.; Simmons, W.L.; Dybvig, K. EPS-I Polysaccharide Protects Mycoplasma pulmonis from Phagocytosis. FEMS Microbiol. Lett. 2013, 338, 155–160. [Google Scholar] [CrossRef]
- Bolland, J.R.; Simmons, W.L.; Daubenspeck, J.M.; Dybvig, K. Mycoplasma polysaccharide protects against complement. Microbiology 2012, 158 Pt 7, 1867–1873. [Google Scholar] [CrossRef]
- Dai, P.; Deng, X.; Liu, P.; Li, L.; Luo, D.; Liao, Y.; Zeng, Y. Mycoplasma genitalium Protein of Adhesion Promotes the Early Proliferation of Human Urothelial Cells by Interacting with RPL35. Pathogens 2021, 10, 1449. [Google Scholar] [CrossRef]
- Hu, Z.; Li, H.; Zhao, Y.; Wang, G.; Shang, Y.; Chen, Y.; Wang, S.; Tian, M.; Qi, J.; Yu, S. NADH oxidase of Mycoplasma synoviae is a potential diagnostic antigen, plasminogen/fibronectin binding protein and a putative adhesin. BMC Vet. Res. 2022, 18, 455. [Google Scholar] [CrossRef]
- Rinaudi, L.; Fujishige, N.A.; Hirsch, A.M.; Banchio, E.; Zorreguieta, A.; Giordano, W. Effects of nutritional and environmental conditions on Sinorhizobium meliloti biofilm formation. Res. Microbiol. 2006, 157, 867–875. [Google Scholar] [CrossRef]
- Nickel, J.C.; Costerton, J.W.; Mclean, R.J.; Olson, M. Bacterial biofilms: Influence on the pathogenesis, diagnosis and treatment of urinary tract infections. J. Antimicrob. Chemother. 1994, 33 (Suppl. A), 31–41. [Google Scholar] [CrossRef]
- Ádám, A.; Pál, Z.; Terhes, G.; Szűcs, M.; Gabay, I.D.; Urbán, E. Culture- and PCR-based detection of BV associated microbiological profile of the removed IUDs and correlation with the time period of IUD in place and the presence of the symptoms of genital tract infection. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 40. [Google Scholar] [CrossRef]
- Kornspan, J.D.; Tarshis, M.; Rottem, S. Adhesion and biofilm formation of Mycoplasma pneumoniae on an abiotic surface. Arch. Microbiol. 2011, 193, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.L.; Daubenspeck, J.M.; Osborne, J.D.; Balish, M.F.; Waites, K.B.; Dybvig, K. Type 1 and type 2 strains of Mycoplasma pneumoniae form different biofilms. Microbiology 2013, 159 Pt 4, 737–747. [Google Scholar] [CrossRef] [PubMed]
- García-Castillo, M.; Morosini, M.I.; Gálvez, M.; Baquero, F.; del Campo, R.; Meseguer, M.-A. Differences in biofilm development and antibiotic susceptibility among clinical Ureaplasma urealyticum and Ureaplasma parvum isolates. J. Antimicrob. Chemother. 2008, 62, 1027–1030. [Google Scholar] [CrossRef]
- Romero, R.; Schaudinn, C.; Kusanovic, J.P.; Gorur, A.; Gotsch, F.; Webster, P.; Nhan-Chang, C.-L.; Erez, O.; Kim, C.J.; Espinozae, J.; et al. Detection of a microbial biofilm in intraamniotic infection. Am. J. Obstet. Gynecol. 2008, 198, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Henrich, B.; Schmitt, M.; Bergmann, N.; Zanger, K.; Kubitz, R.; Häussinger, D.; Pfeffer, K. Mycoplasma salivarium detected in a microbial community with Candida glabrata in the biofilm of an occluded biliary stent. J. Med. Microbiol. 2010, 59 Pt 2, 239–241. [Google Scholar] [CrossRef]
- Kang, T.; Zhou, M.; Yan, X.; Zanger, K.; Kubitz, R.; Häussinger, D.; Pfeffer, K. Biofilm formation and correlations with drug resistance in Mycoplasma synoviae. Vet. Microbiol. 2023, 283, 109777. [Google Scholar] [CrossRef]
