Abstract
Leishmaniasis, caused by the Leishmania parasite, is a neglected public health issue. Leishmania mainly infects macrophages, where metabolic reprogramming shapes their plasticity (M1/M2), affecting the host’s resistance or susceptibility to infection. The development of this infection is influenced by immune responses, with an excessive anti-inflammatory reaction linked to negative outcomes through the modulation of various mediators. Itaconate, produced by the Acod1 gene, is recognized for its anti-inflammatory effects, but its function in leishmaniasis is not well understood. This study aimed to investigate the potential role of itaconate in leishmaniasis. Using transcriptomic data from L. major-infected BMDMs, we assessed the expression dynamics of Il1b and Acod1 and performed pathway enrichment analysis to determine the profile of genes co-expressed with Acod1. Early Acod1 upregulation followed by later Il1b downregulation was noted, indicating a shift towards an anti-inflammatory response. Among the genes co-expressed with Acod1, Ldlr, Hadh, and Src are closely associated with lipid metabolism and the polarization of macrophages towards the M2 phenotype, thereby creating a favorable environment for the survival of Leishmania. Overall, these findings suggest that Acod1 and its co-expressed genes may affect the outcome of Leishmania infection by modulating host metabolism. Accordingly, targeting itaconate-associated pathways could provide a novel therapeutic strategy for leishmaniasis.
1. Introduction
Under inflammatory conditions, the activation of immune cells is characterized by notable metabolic changes. These changes are mediated by the upregulation of specific enzymes that are typically expressed at low levels or that remain inactive under normal conditions [1,2]. Through a variety of processes, including signal transmission, regulation of gene expression, and modification of protein activity, metabolites can impact immune cell function [3,4,5]. The study of immunometabolism, or the interplay between metabolism and the immune response, has recently increased significantly in popularity, and has revealed novel therapeutic possibilities for translational medicine.
Itaconate is an immunometabolite produced by macrophages and other myeloid cells through the enzyme cis-aconitate decarboxylase (ACOD1), also known as immunoresponsive gene 1 (IRG1), which transforms cis-aconitate, an intermediate in the Krebs cycle, into itaconate. Acod1 expression is cell- and tissue-specific; it is expressed at very low levels under normal conditions. However, its expression is upregulated under stress conditions, particularly in response to inflammatory stimuli [6,7,8]. The structural and chemical similarity of itaconate to other metabolites, such as succinate, fumarate, malonate, and phosphoenolpyruvate, has provided insight into its antimicrobial properties. Beyond its role as an antimicrobial metabolite, itaconate has also been shown to exhibit a wide range of immunoregulatory functions [9]. Itaconate has been shown to inhibit succinate dehydrogenase (SDH) activity. Indeed, the addition of itaconate to LPS-activated macrophages impaired SDH activity, leading to decreased expression of inflammatory transcripts [10,11]. Furthermore, in Zika virus-infected mouse neurons, Acod1-dependent itaconate synthesis prevented the growth of the virus by inhibiting SDH activity, supporting its role as anti-inflammatory agent through a succinate-dependent mechanism [12]. Additionally, itaconate impacts glycolysis by inhibiting GAPDH. One study demonstrated that the itaconate derivative 4-octyl itaconate (4-OI) inhibits GAPDH activity, resulting in reduced aerobic glycolysis and decreased production of pro-inflammatory mediators such as IL-1β, nitric oxide synthase (NOS) 2, and TNF in activated macrophages [13]. Itaconate can regulate glycolysis by inhibiting other key metabolic enzymes, such as lactate dehydrogenase A (LDHA) and fructose-6-phosphate 2-kinase [14]. In addition, itaconate has been shown to impact type I interferon (IFN) responses. During influenza A virus infection, itaconate reduces the phosphorylated STAT1 levels in human macrophages, thereby suppressing Cxcl10 expression and demonstrating its immunosuppressive role in regulating IFN-I signaling [15,16]. A significant role of itaconate is in the suppression of the NLRP3 inflammasome, an innate immune component that drives the secretion of proinflammatory cytokines such as IL-1β and IL-18 [17]. Indeed, exogenous itaconate reduced the release of IL-1β from macrophages without affecting its transcription, suggesting direct modulation of NLRP3 activity [18]. On the other hand, Acod1−/− BMDMs stimulated with LPS and ATP produced high levels of IL-1β and IL-18; however, the exact mechanism through which itaconate inhibits inflammasome activation and IL-1β and IL-18 release remains unclear [11,19]. Overall, these studies provide valuable insights into the role of itaconate in modulating immune responses through its anti-inflammatory properties. Although its functions have not been fully explored, the evidence suggests that it plays a crucial role in the response to infections.
Leishmaniasis is a major tropical and subtropical infectious disease and is caused by the protozoan parasite Leishmania. Despite advancements in vaccine development, treatment, and diagnosis, many challenges persist [20,21]. During Leishmania infection, macrophages serve as the main cellular target, housing the parasites within parasitophorous vacuoles (PVs). Metabolic changes in Leishmania-infected macrophages, particularly in M1 and M2 macrophages, have been associated with resistance and susceptibility to the infection, respectively [22]. As an auxotrophic parasite with intricate nutritional requirements, Leishmania manipulates host metabolic pathways to produce essential metabolites for its survival [21,23]. For instance, Leishmania scavenges essential nutrients like arginine, which not only promotes parasite growth but also enables their survival by modulating the immune response of macrophages [24,25,26,27]. Additionally, the activation of host AMPK in Leishmania-infected macrophages, which is associated with a metabolic shift from glycolysis to oxidative metabolism, has been shown to be a key mechanism for parasite survival [23]. Furthermore, during the early stages of infection, Leishmania-infected macrophages exhibit an upregulation in the transcription of several metabolic genes, a process correlated with parasite survival [23,28].
In infectious diseases where pathogens like Leishmania evade the host’s pro-inflammatory mechanisms while exploiting anti-inflammatory pathways to their advantage, targeting the itaconate-mediated response could be a promising therapeutic strategy to improve pathogen clearance. Given the increasing evidence connecting macrophage metabolism to the host’s anti-parasitic responses, this study aimed to elucidate the role of itaconate in the context of leishmaniasis and uncover its potential as a therapeutic target by integrating transcriptomics and bioinformatics data, thereby presenting a highly promising approach for the treatment of the disease.
2. Material and Methods
2.1. Transcriptomic Data
The transcriptome dataset normalized matrix (quantile normalization) was obtained from Gene Expression Omnibus (GEO) dataset GSE31995 [29]. This dataset contains the transcriptomes of murine bone marrow-derived macrophages (BMDMs) infected with L. major promastigotes at different time points. Briefly, the transcriptome dataset was generated using GeneChip Mouse Gene 1.0 ST arrays to analyze the gene expression of BMDMs from BALB/c mice infected with L. major. The samples were collected from non-infected and parasite-infected macrophages at different time points post-infection. A T0 (0h) non-infected condition was used as an additional control. For each biological condition, three independent biological replicates were included. The complete dataset is publicly available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31995 (accessed on 15 December 2024) [30].
