Diversity and Pathogenicity of Colletotrichum Species Causing Coffee Anthracnose in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation, and Purification
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Phylogenetic Analysis
2.4. Morphological Analysis
2.5. Pathogenicity Assay
3. Results
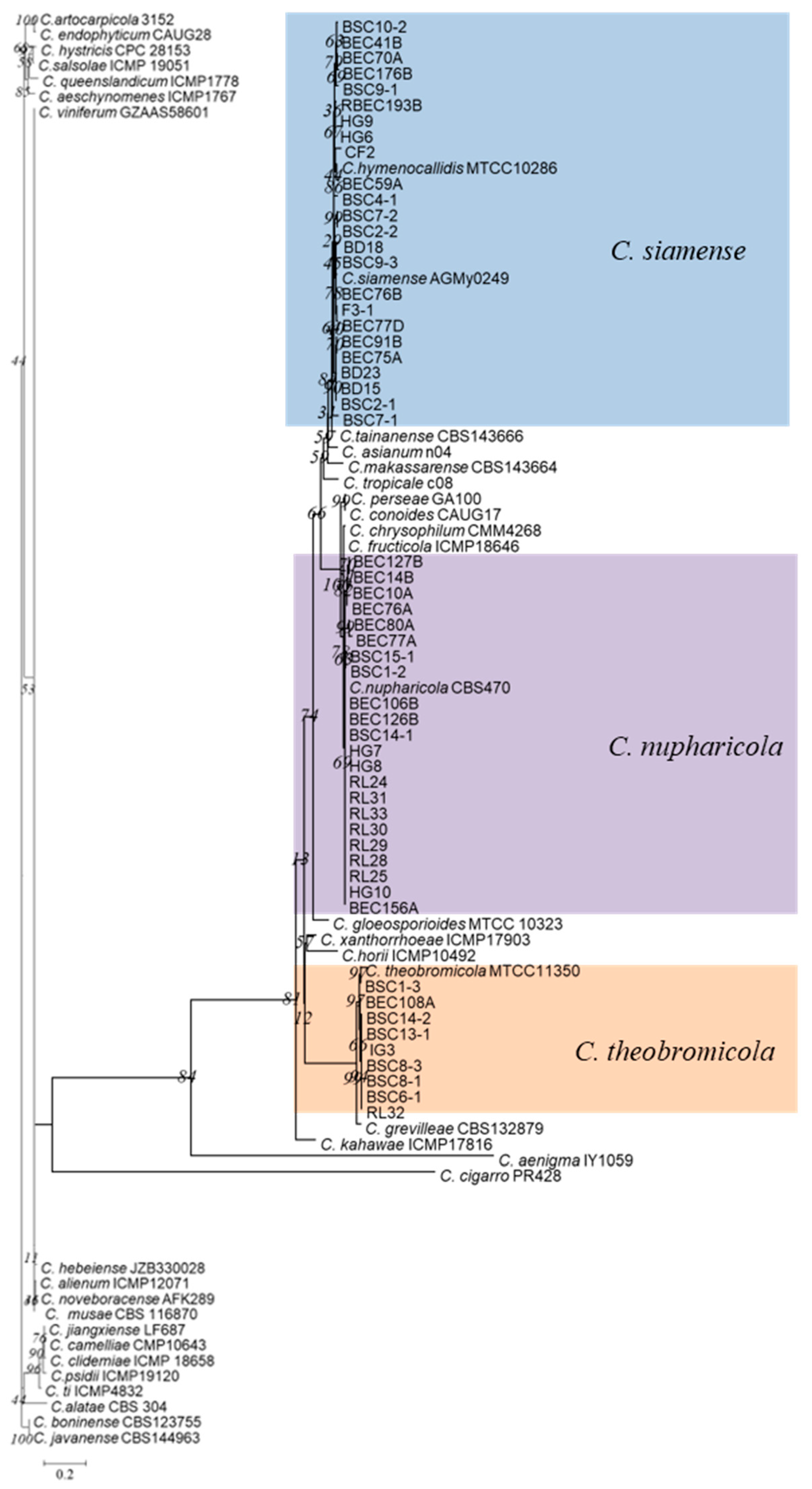
3.1. Phylogenetic Analyses
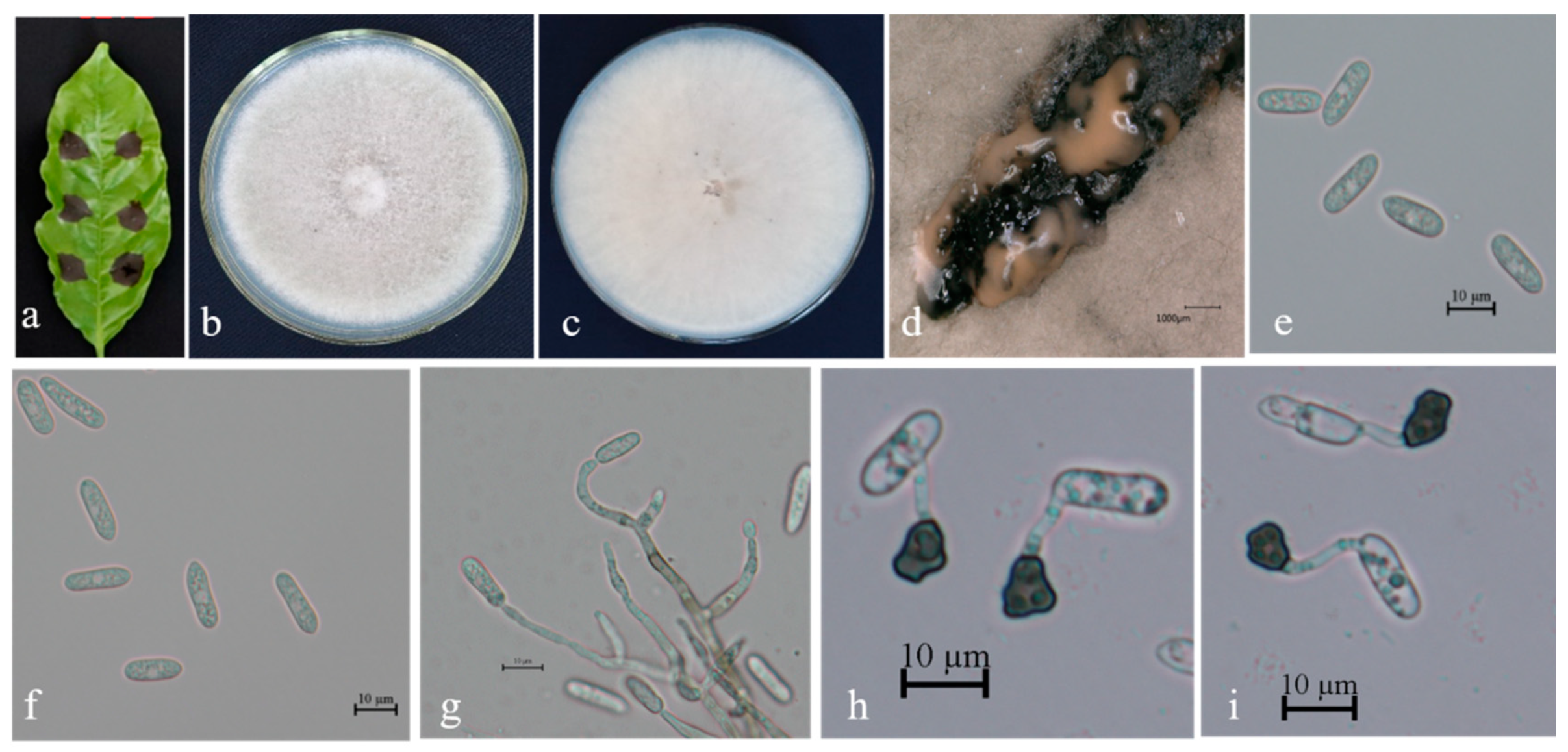
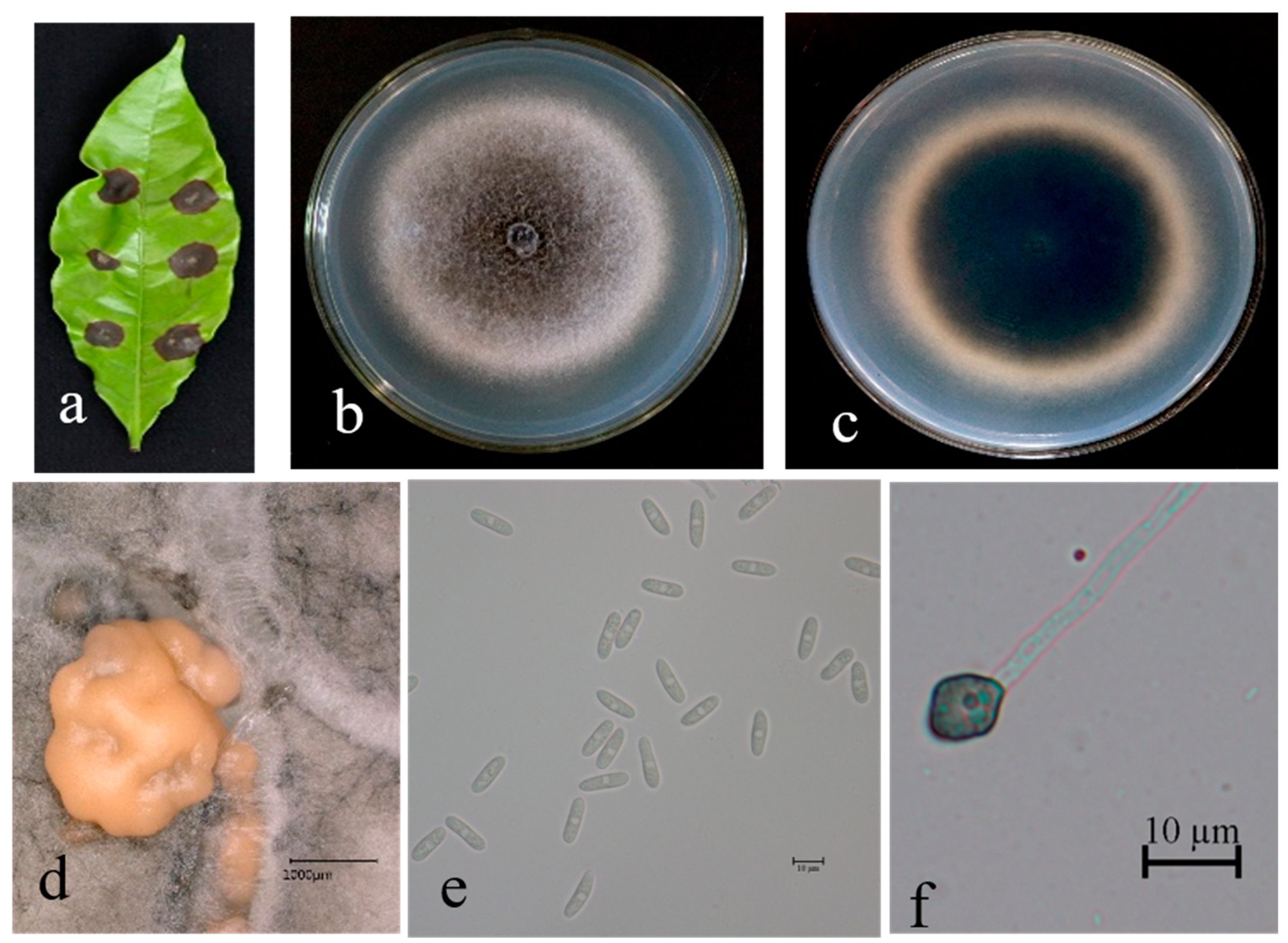
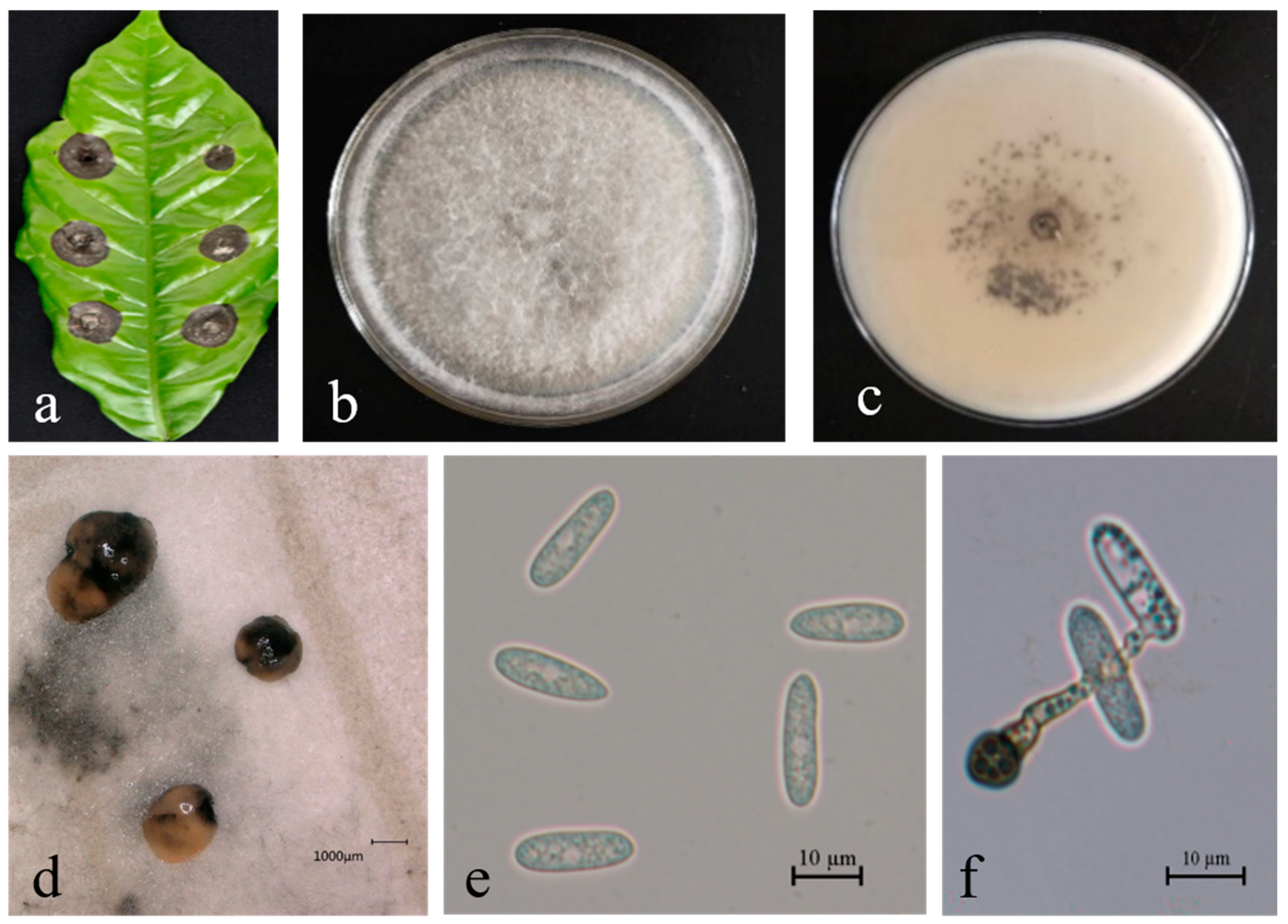
3.2. Taxonomy
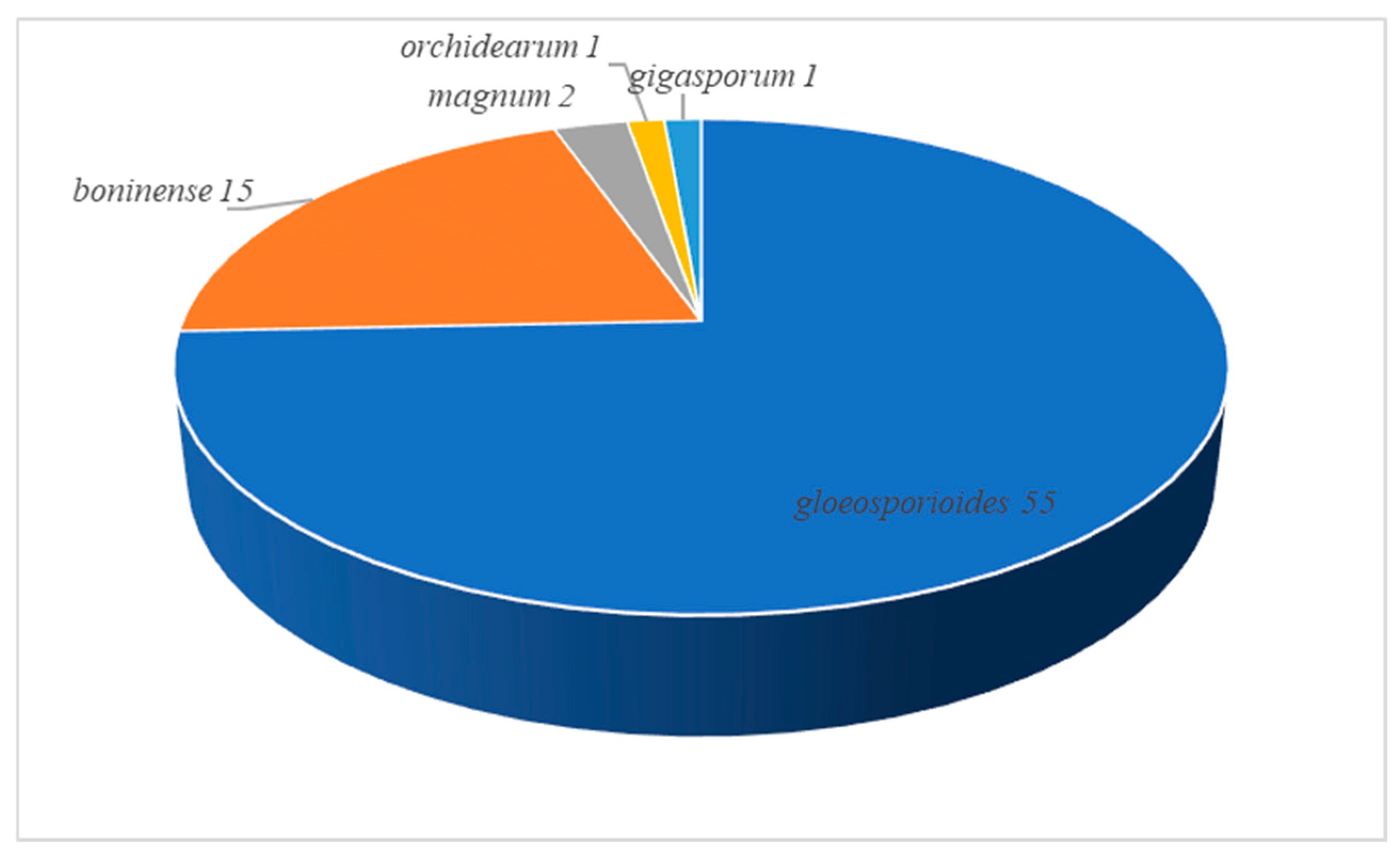
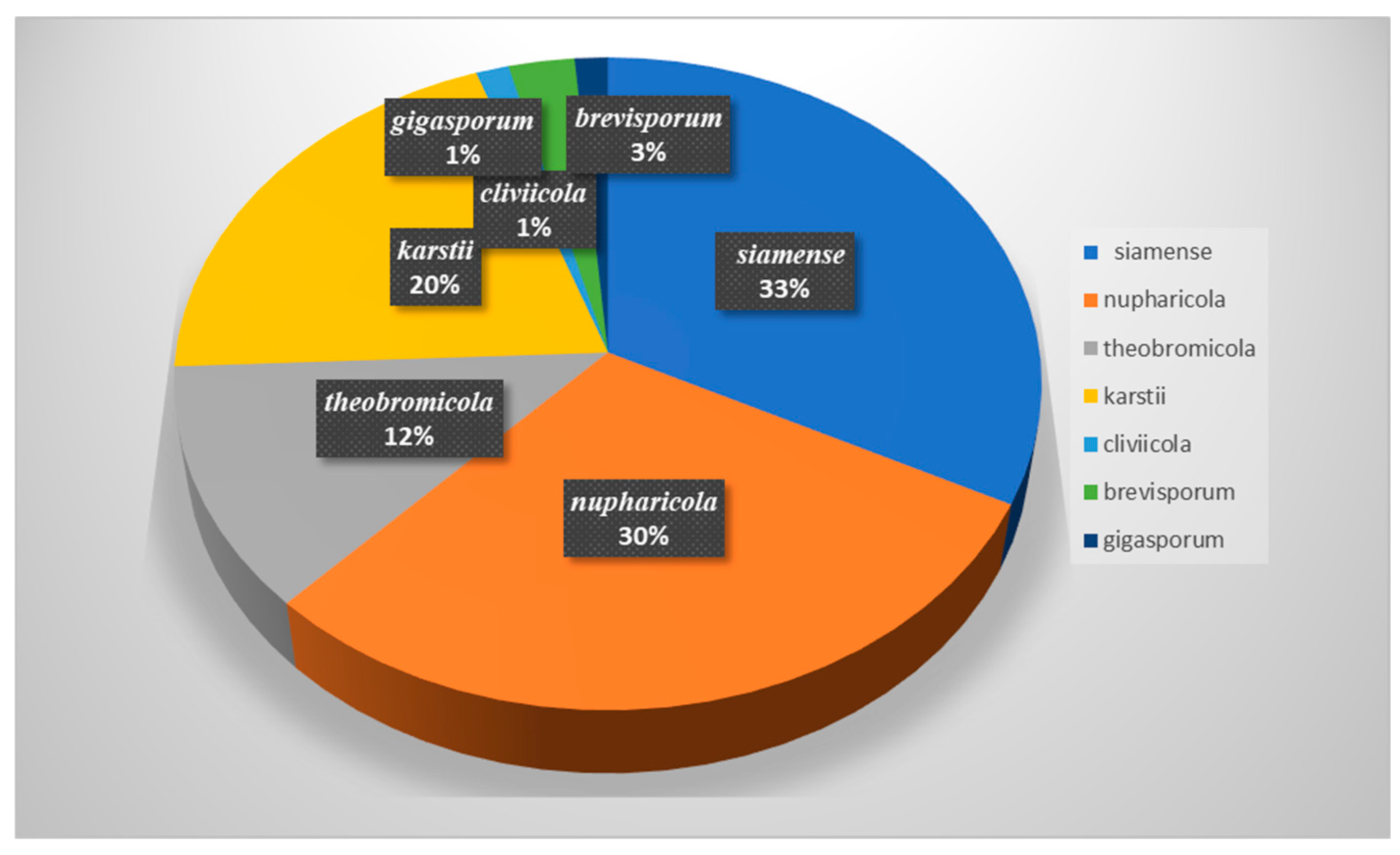
3.3. Species Diversity of Colletotrichum in China
3.4. Pathogenicity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, W.J.; Zhao, J.P.; Hu, R.S.; Dong, Y.P.; Tan, L.H. Differentiation of Chinese robusta coffees according to species, using a combined electronic nose and tongue, with the aid of chemometrics. Food Chem. 2017, 229, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Avelino, J.; Allinne, C.; Cerda, R.; Willocquet, L.; Savary, S. Multiple-disease system in coffee: From crop loss assessment to sustainable management. Annu. Rev. Phytopathol. 2018, 56, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Wan, L.S.; Peng, X.R.; Yu, Y.M.; Zhang, Z.R.; Zhou, L.; Li, Z.R.; Qiu, M.H. Characterization of new Ent-kaurane diterpenoids of Yunnan Arabica coffee beans. Nat. Prod. Bioprospect. 2016, 6, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Vossen, H.A.M.V.D.; Walyaro, D.J. Additional evidence for oligogenic inheritance of durable host resistance to coffee berry disease (Colletotrichum kahawae) in arabica coffee (Coffea arabica L.). Euphytica 2009, 165, 105–111. [Google Scholar] [CrossRef]
- Nguyen, T.H.P.; Säll, T.; Bryngelsson, T.; Liljeroth, E. Variation among Colletotrichum gloeosporioides isolates from infected coffee berries at different locations in Vietnam. Plant Pathol. 2009, 58, 898–909. [Google Scholar] [CrossRef]
