Abstract
Acinetobacter baumannii is one of the most successful and feared nosocomial pathogens. A. baumannii is considered a global threat in the healthcare setting, mainly owing to its ability to acquire multidrug resistance phenotypes. The A. baumannii pathogenesis is guided by its environmental persistence, as well as the production of numerous virulence factors. In several bacteria, the production of pigments, such as melanin, has indeed been linked with virulence and pathogenicity. Melanin is a brownish pigment, rarely observed in A. baumannii, that potentially reduces the susceptibility of the bacteria to host defense mechanisms and environmental insults. This study reports the first outbreak in Europe by pyomelanin-producing A. baumannii strains, in a tertiary-care university hospital in Pisa, Italy. Phenotypic and molecular analyses were performed.
1. Introduction
Acinetobacter baumannii is one of the most successful and feared nosocomial pathogens. It has gained major attention due to its capability to cause ventilator-associated as well as bloodstream infections in critically ill patients, its extensive antimicrobial resistance patterns, and its persistence in the healthcare environment. A. baumannii is considered a global threat in the healthcare setting, mainly owing to its ability to rapidly acquire multi-, extensive-, and even pan-drug-resistant phenotypes [1,2]. Antibiotic resistance has increased during the evolution of this microorganism, mainly because of the acquisition of mobile genetic elements such as transposons, plasmids, and integrons. Much of the spreading of A. baumannii can be directly attributed to the plasticity of its genome, which rapidly mutates when faced with adversity and stress. The Italian Nosocomial Infections Surveillance in Intensive Care Units (ICUs) network (SPINUTI) showed that A. baumannii was the most frequently reported microorganism (16.9%) as the cause of healthcare-associated infections in Italian ICUs during 2010–2011. Also, A. baumannii was responsible for 15% of sepsis in Italian ICUs from 2008 to 2017 [3]. In addition, the majority of isolates are considered to be multidrug-resistant (MDR) [4]. The mortality rate for ventilator-associated pneumonia caused by A. baumannii varies from 40 to 70% [5], while the mortality rate for bloodstream infections ranges from 28 to 43% [6]. For this reason, the World Health Organization (WHO) has included carbapenem-resistant A. baumannii (CRAB) in the critical group of bacteria that pose the greatest threat to human health, prioritizing research and development efforts for new antimicrobial treatments [7]. Over the past decade, the complex mechanisms that led to the emergence of A. baumannii as a feared human pathogen have been unraveled, particularly beyond the canonical drug resistance mechanisms that have been extensively studied in the past [8]. Some authors coined an expression that, in our opinion, describes perfectly the behavior of this microorganism: a ‘persist and resist’ strategy [9]. The A. baumannii pathogenesis is indeed guided by its environmental persistence, including resistance mechanisms to disinfection, desiccation, and oxidative stress; biofilm formation and maintenance; motility, in contrast to its name (‘Acinetobacter’ paradoxically stands for non-motile rod); and the production of numerous virulence factors, such as secretion systems, porins, surface glycoconjugates, and micronutrient acquisition systems, phospholipases, outer membrane vesicles, and serum resistance proteins [10]. In several bacteria, the production of pigments, such as melanin, have been linked with virulence and pathogenicity. Melanin is a brownish substance that potentially reduces the susceptibility of the microbe to host defense mechanisms and environmental insults. Depending on the pathway of synthesis, melanin may be given a different designation, in particular, the term pyomelanin was proposed for the brown pigment produced from tyrosine or phenylalanine through the accumulation of homogentisic acid [11]. Melanin production is a quite rare phenotype in A. baumannii, only three research groups have described this phenomenon [12,13,14]. Moreover, the mechanisms leading to melanin production have not yet been completely unveiled. In some bacteria, and most probably in A. baumannii, this pigment provides protection against oxidative stress and contributes to invasiveness and persistence. Pyomelanin enhances bacterial surface attachment, biofilm formation, extracellular electron transfer, resistance to heavy metals, iron reduction/acquisition, and induces the expression of virulence factors, which increase the adaptive response to environmental stress [15,16]. This study reports the occurrence of A. baumannii strains producing a brown diffusible pigment, visible on Mueller–Hinton agar cultures, which caused an outbreak in a tertiary-care university hospital in Pisa, Italy. Based on whole genome analyses of nine representative strains, we investigated the molecular bases involved in the production of this pigment and the relatedness of pyomelanin-producing A. baumannii (AbauPio+) with autochthonous pyomelanin-non producing A. baumannii strains (AbauPio-). Such an extensive outbreak caused by AbauPio+ has not, to our knowledge, been previously recorded elsewhere. We further investigated the molecular mechanisms beneath pyomelanin production and speculate a pathway involved in a defect in the catabolism of aromatic amino acids. The pyomelanin biosynthetic pathway is well known in Pseudomonas species: Pseudomonas putida metabolizesphenylalanine (Phe) and tyrosine (Tyr) through a peripheral pathway, involving the hydroxylation of Phe to Tyr by a phenylalanine hydroxylase (PhhAB), the conversion of Tyr into 4-hydroxyphenylpyruvate by a tyrosine aminotransferase (TyrB), and the formation of homogentisic acid (HGA) by a 4-hydroxyphenylpyruvate dioxygenase (Hpd) as the central intermediate. HGA is then catabolized by a central catabolic pathway that involves the homogentisate dioxygenase (HmgA), fumarylacetoacetase (HmgB), and maleylacetoacetate isomerase (HmgC), finally yielding fumarate and acetoacetate [17]. So far, mutations or deletions that result in the loss of HmgA function have been described, as well as the overexpression of hmgR, that leads to an accumulation of HGA [18]. The accumulated HGA is then secreted from the cell via the homogentisic acid transport proteins (HatABCDE ABC transporter), where it auto-oxidizes, and self-polymerizes to form pyomelanin [19] (Supplementary Data S3). Thus, we performed an in silico comparative genomic analysis to identify and characterize the genes involved with pyomelanin production and we revealed a defect in the catabolic pathway of fumarate biosynthesis, which leads to the production of pyomelanin via the accumulation of HGA.