2.2. Gene Expression Visualization of Acod1 and Il1b Gene Expression
The expression data for the ACOD1 and IL-1β genes retrieved from the GSE31995 dataset were visualized using GraphPad Prism (v8.0.2). Heatmaps and line graphs with connecting symbols were generated to show the relative expression levels of these genes across the different experimental conditions.
2.3. Identification and Gene Set Enrichment Analysis of Genes Co-Expressed with Upregulated Acod1 Expression
To identify the genes that are co-expressed with Acod1, a correlation analysis was performed using the “limma” package (Linear Model for Microarray, version 3.0.0) using R software (v4.3.3), as previously described by Ritchie and colleagues. Acod1 expression was used as a predictor in this analysis [31].
Positively correlated genes were filtered according to a cut-off of an adjusted p-value < 0.05, which were used for further analysis. The significant positively correlated genes that were used as inputs were Mmp13, Il1rn, Ripk2, Sod2, Car13, Pcdh7, Tgm2, Irak3, Dram1, Src, Itga5, Tfap2e, Sav1, Stx11, Clec4e, Rab11, Fip1, Ldlr, Mmp14, Tmem171, Pgs1, Traf1, Cd69, Tpm4, Gsap, Mcoln2, and Clcn7. These genes were input into EnrichR (https://maayanlab.cloud/Enrichr/, accessed on 15 December 2024) to perform gene set enrichment analysis using the Hallmarks Molecular Signatures Database (Hallmarks MsigDB) to identify significantly enriched pathways [32,33]. Those with an adjusted p-value (also known as q-value) < 0.05 were considered significant. Pathways with higher EnrichR combined scores were visualized.
The significant pathway enrichment results were visualized using the “ggplot” package in Rstudio (v4.3.3) [34]. A bar chart was generated to display the combined score of each pathway. To further explore the relationships between the significant pathways and implicated genes, network visualization was performed using Cytoscape (v3.10.2.) [35].
2.4. Statistical Analysis
The statistical significance of the differences in gene expression between the non-infected and L. major- infected BMDMs across the different time points was analyzed using two-way ANOVA with the appropriate post hoc multiple comparisons in GraphPad Prism (v8.0.2) to assess statistical significance.
3. Results
3.1. Kinetics of Il1b and Acod1 Gene Expression Levels in L. major-Infected Bone Marrow-Derived Macrophages (BMDMs)
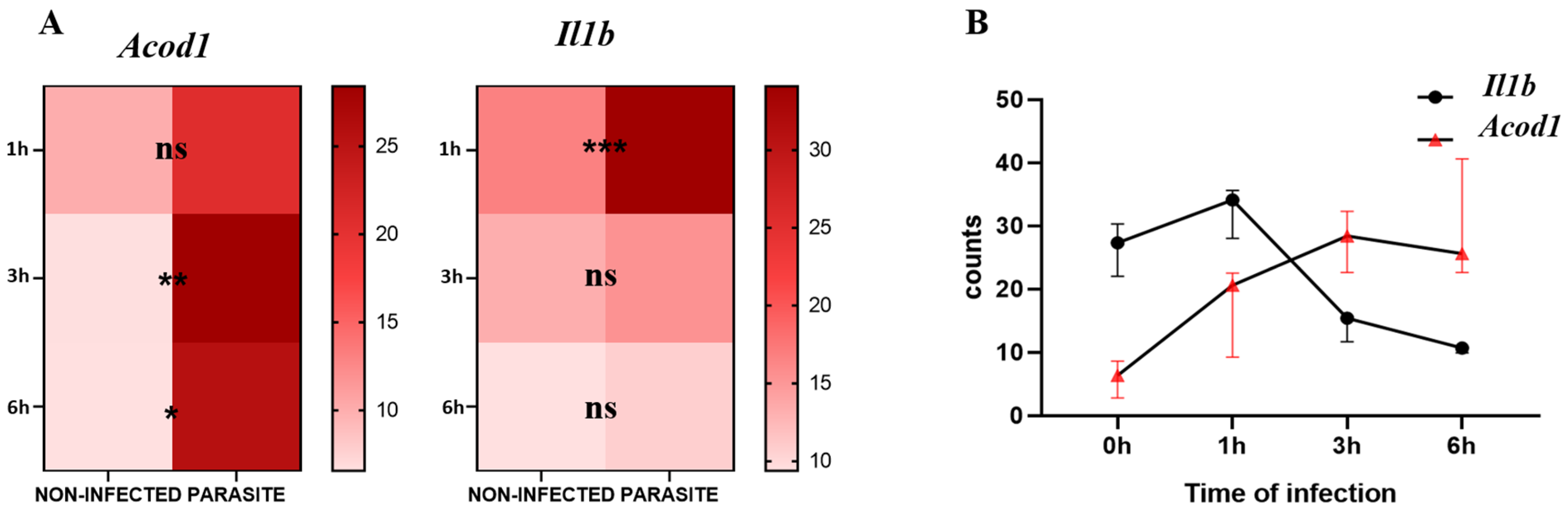
For the analysis of the kinetics of Il1b and Acod1 gene expression, we observed that Il1b was significantly upregulated in infected compared with non-infected BMDMs at 1 h post-infection (p-value = 0.0002) while Acod1 did not show any significant variation at the same timepoint. At 3 h and 6 h post-infection, Il1b expression lost statistical significance, whereas Acod1 showed significant upregulation in L. major-infected macrophages in comparison to non-infected BMDMs at both time points (p-value = 0.0077 and p-value = 0.0121, respectively) (Figure 1A). By visualizing the Il1b and Acod1 gene expression levels on the same graph, we can observe that Il1b expression was prominent at an early time point (1 h), while Acod1 was upregulated at later time points (3 h and 6 h) (Figure 1B).
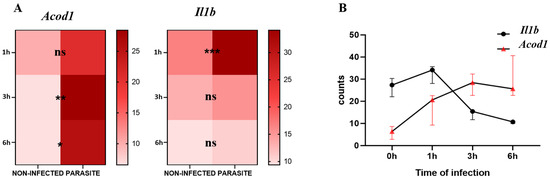
Figure 1.
Acod1 and Il1b gene expression levels from GSE31995 dataset. (A) Expression counts of infected and non-infected BMDMs at 0 h, 1 h, 3 h, and 6 h. (B) Visualization of kinetics of expression of both genes at the three timepoints. ns, not significant; *** p-value < 0.001; ** p-value < 0.01; * p-value ≤ 0.05.