- Zheng, X.L.; He, C.P.; Gao, Y.N.; Zhang, G.N.; Xi, J.G. Biological characteristics and toxicity determination of the Colletotrichum gloeosporioides Penz from Coffea arabica Linn. Chin. J. Trop. Agric. 2015, 35, 94–98. [Google Scholar]
- Abreu, M.S.; Ferreira, J.B.; Martins, F.G. Mancha manteigosa no context do complex Colletotrichum em cafeeiros. In Simposio de Manejo de Plantas: Manejo Fitossanitário do Cafeerio; UFLA: Lavras, Brazil, 2008; pp. 105–126. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretotius, Z.A.; Hammond-Kosack, K.E.; Pietro, A.D.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Guarro, J.; Svidzinski, T.E.; Zaror, L.; Foriaz, M.H.; Gené, J.; Fischman, O. Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioides. J. Clin. Microbiol. 1998, 36, 3060–3065. [Google Scholar] [CrossRef]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009, 39, 45–87. [Google Scholar]
- Shiraishi, A.; Araki-Sasaki, A.; Mitani, A.; Miyamoto, H.; Ohashi, Y.C. Clinical characteristics of keratitis due to Colletotrichum gloeosporioides. J. Ocul. Pharmacol. Ther. 2011, 27, 487–491. [Google Scholar] [CrossRef]
- Shivaprakash, M.R.; Appannanavar, S.B.; Dhaliwal, M.; Gupta, A.; Gupta, S.; Gupta, A.; Chakrabarti, K. Colletotrichum truncatum: An unusual pathogen causing mycotic keratitis and endophthalmitis. J. Clin. Microbiol. 2011, 49, 2894–2898. [Google Scholar] [CrossRef]
- Liu, F.; Cai, L.; Crous, P.W.; Damm, U. The Colletotrichum gigasporum species complex. Persoonia 2014, 33, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Buchta, V.; Nekolová, J.; Jirásková, N.; Bolehovská, R.; Wipler, J.; Hubka, V. Fungal keratitis caused by Colletotrichum dematium: Case study and review. Mycopathologia 2019, 184, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- Cannon, P.F.; Damm, U.; Johnston, P.R.; Weir, B.S. Colletotrichum: Current status and future directions. Stud. Mycol. 