Abstract
Protein glutathionylation is defined as a reversible, ubiquitous post-translational modification, resulting in the formation of mixed disulfides between glutathione and proteins’ cysteine residues. Glutathionylation has been implicated in several cellular mechanisms ranging from protection from oxidative stress to the control of cellular homeostasis and the cell cycle. A significant body of research has examined the multifaceted effects of this post-translational modification under physiological conditions in eukaryotes, with a particular focus on its impact on the development of various diseases in humans. In contrast, the role of glutathionylation in prokaryotic organisms remains to be extensively investigated. However, there has been a recent increase in the number of studies investigating this issue, providing details about the role of glutathione and other related thiols as post-translational modifiers of selected bacterial proteins. It can be concluded that in addition to the classical role of such thiols in protecting against cysteine oxidation and consequent protein inactivation, many more specialized roles of glutathionylation in bacterial pathogenicity, virulence, interspecies competition and survival, and control of gene expression are emerging, and new ones may emerge in the future. In this short review, we aim to summarize the current state-of-the-art in this field of research.
1. Introduction
S-Glutathionylation (GS-ylation) is an important and ubiquitous reversible post-translational modification (PTM) that results in the formation of mixed disulfides between glutathione (GSH) and selected cysteine residues of proteins [1]. It is recognized as one of the most important post-translational mechanisms and regulates various cellular processes under physiological conditions, such as protection against oxidative stress [2]. In eukaryotes, under pathological conditions, deregulated GS-ylation is associated with cardiovascular, pulmonary, and neurodegenerative diseases as well as cancer and diabetes [2].
GSH (L-γ-glutamyl-L-cysteinyl-glycine) is the most abundant eukaryotic and prokaryotic low-molecular-weight thiol, with cellular concentrations in the millimolar range, and is a major regulator of redox status and redox signaling in cells (Figure 1A) [3,4].
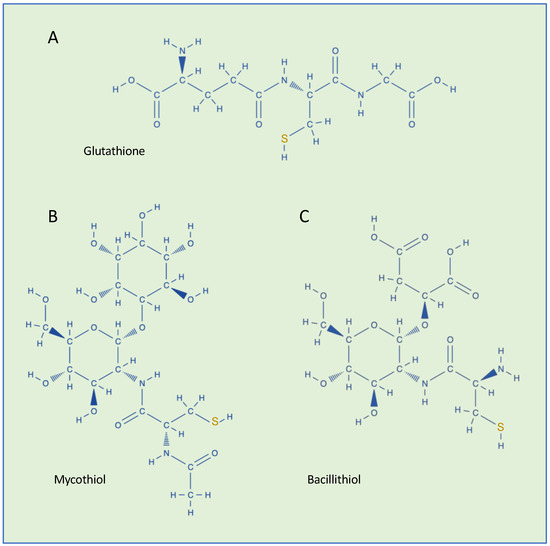
Figure 1.
Chemical structures of low-molecular-weight thiols found in bacteria. (A) Glutathione (GSH, γ-Glu-Cys-Gly), (B) mycothiol (MSH, AcCys-GlcN-Ins), and (C) bacillithiol (BSH, Cys-GlcN-Mal).
GS-ylation has been studied mainly in eukaryotes [2,5,6], including a number of studies focused on unicellular eukaryotic microorganisms such as protozoa, microalgae, and yeasts [7,8,9,10,11,12,13].
Conversely, to date, little is known about GS-ylation in bacteria. In prokaryotes, GSH is widely distributed in Gram-negative aerobic bacteria and in a few Gram-positive strains [14,15]. Like eukaryotes, in addition to its role in the defense of the cell against oxidative stress, GSH participates in many other cellular reactions [14,15]. Some bacterial Gram-positive species that lack the genes and the enzymes necessary to synthesize GSH can acquire thiol from the extracellular environment [16]. In other bacterial species, proteins are post-translationally modified using different thiols. Gram-positive Actinomycetes and Firmicutes use mycothiol and bacillithiol, respectively, as thiol-redox buffers to maintain the reduced state of the cytoplasm (Figure 1B,C) [17]. Under oxidative stress, protein S-bacillithiolation and S-mycothiolation have been observed in these bacteria [17]. Thiols are also found in archaea [18] but, currently, S-thiolation has not been reported in these microorganisms. However, since all aerobic organisms are subject to oxidative stress, S-thiolation is expected to occur also in archaea [18].