3.2. Transcriptomics
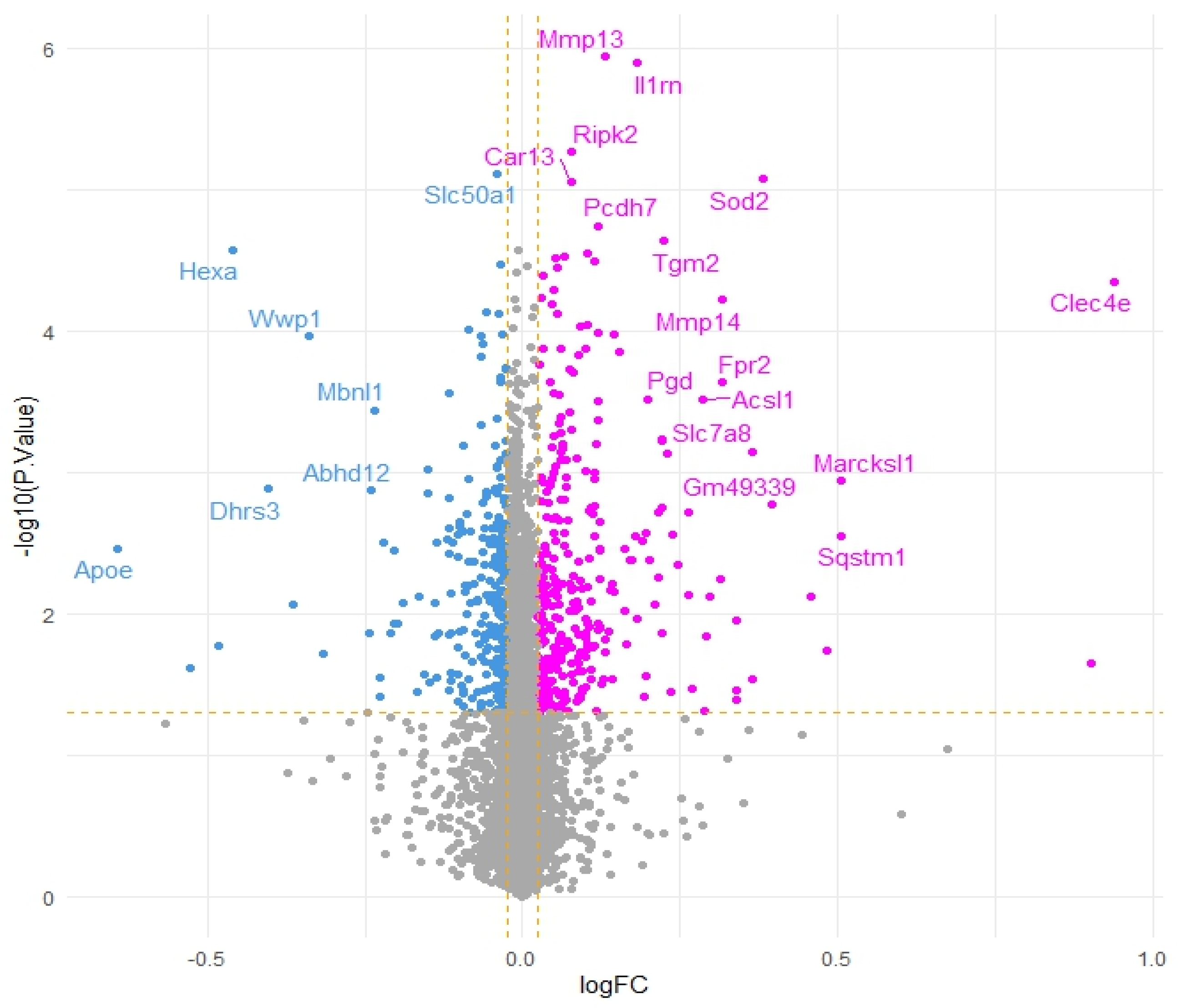
To further corroborate our speculations, genes correlated with Acod1 gene expression upregulation were identified. A total of 14 genes were found to be downregulated and 27 genes were found to be upregulated, while the remaining genes showed no significant variation (Figure 2).
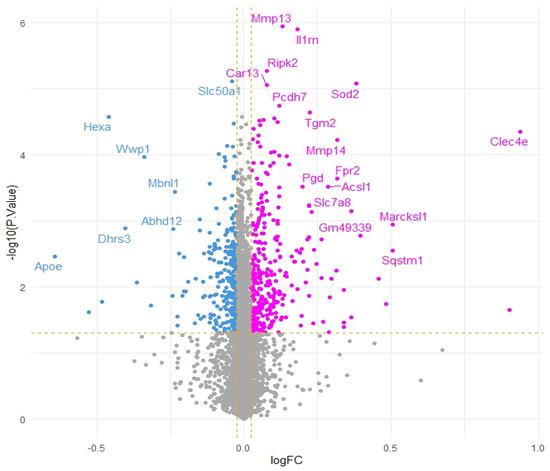
Figure 2.
Volcano plot of genes co-expressed with Acod1 gene expression upregulation (pink dots represent positively correlated genes, blue dots represent negatively correlated genes and grey dots indicate genes that do not show significant correlation. Yellow dashed lines represent thresholds for logFC and −log10 (p-value).
