Metabolome Combined with 16S rDNA Sequencing Reveals a Novel Mechanistic Insight into the Collaboration of Resveratrol and β-Hydroxy-β-Methylbutyric Acid in Regulating the Meat Quality of Tibetan Sheep Through Altering Rumen Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Management of Tibetan Sheep and Sample Collection
2.3. Analysis of Meat Characteristics and Nutritional Quality
2.4. Real-Time Fluorescence Quantitative PCR Analysis
2.5. Determination of AA and Medium- to Long-Chain Fatty Acid Profiles in Meat
2.6. Untargeted Metabolomics of Tibetan Sheep Meat
2.7. Rumen Short-Chain Volatile Fatty Acids Analysis
2.8. Rumen Microbiota Analysis
2.9. Statistical Analysis
3. Results
3.1. Meat Quality
3.2. Genes Expression Related to Muscular Fiber Composition
3.3. AA and Fatty Acid Composition of Meat
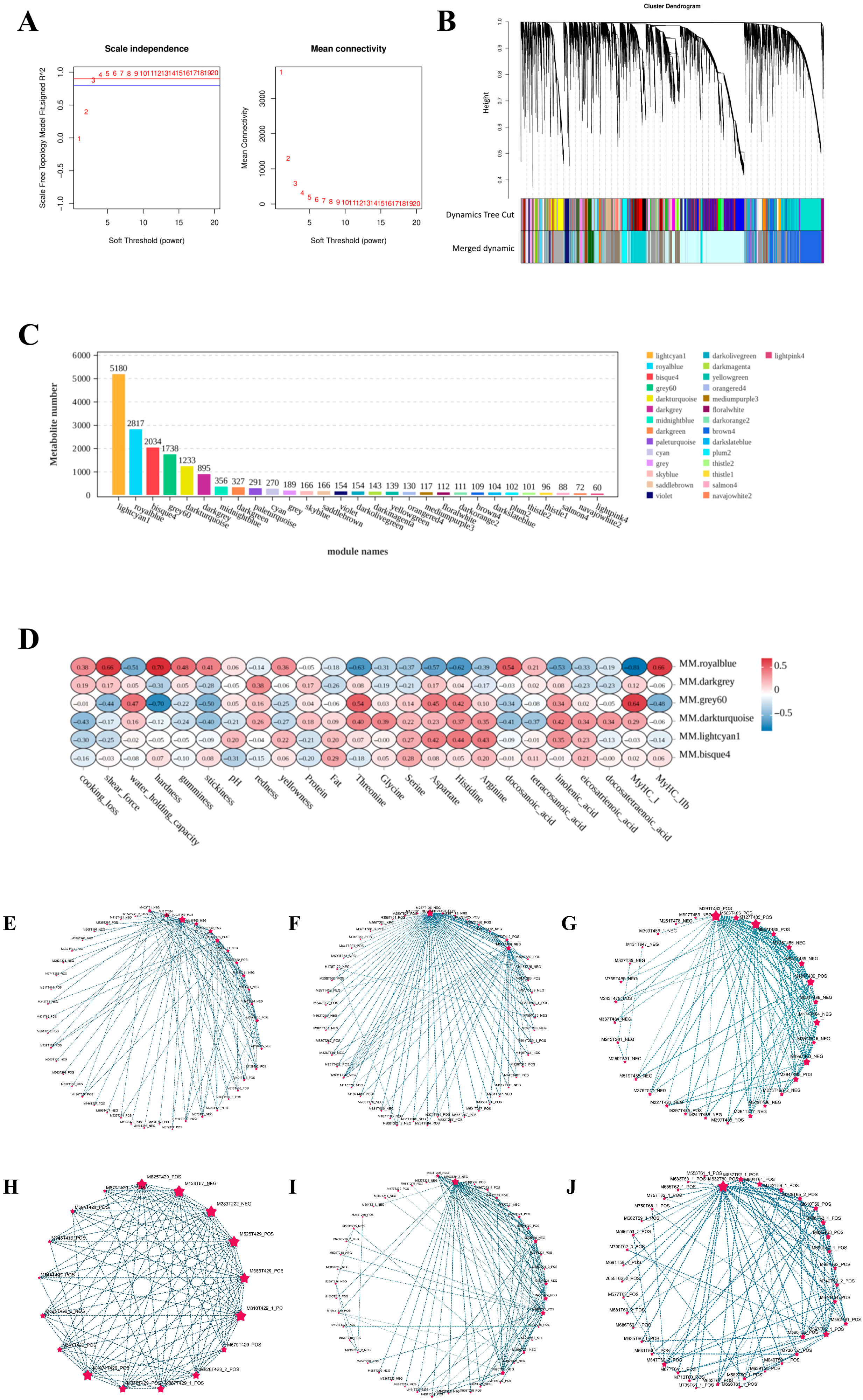
3.4. Untargeted Metabolomic Analysis of Meat
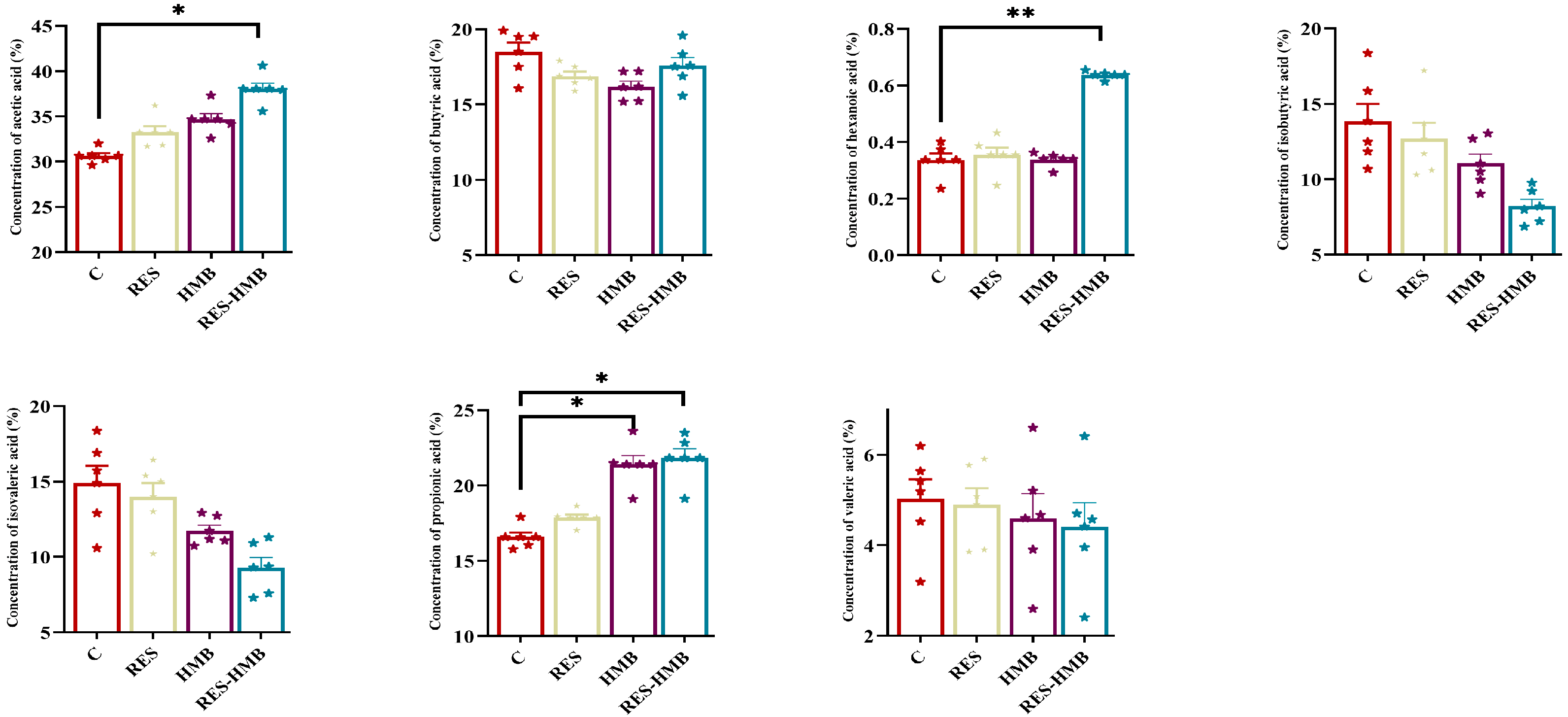
3.5. Rumen Fermentation Parameters
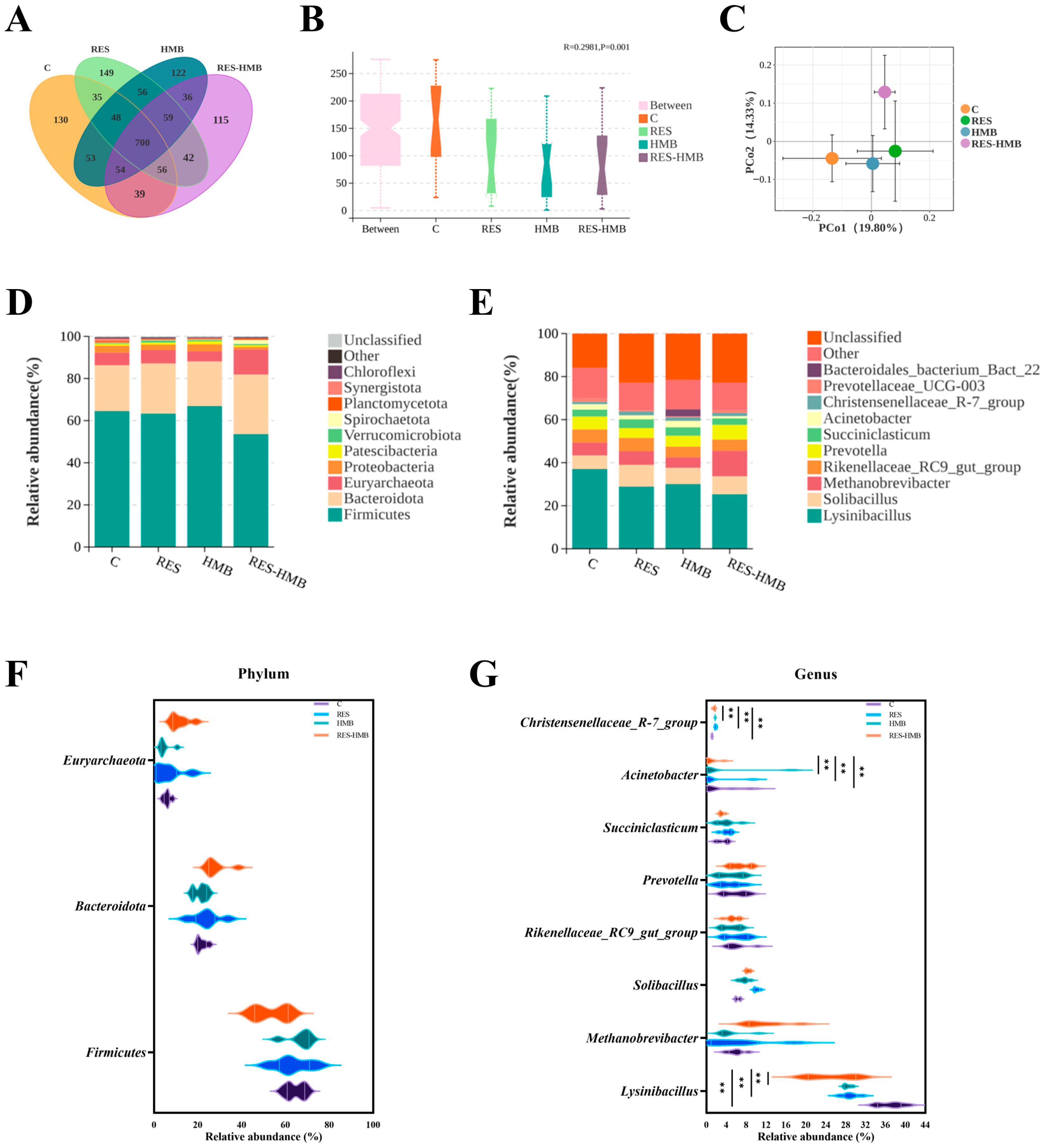
3.6. Ruminal Microbiota Composition
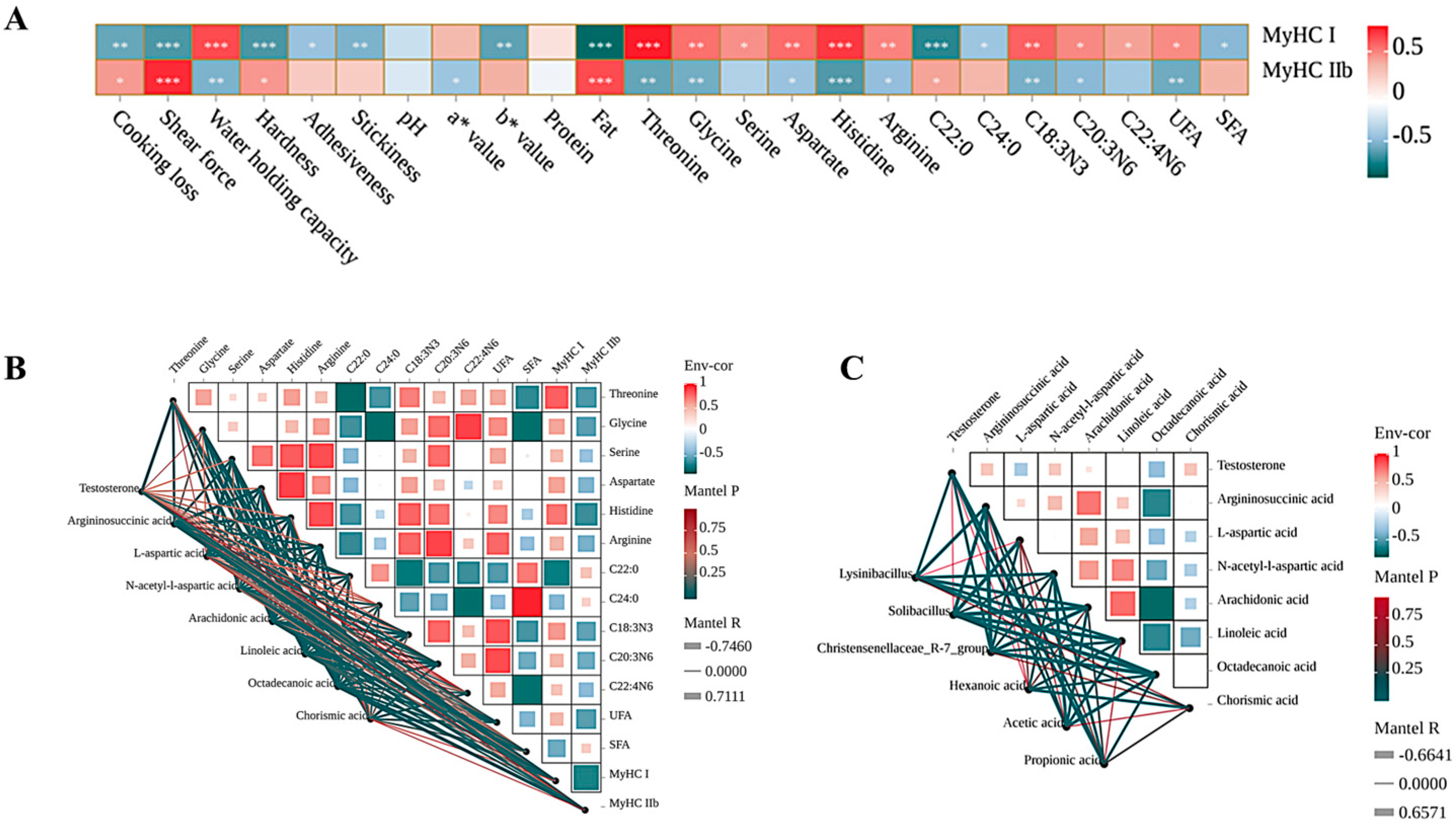
3.7. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCFAs | Branched-chain fatty acids |
| DMs | Differential metabolites |
| EAAs | Essential AAs |
| GC-MS | Gas chromatography-mass spectrometry |
| HMB | β-hydroxy-β-methylbutyric acid |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| LIT | Linear ion trap |
