A Better Understanding of the Clinical and Pathological Changes in Viral Retinitis: Steps to Improve Visual Outcomes
Abstract
1. Introduction
2. Overview of the Retina, Retinal Homeostasis, and Retinal Immune Response Regulation
2.1. Overview of the Retina, Retinal Barriers, and Immune Privilege
2.2. The Innate Immunity
3. Viral Retinitis and Current Animal Models
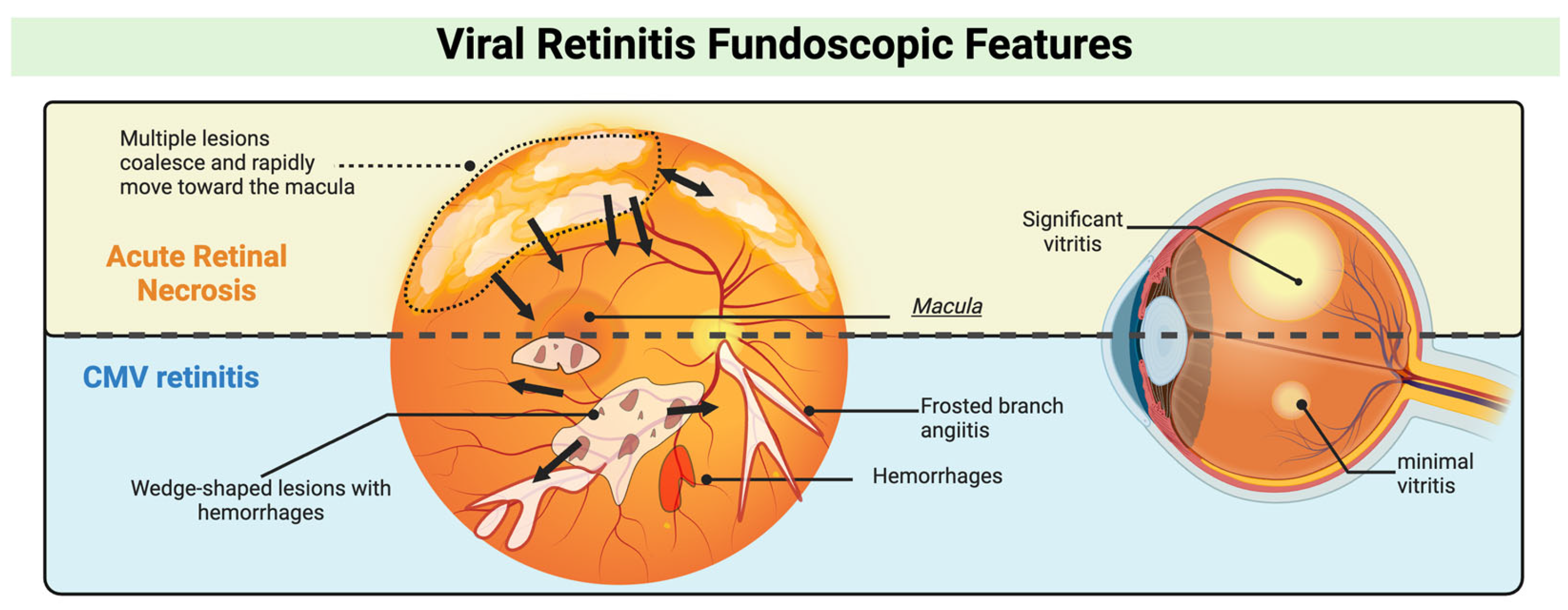
3.1. Clinical Presentations and Management of Viral Retinitis
3.2. Current In Vivo Models for Viral Retinitis
4. Herpes Viruses and Their Roles in Retinitis Progression
4.1. Herpetic Simplex Virus (HSV) and Varicella Zoster Virus (VZV)
4.2. Cytomegalovirus (CMV)
4.3. Innate Immune System Evasion Strategies Utilized by Herpesviruses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conrady, C.D.; Hanson, K.E.; Mehra, S.; Carey, A.; Larochelle, M.; Shakoor, A. The First Case of Trypanosoma cruzi–Associated Retinitis in an Immunocompromised Host Diagnosed With Pan-Organism Polymerase Chain Reaction. Clin. Infect. Dis. 2018, 67, 141–143. [Google Scholar] [CrossRef]
- Blyden, K.M.; Thomas, J.; Emami-Naeini, P.; Fashina, T.; Conrady, C.D.; Albini, T.A.; Carag, J.; Yeh, S. Emerging Infectious Diseases and the Eye: Ophthalmic Manifestations, Pathogenesis, and One Health Perspectives. Int. Ophthalmol. Clin. 2024, 64, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Isada, C.; Miller, R.; Venkat, A.; Chen, R. Viral Retinitis. In Emerging Ocular Infections; Lowder, C.Y., Shrestha, N., Venkat, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 65–77. [Google Scholar]
- Cabrera-Aguas, M.; Khoo, P.; McCluskey, P.; Watson, S.L. Viral Ocular Infections. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 219–233. [Google Scholar]
- Ganatra, J.B.; Chandler, D.; Santos, C.; Kuppermann, B.; Margolis, T.P. Viral causes of the acute retinal necrosis syndrome. Am. J. Ophthalmol. 2000, 129, 166–172. [Google Scholar] [CrossRef]
- Bryant-Hudson, K.; Conrady, C.D.; Carr, D.J. Type I interferon and lymphangiogenesis in the HSV-1 infected cornea–Are they beneficial to the host? Prog. Retin. Eye Res. 2013, 36, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, T.F.; Silvestri, G.; McDowell, C.; Foot, B.; McAvoy, C.E. Acute retinal necrosis in the United Kingdom: Results of a prospective surveillance study. Eye 2012, 26, 370–377, quiz 378. [Google Scholar] [CrossRef]
- Muthiah, M.N.; Michaelides, M.; Child, C.S.; Mitchell, S.M. Acute retinal necrosis: A national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br. J. Ophthalmol. 2007, 91, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Chaiyakunapruk, N.; Lee, S.W.H.; Kulchaitanaroaj, P.; Rayanakorn, A.; Lee, H.; Looker, K.J.; Hutubessy, R.; Gottlieb, S.L. Estimated global and regional economic burden of genital herpes simplex virus infection among 15–49 year-olds in 2016. BMC Glob. Public Health 2024, 2, 42. [Google Scholar] [CrossRef]
- Bruminhent, J.; Autto, S.; Rotjanapan, P.; Ngarmjanyaporn, P.; Bushyakanist, A.; Kirdlarp, S.; O-charoen, P.; Setthaudom, C.; Pisitkun, P. A prospective study of cytomegalovirus-specific cell-mediated immune monitoring and cytomegalovirus infection in patients with active systemic lupus erythematosus receiving immunosuppressants. Open Forum Infect. Dis. 2021, 8, ofab248. [Google Scholar] [CrossRef]
- Menke, B.; Wagner, W.; Song, H.; Thomas, W.; Castillo Almeida, N.E.; Conrady, C.; Yeh, S. Case report: CMV retinitis following local and systemic immunosuppression. Front. Ophthalmol. 2024, 4, 1354104. [Google Scholar] [CrossRef]
- Ude, I.N.; Yeh, S.; Shantha, J.G. Cytomegalovirus retinitis in the highly active anti-retroviral therapy era. Ann. Eye Sci. 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Eong, K.A.; Beatty, S.; Charles, S. Cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Postgrad. Med. J. 1999, 75, 585–590. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain—From eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Patel, B.C.; Tadi, P. Anatomy, Head and Neck: Eye Retina. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Young, R.W. The renewal of photoreceptor cell outer segments. J. Cell Biol. 1967, 33, 61–72. [Google Scholar] [CrossRef]
- Boulton, M. Ageing of the retina and retinal pigment epithelium. In Age-Related Macular Degeneration; Springer: Berlin/Heidelberg, Germany, 2013; pp. 45–63. [Google Scholar]
- Akhtar-Schäfer, I.; Wang, L.; Krohne, T.U.; Xu, H.; Langmann, T. Modulation of three key innate immune pathways for the most common retinal degenerative diseases. EMBO Mol. Med. 2018, 10, e8259. [Google Scholar] [CrossRef] [PubMed]
