A Bioluminescence-Based Serum Bactericidal Assay to Detect Bactericidal Antibodies Against Neisseria meningitidis in Human Sera
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria
2.2. Serum Samples
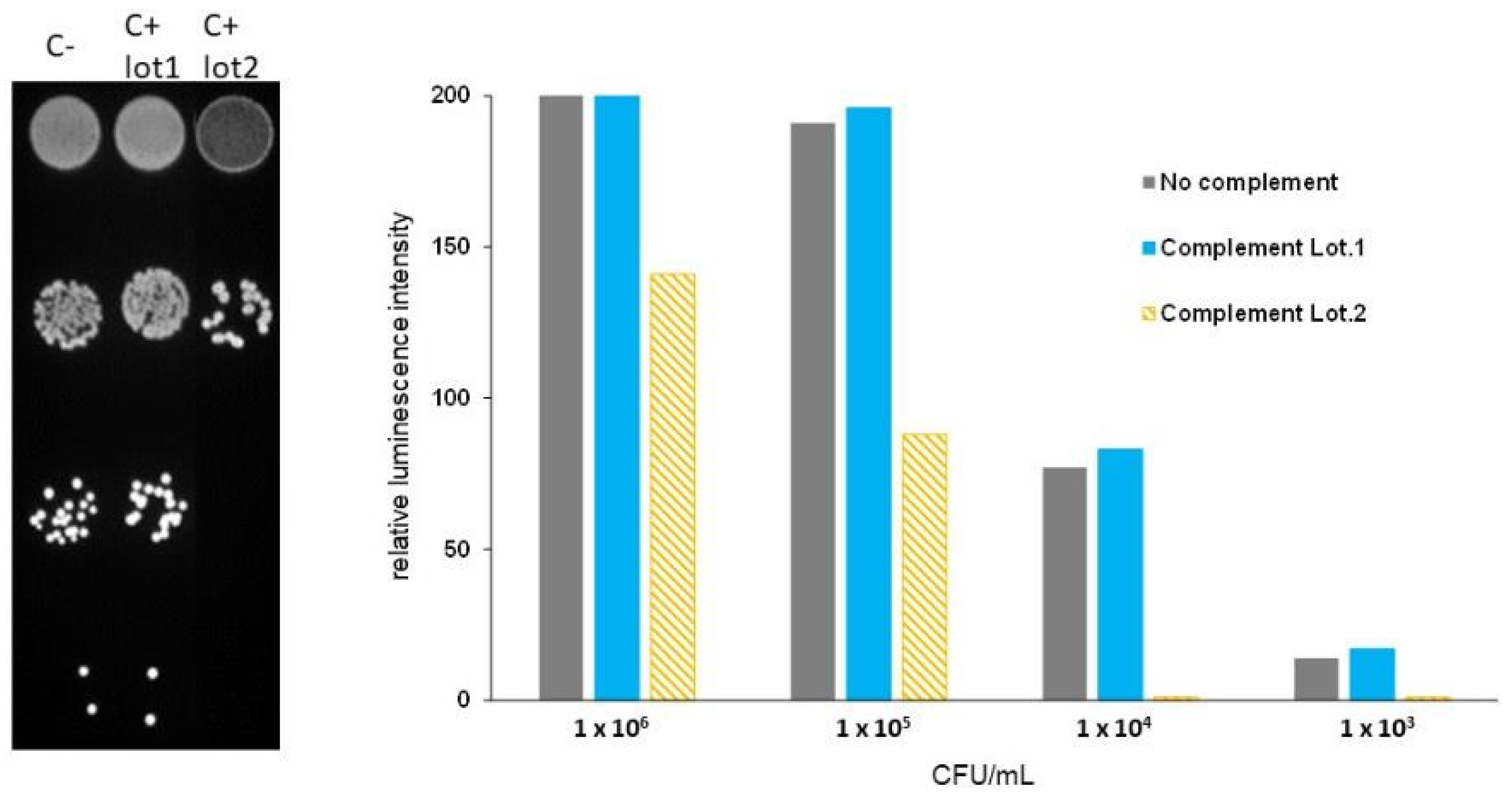
2.3. Complement Source Validation Test
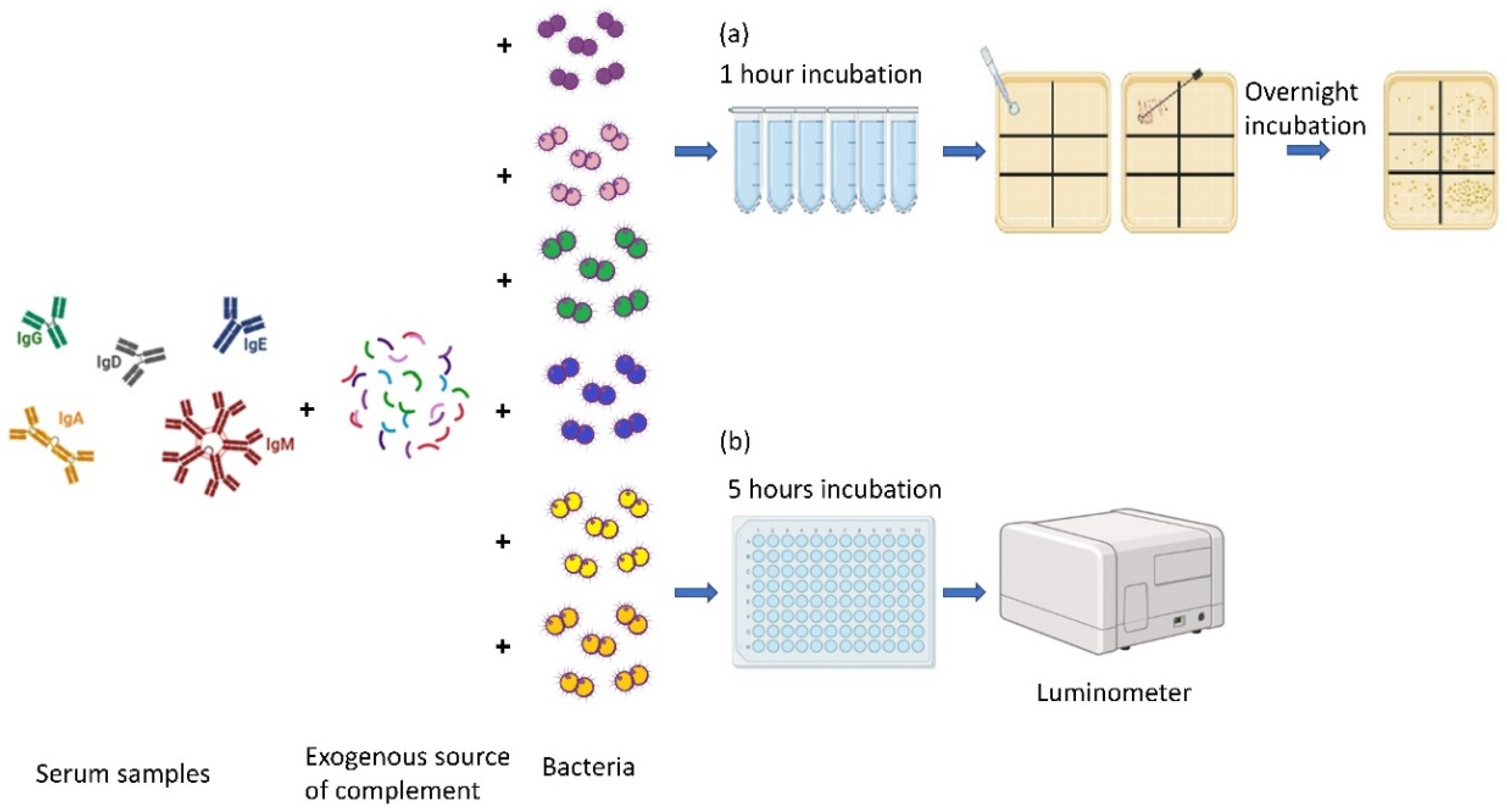
2.4. Conventional SBA
2.5. Bioluminescence SBA (BioLux-SBA)
2.6. Terminal Complement Complex Deposition Assay
2.7. Calculations
3. Results
3.1. Complement Source Validation Assay
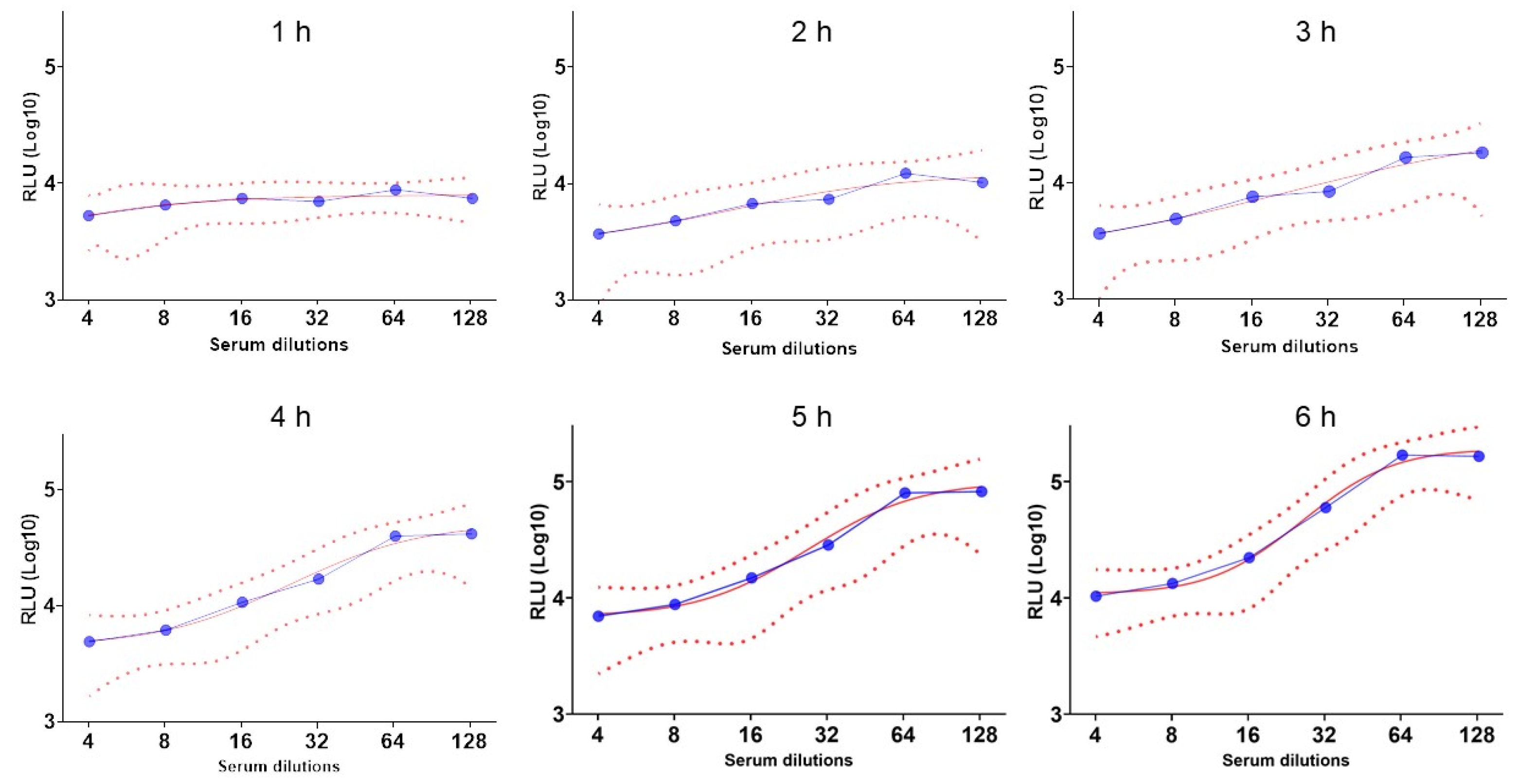
3.2. Set Up the Incubation Time During the BioLux-SBA
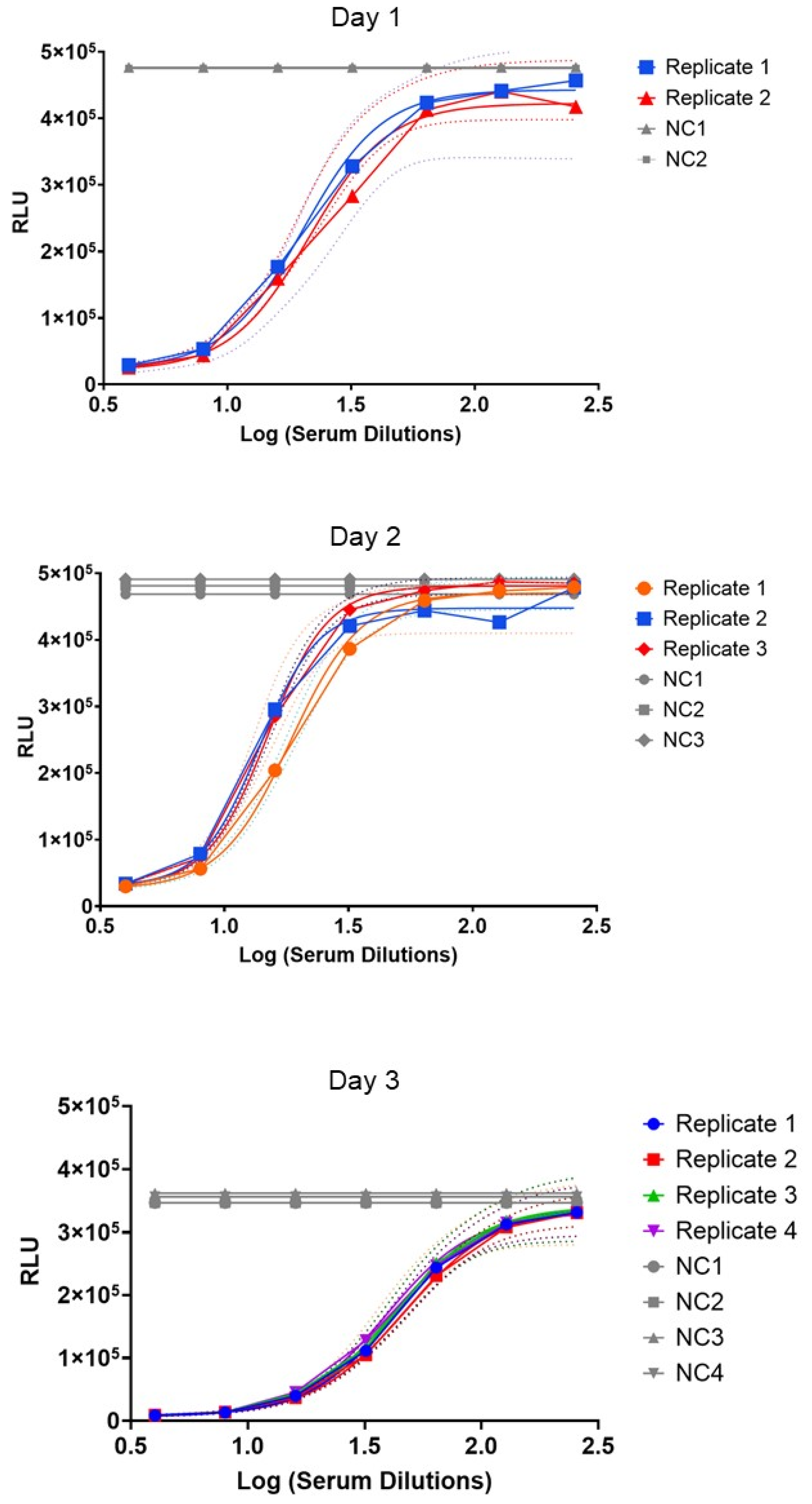
3.3. Repeatability and Intermediate Precision of the BioLux-SBA
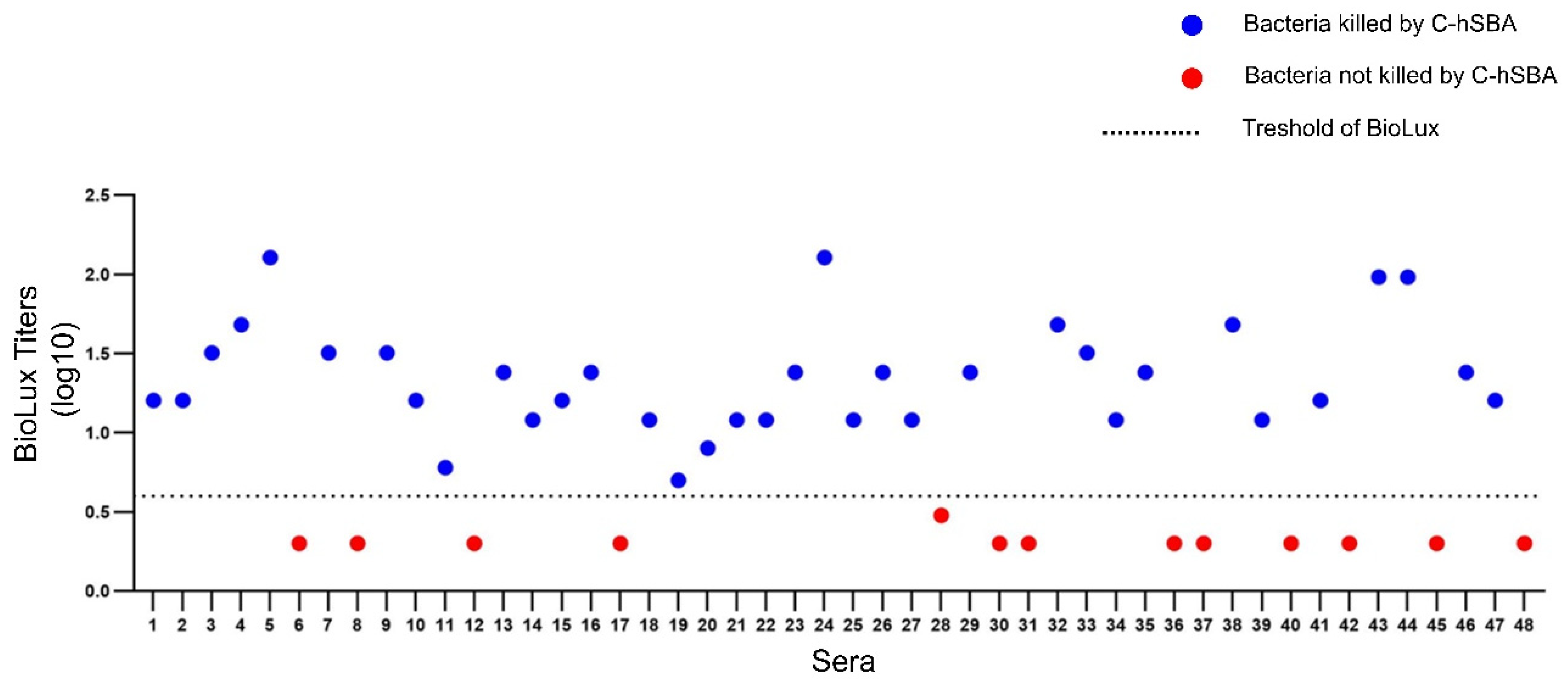
3.4. Correlation Between C-hSBA and BioLux-hSBA
3.5. Defining the Threshold of BioLux-hSBA
3.6. Terminal Complement Complex Deposition Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hollingshead, S.; Tang, C.M. An Overview of Neisseria meningitidis. In Neisseria meningitidis; Methods in Molecular Biology; Humana: New York, NY, USA, 2019; Volume 1969, pp. 1–16. [Google Scholar] [CrossRef]