- Chen, H.; Yu, S.; Hu, M.; Han, X.; Chen, D.; Qiu, X.; Ding, C. Identification of biofilm formation by Mycoplasma gallisepticum. Vet. Microbiol. 2012, 161, 96–103. [Google Scholar] [CrossRef]
- Sokoli, A.; Groebel, K.; Hoelzle, K.; Amselgruber, W.M.; Mateos, J.M.; Schneider, M.K.J.; Ziegler, U.; Felder, K.M.; Hoelzle, L.E. Mycoplasma suis infection results endothelial cell damage and activation: New insight into the cell tropism and pathogenicity of hemotrophic mycoplasma. Vet. Res. 2013, 44, 6. [Google Scholar] [CrossRef]
- Simmons, W.L.; Dybvig, K. Mycoplasma biofilms ex vivo and in vivo. FEMS Microbiol. Lett. 2009, 295, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Catania, S.; Bottinelli, M.; Fincato, A.; Tondo, A.; Matucci, A.; Nai, G.; Righetti, V.; Abbate, F.; Ramírez, A.S.; Gobbo, F.; et al. Pathogenic avian mycoplasmas show phenotypic differences in their biofilm forming ability compared to non-pathogenic species in vitro. Biofilm 2024, 7, 100190. [Google Scholar] [CrossRef]
- Chen, S.; Hao, H.; Yan, X.; Liu, Y.; Chu, Y. Genome-Wide Analysis of Mycoplasma dispar Provides Insights into Putative Virulence Factors and Phylogenetic Relationships. G3 2019, 9, 317–325. [Google Scholar] [CrossRef]
- Simmons, W.L.; Bolland, J.R.; Daubenspeck, J.M.; Dybvig, K. A stochastic mechanism for biofilm formation by Mycoplasma pulmonis. J. Bacteriol. 2007, 189, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- O’loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Bassler, B.L. A quorum-sensing inhibitor blocks pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Payne, G.F.; Bentley, W.E. Quorum sensing communication: Molecularly connecting cells, their neighbors, and even devices. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 447–468. [Google Scholar] [CrossRef]
- Fatoba, A.J.; Okpeku, M.; Adeleke, M.A. Subtractive genomics approach for identification of novel therapeutic drug targets in mycoplasma genitalium. Pathogens 2021, 10, 921. [Google Scholar] [CrossRef]
- Nishi, K.; Gondaira, S.; Hirano, Y.; Ohashi, M.; Sato, A.; Matsuda, K.; Iwasaki, T.; Kanda, T.; Uemura, R.; Higuchi, H. Biofilm characterisation of Mycoplasma bovis co-cultured with Trueperella pyogenes. Vet. Res. 2025, 56, 22. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Perchikov, R.; Cheliukanov, M.; Plekhanova, Y.; Tarasov, S.; Kharkova, A.; Butusov, D.; Arlyapov, V.; Nakamura, H.; Reshetilov, A. Microbial Biofilms: Features of Formation and Potential for Use in Bioelectrochemical Devices. Biosensors 2024, 14, 302. [Google Scholar] [CrossRef]
- Tuson, H.H.; Weibel, D.B. Bacteria–surface interactions. Soft Matter 2013, 9, 4368–4380. [Google Scholar] [CrossRef]
- Simmons, W.L.; Dybvig, K. Catalase Enhances Growth and Biofilm Production of Mycoplasma pneumoniae. Curr. Microbiol. 2015, 71, 190–194. [Google Scholar] [CrossRef]
- Bély, M.; Makovitzky, J. Sensitivity and specificity of Congo red staining according to Romhányi. Comparison with Puchtler’s or Bennhold’s methods. Acta Histochem. 2006, 108, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical techniques to study microbial biofilm on abiotic surfaces: Pros and cons of the main techniques currently in use. Ann. Di Ig. Med. Prev. E Di Comunita 2013, 25, 31–42. [Google Scholar]
