Epidemiological and Antimicrobial Resistance Trends in Bacterial Keratitis: A Hospital-Based 10-Year Study (2014–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains Source
2.2. Strain Isolation
2.3. Antibiotic Susceptibility Testing
2.4. Statistical Analysis
3. Results
3.1. Bacterial Profile Shifts Across the COVID-19 Period
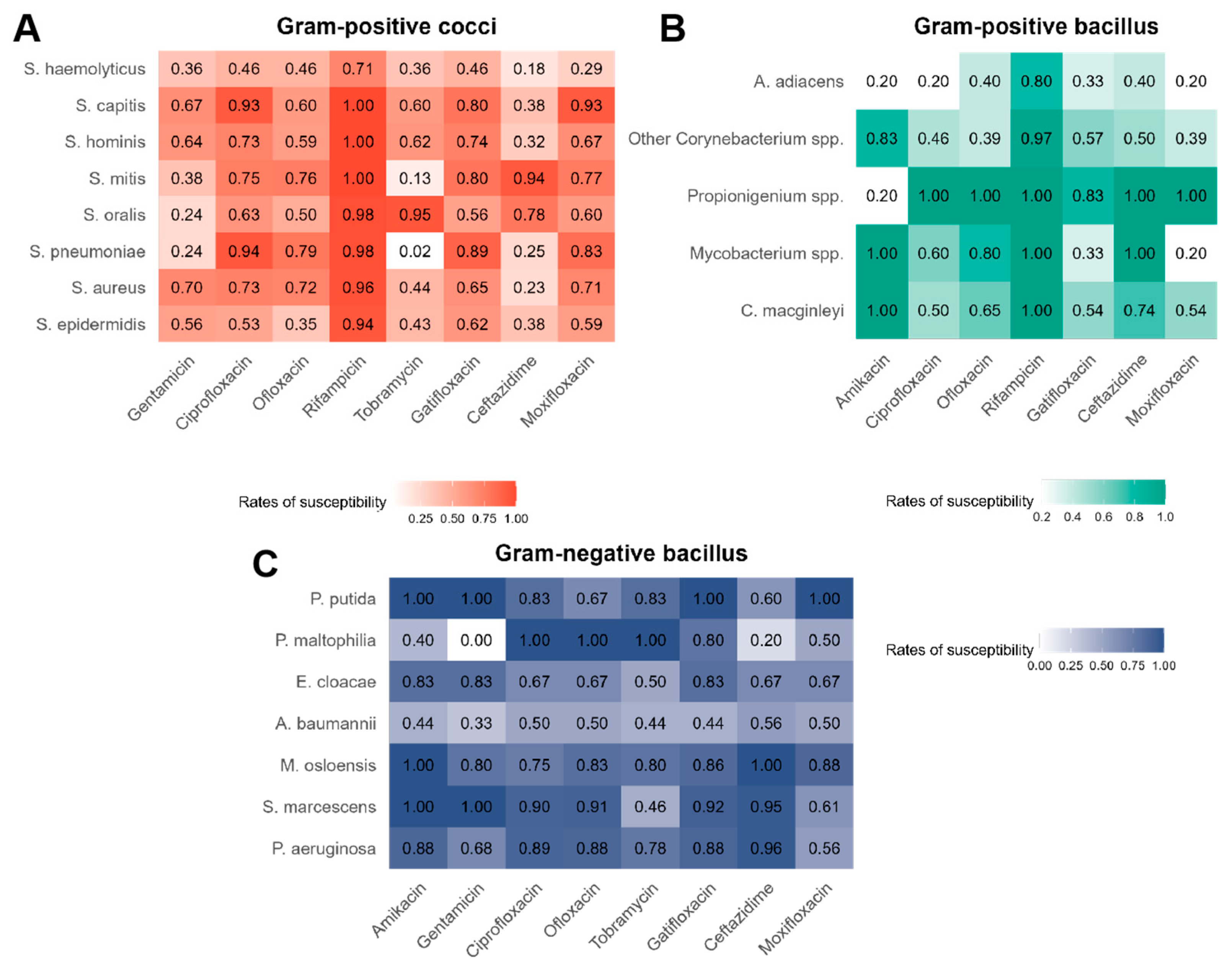
3.2. Overall Antibiotic Susceptibility of Bacteria
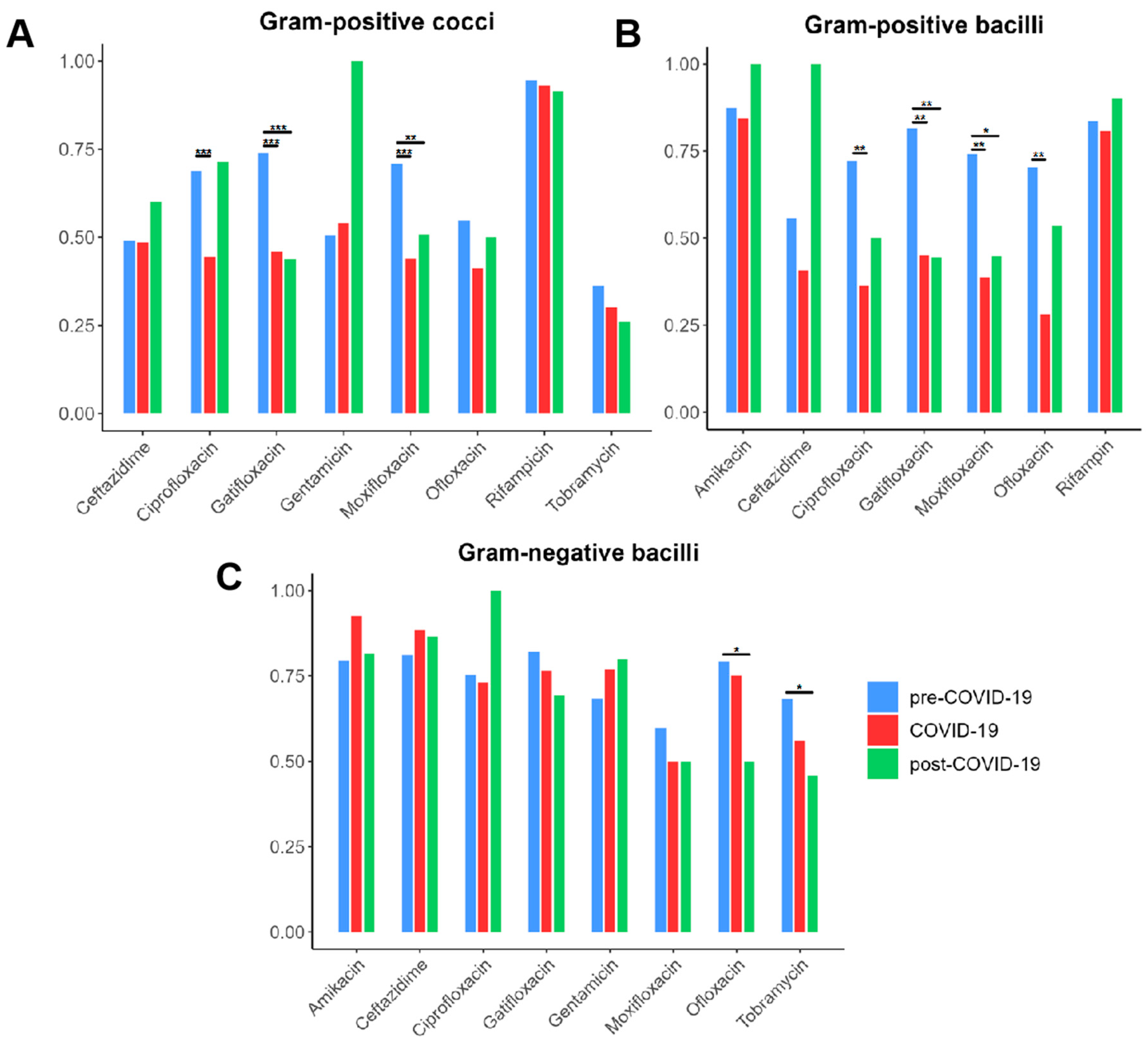
3.3. Trends in Bacterial AMR Across the COVID-19 Pandemic
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| BK | Bacterial keratitis |
| CFU | Colony-Forming Unit |
| CLSI | Clinical and Laboratory Standards Institute |
| COVID-19 | Coronavirus disease 2019 |
| MALDI-TOF-MS | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry |
References
- Cabrera-Aguas, M.; Khoo, P.; Watson, S.L. Infectious Keratitis: A Review. Clin. Experiment. Ophthalmol. 2022, 50, 543–562. [Google Scholar] [CrossRef]
- Ung, L.; Bispo, P.J.M.; Shanbhag, S.S.; Gilmore, M.S.; Chodosh, J. The Persistent Dilemma of Microbial Keratitis: Global Burden, Diagnosis, and Antimicrobial Resistance. Surv. Ophthalmol. 2019, 64, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F. The Epidemiology of Infectious Keratitis. Ocul. Surf. 2023, 28, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-Year Analysis of Incidence, Microbiological Profiles and In Vitro Antimicrobial Susceptibility of Infectious Keratitis: The Nottingham Infectious Keratitis Study. Br. J. Ophthalmol. 