In this paper, we attempt to provide a narrative review of the state of the art in bacterial GS-ylation research, with a particular focus on recent studies highlighting the involvement of protein GS-ylation in cellular mechanisms.
2. Methods
Although this work is a “narrative review” and therefore aims to be mainly descriptive, not involving a systematic search of the literature, the cited articles were retrieved following the guidelines for systematic reviews. We searched articles published up to 13 January 2025 in the PubMed and Web of Science databases, the Google Scholar search engine, and the social network ResearchGate. There were no restrictions on the language or region. We used several keywords for retrieving cited articles, such as: “S-glutathionylation” and “microorganisms or bacteria”. We have retrieved the complete list of abstracts, and the papers that followed the defined criteria for this study were reviewed in full. Additional articles were manually searched by checking the reference lists of already included papers. Two authors (N.A. and L.F.) independently examined all the search results.
3. GS-ylation in Bacteria
3.1. Streptococcus
The Gram-positive bacterium Streptococcus is a genus that encompasses a vast array of species, the majority of which are either commensal or pathogenic for humans and animals. In contrast to S. agalactiae, which employs a bifunctional enzyme encoded by a single gene to synthesize GSH [19], pathogenic streptococci, including S. mutans and S. pneumoniae, lack the genes required for its biosynthesis [20,21]. Consequently, they import GSH from the surrounding environment [16].
In a recent report, the role of GS-ylation in S. mutants, considered to be the major pathogen in the initiation of human dental caries, has been investigated, with a focus on its effect on the interspecies competition within the oral streptococcal biofilm [22]. GS-ylation of a thioredoxin-like protein, at Cys41, protects the bacterium from the toxic effects of ROS derived from hostile bacteria, such as S. sanguinis and S. gordonii, which are pioneer colonizers of the dental plaque that produces high amounts of hydrogen peroxide during aerobic metabolism as a competitive strategy [22].
S. pneumoniae is a facultative anaerobic microorganism that is responsible for the onset of acute bacterial infections [23]. The bacterium is an important agent of community-acquired pneumonia infections in humans, and it is responsible for millions of deaths worldwide. During infections, S. pneumoniae is phagocytosed by host immune cells, which produce ROS as well as the highly reactive oxidant hypochlorous acid within the respiratory burst, thereby killing the microorganisms. The NmlR regulator (Neisseria MeR-like regulator) was identified as being strongly up-regulated in response to hypochlorous acid (HClO) stress in the transcriptome of S. pneumoniae D39 [24]. The NmlR regulator is a transcriptional activator that controls the expression of the nmlR-adhC operon under various stresses in S. pneumoniae [25]. NmlR is related to a large and diverse group of MerR-like regulators [26]. The adhC gene encodes a GSH-dependent alcohol dehydrogenase (AdhC). It has been observed that NmlR is involved in the defense against oxidative stress in S. pneumoniae [24]. In the repressor state, the Cys52 residues of the two NmlR subunits are reduced. Indeed, the basic structure of MerR proteins is a homodimer [27]. During HClO stress, NmlR is either oxidized to form intersubunit disulfides or GS-ylated, resulting in an increased level of AdhC compared to those constitutively produced. Furthermore, the levels of AdhC were found to be low in the S. pneumoniae strain with the NmlR C52A mutation in the presence of HClO. This finding confirms that the conserved Cys52 residue is required for redox-sensing and transcriptional activation by NmlR. Therefore, it was concluded that NmlR functions as a redox-sensing transcriptional activator of the adhC gene under HClO stress, depending on the Cys52 status [24].
3.2. Escherichia coli
Heat shock proteins (Hsps) form a ubiquitous and conserved protein family across prokaryotic and eukaryotic organisms. They play a crucial role in maintaining cellular protein homeostasis and protecting cells from various forms of stress [28]. Hsps function as molecular chaperones within cells, and they also play a pivotal role in regulating cell signaling, cell cycle, and apoptosis [28]. DnaK is the major bacterial Hsp70 [29], and its GS-ylation has been observed in E. coli [30]. It was shown that DnaK becomes inactive under conditions where oxidative stress is accompanied by heat shock and that GS-ylation is one of the factors that contribute to this inactivation. DnaK remains inactive until reducing conditions are restored. Mechanistically, GS-ylation affects the interaction of DnaK with its key partners: DnaJ, GrpE, and σ32. The GS-ylation of DnaK has been observed to result in reversible alterations to the secondary structure and tertiary conformation, which in turn leads to a reduction in the binding ability of the protein. The E. coli DnaK contains a cysteine residue at position 15, which is highly conserved across different species. It has been noted that this residue undergoes GS-ylation in response to oxidative stress [30].