2. Methods
2.1. Epidemiological Context
The Azienda Ospedaliero-Universitaria Pisana is a tertiary-care University Hospital, where almost 50,000 patients are hospitalized every year. There are General wards, Cardiology, Endoscopy, Hematology, Gynecology, Paediatrics, a Neonanal Unit, a Maternity Ward, Surgeries, a Burn Unit, an Emergency department, and nine Intensive Care Units. There is an intense surveillance system for monitoring MDR microorganisms (particularly carbapenemase-producing Enterobacterales, A. baumannii complex, and Stenotrophomonas maltophilia), which consists of a rectal swab analysis (molecular and/or culture) at the admission and, depending on the ward, a weekly or bi-weekly rectal swab analysis during the hospitalization period. On the positive result, an “alert” event is generated by the OpenLIS software v21.1.0 (Engineering Software Laboratory, Belgrade, Serbia, https://www.engserbia.com/) and automatic emails are sent to the ward where the patient is hospitalized, to Microbiology, Infectivology, Infection Control employees, and Medical Direction. Epidemiological data regarding the A. baumannii isolation site, ward, and antimicrobial resistance pattern were extracted using the appropriate query on the OpenLIS software from January 2018 to December 2023.
2.2. Phenotypic and Molecular Characterization of Strains
A total of forty clinical A. baumannii strains, dark in color, on Muller–Hinton agar were isolated from unique patients during May–December 2023. Various biological samples were cultured on common isolation media in routine diagnostics and incubated at 37 °C overnight. Colonies suspected to be AbauPio+ were identified by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS) (Bruker Daltonics GmbH, Bremen, Germany) and cultured on Muller–Hinton agar (Thermo Fisher Scientific, Waltham, MA, USA, https://www.thermofisher.com/). Antimicrobial susceptibility testing was performed by broth microdilution assay panels (Micronaut, Merlin, Germany, https://www.merlin-diagnostika.de/) using the Pickolo—Freedom EVO system (TECAN, Männedorf, Switzerland, https://www.tecan.com/). Cefiderocol inhibition halos were assessed by the disk-diffusion method, according to the manufacturer’s instructions (Thermo Fisher Scientific, https://www.thermofisher.com/). Minimum inhibitory concentrations (MICs) were interpreted according to the latest European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines. The strains were frozen in rich broth with 10% glycerol added for further investigation. The strains’ behavior was evaluated under the exposure to different temperatures of incubation: 25 °C, 30 °C, and 37 °C. The extracellular production of melanin was evaluated by extracting the pigment from cultured AbauPio + agar plates (modified from Loi 2020 [20]). Briefly, small portions of pigmented agar were dissolved in HCl 6 M and incubated for 2 h at room temperature, then, the pellet was washed twice in MilliQ water and resuspended in NaOH 0.5 M. This solution obtained was checked by a spectrophotometer at 200–600 nm to determine peak absorbance. In addition, two couples of AbauPio+ (M-P, F-P) and AbauPio-(M-NP, F-NP) were challenged with hydrogen peroxide (H2O2) agar-diffusion and broth microdilution tests. For the H2O2 agar-diffusion test, 50 µL of hydrogen peroxide was spotted at scalar dilution, from 0.75% to 0.08%, on Mueller–Hinton agar plates previously inoculated with 0.5 McF bacterial suspension. After an overnight incubation at 37 °C, diameters of growth inhibition halos were measured; 50 µL of saline was tested as a negative control. For the H2O2 broth microdilution test, hydrogen peroxide was diluted in Mueller–Hinton broth from 1.5% to 1.46 × 10 −6%. The bacterial suspensions were standardized at 0.5 McF in sterile and then 50 µL was added to 11 mL of Mueller–Hinton broth to obtain a standard inoculum of 5 × 105 CFU/mL. Then, 100 µL of the bacterial suspension was distributed in the wells with the scalar dilution of hydrogen peroxide. After an incubation for 18–20 h at 35 °C, the hydrogen peroxide concentration able to inhibit bacterial growth was identified. Moreover, every well without visible bacterial growth was cultured on blood agar plates to verify the bactericidal activity. Nine strains underwent genomic analyses. Total DNA was extracted from fresh cultures using the QIAamp DNA Mini Kit (Qiagen, https://www.qiagen.com/) according to the manufacturer’s instructions. The concentration and purity of the extracted DNA were determined with a Qubit® 2.0 fluorometer using the dsDNA BR Assay Kit (Life Technologies, Carlsbad, CA, USA). A DNA library was prepared using the Nextera XT DNA Library Preparation Kit (Illumina Inc., San Diego, CA, USA) according to manufacturer’s instructions, and was then run on a MiSeq system (Illumina Inc.) to generate 250 bp paired-end reads. Then, a multivariable Cox regression analysis was performed.
