Facultatively Anaerobic Staphylococci Enable Anaerobic Cutibacterium Species to Grow and Form Biofilms Under Aerobic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Media and Growth Conditions
2.3. Test Tube and Microtiter Plate Biofilm Assays
2.4. NHEK Cell Culture
2.5. Enumeration of Biofilm Bacteria
2.6. Treatment of Biofilms with Enzymes
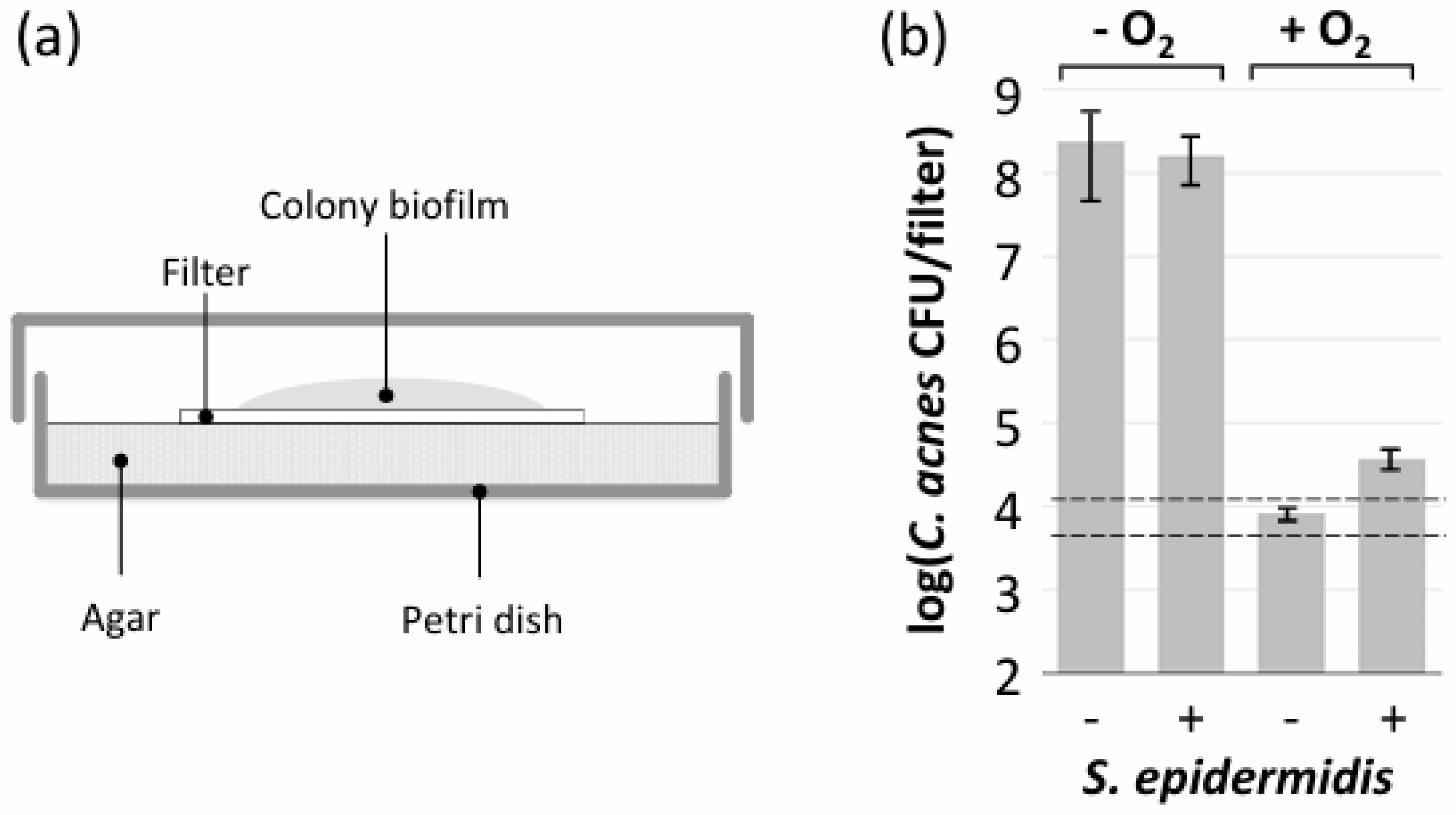
2.7. Colony Biofilm Assay
2.8. Fluorescence Confocal Microscopy
2.9. Statistics and Reproducibility of Results
3. Results
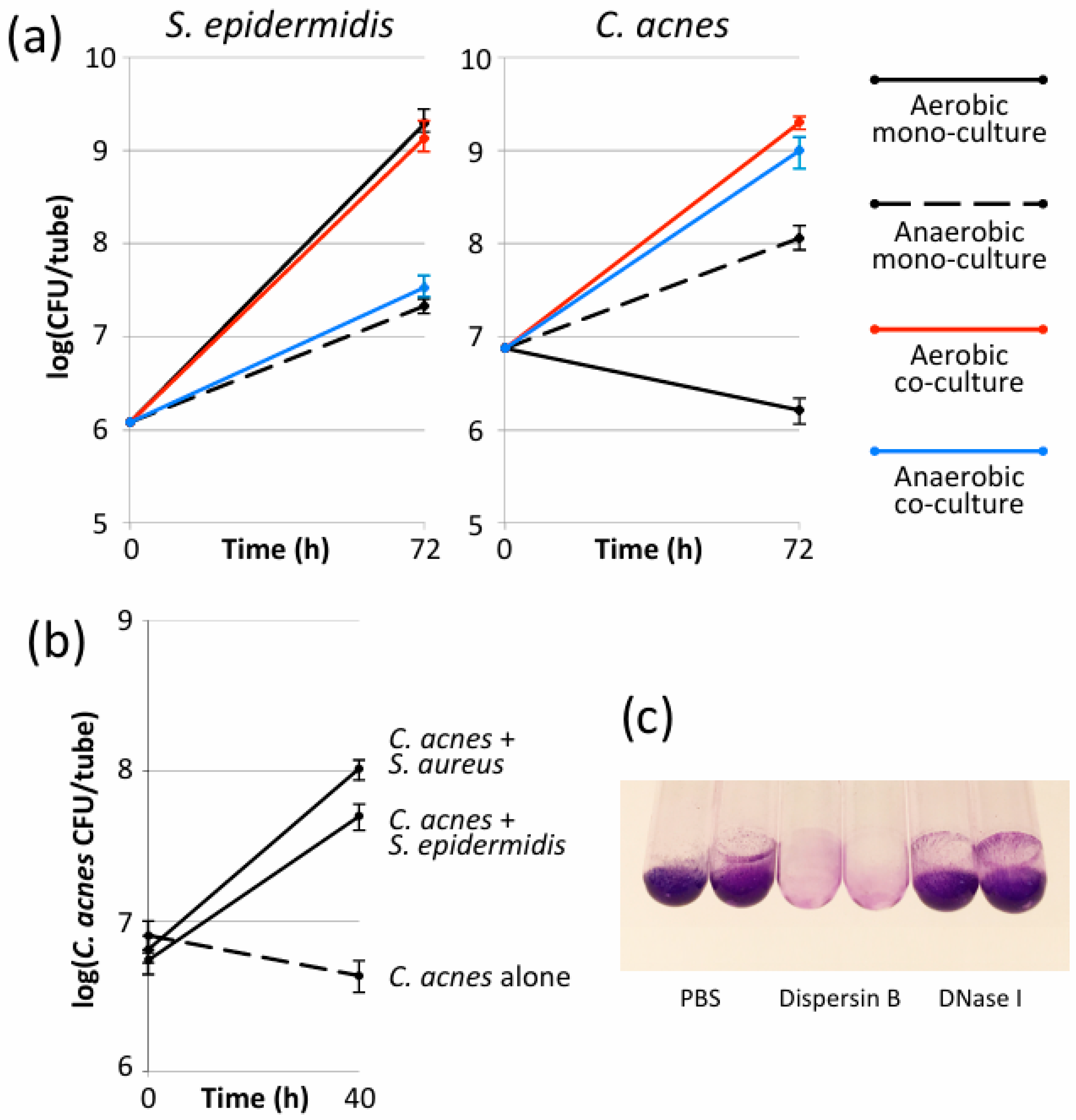
3.1. S. epidermidis Enabled C. acnes to Form Biofilms in Glass Tubes Under Aerobic Conditions
3.2. S. aureus Enabled C. acnes to Form Biofilms in Glass Tubes Under Aerobic Conditions
3.3. Aerobic S. epidermidis/C. acnes Biofilms Contained Poly-N-Acetylglucosamine Exopolysaccharide
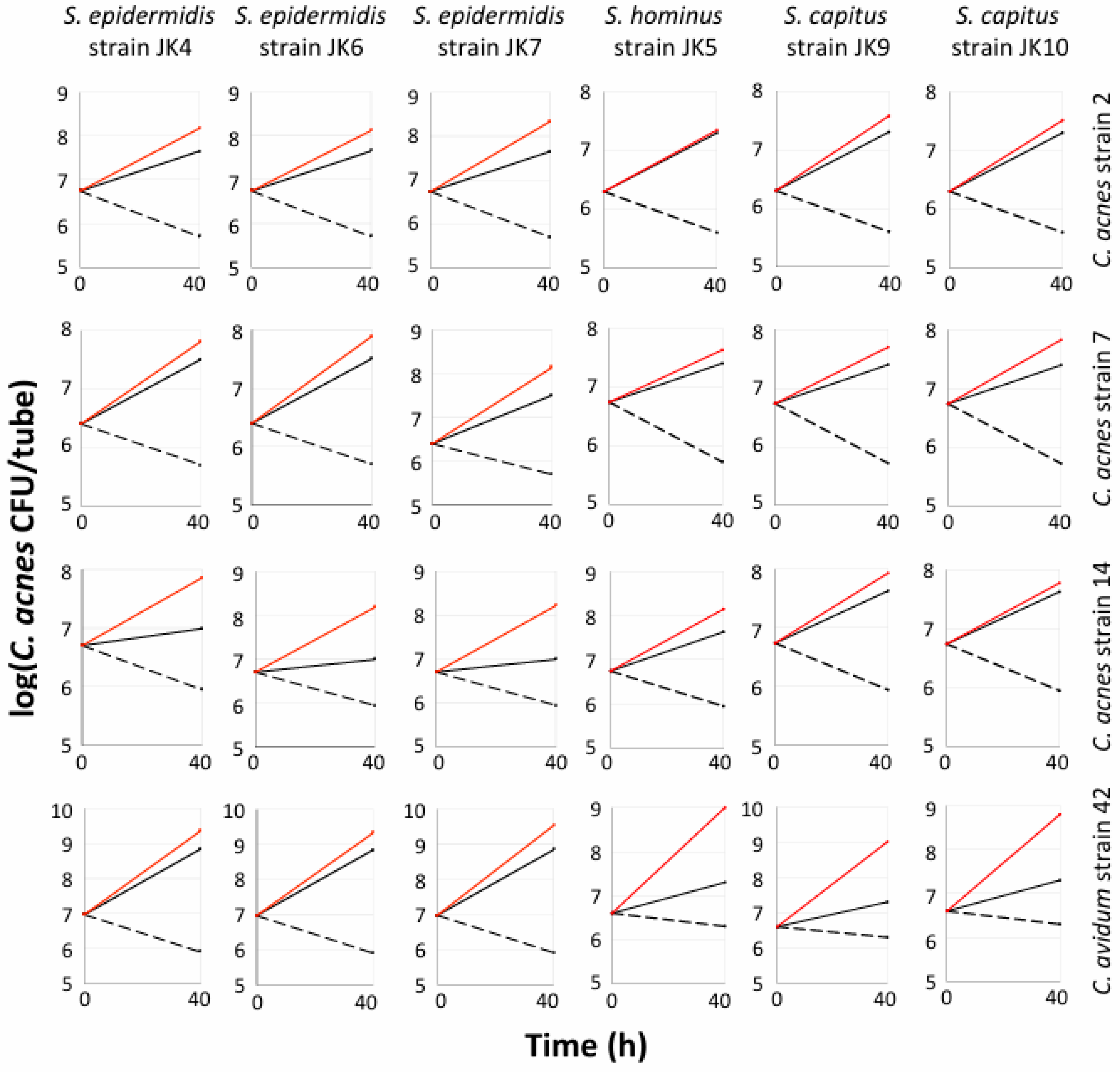
3.4. Multiple Staphylococcal Species Promoted Aerobic Biofilm Formation by C. acnes and C. avidum in Polypropylene Tubes
3.5. Aerobic Growth of C. acnes Depended on Biofilm Formation and Required Live S. epidermidis Cells
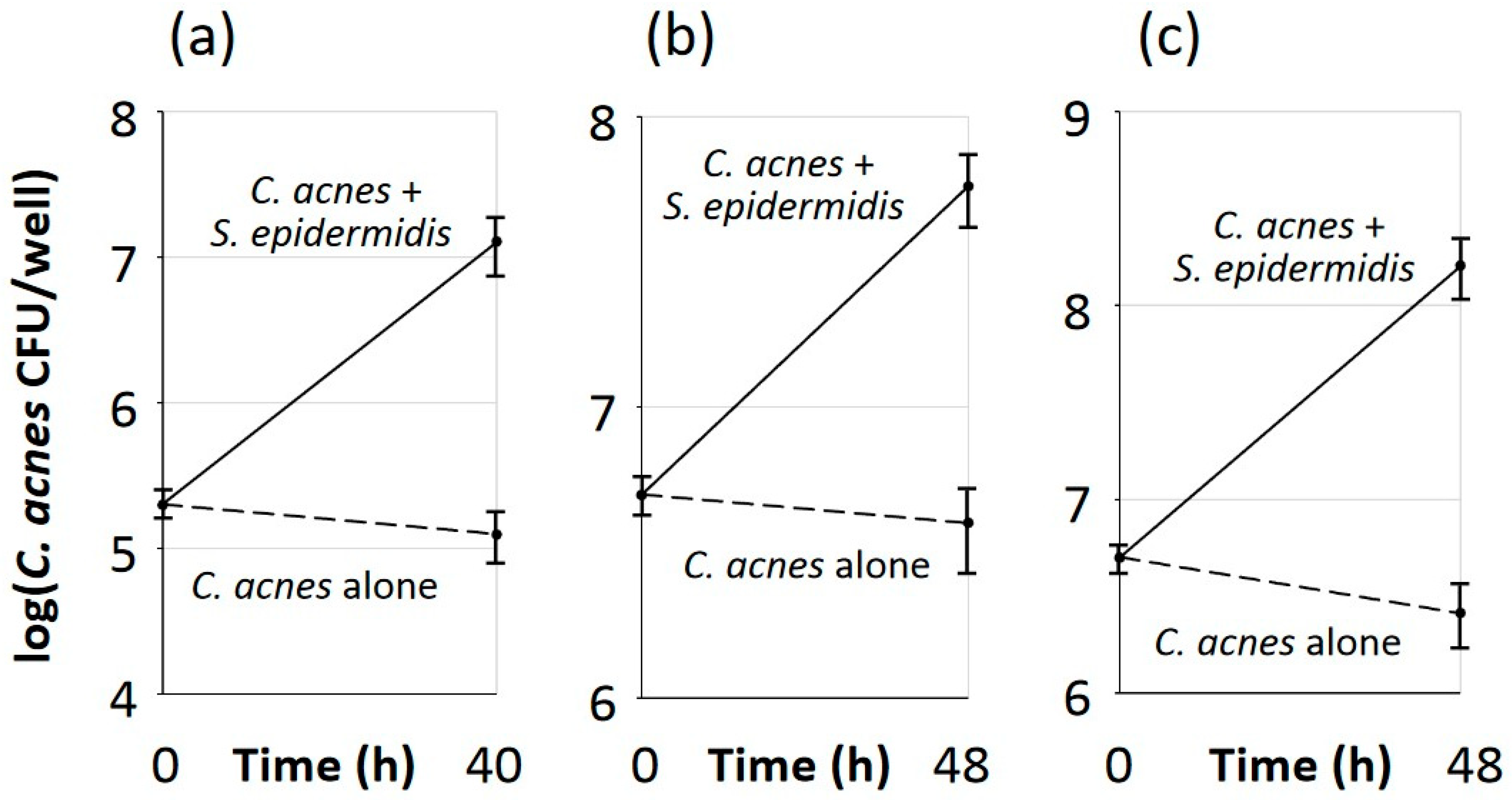
3.6. S. epidermidis Promoted Aerobic Growth of C. acnes in Polystyrene Microtiter Plate Wells but Not in Colony Biofilms
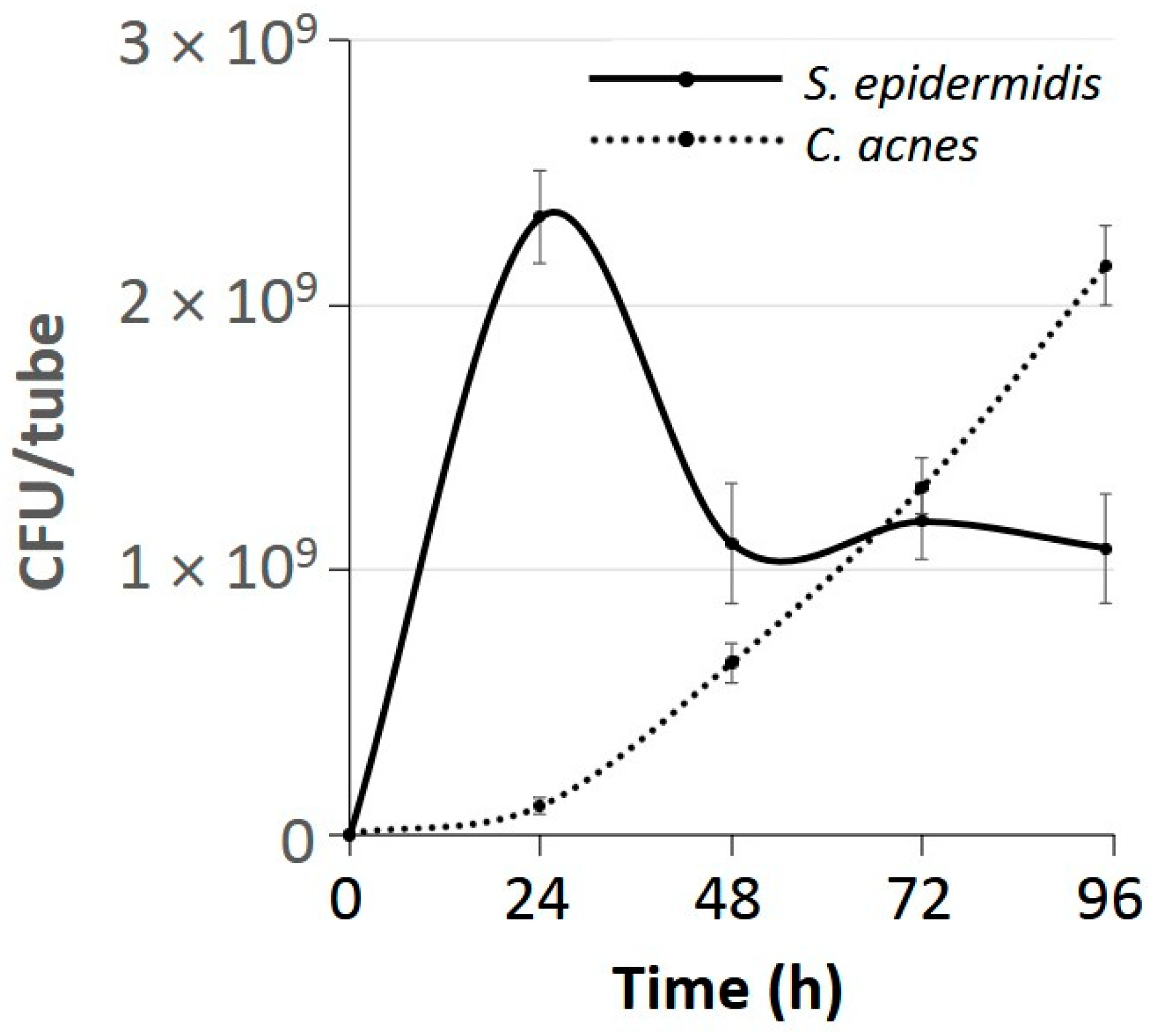
3.7. C. acnes Outcompeted S. epidermidis After 3 Days of Growth Under Aerobic Conditions
3.8. Aerobic C. acnes/S. epidermidis Dual-Species Biofilms Exhibited a Bilayer Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahle, C.M.; Stødkilde, K.; Poehlein, A.; Bömeke, M.; Streit, W.R.; Wenck, H.; Reuter, J.H.; Hüpeden, J.; Brüggemann, H. Interference and co-existence of staphylococci and Cutibacterium acnes within the healthy human skin microbiome. Commun. Biol. 2022, 5, 923. [Google Scholar] [CrossRef] [PubMed]