3.3. Yersinia pestis
Y. pestis is an extremely virulent pathogen that is responsible for the potentially fatal systemic disease known as plague. To avoid phagocytosis, the bacterium is able to suppress the immune response of white blood cells such as macrophages [31]. The V-antigen (LcrV, Low-Calcium Response V protein) is involved in this process. LcrV is a secreted protein that caps the type III secretion machinery. It has been observed that the GS-ylation of LcrV enhances the pathogenicity of Y. pestis in animal models [32]. This was confirmed by the observation that the mutation Cys273Ala improved the animals’ survival [32]. Thus, Y. pestis uses GSH in host tissues to activate a virulence strategy that accelerates disease pathogenesis.
3.4. Salmonella Typhimurium
Salmonella enterica serovar Typhimurium is a Gram-negative primary enteric pathogen that infects both humans and animals [33]. In order to gain a better understanding of the cellular processes underlying infection, the complete proteome of S. Typhimurium was investigated by means of a top-down proteomic approach [34]. Top-down mass spectrometry-based proteomics is able to identify and quantify unique proteoforms by analyzing intact proteins [35], providing a wealth of information on protein isoform switching and changes in PTMs. In the case of S. Typhimurium, top-down proteomics revealed a differential use of protein S-thiolation. In fact, under basal conditions, the bacterium preferentially used GS-ylation, whereas under infectious-like conditions, S. Typhimurium exploited S-cysteinylation. The authors of this study hypothesize that this switch may happen because it is energetically more favorable to synthesize the simpler cysteine moiety, or perhaps because it provides a faster response to changing environmental conditions [34].
3.5. Acidithiobacillus caldus
A. caldus is a Gram-negative, moderately thermophilic, obligately chemolithotrophic microorganism widely used in bio-leaching processes. It has the capability of oxidizing elemental sulfur and a wide range of reduced inorganic sulfur compounds. It uses energy and electrons derived from sulfur oxidation for carbon dioxide fixation and other anabolic processes. Persulfide dioxygenases catalyze the oxidation of glutathione persulfide (GSSH) and higher homologs with sulfite and GSH as products [36]. In the A. caldus strain, persulfide dioxygenase has two surface-exposed cysteines—Cys 87 and Cys 224—involved in catalysis and/or protein stabilization. The GS-ylation of these two cysteines has been reported, suggesting that disulfide formation serves as a protective mechanism against uncontrolled thiol oxidation and the associated loss of enzyme activity [37].
3.6. Listeria monocytogenes
Cholesterol-dependent cytolysins (CDCs) consist of a family of oligomeric pore-forming toxins secreted by a range of pathogenic Gram-positive bacteria as virulence factors responsible for disrupting cellular membranes [38]. CDC listeriolysin O is produced by the facultative intracellular L. monocytogenes and plays a key role in the rapid bacterial escape from the phagolysosome into the cytoplasm where the bacterium reproduces [38]. CDCs mediate membrane binding in part through a conserved C-terminal undecapeptide, which contains a highly conserved and reactive cysteine residue. In CDC listeriolysin O, this Cys residue (Cys484) was shown to be post-translationally modified by GS-ylation [39]. It has been observed that the GS-ylation of Cys484 completely abolishes the activity of the toxin, and the inhibitory effect is fully reversible in the presence of GSH. It is possible that GS-ylation protects the cysteine residue from irreversible oxidation while its reduction is involved in carrying out the toxic activity of the protein. Therefore, GS-ylation may constitute a conserved post-translational regulatory mechanism necessary for the optimal activity of the toxin [39].