- Plafker, S.M.; O’Mealey, G.B.; Szweda, L.I. Chapter Four—Mechanisms for Countering Oxidative Stress and Damage in Retinal Pigment Epithelium. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 298, pp. 135–177. [Google Scholar]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Asp. Med. 2012, 33, 399–417. [Google Scholar] [CrossRef]
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed. Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef] [PubMed]
- Kaestel, C.G.; Lovato, P.; Ødum, N.; Nissen, M.H.; Röpke, C. The immune privilege of the eye: Human retinal pigment epithelial cells selectively modulate T-cell activation in vitro. Curr. Eye Res. 2005, 30, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Zamiri, P.; Masli, S.; Streilein, J.W.; Taylor, A.W. Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3912–3918. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune regulation in the aging retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Mochizuki, M.; Sugita, S.; Kamoi, K. Immunological homeostasis of the eye. Prog. Retin. Eye Res. 2013, 33, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Xu, H. Good news-bad news: The Yin and Yang of immune privilege in the eye. Front. Immunol. 2012, 3, 338. [Google Scholar] [CrossRef] [PubMed]
- Gregerson, D.S. Immune privilege in the retina. Ocul. Immunol. Inflamm. 1998, 6, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Griffith, T.S. A vision of cell death: Insights into immune privilege. Immunol. Rev. 1997, 156, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Metea, M.R.; Newman, E.A. Signalling within the neurovascular unit in the mammalian retina. Exp. Physiol. 2007, 92, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Muoio, V.; Persson, P.B.; Sendeski, M.M. The neurovascular unit—Concept review. Acta Physiol. 2014, 210, 790–798. [Google Scholar] [CrossRef]
- Murakami, Y.; Ishikawa, K.; Nakao, S.; Sonoda, K.H. Innate immune response in retinal homeostasis and inflammatory disorders. Prog. Retin. Eye Res. 2020, 74, 100778. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M.; Forrester, J.V. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009, 28, 348–368. [Google Scholar] [CrossRef] [PubMed]
- Murenu, E.; Gerhardt, M.J.; Biel, M.; Michalakis, S. More than meets the eye: The role of microglia in healthy and diseased retina. Front. Immunol. 2022, 13, 1006897. [Google Scholar] [CrossRef]
- Colonna, M. TREMs in the immune system and beyond. Nat. Rev. Immunol. 2003, 3, 445–453. [Google Scholar] [CrossRef]
- Painter, M.M.; Atagi, Y.; Liu, C.C.; Rademakers, R.; Xu, H.; Fryer, J.D.; Bu, G. TREM2 in CNS homeostasis and neurodegenerative disease. Mol. Neurodegener. 2015, 10, 43. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Raoul, W.; Keller, N.; Rodéro, M.; Behar-Cohen, F.; Sennlaub, F.; Combadière, C. Role of the chemokine receptor CX3CR1 in the mobilization of phagocytic retinal microglial cells. J. Neuroimmunol. 2008, 198, 56–61. [Google Scholar] [CrossRef]
- Paglinawan, R.; Malipiero, U.; Schlapbach, R.; Frei, K.; Reith, W.; Fontana, A. TGFbeta directs gene expression of activated microglia to an anti-inflammatory phenotype strongly focusing on chemokine genes and cell migratory genes. Glia 2003, 44, 219–231. [Google Scholar] [CrossRef]
- De Simone, R.; Ambrosini, E.; Carnevale, D.; Ajmone-Cat, M.A.; Minghetti, L. NGF promotes microglial migration through the activation of its high affinity receptor: Modulation by TGF-beta. J. Neuroimmunol. 2007, 190, 53–60. [Google Scholar] [CrossRef]
- Chen, M.; Xu, H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leucoc. Biol. 2015, 98, 713–725. [Google Scholar] [CrossRef]
- Chan, Y.-H.; Liu, Z.; Bastard, P.; Khobrekar, N.; Hutchison, K.M.; Yamazaki, Y.; Fan, Q.; Matuozzo, D.; Harschnitz, O.; Kerrouche, N.; et al. Human TMEFF1 is a restriction factor for herpes simplex virus in the brain. Nature 2024, 632, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Idorn, M.; Serrero, M.C.; Pan, X.; Thomsen, E.A.; Narita, R.; Maimaitili, M.; Qian, X.; Iversen, M.B.; Reinert, L.S.; et al. TMEFF1 is a neuron-specific restriction factor for herpes simplex virus. Nature 2024, 632, 383–389. [Google Scholar] [CrossRef]
- Serrero, M.C.; Paludan, S.R. Restriction factors regulating human herpesvirus infections. Trends Immunol. 2024, 45, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, K.; Wang, S.; Du, J. Multi-functional BST2/tetherin against HIV-1, other viruses and LINE-1. Front. Cell. Infect. Microbiol. 2022, 12, 979091. [Google Scholar] [CrossRef] [PubMed]