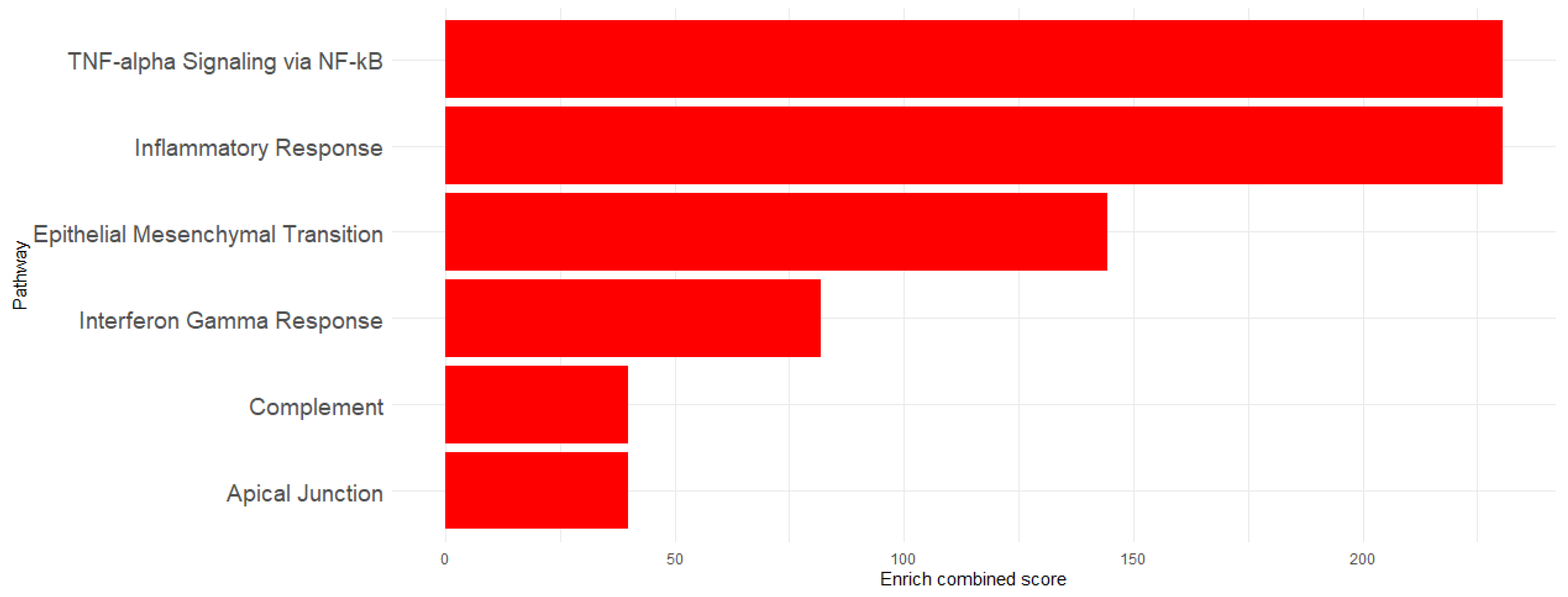
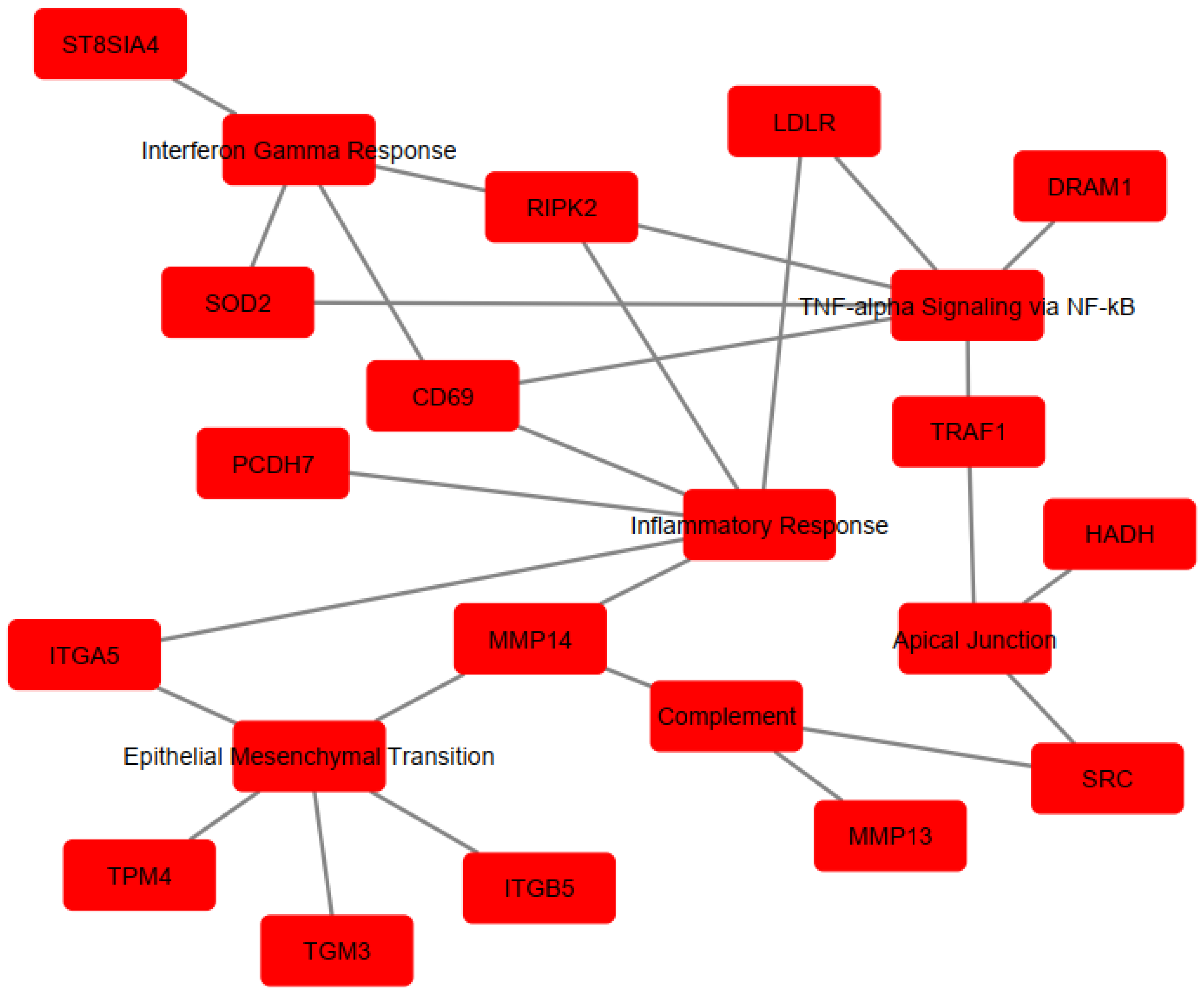
To determine the implication of the upregulated genes, enrichment analysis was performed using Hallmarks MSigDb. The analysis revealed significant enrichment of the following pathways: TNF-α signaling via the NF-κB pathway (EnrichR combined score = 230.6618), Inflammatory Response (EnrichR combined score = 230.6618), Epithelial–Mesenchymal Transition (EnrichR combined score = 144.2694), Interferon-γ Response (EnrichR combined score = 81.89774), Apical Junction (EnrichR combined score = 39.89133), and Complement (EnrichR combined score = 39.89133) (Figure 3). The genes associated with each pathway are detailed in Table 1 and visualized in Figure 4, which shows the network map of the key genes co-expressed with Acod1 and their associated hallmark pathways. In this network, the inflammatory response pathway appears to be the central node, indicating that all the genes in the network are related to this biological process. Several genes in this network are directly or indirectly associated with lipid metabolism, including Ldlr, Hadh, and Src, suggesting that Acod1 upregulation could reshape both metabolic and inflammatory responses during Leishmania infection.
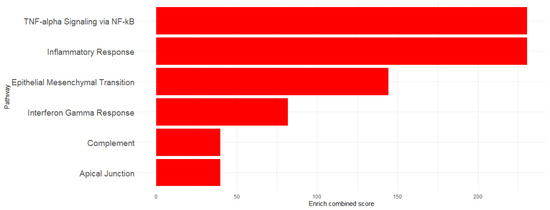
Figure 3.
Enriched pathway visualization using EnrichR combined scores.

Table 1.
EnrichR results using Hallmark Msig_Database.
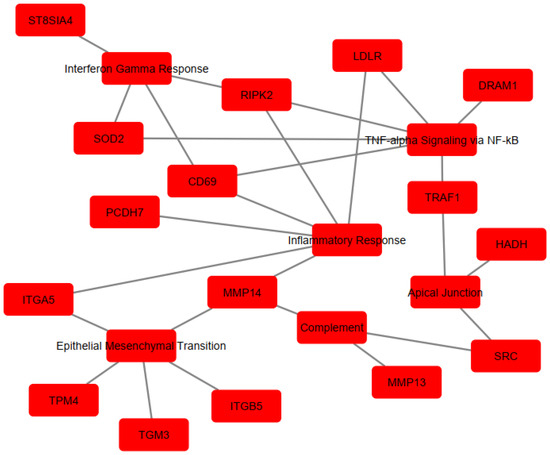
Figure 4.