3.7. Synechocystis sp.
Cyanobacteria is a phylum of the most diverse and widely distributed prokaryotes able to perform oxygenic photosynthesis [40]. Cyanobacterium Synechocystis PCC 6803 possesses a bidirectional [NiFe]-hydrogenase (HoxEFUYH; Hox for hydrogen oxidation), usually coupling protons and electrons/hydrogen interconversion at the [NiFe] active site with NADPH as the electron donor (or vice versa) at the flavin mononucleotide active site [41]. In cyanobacteria, bidirectional hydrogenases are mainly involved in the removal of excess electrons derived from fermentation and photosynthesis, resulting in hydrogen production [42]. The heteropentameric enzyme is composed of the hydrogenase sub-complex (HoxYH) and the diaphorase sub-complex (HoxEFU) (Figure 2A). In addition to principally catalyzing reversible hydrogen oxidation, the [NiFe]-hydrogenase is involved in other processes. For example, under fermentative growth conditions, the production of hydrogen occurs as a means of maintaining redox poise. The hoxEFUYH operon is expressed under the positive control of LexA and AbrB1 regulators, and it is repressed by the negative regulation played by the binding of AbrB2 to its promoter [43,44]. It has been reported that the AbrB2 regulator can be post-translationally controlled through GS-ylation [45]. Indeed, a conserved cysteine residue at position 34 of AbrB2 is involved in the repressor activity (Figure 2B) and this cysteine is the target of GS-ylation, which regulates the binding of the hox promoter [45]. Under oxidative stress, AbrB2 is oxidized and Cys34 is glutathionylated. After recovery from oxidative stress, AbrB2 activity is retrieved by deglutathionylation. Thus, GS-ylation constitutes a significant tool for the regulation of cyanobacterial metabolism under oxidative stress conditions. This was also suggested by a large-scale proteomic analysis of GS-ylation in the cyanobacterium Synechocistis [46].
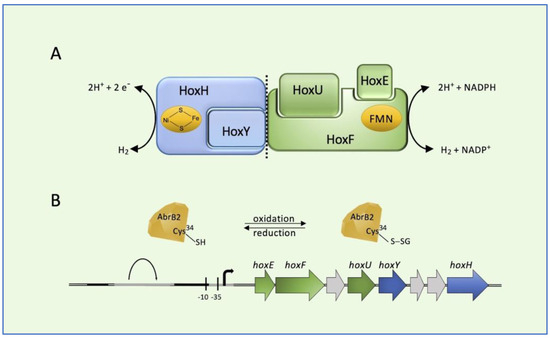
Figure 2.
Bidirectional [NiFe]-hydrogenase (HoxEFUYH; Hox for hydrogen oxidation) from cyanobacterium Synechocystis PCC 6803. (A) Schematic representation of the heteropentameric enzyme and (B) its corresponding genes in the hox locus. AbrB2 (antibiotic resistance) regulator is represented as a diamond. Black bars: LexA (locus for X-ray sensitivity A) binding region; gray bars: AbrB-binding region.
3.8. Glutathionylspermidine S-Thiolation
In Gram-negative bacteria, another defense mechanism against cysteine overoxidation is the formation of mixed disulfides between glutathionylspermidine (Gsp) and the reactive thiols of cysteine residues of proteins [47]. In addition, Gsp was found to display more reduced efficiency than GSH. Gsp synthesis is catalyzed by the Gsp synthetase/amidase through the reaction between the glycine carboxylate of the GSH and spermidine, a polyamine present in both eukaryotic and prokaryotic cells (Figure 3). Gsp synthetase/amidase is a bifunctional enzyme made of two domains that are able to catalyze the synthesis and the hydrolysis of Gsp, respectively (Figure 3). Protein S-thiolation with Gsp was first observed in E. coli [48]. It was also observed that the levels of Gsp S-thiolated proteins were increased by oxidative stress. In the presence of oxidative stress, Gsp amidase activity is inhibited by the oxidation of Cys59 residue. The inhibition of the enzyme activity leads to an increase in the concentration of Gsp followed by an accumulation of Gsp S-thiolate proteins [48]. Gsp S-thiolation has also been detected in Salmonella Typhimurium [49]. Table 1 summarizes the main target proteins undergoing GS-ylation.
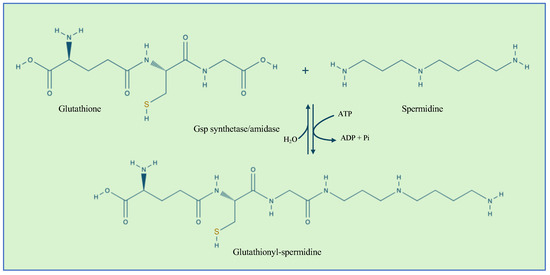
Figure 3.
The reaction catalyzed by Gsp synthetase/amidase. The enzyme contains an N-terminal amidase domain and a C-terminal synthetase domain.

