Emerging Bluetongue Virus Serotype 4 in the Balearic Islands, Spain (2021): Outbreak Investigations and Experimental Infection in Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnosis of Bluetongue Outbreak in the Balearic Island
2.1.1. Sampling by the Official Veterinary Services
2.1.2. Serological Test Flow Chart
2.1.3. Virological Diagnosis Flow Chart
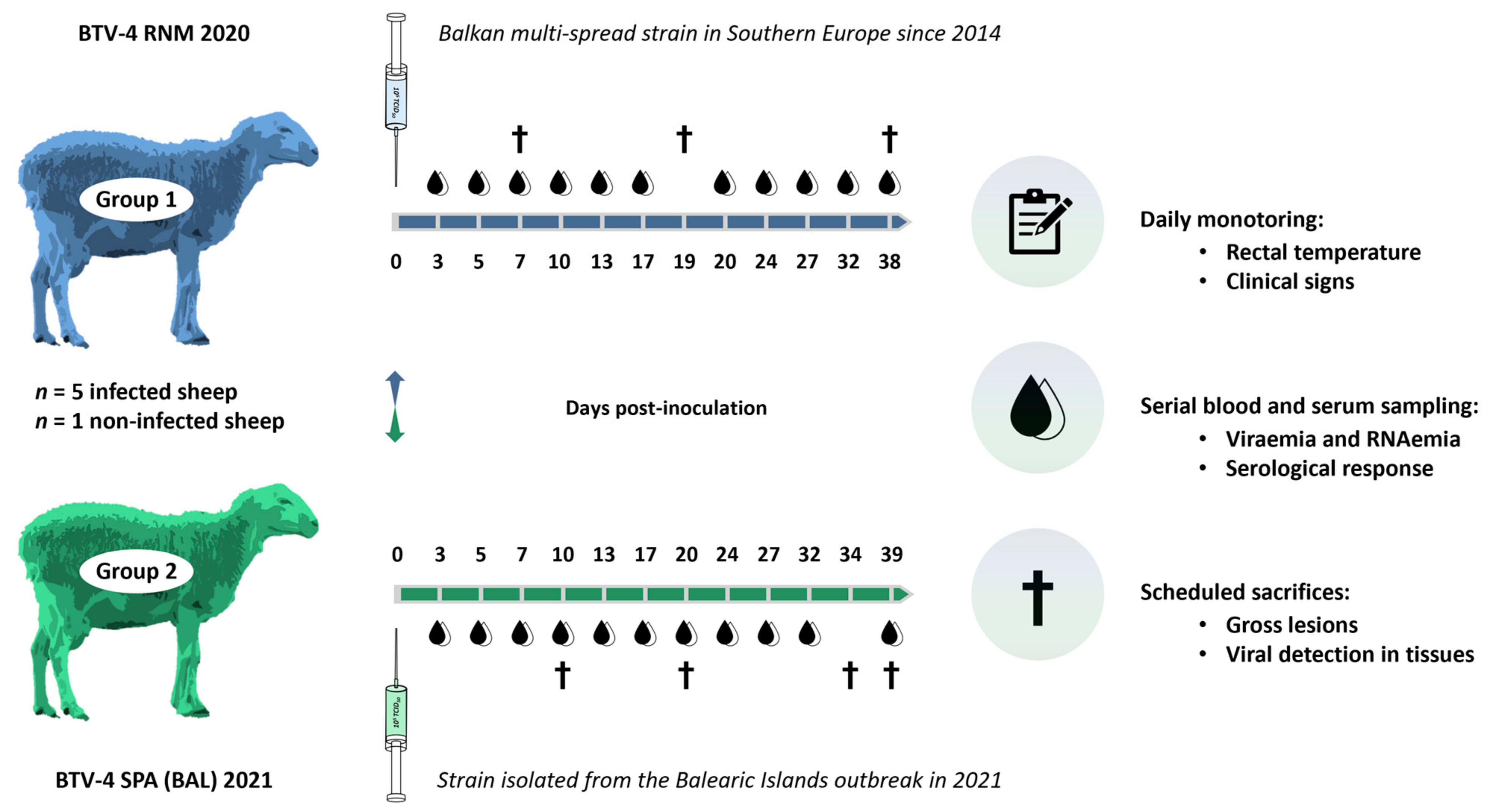
2.2. Experimental Infection of BTV-4 in Sheep
2.2.1. Study Design
2.2.2. Inoculum and Inoculation of Animals
2.2.3. Sampling and Clinical Monitoring of the Animals
2.2.4. Virological Analyses
2.2.5. Analyses of Serological Response
2.2.6. Statistical Analysis
2.3. Diagnostic Methods
2.3.1. ELISA Analysis
2.3.2. Serotyping via Virus Neutralization Test (VNT)
2.3.3. Nucleic Acid Extraction
2.3.4. BTV Genome Detection by Serogroup and Serotype Specific Real Time RT-PCR Methods
2.3.5. Virus Isolation in Cell Culture
2.3.6. Partial Genome Sequencing and Sequencing Analysis
3. Results
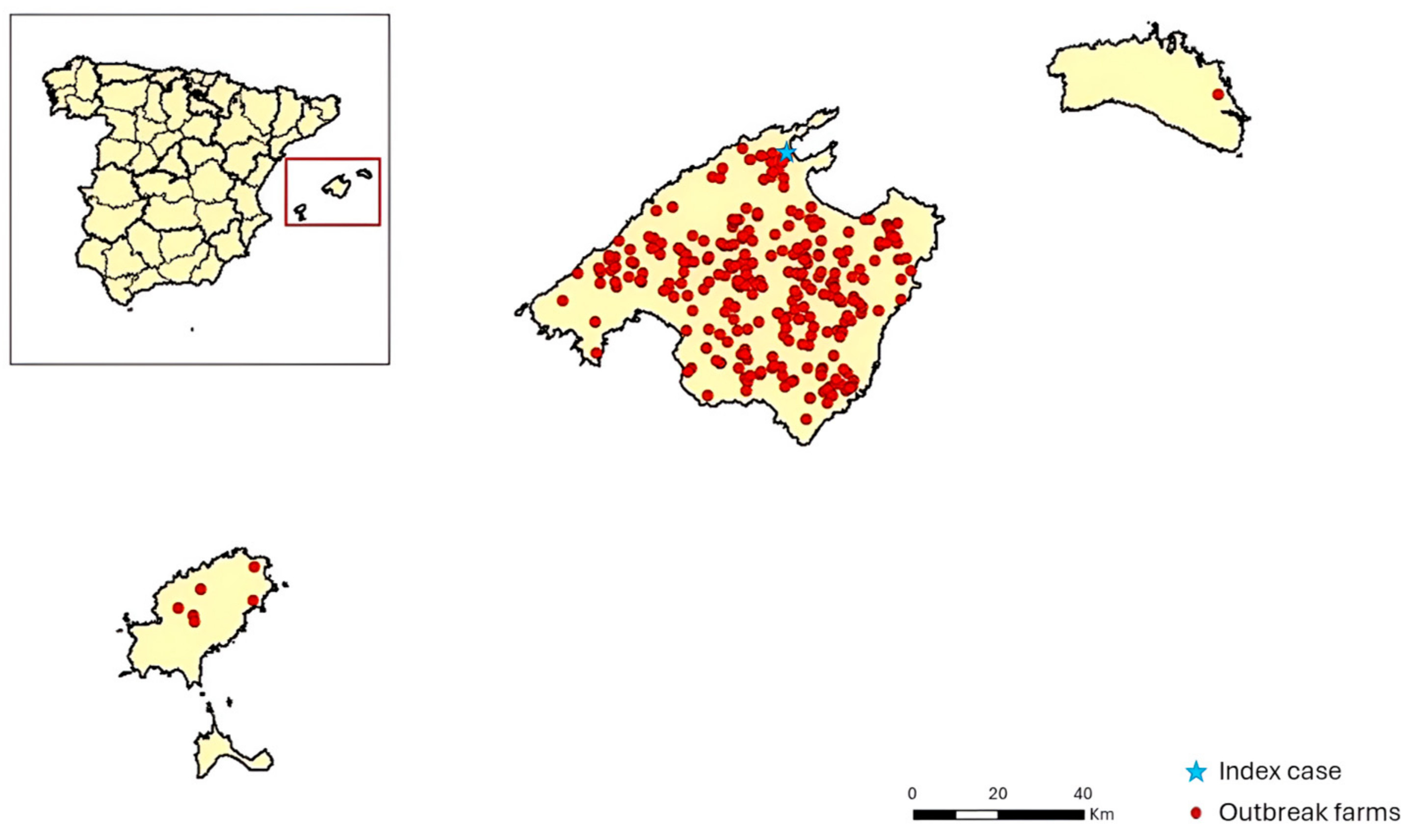
3.1. Timeline and Progression of the Epizootic in the Balearic Islands
3.2. Clinical Signs Observed in Sheep, Cattle, and Goats in the Field During the Outbreak
3.3. Laboratory Diagnosis
3.3.1. Virological Diagnosis
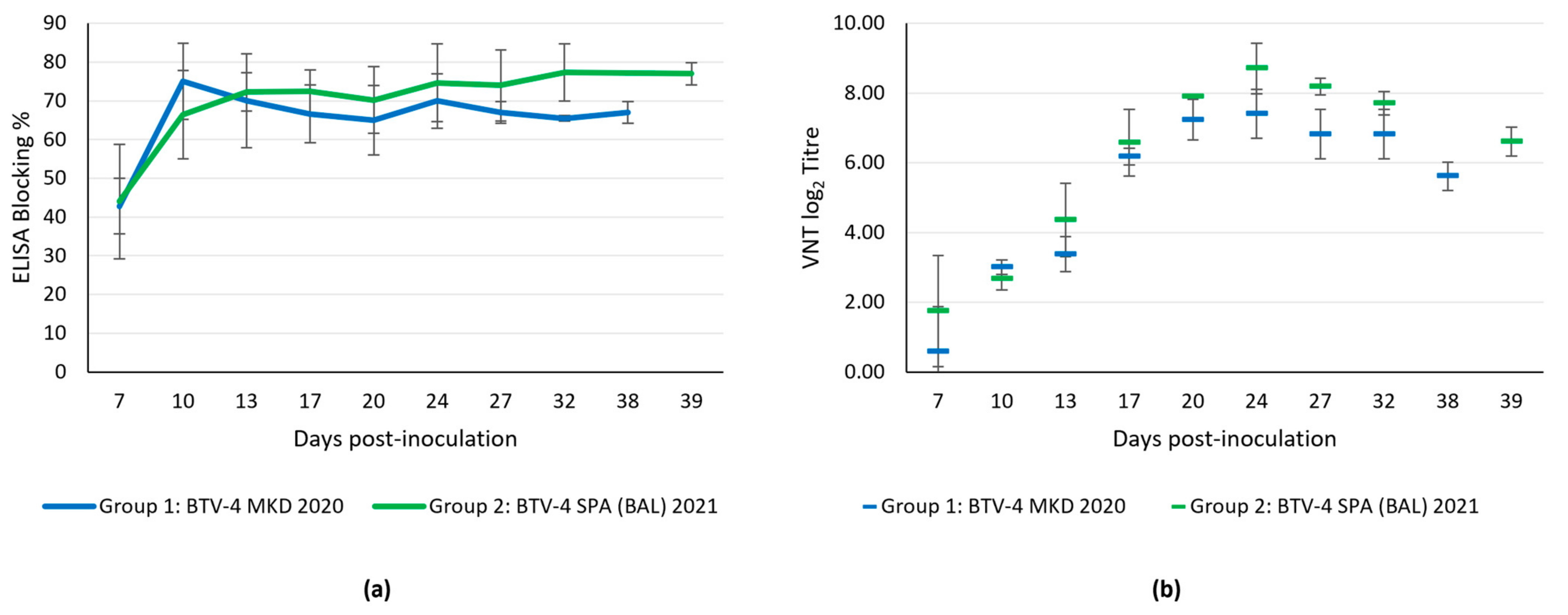
3.3.2. Serological Diagnosis
3.3.3. Post-Vaccination Study
3.3.4. Partial Genome Sequencing
3.4. Experimental Infection with the BTV-4 Strain Detected in the Balearic Islands and Comparison with the Widespread BTV-4 Balkan Strain
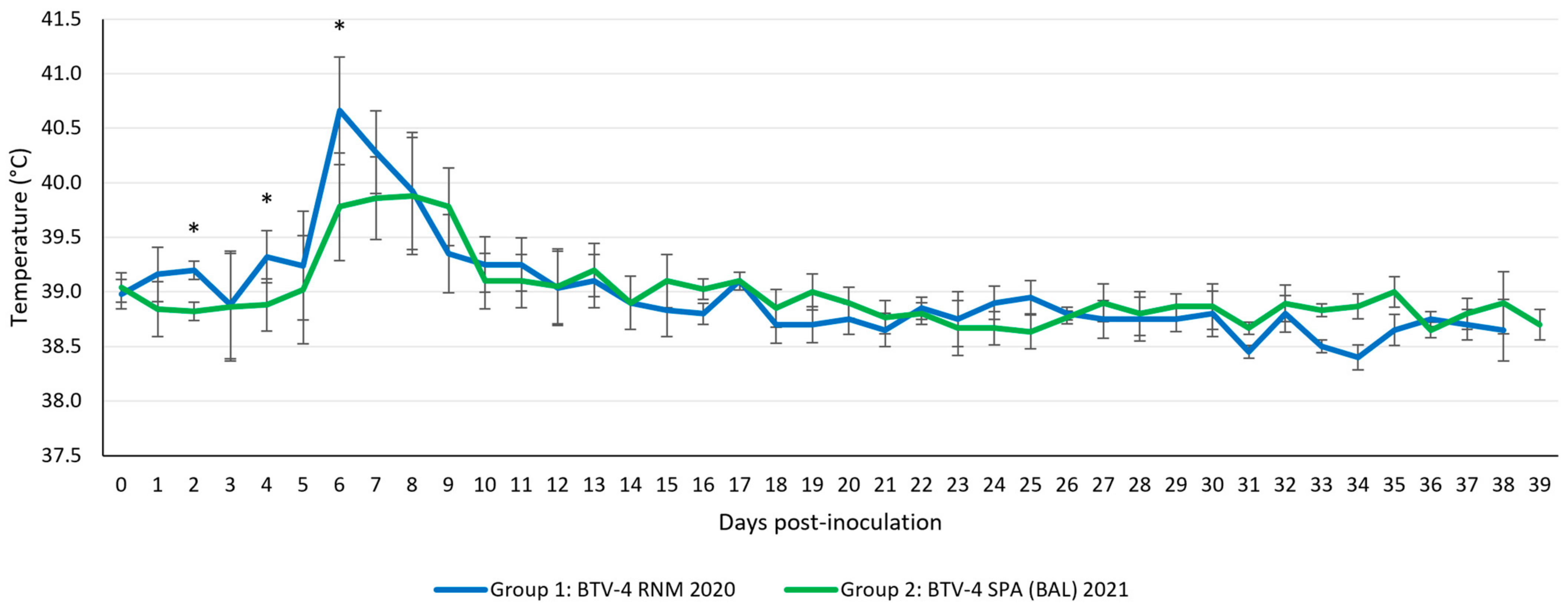