- Fournière, M.; LaVre, T.; Souak, D.; Feuilloley, M.G.J.; Bedoux, G. Staphylococcus epidermidis and Cutibacterium acnes: Two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 2020, 8, 1752. [Google Scholar] [CrossRef]
- Francuzik, W.; Franke, K.; Schumann, R.R.; Heine, G.; Worm, M. Propionibacterium acnes abundance correlates inversely with Staphylococcus aureus: Data from atopic dermatitis skin microbiome. Acta Derm. Venereol. 2018, 98, 490–495. [Google Scholar] [CrossRef]
- Rozas, M.; de Ruijter, A.H.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Berhard Paetzold, B.; Franois Brillet, F. From dysbiosis to healthy skin: Major contributions of Cutibacterium acnes to skin homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Achermann, Y.; Goldstein, E.J.; Coenye, T.; Shirtliff, M.E. Propionibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Bek-Thomsen, M.; Lomholt, H.B.; Kilian, M. Acne is not associated with yet-uncultured bacteria. J. Clin. Microbiol. 2008, 46, 3355–3360. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef]
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The skin microbiome: A new actor in inflammatory acne. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 18–24. [Google Scholar] [CrossRef] [PubMed]
- Tyner, H.; Patel, R. Propionibacterium acnes biofilm—A sanctuary for Staphylococcus aureus? Anaerobe 2016, 40, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Claesen, J.; Spagnolo, J.B.; Ramos, S.F.; Kurita, K.L.; Byrd, A.L.; Aksenov, A.A.; Melnik, A.V.; Wong, W.R.; Wang, S.; Hernandez, R.D.; et al. A Cutibacterium acnes antibiotic modulates human skin microbiota composition in hair follicles. Sci. Transl. Med. 2020, 12, 5445. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; O’Neill, A.M.; Williams, M.R.; Cau, L.; Nakatsuji, T.; Horswill, A.R.; Gallo, R.L. Short chain fatty acids produced by Cutibacterium acnes inhibit biofilm formation by Staphylococcus epidermidis. Sci. Rep. 2020, 10, 21237. [Google Scholar] [CrossRef]