- Wallace, P.K.; Arey, B.; Mahaffee, W.F. Subsurface examination of a foliar biofilm using scanning electron- and focused-ion-beam microscopy. Micron 2011, 42, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Neu, T.R.; Lawrence, J.R. Investigation of microbial biofilm structure by laser scanning microscopy. Adv. Biochem. Eng./Biotechnol. 2014, 146, 1–51. [Google Scholar] [PubMed]
- Tassew, D.D.; Mechesso, A.F.; Park, N.H.; Song, J.-B.; Shur, J.-W.; Park, S.-C. Biofilm formation and determination of minimum biofilm eradication concentration of antibiotics in Mycoplasma hyopneumoniae. J. Vet. Med. Sci. 2017, 79, 1716–1720. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, L.; Yang, F.; Li, J.; Guo, L.; Guo, Y.; He, S. Drug sensitivity and genome-wide analysis of two strains of Mycoplasma gallisepticum with different biofilm intensity. Front. Microbiol. 2023, 14, 1196747. [Google Scholar] [CrossRef] [PubMed]
- Fisunov, G.Y.; Pobeguts, O.V.; Ladygina, V.G.; Zubov, A.I.; Galyamina, M.A.; Kovalchuk, S.I.; Ziganshin, R.K.; DEvsyutina, V.; Matyushkina, D.S.; Butenko, I.O.; et al. Thymidine utilisation pathway is a novel phenotypic switch of Mycoplasma hominis. J. Med. Microbiol. 2022, 71, 001468. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, L.; Zhang, F.; Qiu, X.; Tan, L.; Yu, S.; Cheng, X.; Ding, C. Identification of genes involved in Mycoplasma gallisepticum biofilm formation using mini-Tn4001-SGM transposon mutagenesis. Vet. Microbiol. 2017, 198, 17–22. [Google Scholar] [CrossRef]
- Niu, J.; Yan, M.; Xu, J.; Xu, Y.; Chang, Z.; Sizhu, S. The Resistance Mechanism of Mycoplasma bovis from Yaks in Tibet to Fluoroquinolones and Aminoglycosides. Front. Vet. Sci. 2022, 9, 840981. [Google Scholar] [CrossRef]
- Nagy, E.Z.; Kovács, Á.B.; Wehmann, E.; Bekő, K.; Földi, D.; Bányai, K.; Kreizinger, Z.; Gyuranecz, M. Phenotypic and genetic insights into efflux pump mechanism in Mycoplasma anserisalpingitidis. Front. Microbiol. 2023, 14, 1216893. [Google Scholar] [CrossRef]
- Feng, M.; Schaff, A.C.; Balish, M.F. Mycoplasma pneumoniae biofilms grown in vitro: Traits associated with persistence and cytotoxicity. Microbiology 2020, 166, 629–640. [Google Scholar] [CrossRef]
- Feng, C.; Huang, Y.; Yu, Y.; Duan, G.; Dai, Y.; Dongs, K.; Li, Q. Effects on quinolone resistance due to the biofilm formation activity in Ureaplasma urealyticum. Turk. J. Med. Sci. 2015, 45, 55–59. [Google Scholar] [CrossRef]
- Wang, X.; He, L.; Wu, Z.; Zhong, Q.; Zeng, J.; Yang, W. Analysis of Biofilm Drug Susceptibility of Ureaplasma urealyticum Clinical Isolates. Chin. J. Antibiot. 2019, 44, 856–859. (In Chinese) [Google Scholar]
- Pandelidis, K.; Mccarthy, A.; Chesko, K.L.; Viscardi, R.M. Role of biofilm formation in Ureaplasma antibiotic susceptibility and development of bronchopulmonary dysplasia in preterm neonates. Pediatr. Infect. Dis. J. 2013, 32, 394–398. [Google Scholar] [CrossRef]