2021, 105, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Settle, C.; Morgan, S.J.; Baylis, O.; Ghosh, S. A 10-Year Analysis of Microbiological Profiles of Microbial Keratitis: The North East England Study. Eye 2018, 32, 1416–1417. [Google Scholar] [CrossRef]
- Tomczyk, S.; Taylor, A.; Brown, A.; De Kraker, M.E.A.; El-Saed, A.; Alshamrani, M.; Hendriksen, R.S.; Jacob, M.; Löfmark, S.; Perovic, O.; et al. Impact of the COVID-19 Pandemic on the Surveillance, Prevention and Control of Antimicrobial Resistance: A Global Survey. J. Antimicrob. Chemother. 2021, 76, 3045–3058. [Google Scholar] [CrossRef]
- Lai, C.-C.; Chen, S.-Y.; Ko, W.-C.; Hsueh, P.-R. Increased Antimicrobial Resistance during the COVID-19 Pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Bentivegna, E.; Luciani, M.; Arcari, L.; Santino, I.; Simmaco, M.; Martelletti, P. Reduction of Multidrug-Resistant (MDR) Bacterial Infections during the COVID-19 Pandemic: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 1003. [Google Scholar] [CrossRef]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.-J.; Song, W.; Kim, H.-S.; Kim, H.S.; Kim, J.-S. Impact of COVID-19 on Antimicrobial Consumption and Spread of Multidrug-Resistance in Bacterial Infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef]
