Abstract
Skin cancer, including melanoma and non-melanoma types, presents a significant and growing global health challenge due to its increasing incidence and mortality rates. While conventional treatments such as surgical excision, immunotherapy, and targeted therapies are well-established, microorganism-based approaches represent an innovative and promising alternative. These therapies employ live, genetically engineered, or commensal bacteria, viral vectors, or bacterial components to achieve various therapeutic mechanisms, including tumor targeting, immune system modulation, vascular disruption, competitive exclusion, drug delivery, and direct oncolysis. Despite their potential, these approaches require further investigation to address safety concerns, optimize treatment protocols, and gain a comprehensive understanding of their long-term outcomes.
1. Introduction
Skin cancer is a common disease worldwide with yearly increasing incidence and mortality rates making it a significant public health concern. According to the GLOBOCAN 2022 data, melanoma and non-melanoma skin cancer (NMSC) are the 17th and 5th most common cancers worldwide, respectively. Melanoma had an estimated 331,722 new cases diagnosed and approximately 58,667 deaths, while NMSC had 1,234,533 new cases and 69,416 deaths. Countries with predominantly fair-skinned populations, including North America, Europe, and Oceania, report the highest incidence rates of melanoma and NMSC, with Australia leading globally [1].
The current skin cancer classifications include three major types: melanoma, basal cell carcinoma (BCC), and squamous cell carcinoma (SCC). Melanoma is characterized by the malignant proliferation of melanocytes and is the third most common skin cancer. It is the most metastatic and has the highest mortality rate among skin cancers [2,3]. BCC, the most common type, arises from basal keratinocytes and rarely metastasizes. SCC originates from keratinocytes and often arises from precancerous lesions called actinic keratosis (AK). It is the second most common type and has a higher metastatic potential than BCC [4]. For this paper, skin cancers will be categorized as either melanoma or NMSC.
Other forms of skin cancer include precancerous lesions and less common cutaneous tumors that fall under the umbrella of skin cancer, such as AK, Merkel cell carcinoma (MCC), and cutaneous lymphomas (CL). AK, a common premalignant lesion, involves dysplasia of epidermal keratinocytes [5]. MCC is a rare, aggressive neuroendocrine carcinoma of the skin that has a high risk of metastasis. CLs encompass a group of lymphoproliferative disorders affecting the skin [6]. While risk factors for skin cancer vary by type, they generally include UV radiation exposure, phenotypic characteristics (e.g., fair skin, light hair), family history, and genetic predisposition [7,8].
Current treatments for skin cancer also depend on the type. For melanoma, common treatments include surgical excision along with adjuvant therapies such as immune checkpoint inhibitors (e.g., pembrolizumab), which have been found to have significantly fewer side effects compared to traditional chemotherapies [9]. In contrast, targeted therapies like BRAF inhibitors (e.g., vemurafenib, dabrafenib), which suppress cancer cell proliferation by inhibiting MAPK signaling, are effective but are also known to develop resistance [10]. First-line treatments for BCC and SCC typically involve surgical excision and topical therapies (e.g., imiquimod, 5-fluorouracil) [11]. Standard first-line treatments for both melanoma and NMSC are well-established, but microbial treatments represent a relatively new area of research in skin cancer therapy.
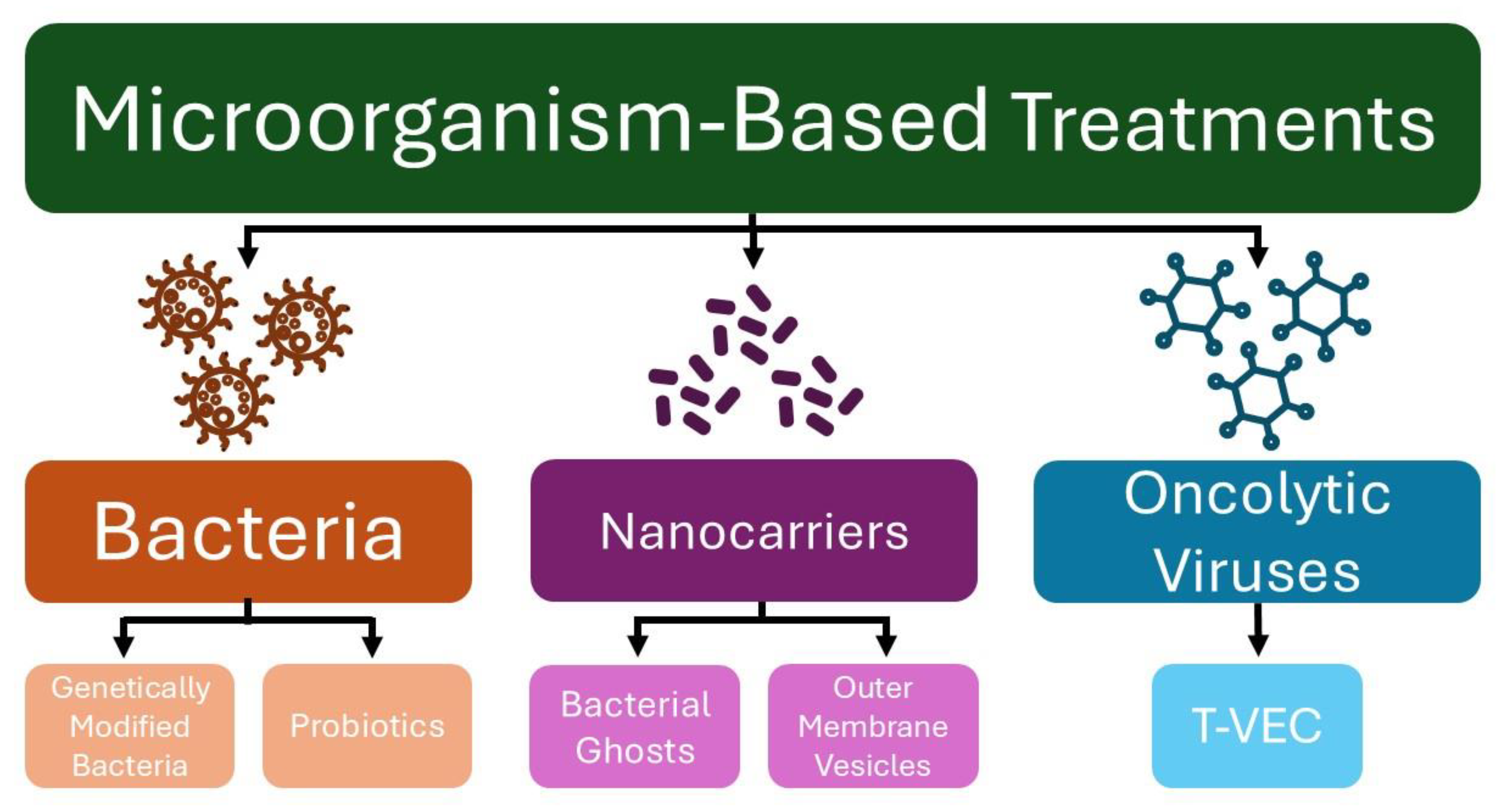
In recent years, microorganisms have been explored as tools for anticancer therapy, serving as vectors for antitumor therapy delivery, vaccines to activate the immune system, and agents to restore or maintain a healthy microbiome (Figure 1) [12]. Although various treatment strategies have been explored to address the rising incidence of skin cancer, the use of microorganisms as therapeutic agents remains relatively underexplored [13]. This paper reviews pre-clinical and clinical studies exploring various microorganism-based treatments for melanoma and NMSC.