- Borrow, R.; Carlone, G.M.; Rosenstein, N.; Blake, M.; Feavers, I.; Martin, D.; Zollinger, W.; Robbins, J.; Aaberge, I.; Granoff, D.M.; et al. Neisseria meningitidis group B correlates of protection and assay standardization—International meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine 2006, 24, 5093–5107. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, S.N.; Campbell, H.; Andrews, N.; Parikh, S.R.; White, J.; Edelstein, M.; Clark, S.A.; Lucidarme, J.; Borrow, R.; Ramsay, M.E. First real world evidence of meningococcal group B vaccine, 4CMenB, protection against meningococcal group W disease; prospective enhanced national surveillance, England. Clin. Infect. Dis. 2021, 73, e1661–e1668. [Google Scholar] [CrossRef] [PubMed]
- Heist, G.D.; Solis-Cohen, S.; Solis-Cohen, M. A study of the virulence of meningococci for man and of human susceptibility to meningococcic infection. J. Immunol. 1922, 7, 1–33. [Google Scholar] [CrossRef]
- Goldschneider, I.; Gotschlich, E.C.; Artenstein, M.S. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 1969, 129, 1307–1326. [Google Scholar] [CrossRef]
- Findlow, J.; Lucidarme, J.; Taha, M.K.; Burman, C.; Balmer, P. Correlates of protection for meningococcal surface protein vaccines: Lessons from the past. Expert Rev. Vaccines 2021, 21, 739–751. [Google Scholar] [CrossRef]
- Findlow, J.; Balmer, P.; Borrow, R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum. Vaccines Immunother. 2019, 15, 2491–2500. [Google Scholar] [CrossRef]