2.3. Software and Tools Used for Genomic Analysis
De novo assembly was performed by SPAdes Genome Assembler tool [21] using UGENE v33 [22], a free open-source software for DNA and protein sequence visualization, alignment, assembly, and annotation, after quality trimming (Qs ≥ 28). Assembled genomes were uploaded to the web tools ResFinder (http://genepi.food.dtu.dk/resfinder) [23] to identify acquired resistance genes as well as to MobileElementFinder (https://pypi.org/project/MobileElementFinder/) [24] to detect mobile genetic elements. Then, the sequences were uploaded on RAST (Rapid Annotation using Subsystem Technology, https://rast.nmpdr.org/), a fully-automated web service for annotating bacterial genomes [25]. RAST was also used for the in silico metabolic reconstruction of pyomelanin-producing A. baumannii and for comparisons both function-based and sequence-based with other A. baumannii genomes. The sequences are available from the NCBI Archive under the Bioproject names PRJNA1007229, PRJNA926509, PRJNA1102832, and PRJNA1102841; accession numbers are reported in Table 1. The DNA sequences of hmgA (NC_016603.1:3098928-3099560), hmgB (NC_016603.1:3097634-3098941), hmgR (NZ_AP014630.1:c3200330-3199053), and hmgC (NM_001046668.1) were used as references for detecting possible gene mutations associated with pyomelanin production. Insertion sequence (IS) elements were identified using the web tool ISfinder (https://isfinder.biotoul.fr/) [26]. The ISAba125 reference sequence (AY751533) was BLASTed (https://blast.ncbi.nlm.nih.gov/) against the whole genome sequence of all the isolates. Manual sequence alignment was performed by MEGA (Molecular Evolutionary Genetics Analysis) software (https://www.megasoftware.net/) [27]. The chewBBACA software v3.3.10 was run in Conda environment for the creation, evaluation, and use of core genome (cg) multilocus sequence typing (MLST) schemas, based on an ad hoc structure including 2390 alleles [28]. The ChewTree tool was used to calculate a phylogenetic tree from chewBBACA alleles on the web tool ARIES (Advanced Research Infrastructure for Experimentation in Genomics—Galaxy Instance at ISS, https://irida.iss.it/irida-phantastic/login) [29]. The tree was visualized by PHYLOViZ (https://www.phyloviz.net/). In addition, 17 genomes of AbauPio- isolated from April 2020 to November 2022 were used as comparators (PRJNA1007229, PRJNA926509).

Table 1.
A. baumannii strains: epidemiological, genotypical, and phenotypical features. M and F are the strains’ names, P stands for pyomelanin-producers; NP stands for non pyomelanin-producers.