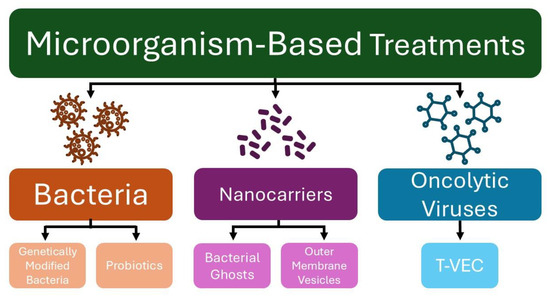
Figure 1.
Microorganism-based treatments for skin cancer. (T-VEC—Talimogene laherparepvec).
2. Treatments
2.1. Bacterium-Based Therapeutics
Bacterium is known for its ability to accumulate and proliferate in specific areas, making it an ideal candidate for initiating an anti-tumor immune response within the tumor microenvironment. Bacterium-based therapeutics utilize live bacteria to treat cancer and diseases in this fashion. There are different mechanisms in which bacterium-based therapeutics can work, including secreting cytotoxic agents, targeting the microenvironment of the tumor, engineering bacterial vectors to express and release tumoricidal proteins, and manipulating viral and bacterial agents [14]. Bacteria and their products were first used and reported by William Coley, who used two different bacteria strains (Streptococcus pyogenes and Serratia marcescens) on unresected tumors. The findings showed that the tumor necrosis factor (TNF-α) secretion increased and helped with tumor regression [15].
Bacteria can be engineered by genetic manipulation or synthetic bioengineering for tumor specificity and to specifically create and deliver anticancer agents based on the clinical need [16]. Attenuated Salmonella typhimurium is very selective to solid tumors, making it an ideal choice for the treatment of melanoma [17]. The expression of IL-2 allows for an enhanced immune response against a tumor which leads to better suppression of melanoma growth compared to non-cytokine-expressing strains [18]. A different strain of genetically engineered Salmonella, AISI-H, uses a dynamic virulence modulation system to heighten its tumor-targeting ability while decreasing off-target toxicity. This specific strain can turn “on” in tumors to allow for the therapeutic effects to take hold and “off” in healthy tissue to avoid unnecessary harm. The AISI-H strain shows exceptional biocompatibility and exhibited notable tumor inhibition and strong immunity activation in a melanoma mouse model [19]. Engineering a non-pathogenic Escherichia coli strain has also been used in tumor treatment which releases CD47—a nanobody antagonist that is an anti-phagocytic receptor that is commonly over-expressed in multiple human cancers including melanoma. By using tumor-colonizing bacteria to deliver CD47, it up-regulates the activation of T cells that infiltrate the tumors, triggers expedited tumor regression, and prevents metastasis [20].
Probiotics are bacteria that, when ingested, provide benefits to the host’s health. The idea of using probiotic bacteria in this way was first suggested in 1906 and has since been extensively studied, providing a greater understanding of their various strains, mechanisms, and benefits [21]. They aid the host by maintaining or restoring a healthy balance of gut and skin microbiota and act against pathogenic microorganisms through a variety of mechanisms, including competitive exclusion, mucin and bacteriocin production, and immune system modulation [22]. Probiotic byproducts known as postbiotics are also used as an alternative to whole probiotic administration [23].
In a study investigating the microbiota composition of patients with NMSC, patients with SCC had a higher frequency of Staphylococcus aureus than healthy patients in biopsies and swab samples from the skin surface. The study also found a tendency for association of S. aureus with AK, but no association with BCC or seborrheic keratosis [24]. S. aureus is thought to promote SCC progression by amplifying the immune response and increasing skin inflammation. It does this by secreting phenol-soluble modulin alpha (PSMα) which stimulates keratinocytes to release cytokines IL-1α and IL-36α, creating a cascade to induce the production of the inflammatory cytokine IL-17 [25].
BCC has a distinct microbiota composition compared to other keratinocyte skin tumors such as SCC and AK which are more similar to each other [26]. The most common microbiota in BCC is β-human papillomavirus (HPV) species type 1, while other β-HPV species are found in high frequency in SCC [27]. Certain β-HPV types produce the viral E6 protein which binds to and destabilizes the histone acetyltransferase p300, reducing its ability to activate transcription of the BRCA1 and BRCA2 DNA repair genes. Consequently, the cell’s ability to repair DNA double-strand breaks from UV damage is impaired [28]. Furthermore, the E6 protein degrades the pro-apoptotic protein Bak in keratinocytes, as well as destabilizes the tumor suppressor p53 [29,30]. All these mechanisms allow the cell to survive UV damage, accumulate UV-induced mutations, and continue to replicate, adding support to the hypothesis that β-HPV plays a role in progressing skin cancer [28].