| LL | Longissimus lumborum |
| Met | Methionine |
| NEAAs | Non-essential AAs |
| OPLS-DA | Orthogonal partial least squares-discriminant analysis |
| OTUs | Operational taxonomic units |
| PCA | Principal component analysis |
| PUFA | Polyunsaturated fatty acid |
| QC | quality control |
| QQQ | Triple quadrupole |
| RES | Resveratrol |
| SCFAs | Short-chain fatty acids |
| SEM | Standard error of the mean |
| SF | Shear force |
| SFAs | Saturated fatty acids |
| UFA | Unsaturated fatty acid |
| VIP | Variable importance in the projection |
| WHC | Water-Holding Capacity |
| WGCNA | Weighted gene co-expression network analysis |
| 3-HPP | 3-hydroxyphenylpropionic acid |
| 4-HPA | 4-hydroxyphenylacetic acid |
References
- Tian, D.; Han, B.; Li, X.; Liu, D.; Zhou, B.; Zhao, C.; Zhang, N.; Wang, L.; Pei, Q.; Zhao, K. Genetic diversity and selection of Tibetan sheep breeds revealed by whole-genome resequencing. Anim. Biosci. 2023, 36, 991–1002. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wang, T.; Zhou, J.; Degen, A.A.; Gou, N.; Li, S.; Bai, Y.; Jing, X.; Wang, W.; Shang, Z. Carcass parameters and meat quality of Tibetan sheep and Small-tailed Han sheep consuming diets of low-protein content and different energy yields. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1010–1023. [Google Scholar]