Cytoscape network visualization of enriched pathways and associate gene sets. Red nodes represent genes co-expressed with Acod1 or their associated pathway, while edges (lines) indicate interactions or associations between them.
The genes are involved in immune and metabolic responses. Specifically, Cd69 and Src are involved in immune cell activation. Traf1, Ripk2, and Dram1 contribute to inflammatory regulation by modulating TNF and NOD-like receptor-mediated signaling and autophagy, respectively. Itga5, Itgb5, Tpm4, and Pcdh7 are associated with cell adhesion/migration, cytoskeletal dynamics, and cell–cell interactions. The St8sia4 gene modulates immune cell interactions through sialylation. In terms of metabolic processes, three genes (Sod2, Ldlr, and Hadh) were implicated in regulating the oxidative stress response and lipid metabolism/cholesterol homeostasis. Other genes, including Mmp14, Mmp13, and Tgm3, are involved in protein cross-linking and extracellular matrix remodeling. Notably, some of these genes play dual roles, affecting “immuno-metabolism”, such as Src, which has been shown to induce lipid synthesis and regulate the cell’s lipid stores [36,37,38].
4. Discussion
Our results showed that Acod1 and Il1b were expressed at different timepoints, with IL-1β being expressed in L. major-infected BMDMs at early timepoints, while Acod1 expression occurred later in the course of the infection. Mainly secreted by macrophages, IL-1β is a potent pro-inflammatory cytokine whose production is partially mediated through NLRP3 inflammasome activation. It plays a crucial role in host defense responses to infection [39,40].
During leishmaniasis, IL-1β production triggers Inducible Nitric Oxide Synthase (iNOS) activation and Nitric Oxide (NO) production, which are responsible for parasite clearance [41]. This suggests that Acod1 expression might mitigate the effects of Il1b expression, thereby mediating an anti-inflammatory response in BMDMs infected with L. major and creating a favorable environment for Leishmania survival. In line with our hypothesis, Palacios et al. demonstrated that itaconic acid abrogated the control of parasite replication in M1 activated macrophages infected with L. infantum [42]. In fact, macrophages are highly plastic cells that respond to their environment through polarization, which plays an essential role in the outcome of Leishmania infections. M1 macrophages are well known as microbicidal immune cells responsible for killing the Leishmania parasite through the secretion of pro-inflammatory cytokines, including Tumor Necrosis Factor-alpha (TNF-α), IL-1β, IL-6, and IFN-γ. This M1 phenotype also promotes the production of Reactive Oxygen Species (ROS) and nitrogen species such as NO [22,43,44,45]. In contrast, M2 macrophages are associated with anti-inflammatory responses. In the context of leishmaniasis, these immunosuppressive cells have been tightly linked to Leishmania survival through the production of IL-10 and TGF-β, while simultaneously inhibiting ROS production [43].
The differential kinetics of Acod1 and Il1b expression in L. major-infected BMDMs may support the hypothesis that itaconate contributes to the progression of leishmaniasis by inhibiting M1 macrophage polarization and potentially driving an M2-mediated response. Few studies have demonstrated the role of itaconate in inhibiting M1 macrophages activation, while its role in M2 macrophages remains largely unknown [46]. Multiple studies have shown that M1 macrophages primarily rely on glycolysis to produce energy, unlike M2 macrophages, which depend on oxidative phosphorylation and lipid metabolism [47]. On the one hand, itaconate has been shown to inhibit glycolysis [46], which may contribute to the inhibition of M1 polarization. On the other hand, itaconate has been implicated in lipid metabolism. One study demonstrated that Acod1-knockout (KO) mice exhibited exacerbated hepatic lipid accumulation during sepsis, improved glucose oxidation, and reduced fatty acid oxidation. Likewise, in vitro treatment of hepatocytes (AML12 cells) with 4-octyl itaconate (4-OI), a derivative of itaconate, promoted mitochondrial fatty acid uptake and clearance by upregulating the expression of oxidative phosphorylation proteins and fatty acid β-oxidation enzymes [48]. Overall, these data indicate that itaconate supports Leishmania survival, potentially by promoting macrophage polarization toward the M2 phenotype through modulation of lipid metabolism.
Gene enrichment analysis of the genes co-expressed with upregulated Acod1 expression in L. major-infected BMDMs, we identified six significant pathways and genes implicated in immune and metabolic processes. Among these, Ldlr, Hadh and Src are implicated in lipid metabolism, which is tightly linked to macrophage polarization.
The Hadh gene encodes short-chain L-3-hydroxyacyl-CoA dehydrogenase (HADH), a crucial enzyme in fatty acid oxidation that mediates the third step of fatty acid oxidation in mitochondria [49]. There are two forms of HADH: the alpha subunit (HADHA) and the beta subunit (HADHB), both of which are integral components of the mitochondrial trifunctional protein (MTP) that facilitates multiple steps in fatty acid metabolism [36]. Due to its critical role in β-oxidation, HADH is thought to be associated with the polarization of macrophages toward the M2 phenotype. To our knowledge, no studies have investigated the involvement of the HADH or β-oxidation in leishmaniasis. However, its role has been studied in the case of Mycobacterium tuberculosis (M. tuberculosis) infections. Although distinct from the Leishmania parasite, both are intracellular pathogens that target macrophages as their host cells. Interestingly, Chandra and colleagues showed that the inhibition of fatty acid oxidation by chemicals, such as trimetazidine (TMZ), which blocks the 3-ketoacyl-CoA thiolase activity of HADHB, restricts the growth of M. tuberculosis. In addition, TMZ treatment of M. tuberculosis-infected BMDMs resulted in mitochondrial ROS (mROS) production, which promotes NADPH oxidase recruitment and autophagy to limit bacterial growth [50]. According to previous reports, ROS are highly produced by M1 macrophages, suggesting that HADH inhibition might control M. tuberculosis infection by promoting the polarization of these pro-inflammatory immune cells.