- Beitari, S.; Ding, S.; Pan, Q.; Finzi, A.; Liang, C. Effect of HIV-1 Env on SERINC5 antagonism. J. Virol. 2017, 91, e02214-16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dai, T.; He, X.; Zhang, Z.; Xie, F.; Wang, S.; Zhang, L.; Zhou, F. The interactions between cGAS-STING pathway and pathogens. Signal Transduct. Target. Ther. 2020, 5, 91. [Google Scholar] [CrossRef]
- Bai, L.; Xu, J.; Zeng, L.; Zhang, L.; Zhou, F. A review of HSV pathogenesis, vaccine development, and advanced applications. Molecular Biomedicine 2024, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Q.; Wang, Y.S. The role of Toll-like receptors in retinal ischemic diseases. Int. J. Ophthalmol. 2016, 9, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.V.; Nagineni, C.N.; Chin, M.S.; Hooks, J.J.; Detrick, B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004, 153, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, D.; Afshar, F.; Kalogeropoulos, C.; Vartholomatos, G.; Lotery, A.J. Diagnostic and therapeutic challenges in acute retinal necrosis; an update. Eye 2024, 38, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Kopplin, L.J.; Thomas, A.S.; Cramer, S.; Kim, Y.H.; Yeh, S.; Lauer, A.K.; Flaxel, C.J. Long-term surgical outcomes of retinal detachment associated with acute retinal necrosis. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Hedayatfar, A.; Ebrahimiadib, N.; Zarei, M.; Ashraf Khorasani, M.; Mahbod, M.; Asgari, S.; Sedaghat, A. Acute retinal necrosis: Clinical manifestation and long-term visual outcomes in a series of polymerase chain reaction–positive patients. Eur. J. Ophthalmol. 2021, 31, 1961–1969. [Google Scholar] [CrossRef]
- Kalogeropoulos, D.; Anastasopoulos, D.; Gartzonika, C.-t.; Zikou, A.K.; Kitsos, G.; Kalogeropoulos, C. Acute retinal necrosis in a patient with HSV-1 encephalitis. BAOJ Ophthalmol. 2017, 2, 9. [Google Scholar]
- De Visser, L.; de Boer, J.H.; Rijkers, G.T.; Wiertz, K.; van den Ham, H.-J.; de Boer, R.; van Loon, A.M.; Rothova, A.; de Groot-Mijnes, D. Cytokines and chemokines involved in acute retinal necrosis. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2139–2151. [Google Scholar] [CrossRef]
- Tabatabaei, S.A.; Cheraqpour, K.; Pour, E.K.; Bohrani Sefidan, B. Long-term prophylaxis in an immunocompetent patient with Cytomegalovirus retinitis: A case report and review of literature. J. Ophthalmic Inflamm. Infect. 2020, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Mishra, A.K.; Verma, A.; Garg, P.; Lnu, S. A Rare Case of Cytomegalovirus Retinitis in a Young Immunocompetent Patient. Cureus 2023, 15, e44948. [Google Scholar] [CrossRef]
- Scherger, S.J.; Molina, K.C.; Palestine, A.G.; Pecen, P.E.; Bajrovic, V. Cytomegalovirus Retinitis in the Modern Era of Solid Organ Transplantation. Transplant. Proc. 2024, 56, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Agarwal, A.; Mahendradas, P.; Lee, C.S.; Gupta, V.; Pavesio, C.E.; Agrawal, R. Viral posterior uveitis. Surv. Ophthalmol. 2017, 62, 404–445. [Google Scholar] [CrossRef] [PubMed]
- Camargo, J.F.; Komanduri, K.V. Emerging concepts in cytomegalovirus infection following hematopoietic stem cell transplantation. Hematol. /Oncol. Stem Cell Ther. 2017, 10, 233–238. [Google Scholar] [CrossRef]
- Sudharshan, S.; Kaleemunnisha, S.; Banu, A.A.; Shrikrishna, S.; George, A.E.; Babu, B.R.; Devaleenal, B.; Kumarasamy, N.; Biswas, J. Ocular lesions in 1,000 consecutive HIV-positive patients in India: A long-term study. J. Ophthalmic Inflamm. Infect. 2013, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Schoenberger, S.D.; Kim, S.J.; Thorne, J.E.; Mruthyunjaya, P.; Yeh, S.; Bakri, S.J.; Ehlers, J.P. Diagnosis and Treatment of Acute Retinal Necrosis: A Report by the American Academy of Ophthalmology. Ophthalmology 2017, 124, 382–392. [Google Scholar] [CrossRef]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives. Biochem. Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef] [PubMed]
- Erice, A. Resistance of human cytomegalovirus to antiviral drugs. Clin. Microbiol. Rev. 1999, 12, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Chou, S. Foscarnet resistance mutations mapping to atypical domains of the cytomegalovirus DNA polymerase gene. Antivir. Res. 2017, 138, 57–60. [Google Scholar] [CrossRef]
- Von Szily, A. Experimental endogenous transmission of infection from bulbus to bulbus. Klin. Monatsbl. Augenheilkd. 1924, 75, 593–602. [Google Scholar]
- Whittum, J.; McCulley, J.P.; Niederkorn, J.Y.; Streilein, J. Ocular disease induced in mice by anterior chamber inoculation of herpes simplex virus. Investig. Ophthalmol. Vis. Sci. 1984, 25, 1065–1073. [Google Scholar]