3.4.1. Clinical Manifestations
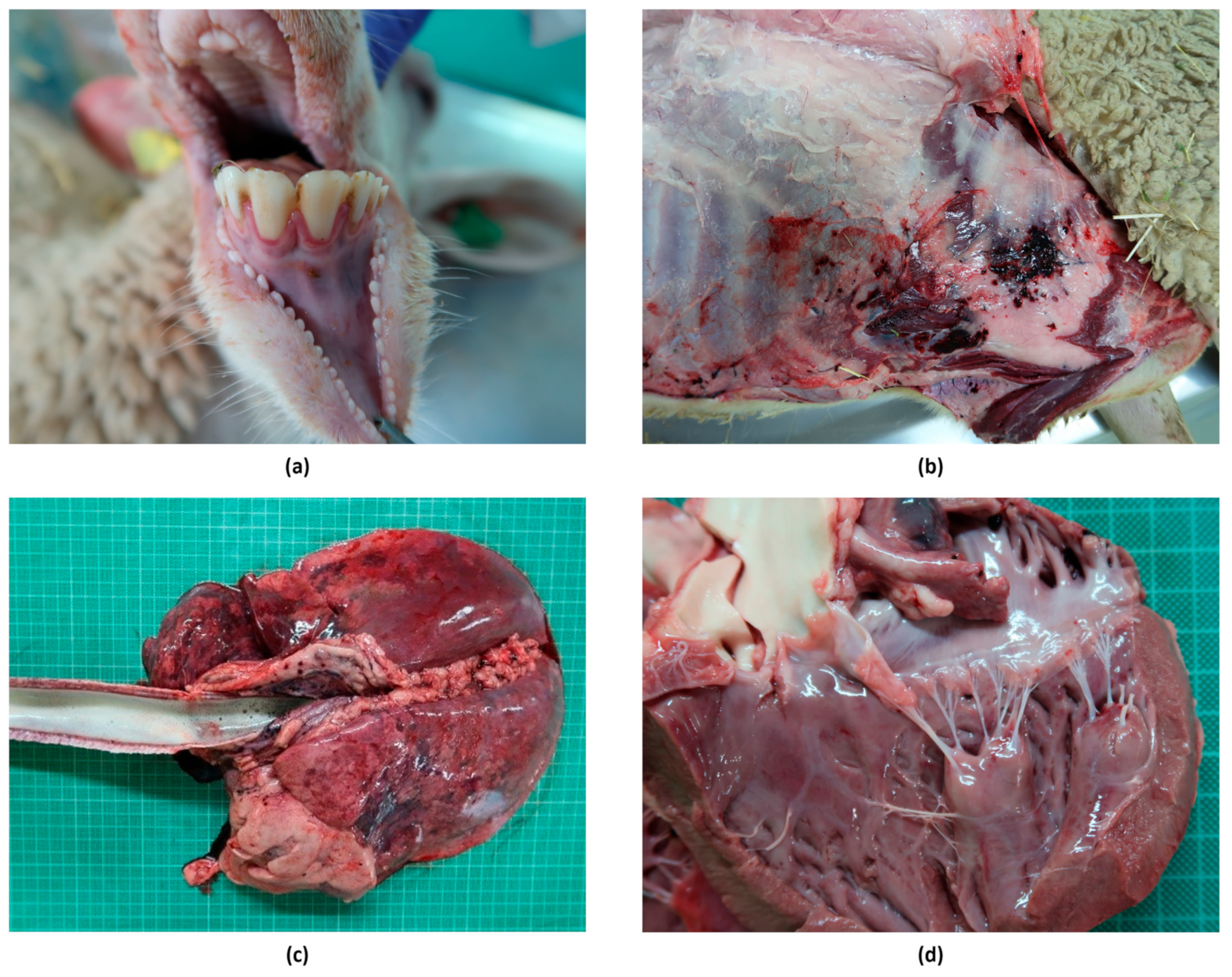
3.4.2. Macroscopic Lesions
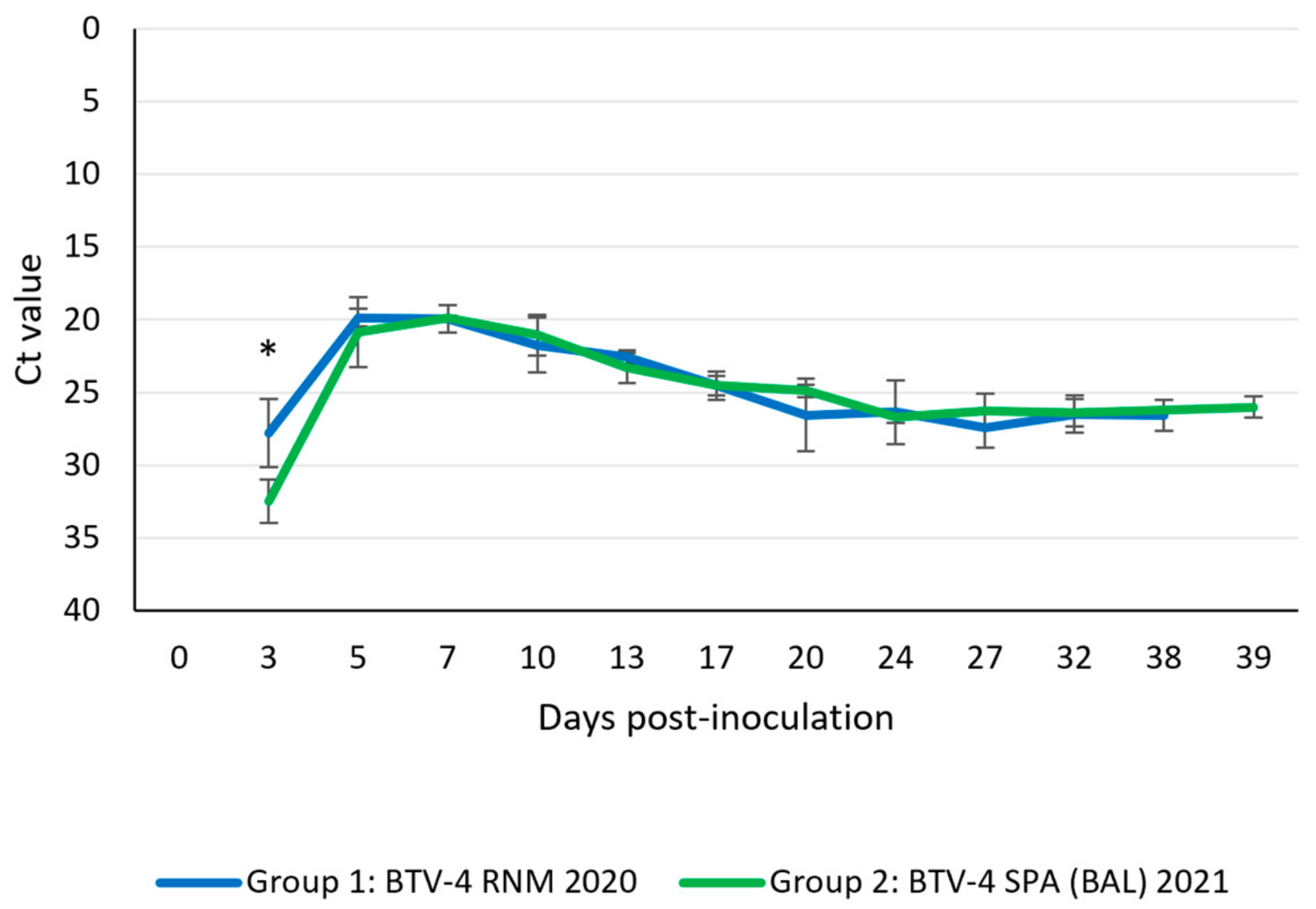
3.4.3. Viral Detection in Blood
3.4.4. Viral Burden in Tissues
3.4.5. Serological Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides Biting Midges, Arboviruses and Public Health in Europe. Antivir. Res. 2013, 100, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Subhadra, S.; Sreenivasulu, D.; Pattnaik, R.; Panda, B.K.; Kumar, S. Bluetongue Virus: Past, Present, and Future Scope. J. Infect. Dev. Ctries. 2023, 17, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Batten, C.A.; Henstock, M.R.; Bin-Tarif, A.; Steedman, H.M.; Waddington, S.; Edwards, L.; Oura, C.A.L. Bluetongue Virus Serotype 26: Infection Kinetics and Pathogenesis in Dorset Poll Sheep. Vet. Microbiol. 2012, 157, 119–124. [Google Scholar] [CrossRef]
- Belbis, G.; Zientara, S.; Bréard, E.; Sailleau, C.; Caignard, G.; Vitour, D.; Attoui, H. Bluetongue Virus: From BTV-1 to BTV-27. In Advances in Virus Research; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 99, pp. 161–197. [Google Scholar]
- Flannery, J.; Frost, L.; Fay, P.; Hicks, H.; Henstock, M.; Smreczak, M.; Orłowska, A.; Rajko-Nenow, P.; Darpel, K.; Batten, C. BTV-14 Infection in Sheep Elicits Viraemia with Mild Clinical Symptoms. Microorganisms 2020, 8, 892. [Google Scholar] [CrossRef]
- Schulz, C.; Sailleau, C.; Bréard, E.; Flannery, J.; Viarouge, C.; Zientara, S.; Beer, M.; Batten, C.; Hoffmann, B. Experimental Infection of Sheep, Goats and Cattle with a Bluetongue Virus Serotype 4 Field Strain from Bulgaria, 2014. Transbound. Emerg. Dis. 2018, 65, e243–e250. [Google Scholar] [CrossRef]