- Thieme, L.; Hartung, A.; Tramm, K.; Klinger-Strobel, M.; Jandt, K.D.; Makarewicz, O.; Pletz, M.W. MBEC Versus MBIC: The Lack of Differentiation between Biofilm Reducing and Inhibitory Effects as a Current Problem in Biofilm Methodology. Biol. Proced. Online 2019, 21, 18. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Qin, L.; Zhu, C.; You, X. Infection strategies of mycoplasmas: Unraveling the panoply of virulence factors. Virulence 2021, 12, 788–817. [Google Scholar] [CrossRef]
- Perez, K.; Mullen, N.; Canter, J.A.; Ley, D.H.; May, M. Phenotypic diversity in an emerging mycoplasmal disease. Microb. Pathog. 2020, 138, 103798. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Y.; Hua, L.; Wei, Y.; Gan, Y.; Chenia, H.Y.; Wang, Y.; Xie, X.; Wang, J.; Liu, M.; et al. Genotyping and biofilm formation of Mycoplasma hyopneumoniae and their association with virulence. Vet. Res. 2022, 53, 95. [Google Scholar] [CrossRef]
- Valdivieso-Garcia, A.; Rosendal, S.; Serebrin, S. Adherence of Mycoplasma mycoides Subspecies mycoides to Cultured Endothelial Cells. Zentralblatt Für Bakteriol. 1989, 272, 210–215. [Google Scholar] [CrossRef]
- Krishnan, M.; Kannan, T.R.; Baseman, J.B. Mycoplasma pneumoniae CARDS Toxin Is Internalized via Clathrin-Mediated Endocytosis. PLoS ONE 2013, 8, e62706. [Google Scholar] [CrossRef]
- Bose, S.; Segovia, J.A.; Somarajan, S.R.; Chang, T.-H.; Kannan, T.R.; Baseman, J.B. ADP-Ribosylation of NLRP3 by Mycoplasma pneumoniae CARDS Toxin Regulates Inflammasome Activity. mBio 2014, 5, e02186. [Google Scholar] [CrossRef] [PubMed]
- Kannan, T.R.; Musatovova, O.; Balasubramanian, S.; Cagle, M.; Jordan, J.L.; Krunkosky, T.M.; Davis, A.; Hardy, R.D.; Baseman, J.B. Mycoplasma pneumoniae Community Acquired Respiratory Distress Syndrome toxin expression reveals growth phase and infection-dependent regulation. Mol. Microbiol. 2010, 76, 1127–1141. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Wu, Z.; Lu, C. Reduced virulence is an important characteristic of biofilm infection of Streptococcus suis. FEMS Microbiol. Lett. 2011, 316, 36–43. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Eterpi, M.; Mcdonnell, G.; Thomas, V. Decontamination efficacy against Mycoplasma. Lett. Appl. Microbiol. 2011, 52, 150–155. [Google Scholar] [CrossRef]
- Macià, M.D.; Del Pozo, J.L.; Díez-Aguilar, M.; Guinea, J. Microbiological diagnosis of biofilm-related infections. Enfermedades Infecc. Y Microbiol. Clin. 2018, 36, 375–381. [Google Scholar] [CrossRef]
- Król, J.E.; Ehrlich, G.D. Using SMART Magnetic Fluids and Gels for Prevention and Destruction of Bacterial Biofilms. Microorganisms 2023, 11, 1515. [Google Scholar] [CrossRef]
- Maes, S.; Vackier, T.; Nguyen Huu, S.; Heyndrickx, M.; Steenackers, H.; Sampers, I.; Raes, K.; Verplaetse, A.; De Reu, K. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 2019, 19, 77. [Google Scholar] [CrossRef]
- Yin, W.; Xu, S.; Wang, Y.; Zhang, Y.; Chou, S.H.; Galperin, M.Y.; He, J. Ways to control harmful biofilms: Prevention, inhibition, and eradication. Crit. Rev. Microbiol. 2021, 47, 57–78. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, A.K.; de Mandal, S.; Shakeel, M.; Bisht, S.S.; Khan, J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front. Microbiol. 2020, 11, 566325. [Google Scholar] [CrossRef]