- Grajewski, R.S.; Li, J.; Wasmuth, S.; Hennig, M.; Bauer, D.; Heiligenhaus, A. Intravitreal treatment with antisense oligonucleotides targeting tumor necrosis factor-α in murine herpes simplex virus type 1 retinitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 231–238. [Google Scholar] [CrossRef]
- Zheng, M.; Fields, M.A.; Liu, Y.; Cathcart, H.; Richter, E.; Atherton, S.S. Neutrophils protect the retina of the injected eye from infection after anterior chamber inoculation of HSV-1 in BALB/c mice. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4018–4025. [Google Scholar] [CrossRef]
- Atherton, S.; Streilein, J. Two waves of virus following anterior chamber inoculation of HSV-1. Investig. Ophthalmol. Vis. Sci. 1987, 28, 571–579. [Google Scholar] [CrossRef]
- Margolis, T.; LaVail, J.; Setzer, P.; Dawson, C. Selective spread of herpes simplex virus in the central nervous system after ocular inoculation. J. Virol. 1989, 63, 4756–4761. [Google Scholar] [CrossRef] [PubMed]
- Vann, V.R.; Atherton, S.S. Neural spread of herpes simplex virus after anterior chamber inoculation. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2462–2472. [Google Scholar]
- Liu, Y.; Sakai, Y.; Minagawa, H.; Toh, Y.; Ishibashi, T.; Inomata, H.; Mori, R. Induction of bilateral retinal necrosis in mice by unilateral intracameral inoculation of a glycoprotein-C deficient clinical isolate of herpes simplex virus type 1. Arch. Virol. 1993, 129, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.W.; Gonzalez, A.; Atherton, S.S. Herpes simplex retinitis in the mouse. Clinicopathologic correlations. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1485–1494. [Google Scholar]
- Freeman, W.R.; Schneiderman, T.E.; Wiley, C.A.; Listhaus, A.D.; Svendsen, P.; Munguia, D.; Bergeron-Lynn, G. An animal model of focal, subacute, viral retinitis. Retina 1993, 13, 214–221. [Google Scholar] [CrossRef]
- Fan, S.; Yoo, J.H.; Park, G.; Yeh, S.; Conrady, C.D. Type I Interferon Signaling Is Critical During the Innate Immune Response to HSV-1 Retinal Infection. Investig. Ophthalmol. Vis. Sci. 2022, 63, 28. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, T.; Oh, J.O.; Minasi, P. In vivo reactivation of latent murine cytomegalovirus in the eye by immunosuppressive treatment. Investig. Ophthalmol. Vis. Sci. 1990, 31, 657–663. [Google Scholar]
- Bale, J.; O’Neil, M.; Lyon, B.; Perlman, S. The pathogenesis of murine cytomegalovirus ocular infection. Anterior chamber inoculation. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1575–1581. [Google Scholar]
- Holland, G.; Fang, E.; Glasgow, B.; Zaragoza, A.; Siegel, L.; Graves, M.; Saxton, E.; Foos, R. Necrotizing retinopathy after intraocular inoculation of murine cytomegalovirus in immunosuppressed adult mice. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2326–2334. [Google Scholar]
- Duan, Y.; Ji, Z.; Atherton, S.S. Dissemination and replication of MCMV after supraciliary inoculation in immunosuppressed BALB/c mice. Investig. Ophthalmol. Vis. Sci. 1994, 35, 1124–1131. [Google Scholar]
- Zhang, M.; Covar, J.; Marshall, B.; Dong, Z.; Atherton, S.S. Lack of TNF-α promotes Caspase-3–independent apoptosis during murine cytomegalovirus retinitis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, J.; Marshall, B.; Xin, H.; Atherton, S.S. Lack of iNOS facilitates MCMV spread in the retina. Investig. Ophthalmol. Vis. Sci. 2007, 48, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Atherton, S.S.; Newell, C.K.; Kanter, M.Y.; Cousins, S.W. Retinitis in euthymic mice following inoculation of murine cytomegalovirus (MCMV) via the supraciliary route. Curr. Eye Res. 1991, 10, 667–677. [Google Scholar] [CrossRef]
- Heinemann, M.-H. Characteristics of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome. Am. J. Med. 1992, 92, S12–S16. [Google Scholar] [CrossRef]
- Romagnani, S.; Del Prete, G.; Manetti, R.; Ravina, A.; Annunziato, F.; De Carli, M.; Mazzetti, M.; Piccinni, M.-P.; D’Elios, M.M.; Parronchi, P. Role of TH1/TH2 cytokines in HIV infection. Immunol. Rev. 1994, 140, 73–92. [Google Scholar] [CrossRef]
- Braun, C.M.; Huang, S.-K.; Bashian, G.G.; Kagey-Sobotka, A.; Lichtenstein, L.M.; Essayan, D.M. Corticosteroid modulation of human, antigen-specific Th1 and Th2 responses. J. Allergy Clin. Immunol. 1997, 100, 400–407. [Google Scholar] [CrossRef]