2012, 73, 181–213. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Damm, U.; Cai, L.; Yan, J.Y. Notes on currently accepted species of Colletotrichum. Mycosphere 2016, 7, 1192–1260. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Crous, P.W. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Chen, Y.J.; Papp, V.; Palla, B.; Papp, D.; Bhunjun, C.S.; Hurdeal, V.G.; Senwanna, C.; Manawasinghe, I.S. One stop shop IV: Taxonomic update with molecular phylogeny for important phytopathogenic genera: 76–100. Fungal Divers. 2020, 103, 87–218. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–114. [Google Scholar] [CrossRef]
- Nguyen, P.T.H.; Pettersson, O.V.; Olsson, P.; Liljeroth, E. Identification of Colletotrichum species associated with anthracnose disease of coffee in Vietnam. Eur. J. Plant Pathol. 2010, 127, 73–87. [Google Scholar] [CrossRef][Green Version]
- Prihastuti, H.; Cai, L.; Chen, H.; Mckenzie, E.H.C.; Hyde, K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009, 39, 89–109. [Google Scholar]
- Liu, F.; Ma, Z.Y.; Hou, L.W.; Diao, Y.Z.; Wu, W.P.; Damm, U.; Song, S.; Cai, L. Updating species diversity of Colletotrichum, with a phylogenomic overview. Stud. Mycol. 2022, 101, 1–56. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.N.; Talhinhas, P.; Várzea, V.; Cai, L.; Paulo, O.S.; Batista, D. Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: An example from coffee (Coffea spp.) hosts. Mycologia 2012, 104, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Cao, X.R.; Xu, X.M.; Che, H.Y.; Swet, J.T.; Luo, D.Q. Characteristics and distribution of Colletotrichum species in coffee plantations in Hainan, China. Plant Pathol. 2019, 6, 1146–1156. [Google Scholar] [CrossRef]
- Cristóbal-Martínez, A.L.; de Jesús Yáǹez-Morales, M.; Solano-Vidal, R. Diversity of Colletotrichum species in coffee (Coffea arabica) plantations in Mexico. Eur. J. Plant Pathol. 2017, 147, 605–614. [Google Scholar] [CrossRef]