- Hayashi, K.; Misawa, T.; Goto, C.; Demizu, Y.; Hara-Kudo, Y.; Kikuchi, Y. The effects of magainin 2-derived and rationally designed antimicrobial peptides on Mycoplasma pneumoniae. PLoS ONE 2022, 17, e0261893. [Google Scholar] [CrossRef]
- Yadav, M.K.; Park, S.W.; Chae, S.W.; Song, J.J. Sinefungin, a natural nucleoside analogue of S-adenosylmethionine, inhibits Streptococcus pneumoniae biofilm growth. BioMed Res. Int. 2014, 2014, 156987. [Google Scholar] [CrossRef]
- Li, T.; Chen, C.; Yu, L.; Ding, W.; Ding, N.; Li, S.; Zhu, C.S. S-Adenosylmethionine Synthetase Is Involved in Biofilm Formation of Mycoplasma pneumoniae. Chin. J. Zoonotic Dis. 2021, 37, 404–409. (In Chinese) [Google Scholar]
- Yong, Y.Y.; Dykes, G.A.; Choo, W.S. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit. Rev. Microbiol. 2019, 45, 201–222. [Google Scholar] [CrossRef]
- Hong-Geller, E.; Micheva-Viteva, S. Small molecule screens to identify inhibitors of infectious disease. In Drug Discovery; IntechOpen: London, UK, 2013. [Google Scholar][Green Version]
- Helmy, Y.A.; Kathayat, D.; Ghanem, M.; Jung, K.; Closs, G., Jr.; Deblais, L.; Srivastava, V.; El-Gazzar, M.; Rajashekara, G. Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens. Vet. Microbiol. 2020, 247, 108799. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial tolerance in biofilms. Microbiol. Spectr. 2015, 3, 10.1128. [Google Scholar] [CrossRef]
- Kathayat, D.; Helmy, Y.A.; Deblais, L.; Rajashekara, G. Novel small molecules affecting cell membrane as potential therapeutics for avian pathogenic Escherichia coli. Sci. Rep. 2018, 8, 15329. [Google Scholar] [CrossRef]
- Hemeg, H.A. Combatting persisted and biofilm antimicrobial resistant bacterial by using nanoparticles. Z. Fur Naturforschung. C J. Biosci. 2022, 77, 365–378. [Google Scholar] [CrossRef]
- Udayagiri, H.; Sana, S.S.; Dogiparthi, L.K.; Vadde, R.; Varma, R.S.; Koduru, J.R.; Ghodake, G.S.; Somala, A.R.; Boya, V.K.N.; Kim, S.C.; et al. Phytochemical fabrication of ZnO nanoparticles and their antibacterial and anti-biofilm activity. Sci. Rep. 2024, 14, 19714. [Google Scholar] [CrossRef]
- Yao, H.; Fan, Y.; Emre, E.S.T.; Li, N.; Ge, M.; Wang, J.; Wei, J. Alginate-modified ZnO anti-planktonic and anti-biofilm nanoparticles for infected wound healing. Int. J. Biol. Macromol. 2024, 280, 135739. [Google Scholar] [CrossRef]
- Awad, N.F.S.; Hashem, Y.M.; Elshater, N.S.; Khalifa, E.; Hamed, R.I.; Nossieur, H.H.; Abd-Allah, E.M.; Elazab, S.T.; Nassan, M.A.; El-Hamid, M.I.A. Therapeutic potentials of aivlosin and/or zinc oxide nanoparticles against mycoplasma gallisepticum and/or ornithobacterium rhinotracheale with a special reference to the effect of zinc oxide nanoparticles on aivlosin tissue residues: An in vivo approach. Poult. Sci. 2022, 101, 101884. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Ramesh, T.; Seshadri, V.D.; Zhu, L. Zinc oxide nanoparticles from corydalis yanhusuo attenuated the mycoplasmal pneumonia in mice through inhibiting the MAPKs signaling pathway. Microb. Pathog. 2020, 147, 104270. [Google Scholar] [CrossRef]