- Dix, R.; Cousins, S. Susceptibility to murine cytomegalovirus retinitis during progression of MAIDS: Correlation with intraocular levels of tumor necrosis factor-α and interferon-γ. Curr. Eye Res. 2004, 29, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Dix, R.D.; Cray, C.; Cousins, S.W. Mice immunosuppressed by murine retrovirus infection (MAIDS) are susceptible to cytomegalovirus retinitis. Curr. Eye Res. 1994, 13, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Roizman, B. Herpesviridae: A brief introduction. Fields Virol. 1990. [Google Scholar]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Speck-Planche, A.; Cordeiro, N.D. Application of Bioinformatics for the search of novel anti-viral therapies: Rational design of anti-herpes agents. Curr. Bioinform. 2011, 6, 81–93. [Google Scholar] [CrossRef]
- Fatahzadeh, M.; Schwartz, R.A. Human herpes simplex virus infections: Epidemiology, pathogenesis, symptomatology, diagnosis, and management. J. Am. Acad. Dermatol. 2007, 57, 737–763. [Google Scholar] [CrossRef]
- Dempsey, M.P.; Conrady, C.D. The Host–Pathogen Interplay: A Tale of Two Stories within the Cornea and Posterior Segment. Microorganisms 2023, 11, 2074. [Google Scholar] [CrossRef]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef]
- Verzosa, A.L.; McGeever, L.A.; Bhark, S.J.; Delgado, T.; Salazar, N.; Sanchez, E.L. Herpes Simplex Virus 1 Infection of Neuronal and Non-Neuronal Cells Elicits Specific Innate Immune Responses and Immune Evasion Mechanisms. Front. Immunol. 2021, 12, 644664. [Google Scholar] [CrossRef]
- Madavaraju, K.; Koganti, R.; Volety, I.; Yadavalli, T.; Shukla, D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2020, 10, 617578. [Google Scholar] [CrossRef]
- Conrady, C.D.; Drevets, D.A.; Carr, D.J. Herpes simplex type I (HSV-1) infection of the nervous system: Is an immune response a good thing? J. Neuroimmunol. 2010, 220, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Spear, P.G. Infections with herpes simplex viruses. N. Engl. J. Med. 1986, 314, 686–691. [Google Scholar] [CrossRef]
- CDC. Clinical Overview of Chickenpox (Varicella). Available online: https://www.cdc.gov/chickenpox/hcp/clinical-overview/index.html (accessed on 1 November 2024).
- Papaloukas, O.; Giannouli, G.; Papaevangelou, V. Successes and challenges in varicella vaccine. Ther. Adv. Vaccines 2014, 2, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.H.; Gilden, D.H.; Cohrs, R.J.; Mahalingam, R.; Nagel, M.A. Varicella zoster virus infection: Clinical features, molecular pathogenesis of disease, and latency. Neurol. Clin. 2008, 26, 675–697. [Google Scholar] [CrossRef]
- Schmid, D.S.; Jumaan, A.O. Impact of varicella vaccine on varicella-zoster virus dynamics. Clin. Microbiol. Rev. 2010, 23, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Solomon, D.; Nussenblatt, R.B.; Palestine, A.G.; Chan, C.-C. Immunocytochemical Staining of Vitreous Cells: Indications, Techniques, and Results. Ophthalmology 1992, 99, 250–256. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Li Puma, D.D.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [Google Scholar] [CrossRef]
- Haynes, R.E.; Azimi, P.H.; Cramblett, H.G. Fatal Herpesvirus hominis (herpes simplex virus) infections in children: Clinical, pathologic, and virologic characteristics. JAMA 1968, 206, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.J.; Soong, S.-J.; Linneman, C.; Liu, C.; Pazin, G.; Alford, C.A. Herpes simplex encephalitis: Clinical assessment. JAMA 1982, 247, 317–320. [Google Scholar] [CrossRef]
- Conrady, C.D.; Zheng, M.; van Rooijen, N.; Drevets, D.A.; Royer, D.; Alleman, A.; Carr, D.J. Microglia and a functional type I IFN pathway are required to counter HSV-1–driven brain lateral ventricle enlargement and encephalitis. J. Immunol. 2013, 190, 2807–2817. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Jouanguy, E.; Ugolini, S.; Smahi, A.; Elain, G.l.; Romero, P.; Segal, D.; Sancho-Shimizu, V.; Lorenzo, L.; Puel, A. TLR3 deficiency in patients with herpes simplex encephalitis. Science 2007, 317, 1522–1527. [Google Scholar] [CrossRef]
- Jambunathan, N.; Clark, C.M.; Musarrat, F.; Chouljenko, V.N.; Rudd, J.; Kousoulas, K.G. Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins gB, gD, gH/gL, gK, and Cellular Receptors Function as Key Players in Membrane Fusion. Viruses 2021, 13, 1849. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.G.; Eisenberg, R.J.; Cohen, G.H. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 2000, 275, 1–8. [Google Scholar] [CrossRef]
- Azeem, A.; Baartman, B.; Conrady, C.D.; Meier, J.L.; El-Herte, R. Herpes simplex virus dissemination with necrotizing hepatitis following Descemet membrane endothelial keratoplasty. BMC Infect. Dis. 2023, 23, 465. [Google Scholar] [CrossRef]
- Farooq, A.V.; Valyi-Nagy, T.; Shukla, D. Mediators and mechanisms of herpes simplex virus entry into ocular cells. Curr. Eye Res. 2010, 35, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Oh, M.J.; Kovacs, M.; Shukla, S.Y.; Valyi-Nagy, T.; Shukla, D. Role for nectin-1 in herpes simplex virus 1 entry and spread in human retinal pigment epithelial cells. FEBS J. 2008, 275, 5272–5285. [Google Scholar] [CrossRef] [PubMed]