There are characteristic bacteria such as Propionibacterium spp. found in healthy skin at a higher frequency compared to S. aureus in SCC and AK [31]. A healthy population of commensal bacteria on the skin is necessary to maintain eubiosis, often by directly inhibiting pathogen growth and enhancing the host’s innate immunity [32]. Staphylococcus epidermis is one of these commensal bacteria species found predominantly on normal human skin [33]. Strains of S. epidermis produce 6-N-hydroxyaminopurine (6-HAP) which selectively inhibits the DNA polymerase activity of tumor cells without affecting normal keratinocytes. 6-HAP acts by competing with adenosine to inhibit DNA synthesis, while it is likely multiple mechanisms occur in nature for the inactivation of 6-HAP in the case of normal skin microbiota that remains unaffected [34]. S. epidermis has also been shown to stimulate the production of IL-17α+ CD8+ T cells which limit pathogen invasion and strengthen the innate barrier immunity of the epidermis [35]. Lipoteichoic acid (LTA) is a product secreted by S. epidermis and other commensal staphylococcal species that increases the production of antimicrobial peptides in mast cells to enhance their infection-fighting ability [36].
In melanoma patients, Trueperella pyogenes and Fusobacterium necrophorum are among the most common bacteria found in the skin microbiome [37]. The Fusobacterium genus is known to sustain inflammation and promote the progression of other cancers, indicated by larger tumors forming more rapidly, and increased proliferation and invasive activity compared to controls [38]. Fusobacterium also acts by creating an immunosuppressed environment via interaction between the bacterial Fap2 protein with human T cell immunoglobulin and ITIM domain (TIGIT) to inhibit natural killer cell cytotoxicity [39].
Several studies show that probiotics can be an effective means of treating both NMSC and melanoma. In NMSC, probiotics generally can neutralize pathogenic microbes by stimulating the host’s mucin and bacteriocin secretion to trap pathogens [40]. Treatment of skin with the probiotic bacteria Lactobacillus reuteri and Lactobacillus rhamnosus, before or concurrent with S. aureus infection, showed significant keratinocyte survival. L. reuteri and L. rhamnosus increased the number of normal primary human epidermal keratinocytes (NHEK) from 8.8% to 53.1% and 42.7%, respectively. L. reuteri inhibited S. aureus adhesion to keratinocytes by competitive exclusion, independent of inhibitory molecule amplification [41]. Although further study is needed, there is also potential for using S. epidermis or its postbiotic 6-HAP as a preventative treatment for skin cancer [34]. In skin tumorigenic mice, treatment with the probiotics Saccharomyces cerevisiae, Bacillus subtilis, and Lactobacillus acidophilus effectively lowered the HPV rate, showing promise for potential treatment of BCC and other NMSCs [42]. Another probiotic, Lactobacillus salivarius REN, has been shown to have anti-tumorigenic activity in human tongue SCC, and the probiotic compound AJ2 inhibited the growth of oral cancer lines in humanized mice models [43,44]. To address UV damage, a common factor in NMSC incidence and progression, the postbiotic LTA can be used to treat UV-stressed skin tumors. Produced by Lactobacillus rhamnosus GG (LGG), LTA can both prevent formation of UV-induced skin tumors and overcome UV-induced skin immunosuppression [45].
Much of the research conducted on probiotics and melanoma is centered on the effects of probiotics on melanoma immunotherapy [46]. Administration of the probiotic Bifidobacterium combined with immunotherapy in a melanoma mouse model was linked to greater tumor outgrowth suppression compared to Bifidobacterium administration alone [47]. A different probiotic, Lactobacillus reuteri FLRE5K1, was studied for its anti-melanoma activity in cell assays and animal models. The results of the study found that L. reuteri FLRE5K1 reduced melanoma incidence by 40% and significantly prolonged survival in tumorigenic mice, likely by stimulating the body’s anti-cancer cytokine production and inhibiting melanoma cell migration [48]. There have also been many studies in recent years exploring the composition of the gut microbiota and understanding how it affects distal tumors. The link between the gut microbiota and melanoma specifically has been well-established, and current research is underway investigating the effectiveness of fecal transplants in melanoma patients [49,50].
The future of bacterium-based therapeutics has many possibilities, especially when considering its use in conjunction with other reliable cancer treatment methods to optimize patient outcomes [51]. Despite the promising potential of bacterium-based therapeutics, however, there are some drawbacks to its use as an agent of tumor-treatment delivery. A primary concern when working with bacteria is that they may trigger a strong immune response in the patient which could result in septic shock. Another potential risk is the body identifying the bacterium as foreign and attacking it, negating the original intent of utilizing bacteria to target the tumors. Additionally, if the bacterium’s targeting-specificity is not honed correctly, it may target and kill healthy cells in the body and trigger early cell death systemically, which can worsen the patient’s prognosis and increase the risk of mortality. Administered bacteria also require correct dosing that varies with the patient; higher dosing can be positively correlated with increased risk of toxicity and decreased anticancer efficacy. These specific concerns, along with the variable stability and consistency of bacterial agents as a whole, create major obstacles for more widespread implementation in cancer treatment [15]. A deeper understanding of the mechanisms employed by these bacteria, along with a more detailed profile of the cancer microbiota, provide promising directions for future research in reducing risks and advancing clinical applications.
2.2. Nanocarriers
Engineered nanomaterials called nanocarriers utilize bacterial and other components to enhance drug delivery. Traditional drug delivery methods often face challenges in reaching targeted sites; however, these barriers can be addressed through the selective modification of nanocarriers for cell-specific targeting [52]. Nanotechnology enables the reinforcement of these drug vehicles’ structural integrity to ensure they reach the intended destination, while also allowing for customization of attributes such as size, charge, and composition to meet treatment needs [53]. Biological nanocarriers derived from Archaea and Bacteria have been explored for drug delivery because they enhance cellular uptake, evade the body’s immune response, can fuse to target cells, and are biodegradable [54].