- Sutton, B.C.; Bailey, J.A.; Jeger, M.J. The genus Glomerella and its anamorph Colletotrichum. Colletotrichum Biol. Pathol. Control 1992, 78, 1–26. [Google Scholar]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.; Waller, J.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Liu, F.; Wang, M.; Damm, U.; Crous, P.W.; Cai, L. Species boundaries in plant pathogenic fungi: A Colletotrichum case study. BMC Evol. Biol. 2016, 16, 81. [Google Scholar] [CrossRef]
- Samson, R.A.; Noonim, P.; Meijer, M.; Houbraken, J.A.M.P.; Frisvad, J.C.; Varga, J. Diagnostic tools to identify black aspergilli. Stud. Mycol. 2007, 59, 129–145. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Johnston, P.R.; Weir, B.S.; Tan, Y.P.; Shivas, R.G.; Crous, P.W. The Colletotrichum boninense species complex. Stud. Mycol. 2012, 73, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Cannon, P.F.; Liu, F.; Barreto, R.W.; Guatimosim, E.; Crous, P.W. The Colletotrichum orbiculare species complex: Important pathogens of field crops and weeds. Fungal Divers. 2013, 61, 29–59. [Google Scholar] [CrossRef]
- Damm, U.; O’Connell, R.J.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum destructivum species complex-hemibiotrophic pathogens of forage and field crops. Stud. Mycol. 2014, 79, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, Q.C.; Zhang, Y.; Li, D.W.; Ye, J.R. Colletotrichum gloeosporioides sensu stricto is a pathogen of leaf anthracnose on evergreen spindle tree (Euonymus japonicus). Plant Dis. 2016, 100, 672–678. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, S.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols a Guide to Methods & Applications; Academic Press: New York, NY, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous as conycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Templeton, M.D.; Rikkerink, E.H.A.; Solon, S.L.; Crowhurst, W.N. Cloning and molecular characterization of the glyceraldehyde-3-phosphate dehydrogenase-encoding gene and cDNA from the plant pathogenic fungus Glomerella cingulata. Gene 1992, 122, 225–230. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetic analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Johnson, D.A.; Carris, L.M.; Rogers, J.D. Morphological and molecular characterization of Colletotrichum nymphaeae and C. nupharicola sp. nov. on water-lilies (Nymphaea and Nuphar). Mycol. Res. 1997, 101, 641–649. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Cai, L.; Hyde, K.D. New species and notes of Colletotrichum on daylilies (Hemerocallis spp.). Trop. Plant Path. 2012, 37, 165–174. [Google Scholar] [CrossRef]
- Damm, U.; Sato, T.; Alizadeh, A.; Groenewald, J.Z.; Crous, P.W. The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Stud. Mycol. 2019, 92, 1–46. [Google Scholar] [CrossRef]
- Noireung, P.; Phoulivong, S.; Liu, F.; Cai, L.; Hyde, K.D. Novel species of Colletotrichum revealed by morphology and molecular analysis. Cryptogam. Mycol. 2012, 33, 347–362. [Google Scholar] [CrossRef]
- Rakotoniriana, E.F.; Rafamantanana, M.; Randriamampionona, D.; Rabemanantsoa, C.; Urveg-Ratsimamanga, S.; Jaziri, M.E. Study in vitro of the impact of endophytic bacteria isolated from Centella asiatica on the disease incidence caused by the hemibiotrophic fungus Colletotrichum higginsianum. Antonie Leeuwenhoek 2013, 103, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Prathibha, V.H.; Rajesh, M.K.; Dinesh, A.; Patil, B.; Daliyamol; Nagaraja, N.R.; Rajkumar; Sabana, A.A.; Gangaraj, K.P.; Thejasri, K.P.; et al. Multi-gene phylogeny and phenotypic analyses revealed an association of different Colletotrichum species with inflorescence dieback and leaf spot of arecanut in India. Physiol. Mol. Plant Pathol. 2024, 134, 102416. [Google Scholar] [CrossRef]