- Schulz, C.; Eschbaumer, M.; Rudolf, M.; König, P.; Keller, M.; Bauer, C.; Gauly, M.; Grevelding, C.G.; Beer, M.; Hoffmann, B. Experimental Infection of South American Camelids with Bluetongue Virus Serotype 8. Vet. Microbiol. 2012, 154, 257–265. [Google Scholar] [CrossRef]
- Matthijnssens, J.; Attoui, H.; Bányai, K.; Brussaard, C.P.D.; Danthi, P.; del Vas, M.; Dermody, T.S.; Duncan, R.; Fāng, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef]
- Samal, S.K.; El-Hussein, A.; Holbrook, F.R.; Beaty, B.J.; Ramig, R.F. Mixed Infection of Culicoides variipennis with Bluetongue Virus Serotypes 10 and 17: Evidence for High Frequency Reassortment in the Vector. J. Gen. Virol. 1987, 68 Pt 9, 2319–2329. [Google Scholar] [CrossRef]
- Saminathan, M.; Singh, K.P.; Khorajiya, J.H.; Dinesh, M.; Vineetha, S.; Maity, M.; Rahman, A.F.; Misri, J.; Malik, Y.S.; Gupta, V.K.; et al. An Updated Review on Bluetongue Virus: Epidemiology, Pathobiology, and Advances in Diagnosis and Control with Special Reference to India. Vet. Q. 2020, 40, 258–321. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Samuel, A.R.; Rao, S.; Attoui, H.; Mertens, P.P.C. Analysis and Phylogenetic Comparisons of Full-Length VP2 Genes of the 24 Bluetongue Virus Serotypes. J. Gen. Virol. 2007, 88, 621–630. [Google Scholar] [CrossRef]