- Wang, Y.; Kuo, S.; Shu, M.; Yu, J.; Huang, S.; Dai, A.; Two, A.; Gallo, R.L.; Huang, C.-M. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: Implications of probiotics in acne vulgaris. Appl. Microbiol. Biotechnol. 2014, 98, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, M.; Steinberg, D.; Meshner, S. Microbial biofilms and the human skin microbiome. NPJ Biofilms Microbiomes 2016, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three faces of biofilms: A microbial lifestyle, a nascent multicellular organism, and an incubator for diversity. NPJ Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Bayston, R.; Ashraf, W.; Barker-Davies, R.; Tucker, E.; Clement, R.; Clayton, J.; Freeman, B.J.C.; Nuradeen, B. Biofilm formation by Propionibacterium acnes on biomaterials in vitro and in vivo: Impact on diagnosis and treatment. J. Biomed. Mater. Res. A 2006, 81, 705–709. [Google Scholar] [CrossRef]
- Jahns, A.C.; Lundskog, B.; Ganceviciene, R.; Palmer, R.H.; Golovleva, I.; Zouboulis, C.C.; McDowell, A.; Patrick, S.; Alexeyev, O.A. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: A case-control study. Br. J. Dermatol. 2012, 167, 50–58. [Google Scholar] [CrossRef]
- Allen, H.B.; Mueller, J.L. A novel finding in atopic dermatitis: Film-producing Staphylococcus epidermidis as an etiology. Int. J. Dermatol. 2011, 50, 992–993. [Google Scholar] [CrossRef]
- Bügner, H.; Mack, D.; Rohde, H. Structural basis of Staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front. Cell Infect. Microbiol. 2015, 5, 14. [Google Scholar] [CrossRef]
- Gannesen, A.V.; Lesouhaitier, O.; Racine, P.J.; Barreau, M.; Netrusov, A.I.; Plakunov, V.K.; Feuilloley, M.G.J. Regulation of monospecies and mixed biofilms formation of skin Staphylococcus aureus and Cutibacterium acnes by human natriuretic peptides. Front. Microbiol. 2018, 9, 2912. [Google Scholar] [CrossRef] [PubMed]