3. Results
3.1. Phenotypic and Epidemiologic Analysis
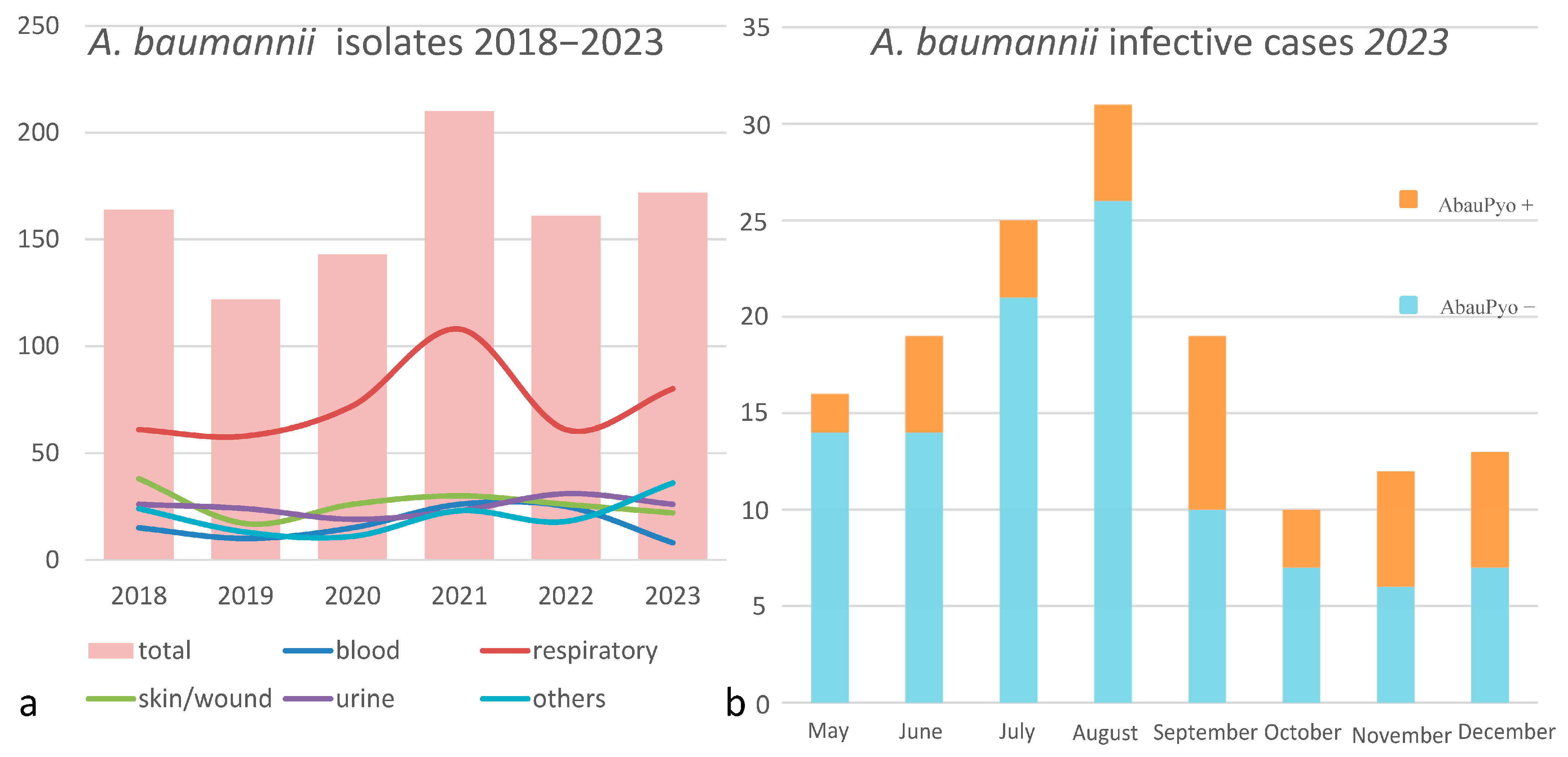
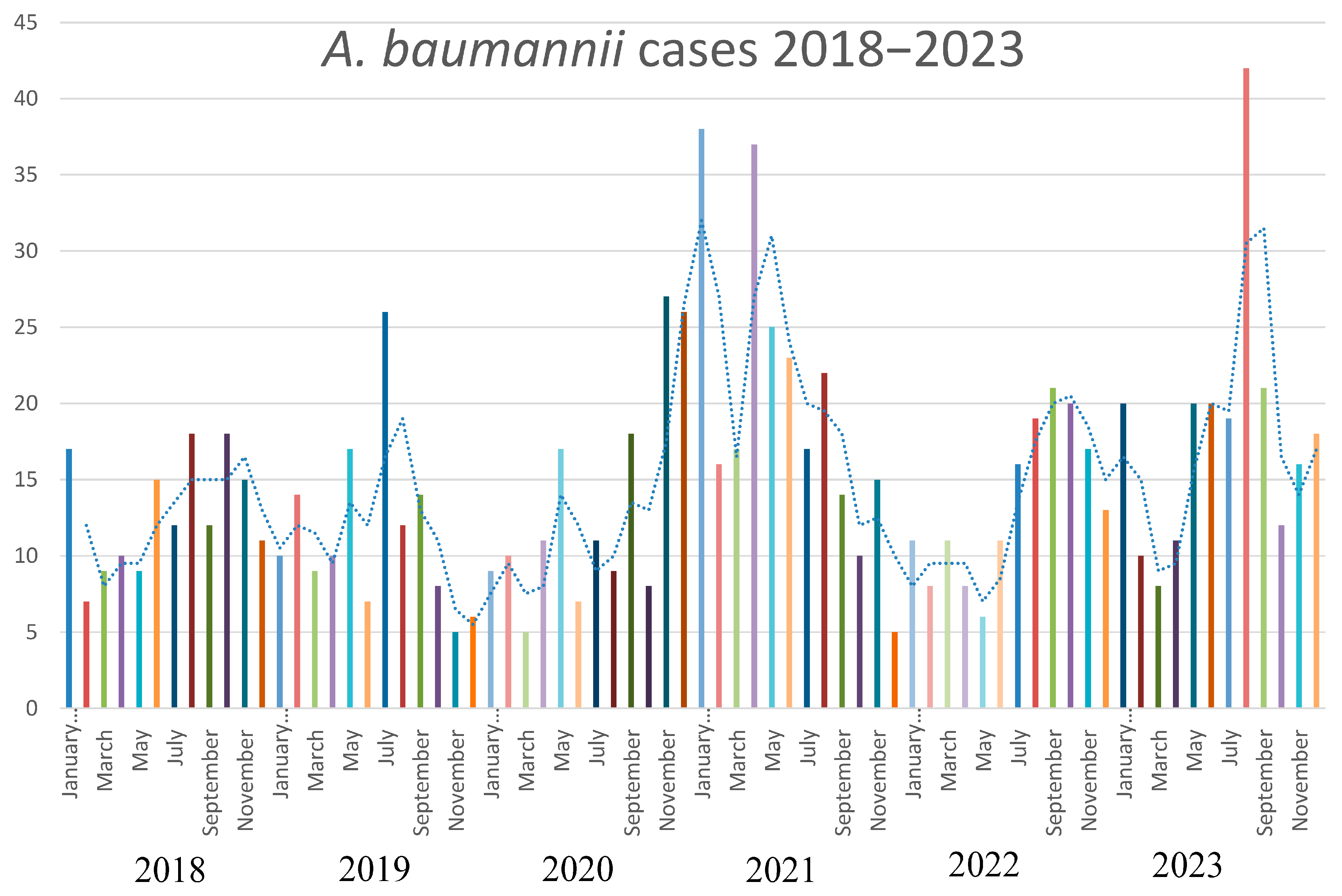
The strains of A. baumannii were correctly identified at the species level by MALDI-TOF MS, with score values above 2.3. Epidemiological data and strains features are reported in Table 1. In relation to their antimicrobial susceptibility pattern, performed by broth microdilution assay, all the strains were shown to be multi-drug-resistant. Regarding the hospital epidemiology, the proportion of A. baumannii isolation was reported in Figure 1a. Figure 1b reports the isolates from infections from May to December 2023, divided by the production of the pigment. The majority of strains were isolated from respiratory samples with an increasing isolation in 2021, both for respiratory samples and for blood cultures. Regarding the susceptibility pattern, the proportion of amikacin-resistant strains went from 67% in 2018 to 90% in 2023, meropenem-resistant strains went from 80% in 2018 to 91% in 2023, while there is an inversion in the proportion of colistin-resistant strains, which went from 4% in 2018 to 1% in 2023. In Figure 2, we reported the epidemiology of all of the A. baumannii isolation from 2018 to 2023, divided by months. As shown, the prevalence is higher in warmer months, except during the COVID-19 pandemic, when all the infection control measures were disrupted. The production of pyomelanin was more evident at 30 °C and 37 °C rather than 25 °C (Supplementary Data S1). In the H2O2 agar-diffusion test, the AbauPio+ strains showed growth at 0.08% of hydrogen peroxide, whereas, at the same hydrogen peroxide concentration, 24 mm of growth inhibition halos were detected for the AbauPio- strains. Differential growth inhibition halos for AbauPio+ and AbauPio- strains are listed in Table 2 (image in Supplementary Data S2). The minimal H2O2 concentration that inhibited the growth of bacteria, evaluated by the H2O2 broth microdilution test, was 0.04% for AbauPio+ strains and 0.00015% for AbauPio-. The bacteriostatic hydrogen peroxide concentrations were also bactericidal. After the extraction, the brown pigment was analyzed by a spectrophotometer and a peak absorption was observed at 215 nm in the UV region, the specific wavelength of melanin [20].
Figure 1.
(a) The proportion of A. baumannii isolation during a 5-year period (2018–2023); the different colored lines represent different sites of isolation. (b) A. baunannii infection cases from May to December 2023, distinguished by the production of the pigment.