- Laing, K.J.; Ouwendijk, W.J.D.; Koelle, D.M.; Verjans, G. Immunobiology of Varicella-Zoster Virus Infection. J. Infect. Dis. 2018, 218, S68–S74. [Google Scholar] [CrossRef] [PubMed]
- Croen, K.D.; Ostrove, J.M.; Dragovic, L.J.; Smialek, J.E.; Straus, S.E. Latent herpes simplex virus in human trigeminal ganglia. N. Engl. J. Med. 1987, 317, 1427–1432. [Google Scholar] [CrossRef]
- Hafezi, W.; Lorentzen, E.U.; Eing, B.R.; Müller, M.; King, N.J.; Klupp, B.; Mettenleiter, T.C.; Kühn, J.E. Entry of herpes simplex virus type 1 (HSV-1) into the distal axons of trigeminal neurons favors the onset of nonproductive, silent infection. PLoS Pathog. 2012, 8, e1002679. [Google Scholar] [CrossRef]
- Aggarwal, A.; Miranda-Saksena, M.; Boadle, R.A.; Kelly, B.J.; Diefenbach, R.J.; Alam, W.; Cunningham, A.L. Ultrastructural visualization of individual tegument protein dissociation during entry of herpes simplex virus 1 into human and rat dorsal root ganglion neurons. J. Virol. 2012, 86, 6123–6137. [Google Scholar] [CrossRef] [PubMed]
- Luxton, G.W.; Haverlock, S.; Coller, K.E.; Antinone, S.E.; Pincetic, A.; Smith, G.A. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 5832–5837. [Google Scholar] [CrossRef] [PubMed]
- Pikkel, Y.Y.; Pikkel, J. Acute Retinal Necrosis in Childhood. Case Rep. Ophthalmol. 2014, 5, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Silva, B.A.; Perova, Z.; Marrone, L.; Masferrer, M.E.; Zhan, Y.; Kaplan, A.; Greetham, L.; Verrechia, V.; Halman, A.; et al. Prefrontal cortical control of a brainstem social behavior circuit. Nat. Neurosci. 2017, 20, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A.; Zhang, J.; Ishimoto, S. Role of retinal vascular endothelial cells in development of CMV retinitis. Trans. Am. Ophthalmol. Soc. 1998, 96, 111–126. [Google Scholar]
- Pereira, L.; Maidji, E.; Tugizov, S.; Jones, T. Deletion mutants in human cytomegalovirus glycoprotein US9 are impaired in cell-cell transmission and in altering tight junctions of polarized human retinal pigment epithelial cells. Scand. J. Infect. Diseases. Suppl. 1995, 99, 82–87. [Google Scholar]
- Scholz, M.; Doerr, H.W.; Cinatl, J. Human cytomegalovirus retinitis: Pathogenicity, immune evasion and persistence. Trends Microbiol. 2003, 11, 171–178. [Google Scholar] [CrossRef]
- Melchjorsen, J.; Matikainen, S.; Paludan, S.R. Activation and evasion of innate antiviral immunity by herpes simplex virus. Viruses 2009, 1, 737–759. [Google Scholar] [CrossRef]
- Griffin, B.D.; Verweij, M.C.; Wiertz, E.J. Herpesviruses and immunity: The art of evasion. Vet. Microbiol. 2010, 143, 89–100. [Google Scholar] [CrossRef]
- Abendroth, A.; Kinchington, P.R.; Slobedman, B. Varicella zoster virus immune evasion strategies. Curr. Top. Microbiol. Immunol. 2010, 342, 155–171. [Google Scholar] [CrossRef]
- Powers, C.; DeFilippis, V.; Malouli, D.; Früh, K. Cytomegalovirus immune evasion. Curr. Top. Microbiol. Immunol. 2008, 325, 333–359. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. TLR signaling. Semin. Immunol. 2007, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Jones, E.A.; Chan, M.; Zhou, S.; Wang, J.; Reed, G.; Bronson, R.; Arnold, M.M.; Knipe, D.M.; Finberg, R.W. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 2004, 101, 1315–1320. [Google Scholar] [CrossRef]
- Wang, J.P.; Bowen, G.N.; Zhou, S.; Cerny, A.; Zacharia, A.; Knipe, D.M.; Finberg, R.W.; Kurt-Jones, E.A. Role of specific innate immune responses in herpes simplex virus infection of the central nervous system. J. Virol. 2012, 86, 2273–2281. [Google Scholar] [CrossRef]
- Lu, X.; Huang, C.; Zhang, Y.; Lin, Y.; Wang, X.; Li, Q.; Liu, S.; Tang, J.; Zhou, L. The Us2 gene product of herpes simplex virus 2 modulates NF-κB activation by targeting TAK1. Sci. Rep. 2017, 7, 8396. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Luo, T.; Wu, F.; Mei, Y.-W.; Peng, J.; Liu, H.; Li, H.-R.; Zhang, S.-L.; Dong, J.-H.; Fang, Y. Involvement of TLR2 and TLR9 in the anti-inflammatory effects of chlorogenic acid in HSV-1-infected microglia. Life Sci. 2015, 127, 12–18. [Google Scholar] [CrossRef]
- Conrady, C.D.; Zheng, M.; Mandal, N.A.; van Rooijen, N.; Carr, D.J. IFN-α-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013, 6, 45–55. [Google Scholar] [CrossRef]