- James, R.S.; Ray, J.; Tan, Y.P.; Shivas, G.R. Colletotrichum siamense, C. theobromicola and C. queenslandicum from several plant species and the identification of C. asianum in the Northern Territory, Australia. Australas. Plant Dis. Notes 2014, 9, 138. [Google Scholar] [CrossRef]
- Damm, U.; Sun, Y.C.; Huang, C.J. Colletotrichum eriobotryae sp. nov. and C. nymphaeae, the anthracnose pathogens of loquat fruit in central Taiwan, and their sensitivity to azoxystrobin. Mycol. Prog. 2020, 19, 367–380. [Google Scholar] [CrossRef]
- Crouch, J.A. Colletotrichum caudatum s.l. is a species complex. IMA Fungus 2014, 5, 17–30. [Google Scholar] [CrossRef]
- Liu, F. Taxonomy and Biodiversity of Colletotrichum. Ph.D. Thesis, University Utrecht, Utrecht, The Netherlands, 2016. [Google Scholar]
- Fu, M.; Crous, P.W.; Bai, Q. Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia 2019, 42, 1–35. [Google Scholar] [CrossRef]
- Zhang, W.; Damm, U.; Crous, P.W.; Groenewald, J.Z.; Li, Y. Anthracnose disease of carpetgrass (Axonopus compressus) caused by Colletotrichum hainanense sp. nov. Plant Dis. 2020, 104, 1744–1750. [Google Scholar] [CrossRef]
- Liu, F.; Weir, B.S.; Damm, U.; Crous, P.W.; Wang, Y.; Liu, B.; Meng, Z.; Cai, L. Unravelling Colletotrichum species associated with Camellia: Employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 2015, 35, 63–86. [Google Scholar] [CrossRef]
- Vieira, W.A.S.; Bezerra, P.A.; da Silva, A.C.; Veloso, J.S.; Câmara, M.P.S.; Doyle, V.P. Optimal markers for the identification of Colletotrichum species. Mol. Phylogenet. Evol. 2020, 143, 10664. [Google Scholar] [CrossRef] [PubMed]
- Takata, Y.; Komine, M.; Uchikawa, K.; Nozawa, S.; Watanabe, K. Pathogenicity of Colletotrichum species isolated from rotten fruit and asymptomatic flowers of loquat in Nagasaki Prefecture, Japan and characterization of C. nagasakiense Takata & Kyoko Watan. sp. nov. Fungal Dis. 2024, 90, 69–81. [Google Scholar]