Archaeosomes are a type of nanocarrier made from the phospholipids of various Archaeobacteria. Phospholipids from this domain have several properties that allow them to withstand extreme environmental conditions, making them ideal candidates for drug-delivery systems [55]. Bacterial ghosts (BGs) are empty, Gram-negative cell envelopes that are nonliving and can be loaded with therapeutic agents. BG are desirable for drug delivery because the outer membrane structures are preserved, and they use the body’s inflammatory response and lysozymes to release the drug contents [56]. Outer membrane vesicles (OMVs) are naturally released vesicles from Gram-negative bacteria used for storing and transporting proteins and metabolites [57]. They contain proteases, sulfatases, and adhesins, allowing them to bypass phagocytosis and enter cells by other means [54].
Archaeosomes have been used in treating other cancers and delivering therapeutic cancer vaccines, however, their application in skin cancer is limited [58,59]. BGs, on the other hand, have been shown to have high recognition and internalization by melanoma tumor cells. Results from a study showed that BGs loaded with plasmid DNA were efficiently internalized and phagocytosed by both antigen-presenting cells and tumor cells with 82% of cells expressing the plasmid-encoded reporter gene [60]. BGs have also shown promise as adjuvants to chemotherapy in melanoma [61], while lysate-loaded BGs have been explored as a potential treatment for skin cancer [62]. OMVs show perhaps the greatest success in using nanocarriers for skin cancer treatment. OMVs, derived from transgenic E. coli and modified to induce the transdermal photo-TRAIL-programmed treatment in melanoma, exhibited effective penetration of the skin and specificity to melanoma. Furthermore, they successfully induced photothermal–photodynamic responses against primary melanoma spheroids and activated TRAIL-induced apoptosis, resulting in the complete eradication of melanoma with little to no side effects [63]. This finding was reinforced by a previous study of OMVs acting via an interferon-γ-mediated mechanism to induce long-term antitumor responses without apparent adverse effects [64]. Amidst these triumphs, limitations such as toxicity risk and variable efficacy still remain [57]. Although additional research is required, nanocarriers are emerging as effective immunotherapeutic agents with prospects for implementation in clinical trials.
2.3. Oncolytic Viruses
Oncolytic viruses (OVs) are viruses, either genetically engineered or naturally occurring, designed to selectively replicate within and destroy cancer cells while sparing normal host tissues [65,66]. Since the mid-1800s, tumor regression has been observed in the presence of incidental viral infections, piquing the interest of researchers and subsequently leading to further investigation of the therapeutic properties of viruses in cancer treatment [67]. OVs combat tumors through three key mechanisms: direct oncolysis, vascular disruption, and immune activation. Through the first mechanism, OVs target and replicate specifically within cancerous endothelial cells, resulting in their destruction while sparing healthy tissue [68]. The second mechanism involves the disruption of tumor vasculature through the delivery of anti-angiogenic genes by oncolytic viruses, which hampers angiogenesis and blood flow to the tumor. Other mechanisms employed to disrupt angiogenesis include targeting the VEGF receptor, E-selectin, and other molecules expressed on endothelial cells especially [69]. This leads to a hypoxic microenvironment, inducing secondary necrotic and apoptotic death in nearby uninfected cells, while amplifying the therapeutic effects of the virus [70]. Finally, OVs stimulate innate and adaptive immune responses by releasing tumor-associated antigens and signals, such as damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). These signals recruit and activate dendritic cells and cytotoxic T cells, enhancing the immune system’s ability to eliminate residual tumor cells [68,71].
The clinical application of OVs is extensive, with melanoma, gastrointestinal, and genitourinary cancers being the most frequently studied in clinical trials [72]. A select number of OVs have been approved for cancer treatment worldwide and have shown significant advantages compared to systemic treatments alone [73]. Talimogene laherparepvec (T-VEC), a genetically modified herpes simplex virus type 1, has been approved in the United States for the treatment of advanced melanoma [65]. Rigvir, an oncolytic virus derived from the ECHO-7 strain of enterovirus, is approved in Latvia, Georgia, Armenia, and Uzbekistan for the treatment of melanoma [74]. H101, a genetically modified adenovirus, has been approved in China as an adjunct to chemotherapy to treat nasopharyngeal carcinoma [75].
Building on their broad clinical applications, oncolytic viruses have demonstrated potential in treating skin cancers, particularly melanoma. The only OV approved for use in the US is T-VEC, which in addition to the aforementioned mechanisms of OVs, has been engineered to be selectively replicated within tumor cells to produce granulocyte–macrophage colony-stimulating factor (GM-CSF) [76]. This induces proliferation, maturation, and migration of dendritic cells, leading to antigen presentation and T cell activation, thus increasing immune response [77]. In the phase III OPTiM trial, T-VEC achieved a durable response rate (DRR) of 16.3% compared to 2.1% with GM-CSF alone and an overall response rate (ORR) of 26.4% versus 5.7% [78,79]. However, in the MASTERKEY-265 trial, T-VEC combined with pembrolizumab (a PD-1 inhibitor used in the treatment of melanoma) failed to show additional clinical benefit compared to pembrolizumab alone [80], thus challenging the efficacy of T-VEC. It should also be noted that while patients with compromised immune systems are at higher risk of developing skin cancer, they are also at higher risk for experiencing adverse effects and complications from T-VEC, considering the fact that it is an active attenuated herpes simplex virus. Complications include herpetic infections, ranging from simple cold sores to severe disseminated infections [81].
The application of OVs for NMSC is currently being explored. One notable study researched the potential use of intralesional T-VEC in patients with cutaneous lymphomas and NMSC, particularly cutaneous SCC and MCC. The results demonstrated a significant reduction of elevation in 84% of tumors and a reduction of redness in 68% of tumors. The non-injected response of tumors observed was 40%, and the overall response in patients treated was 32%, suggesting that T-VEC is a viable treatment option for patients presenting with cutaneous lymphoma and non-melanoma skin cancer [82].
OVs have shown considerable promise as novel treatments for various cancers, including melanoma and NMSC. While the results are promising, further research is needed to optimize treatment protocols, enhance the efficacy of OVs, and understand their long-term effects. Ongoing research is crucial to expanding the therapeutic applications of OVs.