- Abubakar, U.; Al-Anazi, M.; Alanazi, Z.; Rodríguez-Baño, J. Impact of COVID-19 Pandemic on Multidrug Resistant Gram Positive and Gram Negative Pathogens: A Systematic Review. J. Infect. Public Health 2023, 16, 320–331. [Google Scholar] [CrossRef]
- Lima-Fontes, M.; Martinho-Dias, D.; Leuzinger-Dias, M.; Cunha, A.M.; Cardoso, P.N.; Torrão, L.; Moreira, R.; Falcão-Reis, F.; Pinheiro-Costa, J. Microbiological Profile of Infectious Keratitis During COVID-19 Pandemic. Clin. Ophthalmol. 2023, 17, 535–543. [Google Scholar] [CrossRef]
- Butt, G.F.; Recchioni, A.; Moussa, G.; Hodson, J.; Wallace, G.R.; Murray, P.I.; Rauz, S. The Impact of the COVID-19 Pandemic on Microbial Keratitis Presentation Patterns. PLoS ONE 2021, 16, e0256240. [Google Scholar] [CrossRef] [PubMed]
- Haro-Morlett, L.; Vera-Duarte, G.R.; Oliveros-Valdes, F.; Cortes-Moreno, T.N.; Ramirez-Miranda, A.; Navas, A.; Graue-Hernandez, E.O. Effects of the COVID-19 Pandemic on Microbial Keratitis: A 5-Year Comparative Study. Cornea 2024, 1–11. [Google Scholar] [CrossRef]
- Granata, G.; Schiavone, F.; Pipitone, G.; Taglietti, F.; Petrosillo, N. Antibiotics Use in COVID-19 Patients: A Systematic Literature Review. J. Clin. Med. 2022, 11, 7207. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100-Ed33; Performance Standards for Antimicrobial Susceptibility Testing, 33rd Ed. Clinical Laboratory Standards Institute: Wayne, PA, USA, 2023.