- Schwartz-Cornil, I.; Mertens, P.P.C.; Contreras, V.; Hemati, B.; Pascale, F.; Bréard, E.; Mellor, P.S.; MacLachlan, N.J.; Zientara, S. Bluetongue Virus: Virology, Pathogenesis and Immunity. Vet. Res. 2008, 39, 46. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Vögtlin, A.; Hüssy, D.; Jandt, T.; Gobet, H.; Hilbe, M.; Burgener, C.; Schweizer, L.; Häfliger-Speiser, S.; Beer, M.; et al. Putative Novel Atypical Btv Serotype ‘36’ Identified in Small Ruminants in Switzerland. Viruses 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Clavero, M.A. Animal Viral Diseases and Global Change: Bluetongue and West Nile Fever as Paradigms. Front. Genet. 2012, 3, 105. [Google Scholar] [CrossRef]
- Fay, P.C.; Jaafar, F.M.; Batten, C.; Attoui, H.; Saunders, K.; Lomonossoff, G.P.; Reid, E.; Horton, D.; Maan, S.; Haig, D.; et al. Serological Cross-Reactions between Expressed VP2 Proteins from Different Bluetongue Virus Serotypes. Viruses 2021, 13, 1455. [Google Scholar] [CrossRef]
- Martinelle, L.; Dal Pozzo, F.; Thys, C.; De Leeuw, I.; Van Campe, W.; De Clercq, K.; Thiry, E.; Saegerman, C. Assessment of Cross-Protection Induced by a Bluetongue Virus (BTV) Serotype 8 Vaccine towards Other BTV Serotypes in Experimental Conditions. Vet. Res. 2018, 49, 63. [Google Scholar] [CrossRef]
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The Pathology and Pathogenesis of Bluetongue. J. Comp. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/1882 of 3 December 2018. Off. J. Eur. Union 2018, 308, 1–9. [Google Scholar]
- WOAH. Terrestrial Code Online Access. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/ (accessed on 25 October 2024).