- Yu, L.; Lu, M.; Zhang, W.; Alarfaj, A.A.; Hirad, A.H.; Zhang, H. Ameliorative effect of albizia chinensis synthesized ZnO-NPs on mycoplasma pneumoniae infected pneumonia mice model. Microb. Pathog. 2020, 141, 103960. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the Multifunctional Activities of Magnolol. BioMed Res. Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Zhang, W.; Lu, Q.; Guo, Y.; Cao, X.; Shao, H.; Zhai, X.; Luo, Q. Mechanistic insights of magnolol antimicrobial activity against Mycoplasma using untargeted metabolomic analyses. Front. Cell. Infect. Microbiol. 2023, 13, 1325347. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Dumitru, C.D.; Neacșu, I.A.; Oprea, O.C.; Motelica, L.; Voicu Balasea, B.; Ilie, C.-I.; Marinescu, F.; Ripszky, A.; Pituru, S.-M.; Andronescu, E. Biomaterials based on bee products and their effectiveness in soft tissue regeneration. Materials 2025, 18, 2689. [Google Scholar] [CrossRef]
- González-Ochoa, G.; Balderrama-Carmona, A.P.; Erro-Carvajal, J.A.; Erro-Carvajal, J.A.; Soñanez-Organis, J.G.; Zamora-Álvarez, L.A.; Umsza Guez, M.A. Antiviral activity of brazilian propolis from stingless bees against rotavirus. Microorganisms 2025, 13, 1424. [Google Scholar] [CrossRef]
- da Paz, M.M.; Sette, K.M.; Dos Santos, R.E.; Barbosa e Vasconcelos, A.L.; da Costa, D.C.F.; Amaral, A.C.F.; Rodrigues, I.A.; Pereira Rangel, L. Brazilian stingless bee geopropolis exhibit antioxidant properties and anticancer potential against hepatocellular carcinoma cells. Antioxidants 2025, 14, 141. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Pavelić, S.K. Bioactives from bee products and accompanying extracellular vesicles as novel bioactive components for wound healing. Molecules 2021, 26, 3770. [Google Scholar] [CrossRef]
- Miguel, M.G.; Figueiredo, A.C. Propolis and geopropolis volatiles. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 113–136. [Google Scholar]
- Svetikiene, D.; Zamokas, G.; Jokubaite, M.; Marksa, M.; Ivanauskas, L.; Babickaite, L.; Ramanauskiene, K. The comparative study of the antioxidant and antibacterial effects of propolis extracts in veterinary medicine. Vet. Sci. 2024, 11, 375. [Google Scholar] [CrossRef]
- Portal, A.S.; Schiquet, S.; Amaral, P.B.; Krepsky, L.M.; Curban, L.; Rebelo, R.A.; Rau, M.; Althoff, S.L.; Guedes, A.; de Cordova, C.M.M. Composition, antibiofilm, and antibacterial potential of volatile oils from geopropolis of different stingless bees’ species. Chem. Biodivers. 2023, 20, e202300592. [Google Scholar] [CrossRef]
- Roudashti, S.; Zeighami, H.; Mirshahabi, H.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J. Microbiol. Biotechnol. 2017, 33, 50. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Cui, J.; He, Z.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic Effects from Combination of Cryptotanshinone and Fosfomycin Against Fosfomycin-Susceptible and Fosfomycin-Resistant Staphylococcus aureus. Infect. Drug Resist. 2020, 13, 2837–2844. [Google Scholar] [CrossRef] [PubMed]
- Maglennon, G.A.; Cook, B.S.; Deeney, A.S.; Bossé, J.T.; Peters, S.E.; Langford, P.R.; Maskell, D.J.; Tucker, A.W.; Wren, B.W.; Rycroft, A.N.; et al. Transposon mutagenesis in Mycoplasma hyopneumoniae using a novel mariner-based system for generating random mutations. Vet. Res. 2013, 44, 124. [Google Scholar] [CrossRef] [PubMed]