- Piroozmand, A.; Koyama, A.H.; Shimada, Y.; Fujita, M.; Arakawa, T.; Adachi, A. Role of Us3 gene of herpes simplex virus type 1 for resistance to interferon. Int. J. Mol. Med. 2004, 14, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Peri, P.; Mattila, R.K.; Kantola, H.; Broberg, E.; Karttunen, H.S.; Waris, M.; Vuorinen, T.; Hukkanen, V. Herpes simplex virus type 1 Us3 gene deletion influences toll-like receptor responses in cultured monocytic cells. Virol. J. 2008, 5, 140. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Lin, R.; Zheng, C. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J. Virol. 2013, 87, 12814–12827. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Roizman, B. Expression of gamma interferon-dependent genes is blocked independently by virion host shutoff RNase and by US3 protein kinase. J. Virol. 2008, 82, 4688–4696. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ni, L.; Wang, S.; Zheng, C. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-κB activation. J. Virol. 2014, 88, 7941–7951. [Google Scholar] [CrossRef]
- van Lint, A.L.; Murawski, M.R.; Goodbody, R.E.; Severa, M.; Fitzgerald, K.A.; Finberg, R.W.; Knipe, D.M.; Kurt-Jones, E.A. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J. Virol. 2010, 84, 10802–10811. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Ra, E.A.; Lee, T.A.; Choi, H.j.; Lee, E.; Kang, S.; Seo, J.-Y.; Lee, S.; Park, B. HCMV-encoded US7 and US8 act as antagonists of innate immunity by distinctively targeting TLR-signaling pathways. Nat. Commun. 2019, 10, 4670. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, S.; Wang, J. MicroRNA-373 facilitates HSV-1 replication through suppression of type I IFN response by targeting IRF1. Biomed. Pharmacother. 2018, 97, 1409–1416. [Google Scholar] [CrossRef] [PubMed]
- Landais, I.; Pelton, C.; Streblow, D.; DeFilippis, V.; McWeeney, S.; Nelson, J.A. Human Cytomegalovirus miR-UL112-3p Targets TLR2 and Modulates the TLR2/IRAK1/NFκB Signaling Pathway. PLoS Pathog. 2015, 11, e1004881. [Google Scholar] [CrossRef]
- Shirahama, S.; Onoguchi-Mizutani, R.; Kawata, K.; Taniue, K.; Miki, A.; Kato, A.; Kawaguchi, Y.; Tanaka, R.; Kaburaki, T.; Kawashima, H.; et al. Long noncoding RNA U90926 is crucial for herpes simplex virus type 1 proliferation in murine retinal photoreceptor cells. Sci. Rep. 2020, 10, 19406. [Google Scholar] [CrossRef]
- Shirahama, S.; Taniue, K.; Mitsutomi, S.; Tanaka, R.; Kaburaki, T.; Sato, T.; Takeuchi, M.; Kawashima, H.; Urade, Y.; Aihara, M.; et al. Human U90926 orthologous long non-coding RNA as a novel biomarker for visual prognosis in herpes simplex virus type-1 induced acute retinal necrosis. Sci. Rep. 2021, 11, 12164. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef]
- Yamashiro, L.H.; Wilson, S.C.; Morrison, H.M.; Karalis, V.; Chung, J.J.; Chen, K.J.; Bateup, H.S.; Szpara, M.L.; Lee, A.Y.; Cox, J.S.; et al. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat. Commun. 2020, 11, 3382. [Google Scholar] [CrossRef] [PubMed]
- Lio, C.W.; McDonald, B.; Takahashi, M.; Dhanwani, R.; Sharma, N.; Huang, J.; Pham, E.; Benedict, C.A.; Sharma, S. cGAS-STING Signaling Regulates Initial Innate Control of Cytomegalovirus Infection. J. Virol. 2016, 90, 7789–7797. [Google Scholar] [CrossRef] [PubMed]
- Albright, E.R.; Mickelson, C.K.; Kalejta, R.F. Human Cytomegalovirus UL138 Protein Inhibits the STING Pathway and Reduces Interferon Beta mRNA Accumulation during Lytic and Latent Infections. mBio 2021, 12, e02267-21. [Google Scholar] [CrossRef] [PubMed]
- Loiacono, C.M.; Myers, R.; Mitchell, W.J. Neurons differentially activate the herpes simplex virus type 1 immediate-early gene ICP0 and ICP27 promoters in transgenic mice. J. Virol. 2002, 76, 2449–2459. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.H.; Jensen, S.B.; Miettinen, J.J.; Luecke, S.; Prabakaran, T.; Reinert, L.S.; Mettenleiter, T.; Chen, Z.J.; Knipe, D.M.; Sandri-Goldin, R.M.; et al. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. EMBO J. 2016, 35, 1385–1399. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-F.; Zou, H.-M.; Liao, B.-W.; Zhang, H.-Y.; Yang, Y.; Fu, Y.-Z.; Wang, S.-Y.; Luo, M.-H.; Wang, Y.-Y. Human Cytomegalovirus Protein UL31 Inhibits DNA Sensing of cGAS to Mediate Immune Evasion. Cell Host Microbe 2018, 24, 69–80.e64. [Google Scholar] [CrossRef]
- Fabits, M.; Gonçalves Magalhães, V.; Chan, B.; Girault, V.; Elbasani, E.; Rossetti, E.; Saeland, E.; Messerle, M.; Pichlmair, A.; Lisnić, V.J. The cytomegalovirus tegument protein UL35 antagonizes pattern recognition receptor-mediated type I IFN transcription. Microorganisms 2020, 8, 790. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kim, Y.-E.; Stinski, M.F.; Ahn, J.-H.; Song, Y.-J. Human cytomegalovirus IE2 86 kDa protein induces STING degradation and inhibits cGAMP-mediated IFN-β induction. Front. Microbiol. 2017, 8, 1854. [Google Scholar] [CrossRef]