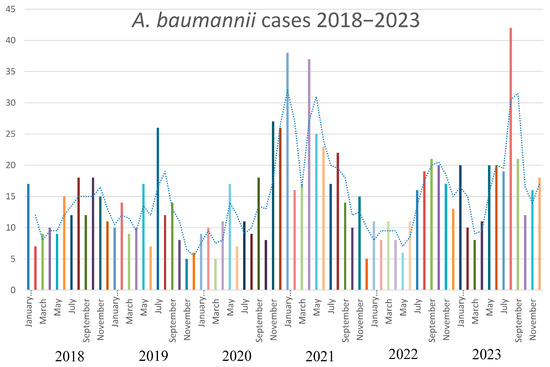
Figure 2.
The epidemiology of A. baumannii during a 5-year period (2018–2023).

Table 2.
Hydrogen peroxide agar-diffusion test: two couples of pigmented (M-P, F-P) and non-pigmented (M-NP, F-NP) A. baumannii strains from the same patients (M and P: each of them had a pigmented and a non-pigmented strain) were tested. Growth inhibition halos for pigmented (MP, FP) and non-pigmented (MNP, FNP) A. baumannii strains are reported (diameters in mm). ✓ stands for “present”.
3.2. Molecular Analysis
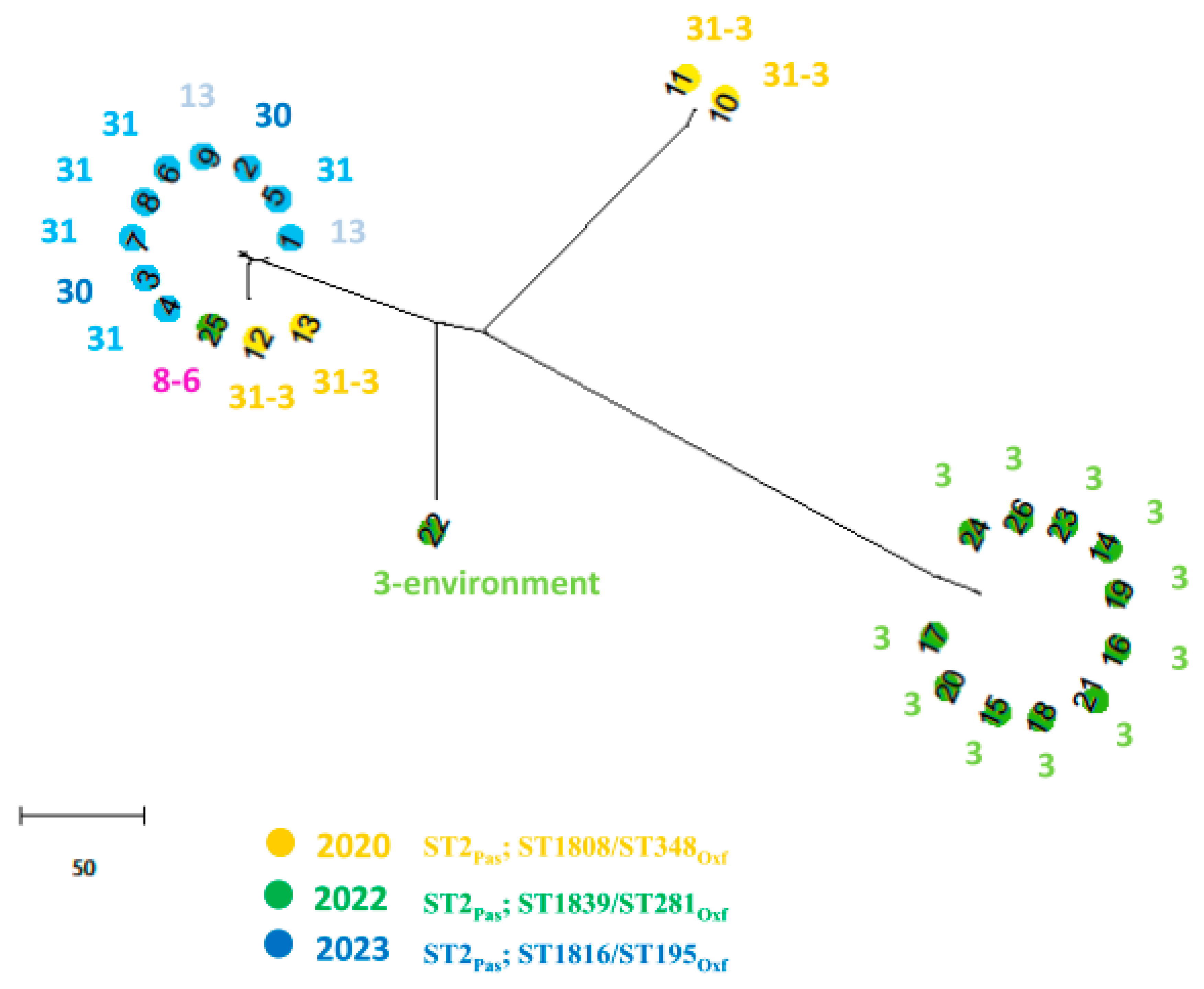
Resistome prediction analyses unveiled different combinations of β-lactamases, including OXA-23, OXA-25, OXA-66, TEM-1D, and ADC-25 enzymes. Several aminoglycoside resistance genes, including aph(6)-Id, aph(3′)-Ia, aph(3″)-Ib, aadA1, armA, and the aac(6′)-Ib-cr gene involved also in fluoroquinolone-resistance, were also detected and are reported in Table 1. In AbauPio- cefiderocol-resistant strains, a missense mutation in PBP3 was detected. Those isolates also harbored a mutation in the TonB-dependent siderophore receptor gene piuA, that showed a frameshift mutation causing a premature stop codon (K384fs). Moreover, the fepA gene, which is the orthologous to pirA, was interrupted by a transposon insertion P635-ISAba125 (IS30 family), and was associated with the resistance to cefiderocol [30]. The core genome whole genome sequencing analysis revealed that the isolate belonged to sequence type 2 (ST) International Clone II, according to the MLST Pasteur database, and to ST1816/ST195, according to the Oxford database (Table 1). The nine AbauPio+, together with seventeen AbauPio- strains, were selected for wgMLST comparison. Figure 3 shows a phylogenetic tree based on the allelic mismatch between these genomes. The different Oxford MLST types are highlighted by different colors: three clonally related clusters of isolates are observed, and in agreement with the Oxford schema classification, except for one sample (number 25, which clustered in an unconventional way). Strain number 22, isolated from the hospital environment, was considered the outgroup. The three clusters are separated according to the timing of isolation (2020, 2022, 2023) and to nosocomial setting, since different wards are involved and the Oxford schema is able to highlight those aspects. ISAba125 is a transposable insertion sequence of 1026nucleotides, a member of the widely distributed IS30 family. In strains number six and seven, the ISAba125 was found in 128 nucleotides upstream of the fumarylacetoacetase gene, in a palindromic region rich in A and T nucleotides resembling a regulatory region, most probably causing a rearrangement of the pyomelanin genes cluster and a misidentification of the site by the RNA polymerase, leading to an accumulation of HGA, which is then secreted from the cell via the HatABCDE ABC transporter, where it auto-oxidizes and self-polymerizes to form pyomelanin. In the remaining AbauPyo+ strains, ISAba125 was found in isolated contigs and the reconstruction of the frame was not possible.