- Mellor, P.S.; Wittmann, E.J. Bluetongue Virus in the Mediterranean Basin 1998–2001. Vet. J. 2002, 164, 20–37. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, B.; Iglesias-Martín, I.; Martínez-Avilés, M.; Sánchez-Vizcaíno, J.M. Orbiviruses in the Mediterranean Basin: Updated Epidemiological Situation of Bluetongue and New Methods for the Detection of BTV Serotype 4. Transbound. Emerg. Dis. 2008, 55, 205–214. [Google Scholar] [CrossRef]
- Gambles, R.M. Bluetongue of Sheep in Cyprus. J. Comp. Pathol. Ther. 1949, 59, 176–190. [Google Scholar] [CrossRef]
- Pérez de Diego, A.C.; Sánchez-Cordón, P.J.; Sánchez-Vizcaíno, J.M. Bluetongue in Spain: From the First Outbreak to 2012. Transbound. Emerg. Dis. 2014, 61, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Mellor, P.S.; Carpenter, S.; Harrup, L.; Baylis, M.; Mertens, P.P.C. Bluetongue in Europe and the Mediterranean Basin: History of Occurrence Prior to 2006. Prev. Vet. Med. 2008, 87, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of Temperate and Tropical Culicoides Midges: Knowledge Gaps and Consequences for Transmission of Culicoides-Borne Viruses. Annu. Rev. Entomol. 2015, 60, 373–392. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Schilling, M.; Sanders, C.; Holding, M.; Johnson, N. Arthropod-Borne Viruses of Human and Animal Importance: Overwintering in Temperate Regions of Europe during an Era of Climate Change. Microorganisms 2024, 12, 1307. [Google Scholar] [CrossRef]
- Zientara, S.; Sánchez-Vizcaíno, J.M. Control of Bluetongue in Europe. Vet. Microbiol. 2013, 165, 33–37. [Google Scholar] [CrossRef]
- Maan, S.; Maan, N.S.; Ross-smith, N.; Batten, C.A.; Shaw, A.E.; Anthony, S.J.; Samuel, A.R.; Darpel, K.E.; Veronesi, E.; Oura, C.A.L.; et al. Sequence Analysis of Bluetongue Virus Serotype 8 from the Netherlands 2006 and Comparison to Other European Strains. Virology 2008, 377, 308–318. [Google Scholar] [CrossRef]
- Toussaint, J.F.; Vandenbussche, F.; Mast, J.; De Meester, L.; Goris, N.; Van Dessel, W.; Vanopdenbosche, E.; Kerkhofs, P.; De Clercq, K.; Zientara, S.; et al. Bluetongue in Northern Europe. Vet. Rec. 2006, 159, 327. [Google Scholar] [CrossRef]
- Orłowska, A.; Trębas, P.; Smreczak, M.; Marzec, A.; Żmudziński, J.F. First Detection of Bluetongue Virus Serotype 14 in Poland. Arch. Virol. 2016, 161, 1969–1972. [Google Scholar] [CrossRef]
- De Clercq, K.; Mertens, P.; De Leeuw, I.; Oura, C.; Houdart, P.; Potgieter, A.C.; Maan, S.; Hooyberghs, J.; Batten, C.; Vandemeulebroucke, E.; et al. Emergence of Bluetongue Serotypes in Europe, Part 2: The Occurrence of a BTV-11 Strain in Belgium. Transbound. Emerg. Dis. 2009, 56, 355–361. [Google Scholar] [CrossRef]
- Holwerda, M.; Santman-Berends, I.M.G.A.; Harders, F.; Engelsma, M.; Vloet, R.P.M.; Dijkstra, E.; Gennip, R.G.P.V.; Mars, M.H.; Spierenburg, M.; Roos, L.; et al. Emergence of Bluetongue Virus Serotype 3, The Netherlands, September 2023. Emerg. Infect. Dis. 2024, 30, 1552–1561. [Google Scholar] [CrossRef]
- Hornyák, Á.; Malik, P.; Marton, S.; Dóró, R.; Cadar, D.; Bányai, K. Emergence of Multireassortant Bluetongue Virus Serotype 4 in Hungary. Infect. Genet. Evol. 2015, 33, 6–10. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; Santman-Berends, I.; van der Heijden, M.G.; Harders, F.; Engelsma, M.; van Gennip, R.G.P.; Maris-Veldhuis, M.A.; Feddema, A.J.; Peterson, K.; Golender, N.; et al. Bluetongue Virus Serotype 12 in Sheep and Cattle in The Netherlands in 2024—A BTV Serotype Reported in Europe for the First Time. Vet. Microbiol. 2025, 301, 110365. [Google Scholar] [CrossRef]
- Breard, E.; Sailleau, C.; Nomikou, K.; Hamblin, C.; Mertens, P.P.C.; Mellor, P.S.; El Harrak, M.; Zientara, S. Molecular Epidemiology of Bluetongue Virus Serotype 4 Isolated in the Mediterranean Basin between 1979 and 2004. Virus Res. 2007, 125, 191–197. [Google Scholar] [CrossRef]
- Sailleau, C.; Breard, E.; Viarouge, C.; Gorlier, A.; Quenault, H.; Hirchaud, E.; Touzain, F.; Blanchard, Y.; Vitour, D.; Zientara, S. Complete Genome Sequence of Bluetongue Virus Serotype 4 That Emerged on the French Island of Corsica in December 2016. Transbound. Emerg. Dis. 2018, 65, e194–e197. [Google Scholar] [CrossRef]
- Sailleau, C.; Breard, E.; Viarouge, C.; Gorlier, A.; Leroux, A.; Hirchaud, E.; Lucas, P.; Blanchard, Y.; Vitour, D.; Grandcollot-Chabot, M.; et al. Emergence of Bluetongue Virus Serotype 4 in Mainland France in November 2017. Transbound. Emerg. Dis. 2018, 65, 1158–1162. [Google Scholar] [CrossRef]
- Celina, S.S.; King, S.; Ashby, M.; Harris, K.; Polo, N.; Alishani, M.; Robaj, A.; Hamidi, A.; Sylejmani, D.; Batten, C.; et al. Re-Emergence of BTV-4 in Sheep Farms in Kosovo, 2020: A Retrospective Study. Transbound. Emerg. Dis. 2023, 2023, 3112126. [Google Scholar] [CrossRef]
- Flannery, J.; King, S.; Rajko-Nenow, P.; Popova, Z.; Krstevski, K.; Djadjovski, I.; Batten, C. Re-Emergence of BTV Serotype 4 in North Macedonia, July 2020. Transbound. Emerg. Dis. 2021, 68, 220–223. [Google Scholar] [CrossRef]
- Saegerman, C.; Berkvens, D.; Mellor, P.S. Bluetongue Epidemiology in the European Union. Emerg. Infect. Dis. 2008, 14, 539–544. [Google Scholar] [CrossRef]
- Hourrigan, J.L.; Klingsporn, A.L. Bluetongue: The Disease in Cattle. Aust. Vet. J. 1975, 51, 170–174. [Google Scholar] [CrossRef]
- Sellers, R.F.; Pedgley, D.E.; Tucker, M.R. Possible Windborne Spread of Bluetongue to Portugal, June–July 1956. Epidemiol. Infect. 1978, 81, 189–196. [Google Scholar] [CrossRef]
- Geering, W.A. Control of Bluetongue in an Epizootic Situation: Australian Plans. Aust. Vet. J. 1975, 51, 220–224. [Google Scholar] [CrossRef]
- Maan, S.; Samuel, A.R.; Maan, N.S.; Attoui, H.; Rao, S.; Mertens, P.P.C. Bluetongue Virus and Disease. Vet. Ital. 2004, 40, 4. [Google Scholar]
- MAPA. Detección del Serotipo 3 del Virus de la Lengua Azul en las Provincias de Badajoz y Huelva (30/09/2024). Available online: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/notala_s3_30092024_tcm30-693678.pdf (accessed on 5 December 2024).
- Barros, S.C.; Henriques, A.M.; Ramos, F.; Luís, T.; Fagulha, T.; Magalhães, A.; Caetano, I.; Santos, F.A.d.; Correia, F.O.; Santana, C.C.; et al. Emergence of Bluetongue Virus Serotype 3 in Portugal (2024). Viruses 2024, 16, 1845. [Google Scholar] [CrossRef]
- MAPA. Programa Nacional de Vigilancia, Control y Erradicación de la Lengua Azul. 2024. Available online: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/programala_tcm30-437541.pdf (accessed on 5 December 2024).
- Real Decreto 148/2023, de 28 de Febrero, Por el Que Se Designa el Laboratorio Nacional de Referencia de Distintas Enfermedades de Los Animales y Se Derogan Diversas Normas de Sanidad Animal. Boletín of Estado 2023, 60, 36236–36240. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2023-6374 (accessed on 30 December 2024).
- EURL. EU Diagnostic Manual for African Horse Sickness and Buetongue. European Union Reference Laboratory for African Horse Sickness and Bluetongue. 2021. Available online: https://www.mapa.gob.es/es/ganaderia/temas/laboratorios-sanidad-genetica/eudiagnosismanualahsbtrev01_tcm30-576045.pdf (accessed on 5 December 2024).