- Wang, B.; Luo, Y.; Wang, Y.; Wang, D.; Hou, Y.; Yao, D.; Tian, J.; Jin, Y. Rumen bacteria and meat fatty acid composition of Sunit sheep reared under different feeding regimens in China. J. Sci. Food Agric. 2021, 101, 1100–1110. [Google Scholar] [CrossRef]
- Xiang, J.; Zhong, L.; Luo, H.; Meng, L.; Dong, Y.; Qi, Z.; Wang, H. A comparative analysis of carcass and meat traits, and rumen bacteria between Chinese Mongolian sheep and Dorper × Chinese Mongolian crossbred sheep. Animal 2022, 16, 100503. [Google Scholar] [CrossRef]
- Holman, D.B.; Gzyl, K.E.; Scott, H.; Prieto, N.; López-Campos, Ó. Associations between the rumen microbiota and carcass merit and meat quality in beef cattle. Appl. Microbiol. Biotechnol. 2024, 108, 287. [Google Scholar] [CrossRef] [PubMed]
- Penner, G.B.; Steele, M.A.; Aschenbach, J.R.; McBride, B.W. Ruminant Nutrition Symposium: Molecular adaptation of ruminal epithelia to highly fermentable diets. J. Anim. Sci. 2011, 89, 1108–1119. [Google Scholar] [CrossRef]
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality-A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef]
- Gionbelli, M.P.; Valadares Filho, S.d.C.; Detmann, E.; Paulino, P.V.R.; Valadares, R.F.D.; Santos, T.R.; Silva, L.F.C.E.; Magalhães, F.A. Intake, performance, digestibility, microbial efficiency and carcass characteristics of growing Nellore heifers fed two concentrate levels. Rev. Bras. Zootec. 