3. Conclusions
The rising incidence of skin cancer underscores the need to explore new treatment modalities. While the use of microorganisms in anticancer therapies is still in its early stages, it presents risks that must be considered (Table 1). A primary concern is the potential for infections and their severe consequences, including death. Achieving the desired therapeutic effect requires a balance between stimulating the host’s immune system and attenuating the microorganism’s activity. Additionally, legal challenges arise due to the limited understanding of microbes’ comprehensive impact on cancer [12]. These challenges highlight the critical need for further research in this field. Despite these obstacles, the successes of the therapies discussed in this review emphasize the importance of continued investigation into utilizing microorganisms and their derivatives for treating skin cancer.

Table 1.
Benefits and risks of different microorganism-based treatments for skin cancer.
Author Contributions
Conceptualization, Y.F. and H.J.O.; writing—original draft preparation, H.J.O., A.W.C. and M.N.K.; writing—review and editing, Y.F. and M.R.W.; visualization, H.J.O.; supervision, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
This study is partially supported by a grant for Yujiang Fang (IOER 112-3119). We would like to express appreciation to Lei Zhao from the Department of Respiratory Medicine, The 2nd People’s Hospital of Hefei and Hefei Hospital Affiliated to Anhui Medical University, Hefei, China for his expertise and help with conceptualization, writing—review and editing, and supervision.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AK | Actinic Keratosis |
| BCC | Basal Cell Carcinoma |
| BG | Bacterial Ghost |
| CL | Cutaneous Lymphoma |
| DAMP | Damage-Associated Molecular Pattern |
| DRR | Durable Response Rate |
| GM-CSF | Granulocyte–Macrophage Colony-Stimulating Factor |
| HPV | Human Papillomavirus |
| LGG | Lactobacillus rhamnosus GG |
| LTA | Lipoteichoic Acid |
| MCC | Merkel Cell Carcinoma |
| NMSC | Non-Melanoma Skin Cancer |
| OMV | Outer Membrane Vesicle |
| ORR | Overall Response Rate |
| OV | Oncolytic Virus |
| PAMP | Pathogen-Associated Molecular Pattern |
| SCC | Squamous Cell Carcinoma |
| TIGIT | T Cell Immunoglobulin and ITIM Domain |
| TNF | Tumor Necrosis Factor |
| T-VEC | Talimogene laherparepvec |
References
- Wang, M.; Gao, X.; Zhang, L. Recent Global Patterns in Skin Cancer Incidence, Mortality, and Prevalence. Chin. Med. J. 2024, 138, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Stătescu, L.; Trandafir, L.M.; Țarcă, E.; Moscalu, M.; Leon Constantin, M.M.; Butnariu, L.I.; Trandafirescu, M.F.; Tîrnovanu, M.C.; Heredea, R.; Pătrașcu, A.V.; et al. Advancing Cancer Research: Current Knowledge on Cutaneous Neoplasia. Int. J. Mol. Sci. 2023, 24, 11176. [Google Scholar] [CrossRef] [PubMed]
- Cocuz, I.G.; Popelea, M.C.; Niculescu, R.; Manea, A.; Sabău, A.-H.; Tinca, A.-C.; Szoke, A.R.; Budin, C.E.; Stoian, A.; Morariu, S.H.; et al. Pathophysiology, Histopathology, and Differential Diagnostics of Basal Cell Carcinoma and Cutaneous Squamous Cell Carcinoma-An Update from the Pathologist’s Point of View. Int. J. Mol. Sci. 2024, 25, 2220. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D.B.; Asgari, M.M.; Bennett, D.D.; Connolly, S.M.; Dellavalle, R.P.; Freeman, E.E.; Goldenberg, G.; Leffell, D.J.; Peschin, S.; Sligh, J.E.; et al. Guidelines of Care for the Management of Actinic Keratosis. J. Am. Acad. Dermatol. 2021, 85, e209–e233. [Google Scholar] [CrossRef]
- Nikolouzakis, T.K.; Falzone, L.; Lasithiotakis, K.; Krüger-Krasagakis, S.; Kalogeraki, A.; Sifaki, M.; Spandidos, D.A.; Chrysos, E.; Tsatsakis, A.; Tsiaoussis, J. Current and Future Trends in Molecular Biomarkers for Diagnostic, Prognostic, and Predictive Purposes in Non-Melanoma Skin Cancer. J. Clin. Med. 2020, 9, 2868. [Google Scholar] [CrossRef]
- Kennedy, C.; Bajdik, C.D.; Willemze, R.; De Gruijl, F.R.; Bouwes Bavinck, J.N. Leiden Skin Cancer Study The Influence of Painful Sunburns and Lifetime Sun Exposure on the Risk of Actinic Keratoses, Seborrheic Warts, Melanocytic Nevi, Atypical Nevi, and Skin Cancer. J. Invest. Dermatol. 2003, 120, 1087–1093. [Google Scholar] [CrossRef]
- Belbasis, L.; Stefanaki, I.; Stratigos, A.J.; Evangelou, E. Non-Genetic Risk Factors for Cutaneous Melanoma and Keratinocyte Skin Cancers: An Umbrella Review of Meta-Analyses. J. Dermatol. Sci. 2016, 84, 330–339. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): A Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Saeed, W.; Shahbaz, E.; Maqsood, Q.; Ali, S.W.; Mahnoor, M. Cutaneous Oncology: Strategies for Melanoma Prevention, Diagnosis, and Therapy. Cancer Control J. Moffitt Cancer Cent. 2024, 31, 10732748241274978. [Google Scholar] [CrossRef]
- Firnhaber, J.M. Basal Cell and Cutaneous Squamous Cell Carcinomas: Diagnosis and Treatment. Am. Fam. Physician 2020, 102, 339–346. [Google Scholar]
- Łukasiewicz, K.; Fol, M. Microorganisms in the Treatment of Cancer: Advantages and Limitations. J. Immunol. Res. 2018, 2018, 2397808. [Google Scholar] [CrossRef] [PubMed]
- Skin Cancer and New Treatment Perspectives: A Review—ClinicalKey. Available online: https://www-clinicalkey-com.dmu.idm.oclc.org/#!/content/playContent/1-s2.0-S0304383514006557?scrollTo=%23hl0001845 (accessed on 21 December 2024).