The Src proto-oncogene, also named c-Src, is a critical non-receptor tyrosine kinase encoded by the SRC gene in humans and contributes to cellular proliferation, differentiation, migration, adhesion, and survival [51]. Likewise, Src is indirectly linked to lipid metabolism: this protein was shown to interact with Lipin-1, an essential enzyme that operates as a phosphatidate phosphatase (PAP) to regulate lipogenesis [38]. In addition to its role in lipogenesis, Lipin-1 plays a crucial role in the oxidation of fatty acids. Schilke et al. showed that Lipin-1 modulates lipid metabolism and oxidative phosphorylation within macrophages through the catabolism of fatty acids and by enhancing β-oxidation in response to diverse pro-resolving stimuli including IL-4, palmitate (a free fatty acid), and apoptotic cellular debris [52]. IL-4 is recognized as a potent polarizing cytokine that polarizes macrophages toward the M2 phenotype. Interestingly, Chandran et al. demonstrated that Lipin-1’s transcriptional co-regulator activity is essential for IL-4-mediated macrophage polarization [53]. In addition to its interaction with Lipin-1, SRC is suspected to be directly involved in driving M2 macrophage polarization. Notably, Xiang Hu et al. observed that pretreatment of BMDMs with SRC inhibitor-1 promoted the expression of M1 macrophage markers in response to IFN-γ and suppressed the expression of M2 macrophage markers in response to IL-4 [54]. In summary, we suggest that the co-expression of Hadh and Src genes with Acod1 in L. major-infected BMDMs may support macrophage polarization toward the M2 phenotype, thereby creating a favorable niche for Leishmania survival within the macrophage.
Ldlr is a cell membrane glycoprotein that functions in binding and internalizing circulating cholesterol-containing lipoprotein particles [37]. Several studies have shown that Ldrl is upregulated during Leishmania infection [30,55,56]. Semini and colleagues showed that infection of BMDMs by both L. major and L. mexicana leads to the upregulation of Ldlr, which was correlated with a significant increase in cholesterol levels in PVs and it was found to form a halo around the parasites. Interestingly, the host cell’s cholesterol was not only trafficked to PVs but also became incorporated into the parasite’s membrane [56]. In another study investigating peritoneal macrophages from Swiss mice infected with L. amazonensis, it was demonstrated that intracellular amastigotes cultured in a cholesterol-free medium were more sensitive to ketoconazole and miconazole (drugs inhibiting ergosterol biosynthesis by Leishmania parasites), suggesting that Leishmania parasites can use cholesterol to replace ergosterol to maintain their membrane properties [57]. Parihar et al. showed that inhibition of cholesterol biosynthesis by simvastatin (HMG-CoA reductase inhibitor) reduced the growth of the L. major amastigote form in primary-culture macrophages. The topical application of simvastatin relieved ear and footpath swelling and ulceration and reduced the parasite burden in both BALB/c and C57BL/6 mice models infected with L. major [58]. These findings suggest that host cholesterol may be used in the construction of the Leishmania parasite’s membrane, affecting its virulence and pathogenicity. In brief, the co-expression of Ldlr with Acod1 in L. major-infected BMDMs suggest that cholesterol is actively internalized by infected macrophages to ensure the survival and proliferation of the parasite within.
Overall, the Acod1 gene, which encodes aconitate decarboxylase, which is responsible for itaconate production, could regulate inflammation by promoting M2 macrophage polarization and inhibiting M1 macrophage polarization. This inhibition may promote Leishmania survival and multiplication within macrophages. Additionally, Src and Hadh, which are co-expressed with Acod1, are likely involved in lipid metabolism processes such as β-oxidation, a characteristic of the M2 macrophage phenotype that may further support Leishmania survival. Likewise, Ldlr also plays a role in the pathogenesis and persistence of Leishmania by ensuring cholesterol uptake.
Although these findings are compelling and have potential, it is crucial to note that our conclusions are based on a bioinformatics analysis. Experimental validation is necessary to confirm the role of itaconate in the context of leishmaniasis.
Overall, these findings suggest that itaconate promotes a favorable intracellular environment for Leishmania survival, both directly and through the co-expression of genes during infection. This highlights itaconate as a potential therapeutic target for treating leishmaniasis.
Author Contributions
Conceptualization, writing—original draft: A.K., H.E.F., D.D., A.A. and K.A.; data curation, visualization, formal analysis: H.E.F. and A.K.; methodology: A.K., H.E.F., D.D., A.A. and K.A.; supervision: K.A.; validation: A.K., H.E.F., D.D., A.A. and K.A.; writing—review and editing: A.K., H.E.F., D.D., A.A., K.A., I.N.I. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used in this study are available online (GEO dataset, https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31995, Date of access: 15 December 2024).
Acknowledgments
We would like to thank Christophe Desterke (affiliated with INSERM UMRS-1311, Faculty of Medicine, University of Paris-Saclay, Villejuif, France) for his valuable technical help and bioinformatics analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kelly, B.; O’Neill, L.A. Metabolic Reprogramming in Macrophages and Dendritic Cells in Innate Immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, C.M.; Holowka, T.; Sun, J.; Blagih, J.; Amiel, E.; DeBerardinis, R.J.; Cross, J.R.; Jung, E.; Thompson, C.B.; Jones, R.G.; et al. Toll-like Receptor–Induced Changes in Glycolytic Metabolism Regulate Dendritic Cell Activation. Blood 2010, 115, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Diskin, C.; Ryan, T.A.J.; O’Neill, L.A.J. Modification of Proteins by Metabolites in Immunity. Immunity 2021, 54, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; O’Neill, L.A.J. Succinate: A Metabolic Signal in Inflammation. Trends Cell Biol. 2014, 24, 313–320. [Google Scholar] [CrossRef]