- Choi, H.J.; Park, A.; Kang, S.; Lee, E.; Lee, T.A.; Ra, E.A.; Lee, J.; Lee, S.; Park, B. Human cytomegalovirus-encoded US9 targets MAVS and STING signaling to evade type I interferon immune responses. Nat. Commun. 2018, 9, 125. [Google Scholar] [CrossRef]
- Barzilai, A.; Zivony-Elbom, I.; Sarid, R.; Noah, E.; Frenkel, N. The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery. J. Virol. 2006, 80, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Koehler, H.S.; Mocarski, E.S.; Dix, R.D. RIPK3 and caspase 8 collaborate to limit herpes simplex encephalitis. PLoS Pathog. 2022, 18, e1010857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Garcia Reino, E.J.; Harschnitz, O.; Guo, H.; Chan, Y.-H.; Khobrekar, N.V.; Hasek, M.L.; Dobbs, K.; Rinchai, D.; Materna, M. Encephalitis and poor neuronal death–mediated control of herpes simplex virus in human inherited RIPK3 deficiency. Sci. Immunol. 2023, 8, eade2860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Zhu, D.; Chen, S.; Liu, M.; Zhao, X.; Jia, R.; et al. Programmed cell death: The battlefield between the host and alpha-herpesviruses and a potential avenue for cancer treatment. Oncotarget 2018, 9, 30704–30719. [Google Scholar] [CrossRef]
- He, S.; Han, J. Manipulation of Host Cell Death Pathways by Herpes Simplex Virus. Curr. Top. Microbiol. Immunol. 2023, 442, 85–103. [Google Scholar] [CrossRef]
- James, S.F.; Mahalingam, R.; Gilden, D. Does apoptosis play a role in varicella zoster virus latency and reactivation? Viruses 2012, 4, 1509–1514. [Google Scholar] [CrossRef]
- Chaudhry, M.Z.; Casalegno-Garduno, R.; Sitnik, K.M.; Kasmapour, B.; Pulm, A.-K.; Brizic, I.; Eiz-Vesper, B.; Moosmann, A.; Jonjic, S.; Mocarski, E.S. Cytomegalovirus inhibition of extrinsic apoptosis determines fitness and resistance to cytotoxic CD8 T cells. Proc. Natl. Acad. Sci. USA 2020, 117, 12961–12968. [Google Scholar] [CrossRef]
- Yu, X.; He, S. The interplay between human herpes simplex virus infection and the apoptosis and necroptosis cell death pathways. Virol. J. 2016, 13, 77. [Google Scholar] [CrossRef]
- Hood, C.; Cunningham, A.L.; Slobedman, B.; Arvin, A.M.; Sommer, M.H.; Kinchington, P.R.; Abendroth, A. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J. Virol. 2006, 80, 1025–1031. [Google Scholar] [CrossRef]
- Guo, H.; Omoto, S.; Harris, P.A.; Finger, J.N.; Bertin, J.; Gough, P.J.; Kaiser, W.J.; Mocarski, E.S. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe 2015, 17, 243–251. [Google Scholar] [CrossRef]
- Deng, Y.; Águeda-Pinto, A.; Brune, W. No Time to Die: How Cytomegaloviruses Suppress Apoptosis, Necroptosis, and Pyroptosis. Viruses 2024, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
- Goldmacher, V.S. vMIA, a viral inhibitor of apoptosis targeting mitochondria. Biochimie 2002, 84, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, A.; Wu, D.; Wang, C.; Huang, M.; Xiong, X.; Jin, L.; Zhou, W.; Qiu, Y.; Zhou, X. Dual inhibition of innate immunity and apoptosis by human cytomegalovirus protein UL37x1 enables efficient virus replication. Nat. Microbiol. 2022, 7, 1041–1053. [Google Scholar] [CrossRef]
- Fletcher-Etherington, A.; Nobre, L.; Nightingale, K.; Antrobus, R.; Nichols, J.; Davison, A.J.; Stanton, R.J.; Weekes, M.P. Human cytomegalovirus protein pUL36: A dual cell death pathway inhibitor. Proc. Natl. Acad. Sci. USA 2020, 117, 18771–18779. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ostermann, E.; Brune, W. A cytomegalovirus inflammasome inhibitor reduces proinflammatory cytokine release and pyroptosis. Nat. Commun. 2024, 15, 786. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, N.M.; Conrady, C.D. A Better Understanding of the Clinical and Pathological Changes in Viral Retinitis: Steps to Improve Visual Outcomes. Microorganisms 2024, 12, 2513. https://doi.org/10.3390/microorganisms12122513
Nguyen NM, Conrady CD. A Better Understanding of the Clinical and Pathological Changes in Viral Retinitis: Steps to Improve Visual Outcomes. Microorganisms. 2024; 12(12):2513. https://doi.org/10.3390/microorganisms12122513
Chicago/Turabian StyleNguyen, Nghi M., and Christopher D. Conrady. 2024. "A Better Understanding of the Clinical and Pathological Changes in Viral Retinitis: Steps to Improve Visual Outcomes" Microorganisms 12, no. 12: 2513. https://doi.org/10.3390/microorganisms12122513
APA StyleNguyen, N. M., & Conrady, C. D. (2024). A Better Understanding of the Clinical and Pathological Changes in Viral Retinitis: Steps to Improve Visual Outcomes. Microorganisms, 12(12), 2513. https://doi.org/10.3390/microorganisms12122513