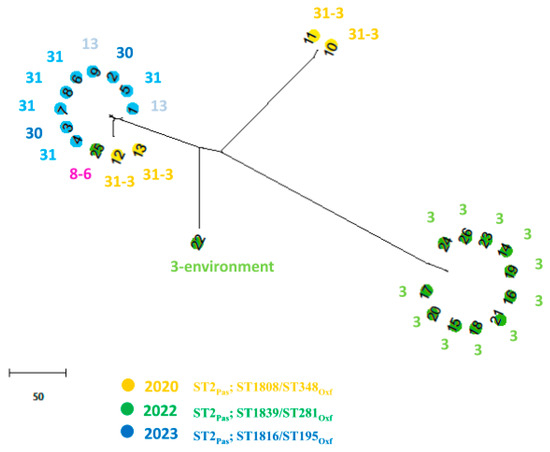
Figure 3.
A phylogenetic tree based on the allelic mismatch between 23 A. baumannii genomes. The chewBBACA software was run in the Conda environment for the creation, evaluation, and use of core genome (cg) multilocus sequence typing (MLST) schemas, based on an ad hoc structure including 2390 alleles. Different colors represent different years of isolation and different sequence types (according to the Oxford schema). Numbers inside the colored dots represent the number of the genome, while the number outside the colored dots represents the number of the ward where the strain was isolated.
4. Discussion
Carbapenem-resistant Acinetobacter baumannii represents one of the pathogens with a higher mortality rate in nosocomial settings [31]. The A. baumannii outbreaks have been associated with high-risk pandemic lineages, named International Clones (ICs), characterized by a high capacity to persist in clinical environments and by presenting a broad antimicrobial resistance profile [32]. The exposure of A. baumannii to the selective pressure of potent antimicrobials, primarily in the ICUs, has gradually led to a global prevalence of A. baumannii strains that are resistant to all β-lactams, including carbapenems. Outbreaks caused by such strains have been identified in several ICUs worldwide and, in many instances, have been associated with strains that are resistant to all available antibacterial agents. Also, in our hospital epidemiology, the proportion of carbapenem-resistant strains is widely distributed, reaching 90% of all the isolations, and strategies for new antimicrobial approaches were attempted [33,34]. Interestingly, an inversion in colistin resistance was registered from 2018 to 2023, most probably because, in our hospital, the use of this antibiotic had decreased in that period, diminishing the selective pressure on the bacteria. Considering the epidemiology from 2018 to 2023, we noticed that the proportion of isolation is higher in warmer months, except during the COVID-19 pandemic, most probably because of the lack of infection control measures. The majority of A. baumannii strains were cultures from respiratory samples with an increase during the COVID-19 pandemic. The same trend was registered also for blood cultures. This may be explained considering the rise in patients admitted to ICUs and subjected to assisted-control ventilation during the COVID-19 pandemic, but also the lack of infection control measures registered in that period. Beyond its environmental persistence and its aptitude to accumulate a large variety of resistance mechanisms, the ability to generate biofilm, the production of a capsule, the presence of lipopolysaccharide and outer-membrane proteins, and the secretion of hydrolytic enzymes have been highlighted as virulence factors of A. baumannii [35]. Melanin is a brownish pigment that has also been linked with the virulence and pathogenicity of microbes able to produce this substance [36]. However, the pigmentation of clinical strains of A. baumannii is quite a rare phenotype [12,13,14] and such outbreaks have not yet been described elsewhere. The overproduction of pyomelanin in A. baumannii has been described as occurring due to changes in the tyrosine metabolic pathway linked to the deletion of the hmgA by ISAba1 [14]. From May to December 2023, the production of a brown diffusible pigment on Muller–Hinton agar was observed in forty isolates identified as A. baumannii, obtained from patients hospitalized in a tertiary-care hospital in Pisa, Italy. The outbreak started in one of the ICUs and overran in several other wards. It was verified that the production of pigment at higher temperatures was evident, as reported by Fonseca at al. (2020) [12], due to the induction of melA (hpd), which is responsible for HGA synthesis, suggesting the role of this biosynthetic pathway in the pyomelanin production in Abaupio+ strains. To explore the molecular basis of pigment production, we investigated the key genes in the pathway of pigment production, focusing right on HGA biosynthesis. The results revealed that ISAba125, an insertion sequence belonging to the IS30 family, inserts upstream of the fumarylacetoacetase gene, possibly resulting in a steric hindrance in the regulatory/promoter region, with consequences of the impossibility of hmgB transcription, the accumulation of HGA, and its conversion into pyomelanin [11]. The impact of pyomelanin on the phenotype of A. baumannii has been studied: Fonseca et al. [12] considered that there might be a connection between pyomelanin production and virulence; Zhao et al. [13] revealed that the pigment may offer a potential protection effect for bacteria under UV radiation. In addition, pyomelanin-producing A. baumannii can show resistance to a wide range of antimicrobial drugs [14], which in turn could become a new challenge in the clinical setting, especially in the ICUs. In our experience, an intense resistance to