- Rambach, G.; Wurzner, R.; Speth, C. Complement: An efficient sword of innate immunity. Contrib. Microbiol. 2008, 15, 78–100. [Google Scholar]
- Heesterbeek, D.A.C.; Angelier, M.L.; Harrison, R.A.; Rooijakkers, S.H.M. Complement and Bacterial Infections: From Molecular Mechanisms to Therapeutic Applications. J. Innate Immun. 2018, 10, 455–464. [Google Scholar] [CrossRef]
- Rosain, J.; Hong, E.; Fieschi, C.; Martins, P.V.; El Sissy, C.; Deghmane, A.E.; Ouachee, M.; Thomas, C.; Launay, D.; de Pontual, L.; et al. Strains Responsible for Invasive Meningococcal Disease in Patients with Terminal Complement Pathway Deficiencies. J. Infect. Dis. 2017, 215, 1331–1338. [Google Scholar] [CrossRef]
- Zipfel, P.F.; Wurzner, R.; Skerka, C. Complement evasion of pathogens: Common strategies are shared by diverse organisms. Mol. Immunol. 2007, 44, 3850–3857. [Google Scholar] [CrossRef]
- El Sissy, C.; Rosain, J.; Vieira-Martins, P.; Bordereau, P.; Gruber, A.; Devriese, M.; de Pontual, L.; Taha, M.K.; Fieschi, C.; Picard, C.; et al. Clinical and Genetic Spectrum of a Large Cohort with Total and Sub-total Complement Deficiencies. Front. Immunol. 2019, 10, 1936. [Google Scholar] [CrossRef]