- Gupta, K.H.; Nowicki, C.; Giurini, E.F.; Marzo, A.L.; Zloza, A. Bacterial-Based Cancer Therapy (BBCT): Recent Advances, Current Challenges, and Future Prospects for Cancer Immunotherapy. Vaccines 2021, 9, 1497. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Mandal, S.M. Bacteria and Bacterial Anticancer Agents as a Promising Alternative for Cancer Therapeutics. Biochimie 2020, 177, 164–189. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-Cancer Interactions: Bacteria-Based Cancer Therapy. Exp. Mol. Med. 2019, 51, 152. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Liu, X.; Min, J.-J.; Tan, W.; Zheng, J.H. Targeted Cancer Immunotherapy with Genetically Engineered Oncolytic Salmonella Typhimurium. Cancer Lett. 2020, 469, 102–110. [Google Scholar] [CrossRef]
- Al-Ramadi, B.K.; Fernandez-Cabezudo, M.J.; El-Hasasna, H.; Al-Salam, S.; Attoub, S.; Xu, D.; Chouaib, S. Attenuated Bacteria as Effectors in Cancer Immunotherapy. Ann. N. Y. Acad. Sci. 2008, 1138, 351–357. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Qiao, L.; Li, C.; Zhang, S.; Yin, X.; Du, Z.; Sun, Y.; Qiu, J.; Chang, X.; et al. Programmable Bacteria with Dynamic Virulence Modulation System for Precision Antitumor Immunity. Adv. Sci. 2024, 11, 2404069. [Google Scholar] [CrossRef]
- Chowdhury, S.; Castro, S.; Coker, C.; Hinchliffe, T.E.; Arpaia, N.; Danino, T. Programmable Bacteria Induce Durable Tumor Regression and Systemic Antitumor Immunity. Nat. Med. 2019, 25, 1057–1063. [Google Scholar] [CrossRef]
- Everlon Cid Rigobelo Probiotics; IntechOpen: London, UK, 2012; ISBN 978-953-51-0776-7.
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Tsilingiri, K.; Barbosa, T.; Penna, G.; Caprioli, F.; Sonzogni, A.; Viale, G.; Rescigno, M. Probiotic and Postbiotic Activity in Health and Disease: Comparison on a Novel Polarised Ex-Vivo Organ Culture Model. Gut 2012, 61, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Kullander, J.; Forslund, O.; Dillner, J. Staphylococcus Aureus and Squamous Cell Carcinoma of the Skin. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Matsumoto, M.; Katayama, Y.; Oguma, R.; Wakabayashi, S.; Nygaard, T.; Saijo, S.; Inohara, N.; Otto, M.; Matsue, H.; et al. Staphylococcus Aureus Virulent PSMα Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe 2017, 22, 667–677.e5. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhan, N.; Pausan, M.R.; Halwachs, B.; Durdević, M.; Windisch, M.; Kehrmann, J.; Patra, V.; Wolf, P.; Boukamp, P.; Moissl-Eichinger, C.; et al. Molecular Profiling of Keratinocyte Skin Tumors Links Staphylococcus Aureus Overabundance and Increased Human β-Defensin-2 Expression to Growth Promotion of Squamous Cell Carcinoma. Cancers 2020, 12, 541. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Regalbuto, E.; Pierucci, F.; Arvia, R.; Mazzoli, S.; Gori, A.; de Giorgi, V. Pattern of HPV Infection in Basal Cell Carcinoma and in Perilesional Skin Biopsies from Immunocompetent Patients. Virol. J. 2012, 9, 309. [Google Scholar] [CrossRef]
- Wallace, N.A.; Robinson, K.; Howie, H.L.; Galloway, D.A. β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation. PLoS Pathog. 2015, 11, e1004687. [Google Scholar] [CrossRef]
- Underbrink, M.P.; Howie, H.L.; Bedard, K.M.; Koop, J.I.; Galloway, D.A. E6 Proteins from Multiple Human Betapapillomavirus Types Degrade Bak and Protect Keratinocytes from Apoptosis after UVB Irradiation. J. Virol. 2008, 82, 10408–10417. [Google Scholar] [CrossRef]
- Wallace, N.A.; Robinson, K.; Galloway, D.A. Beta Human Papillomavirus E6 Expression Inhibits Stabilization of P53 and Increases Tolerance of Genomic Instability. J. Virol. 2014, 88, 6112–6127. [Google Scholar] [CrossRef]
- Wood, D.L.A.; Lachner, N.; Tan, J.-M.; Tang, S.; Angel, N.; Laino, A.; Linedale, R.; Lê Cao, K.-A.; Morrison, M.; Frazer, I.H.; et al. A Natural History of Actinic Keratosis and Cutaneous Squamous Cell Carcinoma Microbiomes. mBio 2018, 9, e01432-18. [Google Scholar] [CrossRef]
- Sanford, J.A.; Gallo, R.L. Functions of the Skin Microbiota in Health and Disease. Semin. Immunol. 2013, 25, 370–377. [Google Scholar] [CrossRef]
- Kloos, W.E.; Musselwhite, M.S. Distribution and Persistence of Staphylococcus and Micrococcus Species and Other Aerobic Bacteria on Human Skin1. Appl. Microbiol. 1975, 30, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Butcher, A.M.; Trzoss, L.L.; Nam, S.-J.; Shirakawa, K.T.; Zhou, W.; Oh, J.; Otto, M.; Fenical, W.; et al. A Commensal Strain of Staphylococcus Epidermidis Protects against Skin Neoplasia. Sci. Adv. 2018, 4, eaao4502. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Linehan, J.L.; Han, S.-J.; Harrison, O.J.; Wilhelm, C.; Conlan, S.; Himmelfarb, S.; Byrd, A.L.; Deming, C.; et al. Commensal–Dendritic-Cell Interaction Specifies a Unique Protective Skin Immune Signature. Nature 2015, 520, 104–108. [Google Scholar] [CrossRef]