- Li, R.; Zhang, P.; Wang, Y.; Tao, K. Itaconate: A Metabolite Regulates Inflammation Response and Oxidative Stress. Oxidative Med. Cell. Longev. 2020, 2020, 5404780. [Google Scholar] [CrossRef]
- Wu, R.; Chen, F.; Wang, N.; Tang, D.; Kang, R. ACOD1 in Immunometabolism and Disease. Cell Mol. Immunol. 2020, 17, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Jamal Uddin, M.; Joe, Y.; Kim, S.-K.; Oh Jeong, S.; Ryter, S.W.; Pae, H.-O.; Chung, H.T. IRG1 Induced by Heme Oxygenase-1/Carbon Monoxide Inhibits LPS-Mediated Sepsis and pro-Inflammatory Cytokine Production. Cell Mol. Immunol. 2016, 13, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-C.; Weng, W.-T.; Scofield, B.A.; Furnas, D.; Paraiso, H.C.; Yu, I.-C.; Yen, J.-H. Immunoresponsive Gene 1 Modulates the Severity of Brain Injury in Cerebral Ischaemia. Brain Commun. 2021, 3, fcab187. [Google Scholar] [CrossRef]
- Wu, R.; Liu, J.; Tang, D.; Kang, R. The Dual Role of ACOD1 in Inflammation. J. Immunol. 2023, 211, 518–526. [Google Scholar] [CrossRef]
- Cordes, T.; Wallace, M.; Michelucci, A.; Divakaruni, A.S.; Sapcariu, S.C.; Sousa, C.; Koseki, H.; Cabrales, P.; Murphy, A.N.; Hiller, K.; et al. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J. Biol. Chem. 2016, 291, 14274–14284. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M.; Nair, S.; Vincent, E.E.; Loginicheva, E.; Cervantes-Barragan, L.; Ma, X.; Huang, S.C.-C.; Griss, T.; et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016, 24, 158–166. [Google Scholar] [CrossRef]
- Daniels, B.P.; Kofman, S.B.; Smith, J.R.; Norris, G.T.; Snyder, A.G.; Kolb, J.P.; Gao, X.; Locasale, J.W.; Martinez, J.; Gale, M.; et al. The Nucleotide Sensor ZBP1 and Kinase RIPK3 Induce the Enzyme IRG1 to Promote an Antiviral Metabolic State in Neurons. Immunity 2019, 50, 64–76.e4. [Google Scholar] [CrossRef]
- Liao, S.-T.; Han, C.; Xu, D.-Q.; Fu, X.-W.; Wang, J.-S.; Kong, L.-Y. 4-Octyl Itaconate Inhibits Aerobic Glycolysis by Targeting GAPDH to Exert Anti-Inflammatory Effects. Nat. Commun. 2019, 10, 5091. [Google Scholar] [CrossRef]
- Sakai, A.; Kusumoto, A.; Kiso, Y.; Furuya, E. Itaconate Reduces Visceral Fat by Inhibiting Fructose 2,6-Bisphosphate Synthesis in Rat Liver. Nutrition 2004, 20, 997–1002. [Google Scholar] [CrossRef]
- Chen, F.; Elgaher, W.a.M.; Winterhoff, M.; Büssow, K.; Waqas, F.H.; Graner, E.; Pires-Afonso, Y.; Casares Perez, L.; de la Vega, L.; Sahini, N.; et al. Citraconate Inhibits ACOD1 (IRG1) Catalysis, Reduces Interferon Responses and Oxidative Stress, and Modulates Inflammation and Cell Metabolism. Nat. Metab. 2022, 4, 534–546. [Google Scholar] [CrossRef]
- Sohail, A.; Iqbal, A.A.; Sahini, N.; Chen, F.; Tantawy, M.; Waqas, S.F.H.; Winterhoff, M.; Ebensen, T.; Schultz, K.; Geffers, R.; et al. Itaconate and Derivatives Reduce Interferon Responses and Inflammation in Influenza A Virus Infection. PLoS Pathog. 2022, 18, e1010219. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Swain, A.; Bambouskova, M.; Kim, H.; Andhey, P.S.; Duncan, D.; Auclair, K.; Chubukov, V.; Simons, D.M.; Roddy, T.P.; Stewart, K.M.; et al. Comparative Evaluation of Itaconate and Its Derivatives Reveals Divergent Inflammasome and Type I Interferon Regulation in Macrophages. Nat. Metab. 2020, 2, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate Is an Anti-Inflammatory Metabolite That Activates Nrf2 via Alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Singh, O.P.; Sundar, S. Developments in Diagnosis of Visceral Leishmaniasis in the Elimination Era. J. Parasitol. Res. 2015, 2015, 239469. [Google Scholar] [CrossRef]
- Assouab, A.; Kihel, A.; Rouahi, M.; Larribau, M.; Karim, Z.; Akarid, K. Cutaneous Leishmaniasis and Iron Metabolism: Current Insights and Challenges. Front. Immunol. 2024, 15, 1488590. [Google Scholar] [CrossRef]
- Almeida, F.S.; Vanderley, S.E.R.; Comberlang, F.C.; de Andrade, A.G.; Cavalcante-Silva, L.H.A.; Silva, E.d.S.; Palmeira, P.H.d.S.; do Amaral, I.P.G.; Keesen, T.S.L. Leishmaniasis: Immune Cells Crosstalk in Macrophage Polarization. Trop. Med. Infect. Dis. 2023, 8, 276. [Google Scholar] [CrossRef]
- Ferreira, C.; Estaquier, J.; Silvestre, R. Immune-Metabolic Interactions between Leishmania and Macrophage Host. Curr. Opin. Microbiol. 2021, 63, 231–237. [Google Scholar] [CrossRef]
- Goldman-Pinkovich, A.; Kannan, S.; Nitzan-Koren, R.; Puri, M.; Pawar, H.; Bar-Avraham, Y.; McDonald, J.; Sur, A.; Zhang, W.-W.; Matlashewski, G.; et al. Sensing Host Arginine Is Essential for Leishmania Parasites’ Intracellular Development. mBio 2020, 11, e02023-20. [Google Scholar] [CrossRef]
- Darif, D.; Nait Irahal, I.; Hammi, I.; Kihel, A.; Kachmar, M.R.; Riyad, M.; Hmimid, F.; Akarid, K. Capparis Spinosa Inhibits Leishmania Major Growth through Nitric Oxide Production in Vitro and Arginase Inhibition in Silico. Exp. Parasitol. 2023, 245, 108452. [Google Scholar] [CrossRef]
- Akarid, K.; Arnoult, D.; Micic-Polianski, J.; Sif, J.; Estaquier, J.; Ameisen, J.C. Leishmania Major. -Mediated Prevention of Programmed Cell Death Induction in Infected Macrophages Is Associated with the Repression of Mitochondrial Release of Cytochrome c. J. Leukoc. Biol. 2004, 76, 95–103. [Google Scholar] [CrossRef]
- Assouab, A.; El Filaly, H.; Akarid, K. Inhibiting Human and Leishmania Arginases Using Cannabis Sativa as a Potential Therapy for Cutaneous Leishmaniasis: A Molecular Docking Study. TropicalMed 2022, 7, 400. [Google Scholar] [CrossRef]
- Rodrigues, V.; André, S.; Maksouri, H.; Mouttaki, T.; Chiheb, S.; Riyad, M.; Akarid, K.; Estaquier, J. Transcriptional Analysis of Human Skin Lesions Identifies Tryptophan-2,3-Deoxygenase as a Restriction Factor for Cutaneous Leishmania. Front. Cell Infect. Microbiol. 2019, 9, 338. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets--Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef]