- Aruta, M.G.; Carducci, M.; Micoli, F.; Necchi, F.; Rossi, O. Increasing the High Throughput of a Luminescence-Based Serum Bactericidal Assay (L-SBA). BioTech 2021, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, A.; Vadivelu, K.; Serino, L.; Neidig, N.; de Wergifosse, B. Endogenous complement human serum bactericidal assay (enc-hSBA) for vaccine effectiveness assessments against meningococcal serogroup B. npj Vaccines 2021, 6, 29. [Google Scholar] [CrossRef]
- Matthias, K.A.; Reveille, A.; Dhara, K.; Lyle, C.S.; Natuk, R.J.; Bonk, B.; Bash, M.C. Development and validation of a standardized human complement serum bactericidal activity assay to measure functional antibody responses to Neisseria gonorrhoeae. Vaccine 2025, 43, 126508. [Google Scholar] [CrossRef]
- Tzeng, Y.L.; Giuntini, S.; Berman, Z.; Sannigrahi, S.; Granoff, D.M.; Stephens, D.S. Neisseria meningitidis Urethritis Outbreak Isolates Express a Novel Factor H Binding Protein Variant That Is a Potential Target of Group B-Directed Meningococcal (MenB) Vaccines. Infect. Immun. 2020, 88, e00462-20. [Google Scholar] [CrossRef] [PubMed]
- Alexander, F.; Brunt, E.; Humphries, H.; Cavell, B.; Leung, S.; Allen, L.; Halkerston, R.; Lesne, E.; Penn, E.; Thomas, S.; et al. Generation of a Universal Human Complement Source by Large-Scale Depletion of IgG and IgM from Pooled Human Plasma. Methods Mol. Biol. 2022, 2414, 341–362. [Google Scholar] [CrossRef] [PubMed]