- Wang, Z.; MacLeod, D.; Di Nardo, A. Commensal-Bacteria LTA Increases Skin Mast Cell Antimicrobial Activity against Vaccinia Viruses. J. Immunol. 2012, 189, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Mekadim, C.; Skalnikova, H.K.; Cizkova, J.; Cizkova, V.; Palanova, A.; Horak, V.; Mrazek, J. Dysbiosis of Skin Microbiome and Gut Microbiome in Melanoma Progression. BMC Microbiol. 2022, 22, 63. [Google Scholar] [CrossRef]
- Yang, Y.; Weng, W.; Peng, J.; Hong, L.; Yang, L.; Toiyama, Y.; Gao, R.; Liu, M.; Yin, M.; Pan, C.; et al. Fusobacterium Nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating TLR4 Signaling to NFκB, Upregulating Expression of microRNA-21. Gastroenterology 2017, 152, 851–866.e24. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium Nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Nwanodi, O. Skin Protective Nutraceuticals: The Current Evidence in Brief. Healthcare 2018, 6, 40. [Google Scholar] [CrossRef]
- Prince, T.; McBain, A.J.; O’Neill, C.A. Lactobacillus Reuteri Protects Epidermal Keratinocytes from Staphylococcus Aureus-Induced Cell Death by Competitive Exclusion. Appl. Environ. Microbiol. 2012, 78, 5119–5126. [Google Scholar] [CrossRef]
- Lee, J.-A.; Ko, J.-H.; Jung, B.-G.; Kim, T.-H.; Hong, J.-I.; Park, Y.-S.; Lee, B.-J. Fermented Prunus Mume with Probiotics Inhibits 7,12-Dimethylbenz[a]Anthracene and 12-O-Tetradecanoyl Phorbol-13-Acetate Induced Skin Carcinogenesis through Alleviation of Oxidative Stress. Asian Pac. J. Cancer Prev. 2013, 14, 2973–2978. [Google Scholar] [CrossRef]
- Wan Mohd Kamaluddin, W.N.F.; Rismayuddin, N.A.R.; Ismail, A.F.; Mohamad Aidid, E.; Othman, N.; Mohamad, N.A.H.; Arzmi, M.H. Probiotic Inhibits Oral Carcinogenesis: A Systematic Review and Meta-Analysis. Arch. Oral Biol. 2020, 118, 104855. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kozlowska, A.K.; Topchyan, P.; Ko, M.-W.; Ohanian, N.; Chiang, J.; Cook, J.; Maung, P.O.; Park, S.-H.; Cacalano, N.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers 2020, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.D.; Campo, V.E.; Cela, E.M.; Morelli, A.E.; Shufesky, W.J.; Tckacheva, O.A.; Leoni, J.; Paz, M.L.; Larregina, A.T.; González Maglio, D.H. Oral Administration of Lipoteichoic Acid from Lactobacillus Rhamnosus GG Overcomes UVB-Induced Immunosuppression and Impairs Skin Tumor Growth in Mice. Eur. J. Immunol. 2019, 49, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Javaherian, M.; Bakhtiari, R.; Ajoudanifar, H.; Shokri, S.; Mirzaie, A. Microbiota, Probiotics and Common Skin Cancer: Association and Therapeutic Application. J. Biol. Res.—Boll. Della Soc. Ital. Biol. Sper. 2022, 95, 10594. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Luo, M.; Hu, M.; Feng, X.; XiaoLi, W.; Dong, D.; Wang, W. Preventive Effect of Lactobacillus Reuteri on Melanoma. Biomed. Pharmacother. 2020, 126, 109929. [Google Scholar] [CrossRef]
- Routy, B.; Jackson, T.; Mählmann, L.; Baumgartner, C.K.; Blaser, M.; Byrd, A.; Corvaia, N.; Couts, K.; Davar, D.; Derosa, L.; et al. Melanoma and Microbiota: Current Understanding and Future Directions. Cancer Cell 2024, 42, 16–34. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giambò, F.; Settanni, C.R.; Cammarota, G.; Gasbarrini, A. Role of Gut Microbiome on Immunotherapy Efficacy in Melanoma. Hum. Vaccines Immunother. 2022, 18, 1926759. [Google Scholar] [CrossRef]
- Huang, X.; Pan, J.; Xu, F.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Bacteria-Based Cancer Immunotherapy. Adv. Sci. 2021, 8, 2003572. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Etter, E.L.; Mei, K.-C.; Nguyen, J. Delivering More for Less: Nanosized, Minimal-Carrier and Pharmacoactive Drug Delivery Systems. Adv. Drug Deliv. Rev. 2021, 179, 113994. [Google Scholar] [CrossRef] [PubMed]
- Moghimipour, E.; Abedishirehjin, S.; Baghbadorani, M.A.; Handali, S. Bacteria and Archaea: A New Era of Cancer Therapy. J. Controlled Release 2021, 338, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zavec, A.B.; Ota, A.; Zupancic, T.; Komel, R.; Ulrih, N.P.; Liovic, M. Archaeosomes Can Efficiently Deliver Different Types of Cargo into Epithelial Cells Grown in Vitro. J. Biotechnol. 2014, 192, 130–135. [Google Scholar] [CrossRef]
- Xie, S.; Li, S.; Zhang, Z.; Chen, M.; Ran, P.; Li, X. Bacterial Ghosts for Targeting Delivery and Subsequent Responsive Release of Ciprofloxacin to Destruct Intracellular Bacteria. Chem. Eng. J. 2020, 399, 125700. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Fonseca, S.; Miquel-Clopés, A.; Cross, K.; Kok, K.-S.; Wegmann, U.; Gil-Cardoso, K.; Bentley, E.G.; Al Katy, S.H.M.; Coombes, J.L.; et al. Bioengineering Commensal Bacteria-Derived Outer Membrane Vesicles for Delivery of Biologics to the Gastrointestinal and Respiratory Tract. J. Extracell. Vesicles 2019, 8, 1632100. [Google Scholar] [CrossRef]
- Karimi, H.; Soleimanjahi, H.; Abdoli, A.; Banijamali, R.S. Combination Therapy Using Human Papillomavirus L1/E6/E7 Genes and Archaeosome: A Nanovaccine Confer Immuneadjuvanting Effects to Fight Cervical Cancer. Sci. Rep. 2020, 10, 5787. [Google Scholar] [CrossRef]