- Piñero-Lambea, C.; Garcia-Ramallo, E.; Martinez, S.; Delgado, J.; Serrano, L.; Lluch-Senar, M. Mycoplasma pneumoniae Genome Editing Based on Oligo Recombineering and Cas9-Mediated Counterselection. ACS Synth. Biol. 2020, 9, 1693–1704. [Google Scholar] [CrossRef]
- Rideau, F.; Le Roy, C.; Sagné, E.; Renaudin, H.; Pereyre, S.; Henrich, B.; Dordet-Frisoni, E.; Citti, C.; Lartigue, C.; Bébéar, C. Random transposon insertion in the Mycoplasma hominis minimal genome. Sci. Rep. 2019, 9, 13554. [Google Scholar] [CrossRef]
- Tseng, C.W.; Kanci, A.; Citti, C.; Rosengarten, R.; Chiu, C.J.; Chen, Z.H.; Geary, S.J.; Browning, G.F.; Markham, P.F. MalF is essential for persistence of Mycoplasma gallisepticum in vivo. Microbiology 2013, 159 Pt 7, 1459–1470. [Google Scholar] [CrossRef]
- Mazzolini, R.; Rodríguez-Arce, I.; Fernández-Barat, L.; Piñero-Lambea, C.; Garrido, V.; Rebollada-Merino, A.; Motos, A.; Torres, A.; Grilló, M.J.; Serrano, L.; et al. Engineered live bacteria suppress Pseudomonas aeruginosa infection in mouse lung and dissolve endotracheal-tube biofilms. Nat. Biotechnol. 2023, 41, 1089–1098. [Google Scholar] [CrossRef]
- Garrido, V.; Piñero-Lambea, C.; Rodriguez-Arce, I.; Paetzold, B.; Ferrar, T.; Weber, M.; Garcia-Ramallo, E.; Gallo, C.; Collantes, M.; Peñuelas, I.; et al. Engineering a genome-reduced bacterium to eliminate Staphylococcus aureus biofilms in vivo. Mol. Syst. Biol. 2021, 17, e10145. [Google Scholar] [CrossRef]
- Alshammari, M.; Ahmad, A.; Alkhulaifi, M.; Al Farraj, D.; Alsudir, S.; Alarawi, M.; Takashi, G.; Alyamani, E. Reduction of biofilm formation of Escherichia coli by targeting quorum sensing and adhesion genes using the CRISPR/Cas9-HDR approach, and its clinical application on urinary catheter. J. Infect. Public Health 2023, 16, 1174–1183. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. JCMA 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host-Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Schilling, J.; Mendling, W. Response of Gardnerella vaginalis biofilm to 5 days of moxifloxacin treatment. FEMS Immunol. Med. Microbiol. 2011, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Alhede, M.; Kragh, K.N.; Qvortrup, K.; Allesen-Holm, M.; van Gennip, M.; Christensen, L.D.; Jensen, P.Ø.; Nielsen, A.K.; Parsek, M.; Wozniak, D.; et al. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS ONE 2011, 6, e27943. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Burgess, A.C.; Cuellar, R.R.; Schwab, N.R.; Balish, M.F. Modelling persistent Mycoplasma pneumoniae biofilm infections in a submerged BEAS-2B bronchial epithelial tissue culture model. J. Med. Microbiol. 2021, 70, 1–10. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. Biofilm-Related Infections in Healthcare: Moving towards New Horizons. Microorganisms 2024, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Lu, D.; Li, M.; Wang, Y. Gene editing tools for mycoplasmas: References and future directions for efficient genome manipulation. Front. Microbiol. 2023, 14, 1191812. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).