- Caron, F.; du Chatelet, I.P.; Leroy, J.P.; Ruckly, C.; Blanchard, M.; Bohic, N.; Massy, N.; Morer, I.; Floret, D.; Delbos, V.; et al. From tailor-made to ready-to-wear meningococcal B vaccines: Longitudinal study of a clonal meningococcal B outbreak. Lancet Infect. Dis. 2011, 11, 455–463. [Google Scholar] [CrossRef]
- Tettelin, H.; Saunders, N.J.; Heidelberg, J.; Jeffries, A.C.; Nelson, K.E.; Eisen, J.A.; Ketchum, K.A.; Hood, D.W.; Peden, J.F.; Dodson, R.J.; et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 2000, 287, 1809–1815. [Google Scholar] [CrossRef]
- Guiddir, T.; Deghmane, A.E.; Giorgini, D.; Taha, M.K. Lipocalin 2 in cerebrospinal fluid as a marker of acute bacterial meningitis. BMC Infect. Dis. 2014, 14, 276. [Google Scholar] [CrossRef]
- Kellogg, D.S., Jr.; Peacock, W.L., Jr.; Deacon, W.E.; Brown, L.; Pirkle, D.I. Neisseria gonorrhoeae. I. Virulence Genetically Linked to Clonal Variation. J. Bacteriol. 1963, 85, 1274–1279. [Google Scholar] [CrossRef]
- Levy, M.; Aouiti Trabelsi, M.; Taha, M.K. Evidence for Multi-Organ Infection During Experimental Meningococcal Sepsis due to ST-11 Isolates in Human Transferrin-Transgenic Mice. Microorganisms 2020, 8, 1456. [Google Scholar] [CrossRef]
- Sevestre, J.; Hong, E.; Delbos, V.; Terrade, A.; Mallet, E.; Deghmane, A.E.; Lemee, L.; Taha, M.K.; Caron, F. Durability of immunogenicity and strain coverage of MenBvac, a meningococcal vaccine based on outer membrane vesicles: Lessons of the Normandy campaign. Vaccine 2017, 35, 4029–4033. [Google Scholar] [CrossRef]