2012, 41, 1243–1252. [Google Scholar]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Fu, S.; Lv, R.; Wang, L.; Hou, H.; Liu, H.; Shao, S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. Saudi J. Biol. Sci. 2018, 25, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Moretón-Lamas, E.; Lago-Crespo, M.; Lage-Yusty, M.A.; López-Hernández, J. Comparison of methods for analysis of resveratrol in dietary vegetable supplements. Food Chem. 2017, 224, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, D.D.; Tu, Y.; Zhang, N.F.; Si, B.W.; Deng, K.D.; Diao, Q.Y. Effect of dietary supplementation with resveratrol on nutrient digestibility, methanogenesis and ruminal microbial flora in sheep. J. Anim. Physiol. Anim. Nutr. 2015, 99, 676–683. [Google Scholar]
- Ma, T.; Wu, W.; Tu, Y.; Zhang, N.; Diao, Q. Resveratrol affects in vitro rumen fermentation, methane production and prokaryotic community composition in a time- and diet-specific manner. Microb. Biotechnol. 2020, 13, 1118–1131. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, Y.; Zhang, S.; Zou, J.; Gao, X.; Song, Y.; Zhang, Y.; Hu, Y.; Huang, Y.; Jiang, Q. The Effect of Dietary Supplementation with Resveratrol on Growth Performance, Carcass and Meat Quality, Blood Lipid Levels and Ruminal Microbiota in Fattening Goats. Foods 2022, 11, 598. [Google Scholar] [CrossRef]