oxidative stress was documented. More interestingly, during the period of AbauPio+ strains isolation, the hospital protocols of wards disinfection involved precisely hydrogen peroxide (Perox, Pestnet, diluted at 1% of H2O2). The study of A. baumannii genomics has provided an expanded view of the adaptation and virulence capacities of this tough bacterial species [37]. The ISAba125 insertion sequence is an expression of this plasticity, and the strains reported here are an emblematic example of their genetic variability. In Abaupio+ strains, ISAba125 inserts upstream of a gene involved in pyomelanin production; in Abaupio- strains, ISAba125 inserts within the fepA gene, causing resistance to cefiderocol [30]. The ability to interfere with gene expression has already been described for ISAba125. This insertion sequence is able to insert upstream of the blaADC gene in the promoter region, causing an overexpression of the gene [38], as well as inside the gene coding for the outer membrane protein CarO OMP, leading to a carbapenem-resistant profile [39]. In addition, ISAba125 has also been associated with gene variants of the blaNDM carbapenemase in the family Enterobacterales [40]. Insertion sequence elements, such asISAba125, could modulate antibiotic resistance depending on the antibiotic treatment [41], as well as virulence factors, such as the production of pyomelanin, as reported here. To our knowledge, this is the first report of a large outbreak caused by ST2Pas;ST1816/ST195Oxf A. baumannii strains, where a biosynthetic pathway compatible with pyomelanin production was investigated. Further studies are required to understand which is the trigger for the production of pyomelanin and to establish the impact of pigment production on the pathogenesis of A. baumannii. In our multivariable Cox regression analysis, there was no statistically relevant difference in 30 days mortality rate between the AbauPio+ group (31.8%) and AbauPio- group (41.9%), also there were no statistically relevant differences between the two groups in the development of bacteremia. However, we prefer not to generalize this concept, since the groups analyzed were too small and therefore not adjusted for sex, age, and therapy; for this reason, we invite other clinicians to study this phenomenon to better clarify the clinical impact of AbauPio+ infections. Finally, due to the association of pyomelanin with virulence in bacteria, the surveillance of pigment production by clinical A. baumannii strains should be implemented and monitored as part of the hospital surveillance programs to contain the dissemination of these strains.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microorganisms13030493/s1, Data S1: Temperatures; Data S2: Hydrogen peroxide; Data S3: Pathway for pyomelanin synthesis.
Author Contributions
A.L. and A.R. performed phenotypical investigations, G.G. investigated epidemiological data, G.T. and M.F. collected clinical data, C.G. performed molecular analysis and drafted the manuscript, and S.B. discussed experimental data and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giammanco, A.; Calà, C.; Fasciana, T.; Dowzicky, M.J. Global Assessment of the Activity of Tigecycline against Multidrug-Resistant Gram-Negative Pathogens between 2004 and 2014 as Part of the Tigecycline Evaluation and Surveillance Trial. mSphere 2017, 2, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.M.; Diene, S.M.; Kempf, M.; Gimenez, G.; Robert, C.; Raoult, D. Real-time sequencing to decipher the molecular mechanism of resistance of a clinical pan-drug-resistant Acinetobacter baumannii isolate from Marseille, France. Antimicrob. Agents Chemother. 2013, 57, 592–596. [Google Scholar] [CrossRef]
- Agodi, A.; Barchitta, M.; Auxilia, F.; Brusaferro, S.; D’Errico, M.M.; Montagna, M.T.; Pasquarella, C.; Tardivo, S.; Arrigoni, C.; Fabiani, L.; et al. Epidemiology of intensive care unit-acquired sepsis in Italy: Results of the SPIN-UTI network. Ann. Ig. 2018, 30, 15–21. [Google Scholar]
- Zarrilli, R.; Bagattini, M.; Migliaccio, A.; Esposito, E.P.; Triassi, M. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in Italy. Ann. Ig. 2021, 33, 401–409. [Google Scholar]
- Garnacho-Montero, J.; Ortiz-Leyba, C.; Fernández-Hinojosa, E.; Aldabó-Pallás, T.; Cayuela, A.; Marquez-Vácaro, J.A.; Garcia-Curiel, A.; Jiménez-Jiménez, F.J. Acinetobacter baumannii ventilator-associated pneumonia: Epidemiological and clinical findings. Intensive Care Med. 2005, 31, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Chopra, T.; Marchaim, D.; Awali, R.A.; Krishna, A.; Johnson, P.; Tansek, R.; Chaudary, K.; Lephart, P.; Slim, J.; Hothi, J.; et al. Epidemiology of bloodstream infections caused by Acinetobacter baumannii and impact of drug resistance to both carbapenems and ampicillin-sulbactam on clinical outcomes. Antimicrob. Agents Chemother. 2013, 57, 6270–6275. [Google Scholar] [CrossRef]
- World Health Statistics 2017: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-156548-6.