- Borrow, R.; Carlone, G.M. Serogroup B and C serum bactericidal assays. Methods Mol. Med. 2001, 66, 289–304. [Google Scholar] [CrossRef]
- Borrow, R.; Aaberge, I.S.; Santos, G.F.; Eudey, T.L.; Oster, P.; Glennie, A.; Findlow, J.; Hoiby, E.A.; Rosenqvist, E.; Balmer, P.; et al. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab. Immunol. 2005, 12, 970–976. [Google Scholar] [CrossRef]
- Borrow, R.; Balmer, P.; Miller, E. Meningococcal surrogates of protection—Serum bactericidal antibody activity. Vaccine 2005, 23, 2222–2227. [Google Scholar] [CrossRef]
- Mak, P.A.; Santos, G.F.; Masterman, K.A.; Janes, J.; Wacknov, B.; Vienken, K.; Giuliani, M.; Herman, A.E.; Cooke, M.; Mbow, M.L.; et al. Development of an automated, high-throughput bactericidal assay that measures cellular respiration as a survival readout for Neisseria meningitidis. Clin. Vaccine Immunol. 2011, 18, 1252–1260. [Google Scholar] [CrossRef]
- Romero-Steiner, S.; Spear, W.; Brown, N.; Holder, P.; Hennessy, T.; Gomez De Leon, P.; Carlone, G.M. Measurement of serum bactericidal activity specific for Haemophilus influenzae type b by using a chromogenic and fluorescent metabolic indicator. Clin. Diagn. Lab. Immunol. 