- Ovcharova, M.A.; Geraskina, O.V.; Danilova, N.D.; Botchkova, E.A.; Martyanov, S.V.; Feofanov, A.V.; Plakunov, V.K.; Gannesen, A.V. Atrial natriuretic peptide affects skin commensal Staphylococcus epidermidis and Cutibacterium acnes dual-species biofilms. Microorganisms 2021, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Ovcharova, M.A.; Schelkunov, M.I.; Geras’kina, O.V.; Makarova, N.E.; Sukhacheva, M.V.; Martyanov, S.V.; Nevolina, E.D.; Zhurina, M.V.; Feofanov, A.V.; Botchkova, E.A.; et al. C-Type natriuretic peptide acts as a microorganism-activated regulator of the skin commensals Staphylococcus epidermidis and Cutibacterium acnes in dual-species biofilms. Biology 2023, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Acosta, E.A.; Little, K.A.; Bratto, B.P.; Lopez, J.G.; Mao, X.; Payne, A.S.; Donia, M.; Devenport, D.; Gitai, Z. Bacterial DNA on the skin surface overrepresents the viable skin microbiome. eLife 2023, 12, RP87192. [Google Scholar] [CrossRef]
- Sheffer-Levi, S.; Rimon, A.; Lerer, V.; Shlomov, T.; Coppenhagen-Glazer, S.; Rakov, C.; Zeiter, T.; Nir-Paz, R.; Hazan, R.; Molho-Pessach, V. Antibiotic susceptibility of Cutibacterium acnes strains isolated from Israeli acne patients. Acta Derm. Venereol. 2020, 100, adv00295. [Google Scholar] [CrossRef]
- Fitz-Gibbon, S.; Tomida, S.; Chiu, B.-H.; Nguyen, L.; Du, C.; Liu, M.; Elashoff, D.; Erfe, M.C.; Loncaric, A.; Kim, J.; et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J. Investig. Dermatol. 2013, 133, 2152–2160. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Perdreau-Remington, F.; Rieg, G.; Mehdi, S.; Perlroth, J.; Bayer, A.S.; Tang, A.W.; Phung, T.O.; Spellberg, B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 2005, 352, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Chaignon, P.; Sadovskaya, I.; Ragunath, C.; Ramasubbu, N.; Kaplan, J.B.; Jabbouri, S. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl. Microbiol. Biotechnol. 2007, 75, 125–132. [Google Scholar] [CrossRef]