- WOAH. Bluetongue (Infection with Bluetongue Virus). In WOAH Terrestrial Manual; WOAH: Paris, France, 2021; pp. 1–23. Available online: http://btv-glue.cvr.gla.ac.uk (accessed on 5 December 2024).
- Kärber, G. Beitrag Zur Kollektiven Behandlung Pharmakologischer Reihenversuche. Naunyn-Schmiedeb. Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Spearman, C. The Method of “Right and Wrong Cases” (Constant Stimuli) without Gauss’s Formulae. Br. J. Psychol. 1904–1920 1908, 2, 227–242. [Google Scholar] [CrossRef]
- Hofmann, M.A.; Renzullo, S.; Mader, M.; Chaignat, V.; Worwa, G.; Thuer, B. Genetic Characterization of Toggenburg Orbivirus, a New Bluetongue Virus, from Goats, Switzerland. Emerg. Infect. Dis. 2008, 14, 1855–1861. [Google Scholar] [CrossRef]
- Mertens, P.P.C.; Maan, N.S.; Prasad, G.; Samuel, A.R.; Shaw, A.E.; Potgieter, A.C.; Anthony, S.J.; Maan, S. Design of Primers and Use of RT-PCR Assays for Typing European Bluetongue Virus Isolates: Differentiation of Field and Vaccine Strains. J. Gen. Virol. 2007, 88, 2811–2823. [Google Scholar] [CrossRef]
- Katz, J.B.; Gustafson, G.A.; Alstad, A.D.; Adler, K.A.; Moser, K.M. Colorimetric Diagnosis of Prolonged Bluetongue Viremia in Sheep, Using an Enzyme-Linked Oligonucleotide Sorbent Assay of Amplified Viral Nucleic Acids. Am. J. Vet. Res. 1993, 54, 2021–2026. [Google Scholar] [CrossRef]
- van Rijn, P.A.; Heutink, R.G.; Boonstra, J.; Kramps, H.A.; van Gennip, R.G.P. Sustained High-Throughput Polymerase Chain Reaction Diagnostics during the European Epidemic of Bluetongue Virus Serotype 8. J. Vet. Diagn. Investig. 2012, 24, 469–478. [Google Scholar] [CrossRef]
- Gethmann, J.; Probst, C.; Conraths, F.J. Economic Impact of a Bluetongue Serotype 8 Epidemic in Germany. Front. Vet. Sci. 2020, 7, 65. [Google Scholar] [CrossRef]
- Brand, S.P.C.; Keeling, M.J. The Impact of Temperature Changes on Vector-Borne Disease Transmission: Culicoides Midges and Bluetongue Virus. J. R. Soc. Interface 2017, 14, 20160481. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, J.; Tildesley, M.; Robbins, A.; Gorsich, E. Parameterisation of a Bluetongue Virus Mathematical Model Using a Systematic Literature Review. Prev. Vet. Med. 2024, 232, 106328. [Google Scholar] [CrossRef]
- Aguilar-Vega, C.; Fernández-Carrión, E.; Lucientes, J.; Sánchez-Vizcaíno, J.M. A Model for the Assessment of Bluetongue Virus Serotype 1 Persistence in Spain. PLoS ONE 2020, 15, e0232534. [Google Scholar] [CrossRef]
- Miranda, M.A.; Borràs, D.; Rincón, C.; Alemany, A. Presence in the Balearic Islands (Spain) of the Midges Culicoides imicola and Culicoides obsoletus Group. Med. Vet. Entomol. 2003, 17, 52–54. [Google Scholar] [CrossRef]
- Katsoulos, P.D.; Giadinis, N.D.; Chaintoutis, S.C.; Dovas, C.I.; Kiossis, E.; Tsousis, G.; Psychas, V.; Vlemmas, I.; Papadopoulos, T.; Papadopoulos, O.; et al. Epidemiological Characteristics and Clinicopathological Features of Bluetongue in Sheep and Cattle, during the 2014 BTV Serotype 4 Incursion in Greece. Trop. Anim. Health Prod. 2016, 48, 469–477. [Google Scholar] [CrossRef]
- BalearsMeteo Balearsmeteo—Informes Mensuales, Anuales y Comparativas de Can Pontico (Pollença). Available online: https://www.balearsmeteo.com/can_pontico/informes_mensuales_anuales.php (accessed on 30 October 2024).
- Carpenter, S.; Wilson, A.; Barber, J.; Veronesi, E.; Mellor, P.; Venter, G.; Gubbins, S. Temperature Dependence of the Extrinsic Incubation Period of Orbiviruses in Culicoides Biting Midges. PLoS ONE 2011, 6, e27987. [Google Scholar] [CrossRef]
- Hilke, J.; Strobel, H.; Woelke, S.; Stoeter, M.; Voigt, K.; Grimm, L.; Meilwes, J.; Punsmann, T.; Blaha, I.; Salditt, A.; et al. A Comparison of Different Vaccination Schemes Used in Sheep Combining Inactivated Bluetongue Vaccines against Serotypes 4 and 8. Vaccine 2019, 37, 5844–5853. [Google Scholar] [CrossRef]
- Savini, G.; Ronchi, G.F.; Leone, A.; Ciarelli, A.; Migliaccio, P.; Franchi, P.; Mercante, M.T.; Pini, A. An Inactivated Vaccine for the Control of Bluetongue Virus Serotype 16 Infection in Sheep in Italy. Vet. Microbiol. 2007, 124, 140–146. [Google Scholar] [CrossRef][Green Version]
- Eschbaumer, M.; Hoffmann, B.; König, P.; Teifke, J.P.; Gethmann, J.M.; Conraths, F.J.; Probst, C.; Mettenleiter, T.C.; Beer, M. Efficacy of Three Inactivated Vaccines against Bluetongue Virus Serotype 8 in Sheep. Vaccine 2009, 27, 4169–4175. [Google Scholar] [CrossRef]
- Aguilar-Vega, C.; Fernández-Carrión, E.; Sánchez-Vizcaíno, J.M. The Possible Route of Introduction of Bluetongue Virus Serotype 3 into Sicily by Windborne Transportation of Infected Culicoides spp. Transbound. Emerg. Dis. 2019, 66, 1665–1673. [Google Scholar] [CrossRef]
- Bibard, A.; Martinetti, D.; Giraud, A.; Picado, A.; Chalvet-Monfray, K.; Porphyre, T. Quantitative Risk Assessment for the Introduction of Bluetongue Virus into Mainland Europe by Long-Distance Wind Dispersal of Culicoides spp.: A Case Study from Sardinia. Risk Anal. 2024, 45, 108–127. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, K.; Hughes, J.; Wash, R.; Kellam, P.; Breard, E.; Zientara, S.; Palmarini, M.; Biek, R.; Mertens, P. Widespread Reassortment Shapes the Evolution and Epidemiology of Bluetongue Virus Following European Invasion. PLOS Pathog. 2015, 11, e1005056. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Tejedor, C. Brief Overview of the Bluetongue Situation in Mediterranean Europe, 1998–2004. Vet. Ital. 2004, 40, 57–60. [Google Scholar] [PubMed]
- Ben Salem, A.; Ben Aicha, E.; Kalthoum, S.; Dhaouadi, A.; Hajlaoui, H.; Bel Haj Mohamed, B.; Ben Slimen, I.; Khalfaoui, W.; Gharbi, R.; Guesmi, K.; et al. Estimation of the Economic Impact of a Bluetongue Serotype 4 Outbreak in Tunisia. Front. Vet. Sci. 2024, 11, 1310202. [Google Scholar] [CrossRef]
- Gondard, M.; Postic, L.; Garin, E.; Turpaud, M.; Vorimore, F.; Ngwa-Mbot, D.; Tran, M.-L.; Hoffmann, B.; Warembourg, C.; Savini, G.; et al. Exceptional Bluetongue Virus (BTV) and Epizootic Hemorrhagic Disease Virus (EHDV) Circulation in France in 2023. Virus Res. 2024, 350, 199489. [Google Scholar] [CrossRef]
- IZS. Bluetongue Serotype 4 Epidemic in Sardinia Region. Available online: https://www.izs.it/IZS/Bluetongue_serotype_4_epidemic_in_Sardinia_Region (accessed on 5 December 2024).