- Wang, C.B.; Jiang, N.; Xue, H.; Piao, C.G.; Li, Y. Colletotrichum chinense sp. nov. from Yucca gloriosa and C. quercicola sp. nov. from Quercus variabilis in China. MycoKeys 2022, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]





| Location | Host Plant | GPS Coordinates | No. of Samples | No. of Isolates |
|---|---|---|---|---|
| Yunnan tropical crop institute demonstration base | Arabica | 22°47′47″; 100°58′59″ | 12 | 9 |
| Nandao River, Yunnan province coffee planting base | Arabica | 22°36′52″; 101°0′38″ | 9 | 6 |
| Malipo, Wenshan, Yunnan coffee planting base | Arabica | 23°12′47″; 104°54′1″ | 10 | 7 |
| Yunnan agricultural germplasm resources nursery | Arabica | 22°37′37″; 100°59′47″ | 17 | 13 |
| Yunnan, Xinghua farm | Arabica | 24°11′41″; 98°10′11″ | 4 | 2 |
| Yunnan, Mangshi, Bandong coffee planting base | Arabica | 24°15′7″; 98°7′28″ | 16 | 3 |
| Yunnan, Mangshi, Hougu coffee base | Arabica | 24°21′38″; 98°27′44″ | 10 | 8 |
| Hainan, Fushan coffee planting base | Robusta/Arabica | 19°49′55″; 109°55′33″ | 8 | 4 |
| Hainan, Baisha coffee planting base | Robusta/Arabica | 19°9′55″; 109°28′39″ | 34 | 22 |
| Locus | Gene | Primers | Sequence (5′-3′) | Reference |
|---|---|---|---|---|
| Internal transcribed spacer regions with intervening 5.8S nrRNA gene | ITS | ITS1 ITS4 | TCCGTAGGTGAACCTGCGG TCCTCCGCTTATTGATATGC | White et al., 1990 [36] |
| Partial actin gene | ACT | ACT-512F ACT-783R | ATGTGCAAGGCCGGTTTCGC TACGAGTCCTTCTGGCCCAT | Carbone et al., 1999 [37] |
| Partial chitin synthase 1 gene | CHS-1 | CHS-79F CHS-354R | TGGGGCAAGGATGCTTGGTTGAAG TGGAAGAACCATCTGTGAGAGTTG | Carbone et al., 1999 [37] |
| Partial glyceraldehyde-3-phosphate dehydrogenase gene | GAPDH | GDF GDR | GCCGTCAACGACCCCTTCATTGA GGGTGGAGTCGTACTTGAGCATGT | Templeton et al., 1992 [38] |
| Partial mating type protein 1-2-1 gene | ApMat | AMF AMR | TCATTCTACGTATGTGCCCG CCAGAAATACACCGAACTTGC | Silva et al., 2012 [24] |