- Roy, A.; Kanhere, M.; Rajarajan, M.; Dureja, R.; Bagga, B.; Das, S.; Sharma, S.; Mohammed, A.; Fernandes, M. Challenges in Management of Microbial Keratitis during COVID-19 Pandemic Related Lockdown: A Comparative Analysis with Pre Pandemic Data. Int. Ophthalmol. 2023, 43, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Bennett, H.G.; Hay, J.; Kirkness, C.M.; Seal, D.V.; Devonshire, P. Antimicrobial Management of Presumed Microbial Keratitis: Guidelines for Treatment of Central and Peripheral Ulcers. Br. J. Ophthalmol. 1998, 82, 137–145. [Google Scholar] [CrossRef]
- Burgos-Blasco, B.; Arriola-Villalobos, P.; Fernandez-Vigo, J.I.; Oribio-Quinto, C.; Ariño-Gutierrez, M.; Diaz-Valle, D.; Benitez-del-Castillo, J.M. Face Mask Use and Effects on the Ocular Surface Health: A Comprehensive Review. Ocul. Surf. 2023, 27, 56–66. [Google Scholar] [CrossRef]
- Lunnemar, P.; Taxbro, K.; Hammarskjöld, F. An Analysis of Central Venous Catheter-Related Bloodstream Infections in Patients Treated in a Swedish COVID-19 Intensive Care Unit. SAGE Open Med. 2024, 12, 20503121241233213. [Google Scholar] [CrossRef]
- Karmarkar, E.N.; Fitzpatrick, T.; Himmelfarb, S.T.; Chow, E.J.; Smith, H.Z.; Lan, K.F.; Matsumoto, J.; Graff, N.R.; DeBolt, C.; Truong, T.; et al. Cluster of Nontoxigenic Corynebacterium diphtheriae Infective Endocarditis and Rising Background C. Diphtheriae Cases—Seattle, Washington, 2020–2023. Clin. Infect. Dis. 2024, 78, 1214–1221. [Google Scholar] [CrossRef]
- Iqbal, N.T.; Khan, H.; Khalid, A.; Mahmood, S.F.; Nasir, N.; Khanum, I.; De Siqueira, I.; Van Voorhis, W. Chronic Inflammation in Post-Acute Sequelae of COVID-19 Modulates Gut Microbiome: A Review of Literature on COVID-19 Sequelae and Gut Dysbiosis. Mol. Med. 2025, 31, 22. [Google Scholar] [CrossRef]
- Galanis, A.; Karampitianis, S.; Vlamis, J.; Karampinas, P.; Vavourakis, M.; Vlachos, C.; Papagrigorakis, E.; Zachariou, D.; Sakellariou, E.; Varsamos, I.; et al. Corynebacterium Striatum Periprosthetic Hip Joint Infection: An Uncommon Pathogen of Concern? Healthcare 2024, 12, 273. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, X.; Lu, Y.; Yao, L.; Liu, A.; Yu, Q.; Jiang, W.; Liang, J.; Li, Y.; Zhou, S.; et al. Pathogen Spectrum and Clinical Characteristics of Lung Cancer Patients: A 10-year Retrospective Study. Int. J. Cancer 2025, 156, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, V.; Malatesta, S.; Carney, T.; Myers, B.; Parry, C.D.H.; Horsburgh, C.R.; Theron, D.; White, L.F.; Warren, R.M.; Jacobson, K.R.; et al. Understanding the Impact of Pandemics on Long-Term Medication Adherence: Directly Observed Therapy in a Tuberculosis Treatment Cohort Pre- and Post-COVID-19 Lockdowns. BMC Infect. Dis. 2024, 24, 1154. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, M.J.; Nazemi, M.; Zeighami, A.; Shahriarirad, R. Changes in Incidence and Clinical Features of Tuberculosis with Regard to the COVID-19 Outbreak in Southern Iran. BMC Infect. Dis. 2024, 24, 1043. [Google Scholar] [CrossRef] [PubMed]
- Venketaraman, V. Editorial: Non-Tuberculous Mycobacteria Infections and COVID-19. Front. Cell. Infect. Microbiol. 2025, 15, 1550277. [Google Scholar] [CrossRef]