- Mack, D.; Siemssen, N.; Laufs, R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: Evidence for functional relation to intercellular adhesion. Infect. Immun. 1992, 60, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.L.; Boles, B.R.; Lauderdale, K.J.; Thoendel, M.; Kavanaugh, J.S.; Horswill, A.R. Fluorescent reporters for Staphylococcus aureus. J. Microbiol. Methods 2009, 77, 251–260. [Google Scholar] [CrossRef]
- Scholz, C.F.P.; Jensen, A.; Lomholt, H.B.; Brüggemann, H.; Kilian, M. A novel high-resolution single locus sequence typing scheme for mixed populations of Propionibacterium acnes in vivo. PLoS ONE 2014, 9, e104199. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Cywes-Bentley, C.; Pier, G.B.; Yakandawala, N.; Sailer, M.; Edwards, M.S.; Kridin, K. Poly-b(1-6)-N-acetyl-D-glucosamine (PNAG) mediates surface attachment, biofilm formation, and biocide resistance in Cutibacterium acnes. Front. Microbiol. 2024, 15, 1386017. [Google Scholar] [CrossRef] [PubMed]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Jabbouri, S.; Sadovskaya, I. Extracellular DNA-dependent biofilm formation by Staphylococcus epidermidis RP62A in response to subminimal inhibitory concentrations of antibiotics. Res. Microbiol. 2011, 162, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Sukhishvili, S.A.; Sailer, M.; Kridin, K.; Ramasubbu, N. Aggregatibacter actinomycetemcomitans dispersin B: The quintessential antibiofilm enzyme. Pathogens 2024, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Chou, W.-L.; Lin, C.-F.; Sung, C.T.; Alalaiwe, A.; Yang, S.-C. Facile biofilm penetration of cationic liposomes loaded with DNase I/proteinase K to eradicate Cutibacterium acnes for treating cutaneous and catheter infections. Int. J. Nanomed. 2021, 16, 8121–8138. [Google Scholar] [CrossRef] [PubMed]