- Caporale, M.; Di Gialleonorado, L.; Janowicz, A.; Wilkie, G.; Shaw, A.; Savini, G.; Van Rijn, P.A.; Mertens, P.; Di Ventura, M.; Palmarini, M. Virus and Host Factors Affecting the Clinical Outcome of Bluetongue Virus Infection. J. Virol. 2014, 88, 10399–10411. [Google Scholar] [CrossRef]
- Coetzee, P.; Van Vuuren, M.; Venter, E.H.; Stokstad, M. A Review of Experimental Infections with Bluetongue Virus in the Mammalian Host. Virus Res. 2014, 182, 21–34. [Google Scholar] [CrossRef]
- Koumbati, M.; Mangana, O.; Nomikou, K.; Mellor, P.S.; Papadopoulos, O. Duration of Bluetongue Viraemia and Serological Responses in Experimentally Infected European Breeds of Sheep and Goats. Vet. Microbiol. 1999, 64, 277–285. [Google Scholar] [CrossRef]
- Eschbaumer, M.; Wäckerlin, R.; Rudolf, M.; Keller, M.; König, P.; Zemke, J.; Hoffmann, B.; Beer, M. Infectious Blood or Culture-Grown Virus: A Comparison of Bluetongue Virus Challenge Models. Vet. Microbiol. 2010, 146, 150–154. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Pleguezuelos, F.J.; Pérez de Diego, A.C.; Gómez-Villamandos, J.C.; Sánchez-Vizcaíno, J.M.; Cerón, J.J.; Tecles, F.; Garfia, B.; Pedrera, M. Comparative Study of Clinical Courses, Gross Lesions, Acute Phase Response and Coagulation Disorders in Sheep Inoculated with Bluetongue Virus Serotype 1 and 8. Vet. Microbiol. 2013, 166, 184–194. [Google Scholar] [CrossRef]
- Flannery, J.; Sanz-Bernardo, B.; Ashby, M.; Brown, H.; Carpenter, S.; Cooke, L.; Corla, A.; Frost, L.; Gubbins, S.; Hicks, H.; et al. Evidence of Reduced Viremia, Pathogenicity and Vector Competence in a Re-Emerging European Strain of Bluetongue Virus Serotype 8 in Sheep. Transbound. Emerg. Dis. 2019, 66, 1177–1185. [Google Scholar] [CrossRef]
- Westrich, J.A.; McNulty, E.E.; Stoltz, M.; Sherman, T.J.; Carpenter, M.; Burton, M.; Nalls, A.; Rubio, H.S.; Sandoval, A.; Mayo, C.; et al. Immunological and Pathogenic Differences of Two Experimental Bluetongue Virus Serotype Infections Evaluated in Two Disparate Host Species. Viruses 2024, 16, 1593. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Crafford, J.E.; Vernau, W.; Gardner, I.A.; Goddard, A.; Guthrie, A.J.; Venter, E.H. Experimental Reproduction of Severe Bluetongue in Sheep. Vet. Pathol. 2008, 45, 310–315. [Google Scholar] [CrossRef]
- Pérez de Diego, A.C.; Athmaram, T.N.; Stewart, M.; Rodríguez-Sánchez, B.; Sánchez-Vizcaíno, J.M.; Noad, R.; Roy, P. Characterization of Protection Afforded by a Bivalent Virus-Like Particle Vaccine against Bluetongue Virus Serotypes 1 and 4 in Sheep. PLoS ONE 2011, 6, e26666. [Google Scholar] [CrossRef]
- Bonneau, K.R.; DeMaula, C.D.; Mullens, B.A.; MacLachlan, N.J. Duration of Viraemia Infectious to Culicoides sonorensis in Bluetongue Virus-Infected Cattle and Sheep. Vet. Microbiol. 2002, 88, 115–125. [Google Scholar] [CrossRef]
- Brewer, A.W.; MacLachlan, N.J. Ultrastructural Characterization of the Interaction of Bluetongue Virus with Bovine Erythrocytes in Vitro. Vet. Pathol. 1992, 29, 356–359. [Google Scholar] [CrossRef]
- MacLachlan, N.J.; Nunamaker, R.A.; Katz, J.B.; Sawyer, M.M.; Akita, G.Y.; Osburn, B.I.; Tabachnick, W.J. Detection of Bluetongue Virus in the Blood of Inoculated Calves: Comparison of Virus Isolation, PCR Assay, and in Vitro Feeding of Culicoides variipennis. Arch. Virol. 1994, 136, 1–8. [Google Scholar] [CrossRef]
- Singer, R.S.; MacLachlan, N.J.; Carpenter, T.E. Maximal Predicted Duration of Viremia in Bluetongue Virus—Infected Cattle. J. Vet. Diagn. Investig. 2001, 13, 43–49. [Google Scholar] [CrossRef]
- Pini, A. Study on the Pathogenesis of Bluetongue: Replication of the Virus in the Organs of Infected Sheep. Onderstepoort J. Vet. Res. 1976, 43, 159–164. [Google Scholar]
- Westrich, J.A.; McNulty, E.E.; Carpenter, M.; Burton, M.; Reed, K.; Nalls, A.; Sandoval, A.; Mayo, C.; Mathiason, C.K. Monitoring Longitudinal Immunological Responses to Bluetongue Virus 17 in Experimentally Infected Sheep. Virus Res. 2023, 338, 199246. [Google Scholar] [CrossRef]
- Daniels, P.W.; Sendow, I.; Pritchard, L.I.; Sukarsih; Eaton, B.T. Regional Overview of Bluetongue Viruses in South-East Asia: Viruses, Vectors and Surveillance. Vet. Ital. 2004, 40, 94–100. [Google Scholar] [PubMed]
- Chiuya, T.; Fèvre, E.M.; Okumu, N.O.; Abdi, A.M.; Junglen, S.; Borgemeister, C. Exposure to Arboviruses in Cattle: Seroprevalence of Rift Valley Fever, Bluetongue, and Epizootic Hemorrhagic Disease Viruses and Risk Factors in Baringo County, Kenya. Pathogens 2024, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Medrouh, B.; Abdelli, A.; Belkessa, S.; Ouinten, Y.; Brahimi, M.; Hakem, A.; Kernif, T.; Singer, S.M.; Ziam, H.; Tsaousis, A.D.; et al. Seroprevalence and Risk Factors of Bluetongue Virus in Domestic Cattle, Sheep, Goats and Camels in Africa: A Systematic Review and Meta-Analysis. Vet. Q. 2024, 44, 1–12. [Google Scholar] [CrossRef]

| Serotype/Topotype | Primers (F/R) and Probe (P) | Sequence 5′-3′ | Product Size (pb) | Validated to Detect |
|---|---|---|---|---|
| BTV-1 w.med | BTV-1 w.med (F) | GACGATGCCGCGTATGGT | 135 | Western Mediterranean strains |