- Rabhi, I.; Rabhi, S.; Ben-Othman, R.; Rasche, A.; Consortium, S.; Daskalaki, A.; Trentin, B.; Piquemal, D.; Regnault, B.; Descoteaux, A.; et al. Transcriptomic Signature of Leishmania Infected Mice Macrophages: A Metabolic Point of View. PLoS Neglected Trop. Dis. 2012, 6, e1763. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Wickham, H. Manipulating Data. In ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009; pp. 157–175. ISBN 978-0-387-98141-3. [Google Scholar]
- Cline, M.S.; Smoot, M.; Cerami, E.; Kuchinsky, A.; Landys, N.; Workman, C.; Christmas, R.; Avila-Campilo, I.; Creech, M.; Gross, B.; et al. Integration of Biological Networks and Gene Expression Data Using Cytoscape. Nat. Protoc. 2007, 2, 2366–2382. [Google Scholar] [CrossRef]
- Wang, X.; Song, H.; Liang, J.; Jia, Y.; Zhang, Y. Abnormal Expression of HADH, an Enzyme of Fatty Acid Oxidation, Affects Tumor Development and Prognosis (Review). Mol. Med. Rep. 2022, 26, 355. [Google Scholar] [CrossRef]
- Go, G.-W.; Mani, A. Low-Density Lipoprotein Receptor (LDLR) Family Orchestrates Cholesterol Homeostasis. Yale J. Biol. Med. 2012, 85, 19–28. [Google Scholar]
- Lin, S.-Y.; Lin, S.-C. SRC Promotes Lipogenesis: Implications for Obesity and Breast Cancer. Mol. Cell. Oncol. 2021, 8, 1866975. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the Mechanism of IL-1β Secretion. Cytokine Growth Factor. Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Kihel, A.; Hammi, I.; Darif, D.; Lemrani, M.; Riyad, M.; Guessous, F.; Akarid, K. The Different Faces of the NLRP3 Inflammasome in Cutaneous Leishmaniasis: A Review. Cytokine 2021, 147, 155248. [Google Scholar] [CrossRef]
- Lima-Junior, D.S.; Costa, D.L.; Carregaro, V.; Cunha, L.D.; Silva, A.L.N.; Mineo, T.W.P.; Gutierrez, F.R.S.; Bellio, M.; Bortoluci, K.R.; Flavell, R.A.; et al. Inflammasome-Derived IL-1β Production Induces Nitric Oxide–Mediated Resistance to Leishmania. Nat. Med. 2013, 19, 909–915. [Google Scholar] [CrossRef]
- Palacios, G.; Vega-García, E.; Valladares, B.; Pérez, J.A.; Dorta-Guerra, R.; Carmelo, E. Gene Expression Profiling of Classically Activated Macrophages in Leishmania Infantum Infection: Response to Metabolic Pre-Stimulus with Itaconic Acid. TropicalMed 2023, 8, 264. [Google Scholar] [CrossRef]
- Costa-da-Silva, A.C.; Nascimento, D.D.O.; Ferreira, J.R.M.; Guimarães-Pinto, K.; Freire-de-Lima, L.; Morrot, A.; Decote-Ricardo, D.; Filardy, A.A.; Freire-de-Lima, C.G. Immune Responses in Leishmaniasis: An Overview. TropicalMed 2022, 7, 54. [Google Scholar] [CrossRef]
- Tomiotto-Pellissier, F.; Bortoleti, B.T.D.S.; Assolini, J.P.; Gonçalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage Polarization in Leishmaniasis: Broadening Horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef] [PubMed]
- Maksouri, H.; Dang, P.M.-C.; Rodrigues, V.; Estaquier, J.; Riyad, M.; Akarid, K. Moroccan Strains of Leishmania Major and Leishmania Tropica Differentially Impact on Nitric Oxide Production by Macrophages. Parasit. Vectors 2017, 10, 506. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Kong, W.; Zeng, T. Itaconate: A Potent Macrophage Immunomodulator. Inflammation 2023, 46, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Deng, Q.; Hu, Y.; Dong, J.; Wang, W.; Wang, Y.; Li, C. Macrophage Polarization, Metabolic Reprogramming, and Inflammatory Effects in Ischemic Heart Disease. Front. Immunol. 2022, 13, 934040. [Google Scholar] [CrossRef]
- Mainali, R.; Buechler, N.; Otero, C.; Edwards, L.; Key, C.-C.; Furdui, C.; Quinn, M.A. Itaconate Stabilizes CPT1a to Enhance Lipid Utilization during Inflammation. eLife 2024, 12, RP92420. [Google Scholar] [CrossRef]
- Popa, F.I.; Perlini, S.; Teofoli, F.; Degani, D.; Funghini, S.; La Marca, G.; Rinaldo, P.; Vincenzi, M.; Antoniazzi, F.; Boner, A.; et al. 3-Hydroxyacyl-Coenzyme A Dehydrogenase Deficiency: Identification of a New Mutation Causing Hyperinsulinemic Hypoketotic Hypoglycemia, Altered Organic Acids and Acylcarnitines Concentrations. In JIMD Reports—Case and Research Reports, 2011/2; SSIEM, Ed.; JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 71–77. ISBN 978-3-642-24757-6. [Google Scholar]
- Chandra, P.; He, L.; Zimmerman, M.; Yang, G.; Köster, S.; Ouimet, M.; Wang, H.; Moore, K.J.; Dartois, V.; Schilling, J.D.; et al. Inhibition of Fatty Acid Oxidation Promotes Macrophage Control of Mycobacterium Tuberculosis. mBio 2020, 11, e01139-20. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Iida, M.; Dunn, E.F. The Role of Src in Solid Tumors. Oncologist 2009, 14, 667–678. [Google Scholar] [CrossRef]
- Schilke, R.M.; Blackburn, C.M.R.; Rao, S.; Krzywanski, D.M.; Finck, B.N.; Woolard, M.D. Macrophage-Associated Lipin-1 Promotes β-Oxidation in Response to Proresolving Stimuli. Immunohorizons 2020, 4, 659–669. [Google Scholar] [CrossRef]
- Chandran, S.; Schilke, R.M.; Blackburn, C.M.R.; Yurochko, A.; Mirza, R.; Scott, R.S.; Finck, B.N.; Woolard, M.D. Lipin-1 Contributes to IL-4 Mediated Macrophage Polarization. Front. Immunol. 2020, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, H.; Han, C.; Cao, X. Src Promotes Anti-Inflammatory (M2) Macrophage Generation via the IL-4/STAT6 Pathway. Cytokine 2018, 111, 209–215. [Google Scholar] [CrossRef]
- Fortéa, J.O.Y.; De La Llave, E.; Regnault, B.; Coppée, J.-Y.; Milon, G.; Lang, T.; Prina, E. Transcriptional Signatures of BALB/c Mouse Macrophages Housing Multiplying Leishmania Amazonensis Amastigotes. BMC Genom. 2009, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Semini, G.; Paape, D.; Paterou, A.; Schroeder, J.; Barrios-Llerena, M.; Aebischer, T. Changes to Cholesterol Trafficking in Macrophages by Leishmania Parasites Infection. Microbiologyopen 2017, 6, e00469. [Google Scholar] [CrossRef]
- Andrade-Neto, V.V.; Manso, P.P.D.A.; Pereira, M.G.; De Cicco, N.N.T.; Atella, G.C.; Pelajo-Machado, M.; Menna-Barreto, R.F.S.; Torres-Santos, E.C. Host Cholesterol Influences the Activity of Sterol Biosynthesis Inhibitors in Leishmania Amazonensis. Mem. Inst. Oswaldo Cruz 2022, 117, e220407. [Google Scholar] [CrossRef]
- Parihar, S.P.; Hartley, M.-A.; Hurdayal, R.; Guler, R.; Brombacher, F. Topical Simvastatin as Host-Directed Therapy against Severity of Cutaneous Leishmaniasis in Mice. Sci. Rep. 2016, 6, 33458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).