Table 1.
GS-ylations and their functional role in bacteria.
4. Conclusions and Future Directions
The survey of the literature that we performed in the preparation of this short review revealed, on the one hand, that GS-ylation is a common mechanism to control protein function in bacteria belonging to different phyla but, on the other hand, still little is known on this PTM in prokaryotes when compared to eukaryotes. Given the vulnerability of proteins’ cysteine residues to conditions of oxidative stress, it is not surprising that one of the main roles of this modifier in bacteria, either synthesized by the microorganism itself or imported from the environment, is to provide protection from cysteine oxidation and consequent protein inactivation. In this regard, GSH is not the only resource used by bacteria, which can also in some cases rely on different thiols like mycothiol and bacillithiol or on the shorter version of GSH, i.e., gamma-glutamylcysteine, which is used by selected Gram-positive bacteria [18]. However, a number of studies surveyed here have also highlighted more specialized roles for GS-ylation in influencing important cellular events, ranging from the control of the DnaK interactome in E. coli to the control of phagocytosis suppression in Y. pestis and the escape from the phagolysosome of L. monocytogenes—events that underlie a considerable role of this PTM in virulence—to the control of gene transcription in cyanobacteria.
It is important to underline that many of these interesting studies that shed light on the specialized roles of GS-ylation in bacterial pathogenicity and interspecies competition and survival are quite recent and testify that this field of research is active and lively. Whole proteome PTM analysis of many different bacteria in various conditions, using mass spectrometry methods, are performed routinely today and hold the promise to identify new proteins and new mechanisms that are affected by GS-ylation in bacteria and enhance our understanding of the physiological and pathological roles played by this important PTM in bacteria.
GS-ylation in bacteria is a promising and under-explored field with many future directions, as a complete understanding of the functions of this PTM in bacteria has not yet been achieved. We have reviewed here several recent discoveries on the role of GS-ylation in oxidative stress response, virulence regulation, biofilm formation, and adaptation to environmental stress. These findings, and possibly others that will emerge from future research in this field, promise to allow for the identification of new therapeutic targets for the control of bacterial infections, especially in the face of increasing antibiotic resistance, and we believe that specific inhibitors or activators of GS-ylation pathways in different bacteria will be developed in the near future for this purpose.
Author Contributions
N.A. and L.F. wrote the manuscript. M.M. carried out the bibliography and prepared the table and figures. V.D.L. critically read the manuscript before submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partly supported by grants from the University of Chieti-Pescara “G. d’Annunzio” (to L.F. and N.A.).
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ramazi, S.; Zahiri, J. Post-Translational Modifications in Proteins: Resources, Tools and Prediction Methods. Database 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Federici, L.; Masulli, M.; De Laurenzi, V.; Allocati, N. The Role of S-Glutathionylation in Health and Disease: A Bird’s Eye View. Nutrients 2024, 16, 2753. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.W.K.; Gan, Y.-H. New Roles for Glutathione: Modulators of Bacterial Virulence and Pathogenesis. Redox Biol. 2021, 44, 102012. [Google Scholar] [CrossRef] [PubMed]