2004, 11, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.A.; Ram, S. Meningococcal disease and the complement system. Virulence 2014, 5, 98–126. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantoni, G.; Deghmane, A.-E.; Caron, F.; Taha, M.-K. A Bioluminescence-Based Serum Bactericidal Assay to Detect Bactericidal Antibodies Against Neisseria meningitidis in Human Sera. Microorganisms 2025, 13, 595. https://doi.org/10.3390/microorganisms13030595
Fantoni G, Deghmane A-E, Caron F, Taha M-K. A Bioluminescence-Based Serum Bactericidal Assay to Detect Bactericidal Antibodies Against Neisseria meningitidis in Human Sera. Microorganisms. 2025; 13(3):595. https://doi.org/10.3390/microorganisms13030595
Chicago/Turabian StyleFantoni, Giulia, Ala-Eddine Deghmane, François Caron, and Muhamed-Kheir Taha. 2025. "A Bioluminescence-Based Serum Bactericidal Assay to Detect Bactericidal Antibodies Against Neisseria meningitidis in Human Sera" Microorganisms 13, no. 3: 595. https://doi.org/10.3390/microorganisms13030595
APA StyleFantoni, G., Deghmane, A.-E., Caron, F., & Taha, M.-K. (2025). A Bioluminescence-Based Serum Bactericidal Assay to Detect Bactericidal Antibodies Against Neisseria meningitidis in Human Sera. Microorganisms, 13(3), 595. https://doi.org/10.3390/microorganisms13030595