| BTV-1 w.med (R) | TTAGCTTTGCATCCTTTTCAAAA | |||
| BTV-1 w.med (P) | FAM-TAGAACGTTACCTTCTGATTTT-MGB | |||
| BTV-8 eur | BTV-8 eur (F) | TGCGCACGATATYCGAATT | 66 | European strains |
| BTV-8 eur (R) | GACGTCAGCCCAAAACGATT | |||
| BTV-8 eur (P) | FAM-TTGTACGCTCCAACYTCCAAAGGTA-BHQ1 | |||
| BTV-4 w.med | BTV-4 w.med (F) | AACACGTATTTATTGTCCTCCAATTG | 59 | Western Mediterranean strains |
| BTV-4 w.med (R) | AGCTTGCGGCCGGAAT | |||
| BTV-4 w.med (P) | FAM-CGTTCCCGTTGACCG-MGB | |||
| BTV-4 ext | BTV-4 ext (F) | AACACRTAYTTATTGTCYTCYAATTG | 59 | Western Mediterranean and Balkan strains |
| BTV-4 ext (R) | AGCTTGCGGCCGGAAT | |||
| BTV-4 ext (P) | FAM-CGTTCCCGTTGACCG-MGB |
| Viral Segment Material ID (AN) | Length (pb) | nt Position in BTV-4/GRE2014/08 (AN) | % | Strain | AN |
|---|---|---|---|---|---|
| Partial seg-2 isolate BTV-4 SPA 2021/01 VP2 (MZ919337.1) | 1126 | 767 to 1892 (MT879212.1) | 99.73 | BTV-4/21-03 CORSICA 2021 | PP262563.1 |
| 98.05 | BTV-4/16-03 CORSICA 2016 | KY654329.1 | |||
| 98.05 | BTV-4/KOS2014/01 | OP186417.1 | |||
| 98.05 | BTV-4/GRE2014/08 | MT879212.1 | |||
| Partial seg-5 isolate BTV-4 SPA 2021/01 NS1 (MZ919338.1) | 260 | 22 to 281 (MT879215.1) | 100 | BTV-4/21-03 CORSICA 2021 | PP262566.1 |
| 98.85 | BTV 17/O.aries-tc/ZAF/2014 | MG255486.1 | |||
| 98.85 | BTV 5/O.aries-tc/ZAF/2011 | MG255456.1 | |||
| 98.85 | BTV-4 isolate SPA2010/01 | KP821432.1 | |||
| Partial seg-10 isolate BTV-4 SPA 2021/01 NS3 (MZ919339.1) | 760 | 3 to 762 (MT879220.1) | 99.87 | BTV-4/21-03 CORSICA 2021 | PP262571.1 |
| 98.55 | BTV-1 TUN2007/01 | KP821975.1 | |||
| 98.55 | BTV-1 MOR2007/01 | KP821975.1 | |||
| 98.55 | BTV-1 LIB2007/06 | KP821973.1 |
| Clinical Sings | Group 1 | Group 2 | ||
|---|---|---|---|---|
| Onset dpi | n | Onset dpi | n | |
| Hyperemic and congestive mucous membranes | 5–6 | 5/5 | 3–13 | 5/5 |
| Fever | 6–9 | 5/5 | 6–9 | 3/5 |
| Serous nasal discharge | 5–6 | 4/5 | 5–7 | 4/5 |
| Dyspnoea | 6 | 4/5 | 6–24 | 3/5 |
| Increase in size on retropharyngeal and submandibular lymph nodes | 6–28 | 2/5 | 7–17 | 4/5 |
| Neck edema | 11–20 | 3/4 | 11–24 | 3/4 |
| Weakness and apathy | 11–28 | 2/4 | 12–26 | 2/4 |
| Muzzle edema | 18 | 1/3 | 17 | 2/4 |
| Lameness | 11 | 1/4 | 11 | 2/4 |
| Ulcers in the oral and nasal mucosa | 17 | 1/3 | 11 | 1/4 |
| Ptyalism | 0/5 | 3 | 1/5 | |
| Virus Isolation in Blood (Positive or Negative; Number of Positive Animals/Total Animals) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | 3 dpi | 5 dpi | 7 dpi | 10 dpi | 13 dpi | 17 dpi | 20 dpi | 24 dpi | 27 dpi | 32 dpi | 38 dpi |
| +; 1/5 | +; 5/5 | +; 4/5 | +; 3/4 | +; 1/3 | −; 0/3 | −; 0/2 | −; 0/2 | −; 0/2 | −; 0/2 | −; 0/2 | |
| Group 2 | 3 dpi | 5 dpi | 7 dpi | 10 dpi | 13 dpi | 17 dpi | 20 dpi | 24 dpi | 27 dpi | 32 dpi | 39 dpi |
| +; 1/5 | +; 5/5 | +; 5/5 | +; 4/5 | −; 0/4 | −; 0/4 | −; 0/3 | −; 0/3 | −; 0/3 | −; 0/3 | −; 0/2 | |
| Group | Dpi | Liver | Lung | Heart | Spleen | Kidney | Mes. l.n. | Med. l.n. |
|---|---|---|---|---|---|---|---|---|
| Group 1 | 7 | +++ | + * | +++ | +++ * | ++ | ++ | ++ |
| 12 | +++ | +++ | +++ | +++ | ++ | ++ | ++ | |
| 19 | ++ | ++ | + | +++ | ++ | + | + | |
| 38 | + | + | − | ++ | + | + | + | |
| Group 2 | 10 | +++ | +++ | +++ | +++ * | +++ | +++ | ++ |
| 20 | ++ | ++ | + | +++ | + | ++ | ++ | |
| 34 | ++ | + | + | ++ | + | + | ++ | |
| 39 | + | + | + | ++ | + | − | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Trancón, D.; Valero-Lorenzo, M.; Ruano, M.J.; Fernández-Pacheco, P.; García-Villacieros, E.; Tena-Tomás, C.; López-Herranz, A.; Morales, J.; Martí, B.; Jiménez-Clavero, M.Á.; et al. Emerging Bluetongue Virus Serotype 4 in the Balearic Islands, Spain (2021): Outbreak Investigations and Experimental Infection in Sheep. Microorganisms 2025, 13, 411. https://doi.org/10.3390/microorganisms13020411
Romero-Trancón D, Valero-Lorenzo M, Ruano MJ, Fernández-Pacheco P, García-Villacieros E, Tena-Tomás C, López-Herranz A, Morales J, Martí B, Jiménez-Clavero MÁ, et al. Emerging Bluetongue Virus Serotype 4 in the Balearic Islands, Spain (2021): Outbreak Investigations and Experimental Infection in Sheep. Microorganisms. 2025; 13(2):411. https://doi.org/10.3390/microorganisms13020411
Chicago/Turabian StyleRomero-Trancón, David, Marta Valero-Lorenzo, María José Ruano, Paloma Fernández-Pacheco, Elena García-Villacieros, Cristina Tena-Tomás, Ana López-Herranz, Jorge Morales, Bartolomé Martí, Miguel Ángel Jiménez-Clavero, and et al. 2025. "Emerging Bluetongue Virus Serotype 4 in the Balearic Islands, Spain (2021): Outbreak Investigations and Experimental Infection in Sheep" Microorganisms 13, no. 2: 411. https://doi.org/10.3390/microorganisms13020411
APA StyleRomero-Trancón, D., Valero-Lorenzo, M., Ruano, M. J., Fernández-Pacheco, P., García-Villacieros, E., Tena-Tomás, C., López-Herranz, A., Morales, J., Martí, B., Jiménez-Clavero, M. Á., Cáceres-Garrido, G., Agüero, M., & Villalba, R. (2025). Emerging Bluetongue Virus Serotype 4 in the Balearic Islands, Spain (2021): Outbreak Investigations and Experimental Infection in Sheep. Microorganisms, 13(2), 411. https://doi.org/10.3390/microorganisms13020411