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Ohyama, A. Characterization of “pyomelanin”-producing strains of Pseudomonas aeruginosa. Int. J. Syst. Bacteriol. 1972, 22, 53–64. [Google Scholar] [CrossRef]
- Fonseca, É.; Freitas, F.; Caldart, R.; Morgado, S.; Vicente, A.C. Pyomelanin biosynthetic pathway in pigment-producer strains from the pandemic Acinetobacter baumannii IC-5. Mem. Inst. Oswaldo Cruz. 2020, 115, e200371. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, H.; Yao, Y.; Zhang, L.; Zhou, Z.; Leptihn, S.; Yu, Y.; Hua, X.; Fu, Y. Description of a Rare Pyomelanin-Producing Carbapenem-Resistant Acinetobacter baumannii Strain Coharboring Chromosomal OXA-23 and NDM-1. Microbiol. Spectr. 2022, 10, e0214422. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Souza, T.; Martins, N.; Maia, F.; Frases, S.; Bonelli, R.R.; Riley, L.W.; Moreira, B.M. Pyomelanin production: A rare phenotype in Acinetobacter baumannii. J. Med. Microbiol. 2014, 63, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Chatfield, C.H.; Cianciotto, N.P. The secreted pyomelanin pigment of Legionella pneumophila confers ferric reductase activity. Infect. Immun. 2007, 75, 4062–4070. [Google Scholar] [CrossRef]
- Valeru, S.P.; Rompikuntal, P.K.; Ishikawa, T.; Vaitkevicius, K.; Sjöling, A.; Dolganov, N.; Zhu, J.; Schoolnik, G.; Wai, S.N. Role of melanin pigment in expression of Vibrio cholerae virulence factors. Infect. Immun. 2009, 77, 935–942. [Google Scholar] [CrossRef]
- Arias-Barrau, E.; Olivera, E.R.; Luengo, J.M.; Fernández, C.; Galán, B.; García, J.L.; Díaz, E.; Miñambres, B. The homogentisate pathway a central catabolic pathway involved in the degradation of L-phenylalanine, L-tyrosine, and 3-hydroxyphenylacetate in Pseudomonas putida. J. Bacteriol. 2004, 186, 5062–5077. [Google Scholar] [CrossRef]
- Ranjan, V.K.; Saha, T.; Mukherjee, S.; Chakraborty, R. Draft genome sequence of a novel bacterium, Pseudomonas sp strain MR 02, capable of pyomelanin production, isolated from the Mahananda River at Siliguri, West Bengal, India. Genome Announc. 2018, 6, e01443-17. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.C.; Newman, D.K. A putative ABC transporter, hatABCDE, is among molecular determinants of pyomelanin production in Pseudomonas aeruginosa. J. Bacteriol. 2010, 192, 5962–5971. [Google Scholar] [CrossRef]
- Loi, J.; Yi, T.S.; Pinsa, A.; Mulyana, S.; Singaretnam, L.G.; Riwanto, M.; Chang, J. Pyomelanin production from a marine isolate of Acinetobacter spp. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 2250–2259. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; UGENE team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Siguier, P.; Perochon, J.; Lestrade, L.; Mahillon, J.; Chandler, M. ISfinder: The reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006, 34, D32–D36. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Machado, M.P.; Silva, D.N.; Rossi, M.; Moran-Gilad, J.; Santos, S.; Ramirez, M.; Carriço, J.A. chewBBACA: A complete suite for gene-by-gene schema creation and strain identification. Microb. Genom. 2018, 4, e000166. [Google Scholar] [CrossRef]
- Knijn, A.; Michelacci., V.; Orsini, M.; Morabito, S. Advanced Research Infrastructure for Experimentation in genomicS (ARIES): A lustrum of Galaxy experience. bioRxiv 2020. [Google Scholar] [CrossRef]
- Giordano, C.; Barnini, S. Glycine restores the sensitivity to antibiotics in multidrug-resistant bacteria. Microbiol. Spectr. 2024, 12, e0016424. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Carbonara, S.; Marino, A.; Di Caprio, G.; Carretta, A.; Mularoni, A.; Mariani, M.F.; Maraolo, A.E.; Scotto, R.; et al. Mortality attributable to bloodstream infections caused by different carbapenem-resistant Gram negative bacilli: Results from a nationwide study in Italy (ALARICO Network). Clin. Infect. Dis. 2023, 76, 2059–2069. [Google Scholar] [CrossRef]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, G.; Giordano, C.; Leonildi, A.; Riccardi, N.; Galfo, V.; Limongi, F.; Nicastro, M.; Barnini, S.; Falcone, M. Salvage therapy with sulbactam/durlobactam against cefiderocol-resistant Acinetobacter baumannii in a critically ill burn patient: Clinical challenges and molecular characterization. JAC Antimicrob. Resist. 2023, 5, dlad078. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Nicastro, M.; Leonildi, A.; Vecchione, A.; Casella, C.; Forfori, F.; Malacarne, P.; Guarracino, F.; Barnini, S.; et al. Cefiderocol as Rescue Therapy for Acinetobacter baumannii and Other Carbapenem-resistant Gram-negative Infections in Intensive Care Unit Patients. Clin. Infect. Dis. 2021, 72, 2021–2024. [Google Scholar] [CrossRef]
- Roca, I.; Espinal, P.; Vila-Farrés, X.; Vila, J. The Acinetobacter baumannii oxymoron: Commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 2012, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Casadevall, A. The contribution of melanin to microbial pathogenesis. Cell Microbiol. 2003, 5, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Imperi, F.; Antunes, L.C.; Blom, J.; Villa, L.; Iacono, M.; Visca, P.; Carattoli, A. The genomics of Acinetobacter baumannii: Insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life 2011, 63, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Hèritier, C.; Poirel, L.; Nordmann, P. Cephalosporinase overexpression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 2006, 12, 123–130. [Google Scholar] [CrossRef]
- Mussi, M.A.; Limansky, A.S.; Viale, A.M. Acquisition of resistance to carbapenems in multidrug-resistant clinical strains of Acinetobacter baumannii: Natural insertional inactivation of a gene encoding a member of a novel family of b-barrel outer membrane proteins. Antimicrob. Agents Chemother. 2005, 49, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Hornsey, M.; Phee, L.; Wareham, D.W. A novel variant, NDM-5, of the New Delhi metallo-b-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob. Agents Chemother. 2011, 55, 5952–5954. [Google Scholar] [CrossRef]
- Lopes, B.S.; Amyes, S.G.B. Role of ISAba1 and ISAba125 in governing the expression of blaADC in clinically relevant Acinetobacter baumannii strains resistant to cephalosporins. J. Med. Microbiol. 2012, 61, 1103–1108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).