- Scirè, A.; Cianfruglia, L.; Minnelli, C.; Bartolini, D.; Torquato, P.; Principato, G.; Galli, F.; Armeni, T. Glutathione Compartmentalization and Its Role in Glutathionylation and Other Regulatory Processes of Cellular Pathways. BioFactors 2019, 45, 152–168. [Google Scholar] [CrossRef]
- Pingarron-Cardenas, G.; Onkokesung, N.; Goldberg-Cavalleri, A.; Lange, G.; Dittgen, J.; Edwards, R. Selective Herbicide Safening in Dicot Plants: A Case Study in Arabidopsis. Front. Plant Sci. 2024, 14, 1335764. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; He, Y.-Q.; Ye, T.-T.; Huang, X.; Wu, H.; Ma, T.-X.; Pritchard, H.W.; Wang, X.-F.; Xue, H. Glutathionylation of a Glycolytic Enzyme Promotes Cell Death and Vigor Loss During Aging of Elm Seeds. Plant Physiol. 2024, 195, 2596–2616. [Google Scholar] [CrossRef]
- Pretzel, J.; Gehr, M.; Eisenkolb, M.; Wang, L.; Fritz-Wolf, K.; Rahlfs, S.; Becker, K.; Jortzik, E. Characterization and Redox Regulation of Plasmodium falciparum Methionine Adenosyltransferase. J. Biochem. 2016, 160, 355–367. [Google Scholar] [CrossRef]
- Schipper, S.; Wu, H.; Furdui, C.M.; Poole, L.B.; Delahunty, C.M.; Park, R.; Yates, J.R.; Becker, K.; Przyborski, J.M. Identification of Sulfenylation Patterns in Trophozoite Stage Plasmodium falciparum Using a Non-Dimedone Based Probe. Mol. Biochem. Parasitol. 2021, 242, 111362. [Google Scholar] [CrossRef]
- Xing, C.; Li, J.; Lam, S.M.; Yuan, H.; Shui, G.; Yang, J. The Role of Glutathione-Mediated Triacylglycerol Synthesis in the Response to Ultra-High Cadmium Stress in Auxenochlorella protothecoides. J. Environ. Sci. 2021, 108, 58–69. [Google Scholar] [CrossRef]
- Gergondey, R.; Garcia, C.; Serre, V.; Camadro, J.M.; Auchère, F. The Adaptive Metabolic Response Involves Specific Protein Glutathionylation during the Filamentation Process in the Pathogen Candida albicans. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2016, 1862, 1309–1323. [Google Scholar] [CrossRef]
- Shino, S.; Nasuno, R.; Takagi, H. S-Glutathionylation of Fructose-1,6-Bisphosphate Aldolase Confers Nitrosative Stress Tolerance on Yeast Cells via a Metabolic Switch. Free Radic. Biol. Med. 2022, 193, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Christiaens, R.; Janssens, P.; Collin, S. Unexpected Behavior of a Maltose-Negative Saccharomyces cerevisiae Yeast: Higher Release of Polyfunctional Thiols from Glutathionylated Than from Cysteinylated S-Conjugates. Fermentation 2024, 10, 276. [Google Scholar] [CrossRef]
- Vicente, J.; Kiene, F.; Fracassetti, D.; De Noni, I.; Shemehen, R.; Tarasov, A.; Dobrydnev, A.V.; Marquina, D.; Santos, A.; Rauhut, D.; et al. Precursors Consumption Preferences and Thiol Release Capacity of the Wine Yeasts Saccharomyces cerevisiae, Torulaspora delbrueckii, and Lachancea thermotolerans. Int. J. Food Microbiol. 2024, 425, 110858. [Google Scholar] [CrossRef]
- Allocati, N.; Federici, L.; Masulli, M.; Di Ilio, C. Glutathione Transferases in Bacteria: Bacterial GSTs. FEBS J. 2009, 276, 58–75. [Google Scholar] [CrossRef]
- Allocati, N.; Federici, L.; Masulli, M.; Di Ilio, C. Distribution of Glutathione Transferases in Gram-Positive Bacteria and Archaea. Biochimie 2012, 94, 588–596. [Google Scholar] [CrossRef]
- Sikanyika, M.; Aragão, D.; McDevitt, C.A.; Maher, M.J. The Structure and Activity of the Glutathione Reductase from Streptococcus pneumoniae. Acta Crystallogr. F Struct. Biol. Commun. 2019, 75, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox Regulation by Reversible Protein S-Thiolation in Gram-Positive Bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Maupin-Furlow, J.A. Redox and Thiols in Archaea. Antioxidants 2020, 9, 381. [Google Scholar] [CrossRef]