- Gannesen, A.V.; Zdorovenko, E.L.; Botchkova, E.A.; Hardouin, J.; Massier, S.; Kopitsyn, D.S.; Gorbachevskii, M.V.; Kadykova, A.A.; Shashkov, A.S.; Zhurina, M.V.; et al. Composition of the biofilm matrix of Cutibacterium acnes acneic strain RT5. Front. Microbiol. 2019, 10, 1284. [Google Scholar] [CrossRef]
- Jahns, A.C.; Eilers, H.; Alexeyev, O.A. Transcriptomic analysis of Propionibacterium acnes biofilms in vitro. Anaerobe 2016, 42, 111–118. [Google Scholar] [CrossRef]
- Kuehnast, T.; Cakar, F.; Weinhäupl, T.; Pilz, A.; Selak, S.; Schmidt, M.A.; Rüter, C.; Schild, S. Comparative analyses of biofilm formation among different Cutibacterium acnes isolates. Int. J. Med. Microbiol. 2018, 308, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.I.; Nagahori, R.; Yamada, S.; Sugimoto, S.; Sato, C.; Sato, M.; Iwase, T.; Hashimoto, K.; Mizunoe, Y. The composition and structure of biofilms developed by Propionibacterium acnes isolated from cardiac pace-maker devices. Front. Microbiol. 2018, 9, 182. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nature Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- Tilles, G. Acne pathogenesis: History of concepts. Dermatology 2014, 229, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Bartnicka, D.; Karkowska-Kuleta, J.; Zawrotniak, M.; Satała, D.; Michalik, K.; Zielinska, G.; Bochenska, O.; Kozik, A.; Ciaston, I.; Koziel, J.; et al. Adhesive protein-mediated cross-talk between Candida albicans and Porphyromonas gingivalis in dual species biofilm protects the anaerobic bacterium in unfavorable oxic environment. Sci. Rep. 2019, 9, 4376. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Lemoine, V.; Hoogenkamp, M.A.; Girardot, M.; Krom, B.P.; Imbert, C. Candida albicans enhances initial biofilm growth of Cutibacterium acnes under aerobic conditions. Biofouling 2019, 35, 350–360. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Marsh, P.D.; Allison, C.; Schilling, K.M. Effect of oxygen, inoculum composition and flow rate on development of mixed-culture oral biofilms. Microbiology 1996, 142, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.I.; Zilm, P.S.; Rogers, A.H. Fusobacterium nucleatum supports the growth of Porphyromonas gingivalis in oxygenated and carbon-dioxide-depleted environments. Microbiology 2002, 148, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.P.; Cowley, E.S.; Nobile, C.J.; Hartooni, N.; Newman, D.K.; Johnson, A.D. Anaerobic bacteria grow within Candida albicans biofilms and induce biofilm formation in suspension cultures. Curr. Biol. 2014, 24, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Janus, M.M.; Crielaard, W.; Volgenant, C.M.C.; van der Veen, M.H.; Brandt, B.W.; Krom, B.P. Candida albicans alters the bacterial microbiome of early in vitro oral biofilms. J. Oral. Microbiol. 2017, 9, 1270613. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.C.; Cheung, G.S.P.; Chang, J.W.W.; Zhang, C.; Lee, A.H.C. Enterococcus faecalis shields Porphyromonas gingivalis in dual-species biofilm in oxic condition. Microorganisms 2022, 10, 1729. [Google Scholar] [CrossRef] [PubMed]


| Species | Strain | Characteristics * | Reference |
|---|---|---|---|
| C. acnes | 2 | Moderate acne, SLST A1, Ermr | [25] |
| C. acnes | 7 | Moderate acne, SLST C1, Ermr | [25] |
| C. acnes | 14 | Mild acne, SLST D1, Ermr | [25] |
| C. acnes | HL086PA1 | Severe acne, SLST E4, Ermr | [26] |
| C. avidum | 42 | Acne patient, Ermr | [25] |
| S. aureus | JE2 | Necrotizing fasciitis, MRSA, USA300 clone, Erms | [27] |