- Bouhamdani, N.; Comeau, D.; Bourque, C.; Saulnier, N. Encephalic Nocardiosis after Mild COVID-19: A Case Report. Front. Neurol. 2023, 14, 1137024. [Google Scholar] [CrossRef]
- Stamos, D.B.; Barajas-Ochoa, A.; Raybould, J.E. Nocardia Pseudobrasiliensis Co-Infection in SARS-CoV-2 Patients. Emerg. Infect. Dis. 2023, 29, 696–700. [Google Scholar] [CrossRef]
- Sakalauskienė, G.V.; Malcienė, L.; Stankevičius, E.; Radzevičienė, A. Unseen Enemy: Mechanisms of Multidrug Antimicrobial Resistance in Gram-Negative ESKAPE Pathogens. Antibiotics 2025, 14, 63. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Liu, J.; Fu, P.; Wang, Y.-X.; Fan, P.-P.; Zhou, J.-L.; Xiang, X.-Q.; Shen, H.-L.; Liu, T.-Y.; Zhang, Y.-Y.; et al. Epidemiological Profile and Antimicrobial Resistance Trends of Staphylococcus Aureus in Chinese Pediatric Intensive Care Units from 2016 to 2022: A Multi-Center Retrospective Study. BMC Infect. Dis. 2025, 25, 298. [Google Scholar] [CrossRef]
- Cheng, Z.; Shi, Q.; Peng, B.; Zhang, Z.; Wei, Z.; Wang, Z.; Zhang, Y.; Chen, K.; Xu, X.; Lu, X.; et al. Risk Factors, Clinical Characteristics, and Antibiotic Susceptibility Patterns of Streptococcal Keratitis: An 18-Year Retrospective Study from a Tertiary Hospital in China. Antibiotics 2024, 13, 1190. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. In Virulence Mechanisms of Bacterial Pathogens, 5th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kato, N.; Haruta, M.; Arai, R.; Sato, K.; Furushima, K.; Yokomizo, K.; Okuno, M.; Yamamoto, T.; Ogura, Y.; Yoshida, S. Relationship Between Fluoroquinolone Resistance and Mutations in the Quinolone Resistance-Determining Region in Corynebacterium Macginleyi. Invest. Ophthalmol. Vis. Sci. 2024, 65, 38. [Google Scholar] [CrossRef] [PubMed]
- Sulis, G.; Daniels, B.; Kwan, A.; Gandra, S.; Daftary, A.; Das, J.; Pai, M. Antibiotic Overuse in the Primary Health Care Setting: A Secondary Data Analysis of Standardised Patient Studies from India, China and Kenya. BMJ Glob. Health 2020, 5, e003393. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of Drug Resistance: Quinolone Resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of Antibiotics and Antibiotic Resistance, and Their Impacts on Drug Development: A Narrative Review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Li, W.; Gao, M.; Yu, J. Rising Prevalence and Drug Resistance of Corynebacterium Striatum in Lower Respiratory Tract Infections. Front. Cell. Infect. Microbiol. 2025, 14, 1526312. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; Van Kleef, E.; et al. Antimicrobial Resistance and COVID-19: Intersections and Implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Levy, S. Antibiotic Resistance—the Problem Intensifies. Adv. Drug Deliv. Rev. 2005, 57, 1446–1450. [Google Scholar] [CrossRef]
- Madduri, B.; Mohan, N.; Fernandez, M.; Joseph, J. Microbial Keratitis at a Single Tertiary Eye Care in Andhra Pradesh: A 12-Year Analysis of Microbiological Profile and In Vitro Susceptibility of Bacterial Isolates. Oman J. Ophthalmol. 2024, 17, 352–356. [Google Scholar] [CrossRef]

| Bacteria | Total N (%) | Pre-COVID-19 N (%) | COVID-19 N (%) | Post-COVID-19 N (%) | p Value |
|---|---|---|---|---|---|
| Gram-positive cocci | 674 (62.9%) | 460 (69.8%) | 110 (54.7%) | 104 (49.3%) | <0.001 * |