| Species | Isolates | Variety | Arabica | Robusta | ||
|---|---|---|---|---|---|---|
| Young Leaf | Mature Leaf | Young Leaf | Mature Leaf | |||
| C. brevisporum | BEC92 | Arabica | 16.7 | 8.5 | 0 | 0 |
| BSC15-2 | Robusta | 33.3 | 25 | 50 | 33.3 | |
| C. cliviicola | FS4-2 | Robusta | 25 | 16.7 | 50 | 16.7 |
| C. gigasporum | BEC191A | Arabica | 75 | 66.7 | 50 | 50 |
| C. karstii | Bai1 | Robusta | 83.3 | 75 | 100 | 66.7 |
| BEC26C | Arabica | 33.3 | 16.7 | 50 | 33.3 | |
| C. nupharicola | BSC1-2 | Robusta | 100 | 50 | 75 | 75 |
| BEC106B | Arabica | 33.3 | 33.3 | 50 | 33.3 | |
| C. siamense | BSC4-1 | Robusta | 50 | 75 | 75 | 100 |
| HG 9 | Arabica | 75 | 50 | 50 | 50 | |
| C. theobromicola | BSC1-3 | Robusta | 75 | 100 | 100 | 100 |
| IG3 | Arabica | 33.3 | 16.7 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, W.; Hu, X.; He, C.; Liang, Y.; Huang, X.; Yi, K.; Wu, W. Diversity and Pathogenicity of Colletotrichum Species Causing Coffee Anthracnose in China. Microorganisms 2025, 13, 512. https://doi.org/10.3390/microorganisms13030512
Lu Y, Zhang W, Hu X, He C, Liang Y, Huang X, Yi K, Wu W. Diversity and Pathogenicity of Colletotrichum Species Causing Coffee Anthracnose in China. Microorganisms. 2025; 13(3):512. https://doi.org/10.3390/microorganisms13030512
Chicago/Turabian StyleLu, Ying, Weiyi Zhang, Xiaoli Hu, Chunping He, Yanqiong Liang, Xing Huang, Kexian Yi, and Weihuai Wu. 2025. "Diversity and Pathogenicity of Colletotrichum Species Causing Coffee Anthracnose in China" Microorganisms 13, no. 3: 512. https://doi.org/10.3390/microorganisms13030512
APA StyleLu, Y., Zhang, W., Hu, X., He, C., Liang, Y., Huang, X., Yi, K., & Wu, W. (2025). Diversity and Pathogenicity of Colletotrichum Species Causing Coffee Anthracnose in China. Microorganisms, 13(3), 512. https://doi.org/10.3390/microorganisms13030512