- Benvegnu, T.; Lemiègre, L.; Cammas-Marion, S. New Generation of Liposomes Called Archaeosomes Based on Natural or Synthetic Archaeal Lipids as Innovative Formulations for Drug Delivery. Recent Pat. Drug Deliv. Formul. 2009, 3, 206–220. [Google Scholar] [CrossRef]
- Kudela, P.; Koller, V.J.; Lubitz, W. Bacterial Ghosts (BGs)—Advanced Antigen and Drug Delivery System. Vaccine 2010, 28, 5760–5767. [Google Scholar] [CrossRef]
- Groza, D.; Gehrig, S.; Kudela, P.; Holcmann, M.; Pirker, C.; Dinhof, C.; Schueffl, H.H.; Sramko, M.; Hoebart, J.; Alioglu, F.; et al. Bacterial Ghosts as Adjuvant to Oxaliplatin Chemotherapy in Colorectal Carcinomatosis. Oncoimmunology 2018, 7, e1424676. [Google Scholar] [CrossRef]
- Luo, M.; Chen, X.; Gao, H.; Yang, F.; Chen, J.; Qiao, Y. Bacteria-Mediated Cancer Therapy: A Versatile Bio-Sapper with Translational Potential. Front. Oncol. 2022, 12, 980111. [Google Scholar] [CrossRef]
- Peng, L.-H.; Wang, M.-Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.-T.; Yuan, T.-J.; Jiang, Z.-H.; Gao, J.-Q.; Ning, X.-H. Engineering Bacterial Outer Membrane Vesicles as Transdermal Nanoplatforms for Photo-TRAIL–Programmed Therapy against Melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Park, H.T.; Dinh, N.T.H.; Choi, S.J.; Lee, J.; Kim, J.H.; Lee, S.-W.; Gho, Y.S. Bacterial Outer Membrane Vesicles Suppress Tumor by Interferon-γ-Mediated Antitumor Response. Nat. Commun. 2017, 8, 626. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic Virus Therapy: A New Era of Cancer Treatment at Dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Jhawar, S.R.; Thandoni, A.; Bommareddy, P.K.; Hassan, S.; Kohlhapp, F.J.; Goyal, S.; Schenkel, J.M.; Silk, A.W.; Zloza, A. Oncolytic Viruses—Natural and Genetically Engineered Cancer Immunotherapies. Front. Oncol. 2017, 7, 202. [Google Scholar] [CrossRef]
- Kelly, E.; Russell, S.J. History of Oncolytic Viruses: Genesis to Genetic Engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef]
- Guo, Z.S.; Liu, Z.; Bartlett, D.L. Oncolytic Immunotherapy: Dying the Right Way Is a Key to Eliciting Potent Antitumor Immunity. Front. Oncol. 2014, 4, 74. [Google Scholar] [CrossRef]
- Merchan, J.; Toro Bejarano, M. Targeting Tumor Vasculature through Oncolytic Virotherapy: Recent Advances. Oncolytic Virother. 2015, 169, 169–181. [Google Scholar] [CrossRef]
- Thorne, S.H.; Tam, B.Y.Y.; Kirn, D.H.; Contag, C.H.; Kuo, C.J. Selective Intratumoral Amplification of an Antiangiogenic Vector by an Oncolytic Virus Produces Enhanced Antivascular and Anti-Tumor Efficacy. Mol. Ther. 2006, 13, 938–946. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMP s and DAMP s: Signal 0s That Spur Autophagy and Immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Macedo, N.; Miller, D.M.; Haq, R.; Kaufman, H.L. Clinical Landscape of Oncolytic Virus Research in 2020. J. Immunother. Cancer 2020, 8, e001486. [Google Scholar] [CrossRef]
- Xie, R.; Bi, X.; Shang, B.; Zhou, A.; Shi, H.; Shou, J. Efficacy and Safety of Oncolytic Viruses in Advanced or Metastatic Cancer: A Network Meta-Analysis. Virol. J. 2021, 18, 158. [Google Scholar] [CrossRef] [PubMed]
- Alberts, P.; Tilgase, A.; Rasa, A.; Bandere, K.; Venskus, D. The Advent of Oncolytic Virotherapy in Oncology: The Rigvir® Story. Eur. J. Pharmacol. 2018, 837, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and Its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Ismail, R.; Puzanov, I. Intratumoral Immunotherapy—Update 2019. Oncologist 2020, 25, e423–e438. [Google Scholar] [CrossRef]
- Perales, M.-A.; Yuan, J.; Powel, S.; Gallardo, H.F.; Rasalan, T.S.; Gonzalez, C.; Manukian, G.; Wang, J.; Zhang, Y.; Chapman, P.B.; et al. Phase I/II Study of GM-CSF DNA as an Adjuvant for a Multipeptide Cancer Vaccine in Patients with Advanced Melanoma. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 2022–2029. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Collichio, F.A.; Amatruda, T.; Senzer, N.N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Doleman, S.; Ye, Y.; et al. OPTiM: A Randomized Phase III Trial of Talimogene Laherparepvec (T-VEC) versus Subcutaneous (SC) Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) for the Treatment (Tx) of Unresected Stage IIIB/C and IV Melanoma. J. Clin. Oncol. 2013, 31, LBA9008. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined with Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- DailyMed—IMLYGIC- Talimogene Laherparepvec Injection, Suspension. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=64ffb680-ea8c-42fc-9649-9e8c0eb77ddb (accessed on 28 January 2025).
- Ramelyte, E.; Pawlik, L.; Turko, P.; Sella, F.; Roshardt Prieto, N.M.; Stäger, R.; Maul, J.-T.; Dummer, R. Intralesional Oncolytic Virotherapy with Talimogene Laherparepvec in Patients with Cutaneous Lymphomas and Non-Melanoma Skin Cancers. J. Clin. Oncol. 2023, 41, 9581. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).