| Staphylococcus epidermidis | 285 (26.6%) | 205 (31.1%) | 43 (21.4%) | 37 (17.5%) | <0.001 * |
| Staphylococcus aureus | 54 (5.0%) | 33 (5.0%) | 10 (5.0%) | 11 (5.2%) | 0.992 |
| Streptococci spp. | 202 (18.9%) | 133 (20.2%) | 33 (16.4%) | 36 (17.1%) | 0.371 |
| Abiotrophia spp. | 11 (1.0%) | 8 (1.2%) | 2 (1%) | 1 (0.5%) | 0.649 |
| Enterococcus | 10 (0.9%) | 2 (0.3%) | 6 (3.0%) | 2 (0.9%) | 0.003 * |
| Kocuria spp. | 7 (0.7%) | 5 (0.8%) | 0 (0%) | 2 (0.9%) | 0.424 |
| Micrococci | 4 (0.4%) | 1 (0.2%) | 0 (0%) | 3 (1.4%) | 0.020 * |
| Other Gram-positive cocci | 101 (9.4%) | 73 (11.1%) | 16 (8.0%) | 12 (5.7%) | 0.048 |
| Gram-positive bacilli | 139 (13.0%) | 57 (8.6%) | 37 (18.4%) | 45 (21.3%) | <0.001 * |
| Corynebacterium spp. | 84 (7.8%) | 28 (4.2%) | 22 (10.9%) | 34 (16.1%) | <0.001 * |
| Nocardia spp. | 12 (1.1%) | 4 (0.6%) | 4 (2.0%) | 4 (1.9%) | 0.130 |
| Mycobacterium | 9 (0.8%) | 2 (0.3%) | 6 (3.0%) | 1 (0.5%) | 0.001 * |
| Other Gram-positive bacilli | 34 (3.2%) | 23 (3.5%) | 5 (2.5%) | 6 (2.8%) | 0.742 |
| Gram-negative bacilli | 252 (23.5%) | 139 (21.1%) | 54 (26.9%) | 59 (28%) | 0.057 |
| Pseudomonas spp. | 84 (7.8%) | 48 (7.3%) | 18 (9.0%) | 18 (8.5%) | 0.681 |
| Klebsiella spp. | 21 (2%) | 14 (2.1%) | 3 (1.5%) | 4 (1.9%) | 0.850 |
| Serratia spp. | 28 (2.6%) | 14 (2.1%) | 9 (4.5%) | 5 (2.4%) | 0.182 |
| Moraxella spp. | 25 (2.3%) | 10 (1.5%) | 6 (3.0%) | 9 (4.3%) | 0.056 |
| Acinetobacter spp. | 17 (1.6%) | 12 (1.8%) | 1 (0.5%) | 4 (1.9%) | 0.389 |
| Haemophilus spp. | 8 (0.7%) | 4 (0.6%) | 1 (0.5%) | 3 (1.4%) | 0.441 |
| Enterobacter spp. | 8 (0.7%) | 5 (0.8%) | 3 (1.5%) | 0 (0%) | 0.213 |
| Other Gram-negative bacilli | 61 (5.7%) | 32 (4.9%) | 13 (6.5%) | 16 (7.6%) | 0.288 |
| Gram-negative cocci | 6 (0.6%) | 3 (0.5%) | 0 (0%) | 3 (1.4%) | 0.130 |
| Total | 1071 (100%) | 659 (100%) | 201 (100%) | 211 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Mao, D.; Zhang, Z.; Qudsi, A.I.; Wei, M.; Cheng, Z.; Zhang, Y.; Wang, Z.; Chen, K.; Xu, X.; et al. Epidemiological and Antimicrobial Resistance Trends in Bacterial Keratitis: A Hospital-Based 10-Year Study (2014–2024). Microorganisms 2025, 13, 670. https://doi.org/10.3390/microorganisms13030670
Shi Q, Mao D, Zhang Z, Qudsi AI, Wei M, Cheng Z, Zhang Y, Wang Z, Chen K, Xu X, et al. Epidemiological and Antimicrobial Resistance Trends in Bacterial Keratitis: A Hospital-Based 10-Year Study (2014–2024). Microorganisms. 2025; 13(3):670. https://doi.org/10.3390/microorganisms13030670
Chicago/Turabian StyleShi, Qingquan, Deshuo Mao, Zijun Zhang, Ahyan Ilman Qudsi, Mingda Wei, Zhen Cheng, Yang Zhang, Zhiqun Wang, Kexin Chen, Xizhan Xu, and et al. 2025. "Epidemiological and Antimicrobial Resistance Trends in Bacterial Keratitis: A Hospital-Based 10-Year Study (2014–2024)" Microorganisms 13, no. 3: 670. https://doi.org/10.3390/microorganisms13030670
APA StyleShi, Q., Mao, D., Zhang, Z., Qudsi, A. I., Wei, M., Cheng, Z., Zhang, Y., Wang, Z., Chen, K., Xu, X., Lu, X., & Liang, Q. (2025). Epidemiological and Antimicrobial Resistance Trends in Bacterial Keratitis: A Hospital-Based 10-Year Study (2014–2024). Microorganisms, 13(3), 670. https://doi.org/10.3390/microorganisms13030670