- Walker, E.A.; Port, G.C.; Caparon, M.G.; Janowiak, B.E. Glutathione Synthesis Contributes to Virulence of Streptococcus agalactiae in a Murine Model of Sepsis. J. Bacteriol. 2019, 201, 10-1128. [Google Scholar] [CrossRef]
- Potter, A.J.; Trappetti, C.; Paton, J.C. Streptococcus pneumoniae Uses Glutathione To Defend against Oxidative Stress and Metal Ion Toxicity. J. Bacteriol. 2012, 194, 6248–6254. [Google Scholar] [CrossRef]
- Sperandio, B.; Gautier, C.; Pons, N.; Ehrlich, D.S.; Renault, P.; Guédon, E. Three Paralogous LysR-Type Transcriptional Regulators Control Sulfur Amino Acid Supply in Streptococcus mutans. J. Bacteriol. 2010, 192, 3464–3473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Zhang, C.; Li, C.; Zhou, J.; Xu, X.; Peng, X.; Zhou, X. S-Glutathionylation Proteome Profiling Reveals a Crucial Role of a Thioredoxin-like Protein in Interspecies Competition and Cariogenecity of Streptococcus mutans. PLoS Pathog. 2020, 16, e1008774. [Google Scholar] [CrossRef] [PubMed]
- Loughran, A.J.; Orihuela, C.J.; Tuomanen, E.I. Streptococcus pneumoniae: Invasion and Inflammation. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, V.N.; Linzner, N.; Busche, T.; Said, N.; Weise, C.; Kalinowski, J.; Wahl, M.C.; Antelmann, H. The MerR -family Regulator NmlR Is Involved in the Defense against Oxidative Stress in Streptococcus pneumoniae. Mol. Microbiol. 2023, 119, 191–207. [Google Scholar] [CrossRef]
- Potter, A.J.; Kidd, S.P.; McEwan, A.G.; Paton, J.C. The MerR/NmlR Family Transcription Factor of Streptococcus pneumoniae Responds to Carbonyl Stress and Modulates Hydrogen Peroxide Production. J. Bacteriol. 2010, 192, 4063–4066. [Google Scholar] [CrossRef]
- McEwan, A.G.; Djoko, K.Y.; Chen, N.H.; Couñago, R.L.M.; Kidd, S.P.; Potter, A.J.; Jennings, M.P. Novel Bacterial MerR-like Regulators. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 58, pp. 1–22. ISBN 978-0-12-381043-4. [Google Scholar]
- Schumacher, M.A.; Den Hengst, C.D.; Bush, M.J.; Le, T.B.K.; Tran, N.T.; Chandra, G.; Zeng, W.; Travis, B.; Brennan, R.G.; Buttner, M.J. The MerR-like Protein BldC Binds DNA Direct Repeats as Cooperative Multimers to Regulate Streptomyces Development. Nat. Commun. 2018, 9, 1139. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Mayer, M.P. The Hsp70-Chaperone Machines in Bacteria. Front. Mol. Biosci. 2021, 8, 694012. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Wu, S.; Gong, W.; Chen, C.; Perrett, S. Glutathionylation of the Bacterial Hsp70 Chaperone DnaK Provides a Link between Oxidative Stress and the Heat Shock Response. J. Biol. Chem. 2016, 291, 6967–6981. [Google Scholar] [CrossRef]
- Heesemann, J.; Sing, A.; Trülzsch, K. Yersinia’s Stratagem: Targeting Innate and Adaptive Immune Defense. Curr. Opin. Microbiol. 2006, 9, 55–61. [Google Scholar] [CrossRef]
- Mitchell, A.; Tam, C.; Elli, D.; Charlton, T.; Osei-Owusu, P.; Fazlollahi, F.; Faull, K.F.; Schneewind, O. Glutathionylation of Yersinia pestis LcrV and Its Effects on Plague Pathogenesis. mBio 2017, 8, e00646-17. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, A.; Vila, J. Salmonella enterica Serovar Typhimurium Skills To Succeed in the Host: Virulence and Regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef] [PubMed]
- Ansong, C.; Wu, S.; Meng, D.; Liu, X.; Brewer, H.M.; Deatherage Kaiser, B.L.; Nakayasu, E.S.; Cort, J.R.; Pevzner, P.; Smith, R.D.; et al. Top-down Proteomics Reveals a Unique Protein S-Thiolation Switch in Salmonella typhimurium in Response to Infection-like Conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 10153–10158. [Google Scholar] [CrossRef]
- Brown, K.A.; Melby, J.A.; Roberts, D.S.; Ge, Y. Top-down Proteomics: Challenges, Innovations, and Applications in Basic and Clinical Research. Expert. Rev. Proteom. 2020, 17, 719–733. [Google Scholar] [CrossRef]