| S. capitis | JK9 | Healthy subject, Erms | Laboratory strain |
| S. capitis | JK10 | Healthy subject, Erms | Laboratory strain |
| S. epidermidis | 5 | Implant infection, Erms | [28] |
| S. epidermidis | 1457 | Implant infection, Erms | [29] |
| S. epidermidis | 1457/pCM29-GFP | Strain 1457 expressing GFP, Erms | [30] |
| S. epidermidis | JK4 | Healthy subject, Erms | Laboratory strain |
| S. epidermidis | JK6 | Healthy subject, Erms | Laboratory strain |
| S. epidermidis | JK7 | Healthy subject, Erms | Laboratory strain |
| S. hominis | JK5 | Healthy subject, Erms | Laboratory strain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, J.B.; Assa, M.; Mruwat, N.; Sailer, M.; Regmi, S.; Kridin, K. Facultatively Anaerobic Staphylococci Enable Anaerobic Cutibacterium Species to Grow and Form Biofilms Under Aerobic Conditions. Microorganisms 2024, 12, 2601. https://doi.org/10.3390/microorganisms12122601
Kaplan JB, Assa M, Mruwat N, Sailer M, Regmi S, Kridin K. Facultatively Anaerobic Staphylococci Enable Anaerobic Cutibacterium Species to Grow and Form Biofilms Under Aerobic Conditions. Microorganisms. 2024; 12(12):2601. https://doi.org/10.3390/microorganisms12122601
Chicago/Turabian StyleKaplan, Jeffrey B., Michael Assa, Noor Mruwat, Miloslav Sailer, Suresh Regmi, and Khalaf Kridin. 2024. "Facultatively Anaerobic Staphylococci Enable Anaerobic Cutibacterium Species to Grow and Form Biofilms Under Aerobic Conditions" Microorganisms 12, no. 12: 2601. https://doi.org/10.3390/microorganisms12122601
APA StyleKaplan, J. B., Assa, M., Mruwat, N., Sailer, M., Regmi, S., & Kridin, K. (2024). Facultatively Anaerobic Staphylococci Enable Anaerobic Cutibacterium Species to Grow and Form Biofilms Under Aerobic Conditions. Microorganisms, 12(12), 2601. https://doi.org/10.3390/microorganisms12122601