- Chen, L.; Ren, Y.; Lin, J.; Liu, X.; Pang, X.; Lin, J. Acidithiobacillus caldus Sulfur Oxidation Model Based on Transcriptome Analysis between the Wild Type and Sulfur Oxygenase Reductase Defective Mutant. PLoS ONE 2012, 7, e39470. [Google Scholar] [CrossRef]
- Rühl, P.; Haas, P.; Seipel, D.; Becker, J.; Kletzin, A. Persulfide Dioxygenase From Acidithiobacillus caldus: Variable Roles of Cysteine Residues and Hydrogen Bond Networks of the Active Site. Front. Microbiol. 2018, 9, 1610. [Google Scholar] [CrossRef]
- Morton, C.J.; Sani, M.-A.; Parker, M.W.; Separovic, F. Cholesterol-Dependent Cytolysins: Membrane and Protein Structural Requirements for Pore Formation: Focus Review. Chem. Rev. 2019, 119, 7721–7736. [Google Scholar] [CrossRef]
- Portman, J.L.; Huang, Q.; Reniere, M.L.; Iavarone, A.T.; Portnoy, D.A. Activity of the Pore-Forming Virulence Factor Listeriolysin O Is Reversibly Inhibited by Naturally Occurring S-Glutathionylation. Infect. Immun. 2017, 85, e00959-16. [Google Scholar] [CrossRef]
- Walter, J.M.; Coutinho, F.H.; Dutilh, B.E.; Swings, J.; Thompson, F.L.; Thompson, C.C. Ecogenomics and Taxonomy of Cyanobacteria Phylum. Front. Microbiol. 2017, 8, 2132. [Google Scholar] [CrossRef]
- McIntosh, C.L.; Germer, F.; Schulz, R.; Appel, J.; Jones, A.K. The [NiFe]-Hydrogenase of the Cyanobacterium Synechocystis sp. PCC 6803 Works Bidirectionally with a Bias to H2 Production. J. Am. Chem. Soc. 2011, 133, 11308–11319. [Google Scholar] [CrossRef]
- Horch, M.; Lauterbach, L.; Lenz, O.; Hildebrandt, P.; Zebger, I. NAD(H)-Coupled Hydrogen Cycling—Structure-Function Relationships of Bidirectional [NiFe] Hydrogenases. FEBS Lett. 2012, 586, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Lindblad, P. Transcriptional Regulation of the Cyanobacterial Bidirectional Hox-Hydrogenase. Dalton Trans. 2009, 45, 9990–9996. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, J.; Saenkham, P.; Sakr, S.; Leplat, C.; Ortega-Ramos, M.; Bottin, H.; Cournac, L.; Cassier-Chauvat, C.; Chauvat, F. The AbrB2 Autorepressor, Expressed from an Atypical Promoter, Represses the Hydrogenase Operon To Regulate Hydrogen Production in Synechocystis Strain PCC6803. J. Bacteriol. 2012, 194, 5423–5433. [Google Scholar] [CrossRef] [PubMed]
- Sakr, S.; Dutheil, J.; Saenkham, P.; Bottin, H.; Leplat, C.; Ortega-Ramos, M.; Aude, J.-C.; Chapuis, V.; Guedeney, G.; Decottignies, P.; et al. The Activity of the Synechocystis PCC6803 AbrB2 Regulator of Hydrogen Production Can Be Post-Translationally Controlled through Glutathionylation. Int. J. Hydrogen Energy 2013, 38, 13547–13555. [Google Scholar] [CrossRef]
- Chardonnet, S.; Sakr, S.; Cassier-Chauvat, C.; Le Maréchal, P.; Chauvat, F.; Lemaire, S.D.; Decottignies, P. First Proteomic Study of S-Glutathionylation in Cyanobacteria. J. Proteome Res. 2015, 14, 59–71. [Google Scholar] [CrossRef]
- Lin, J.; Chiang, B.-Y.; Chou, C.-C.; Chen, T.-C.; Chen, Y.-J.; Chen, Y.-J.; Lin, C.-H. Glutathionylspermidine in the Modification of Protein SH Groups: The Enzymology and Its Application to Study Protein Glutathionylation. Molecules 2015, 20, 1452–1474. [Google Scholar] [CrossRef]
- Chiang, B.-Y.; Chen, T.-C.; Pai, C.-H.; Chou, C.-C.; Chen, H.-H.; Ko, T.-P.; Hsu, W.-H.; Chang, C.-Y.; Wu, W.-F.; Wang, A.H.-J.; et al. Protein S-Thiolation by Glutathionylspermidine (Gsp). J. Biol. Chem. 2010, 285, 25345–25353. [Google Scholar] [CrossRef]
- Nair, A.V.; Singh, A.; Rajmani, R.S.; Chakravortty, D. Salmonella Typhimurium Employs Spermidine to Exert Protection against ROS-Mediated Cytotoxicity and Rewires Host Polyamine Metabolism to Ameliorate Its Survival in Macrophages. Redox Biol. 2024, 72, 103151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).