- Yu, Q.; Fang, C.; Ma, Y.; He, S.; Ajuwon, K.M.; He, J. Dietary resveratrol supplement improves carcass traits and meat quality of Pekin ducks. Poult. Sci. 2021, 100, 100802. [Google Scholar] [CrossRef]
- Liao, Q.; Wu, T.; Fu, Q.; Wang, P.; Zhao, Y.; Li, Y.; Xiao, H.; Zhou, L.; Song, Z. Effects of Dietary Inclusion of β-Hydroxy-β-Methylbutyrate on Growth Performance, Fat Deposition, Bile Acid Metabolism, and Gut Microbiota Function in High-Fat and High-Cholesterol Diet-Challenged Layer Chickens. Curr. Issues Mol. Biol. 2022, 44, 3413–3427. [Google Scholar]
- Kaczka, P.; Michalczyk, M.M.; Jastrząb, R.; Gawelczyk, M.; Kubicka, K. Mechanism of Action and the Effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Different Types of Physical Performance—A Systematic Review. J. Hum. Kinet. 2019, 68, 211–222. [Google Scholar] [CrossRef]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Silva, V.R.; Belozo, F.L.; Micheletti, T.O.; Conrado, M.; Stout, J.R.; Pimentel, G.D.; Gonzalez, A.M. β-hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: A systematic review. Nutr. Res. 2017, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- McCollum, M.Q.; Vázquez-Añón, M.; Dibner, J.J.; Webb, K.E., Jr. Absorption of 2-hydroxy-4-(methylthio)butanoic acid by isolated sheep ruminal and omasal epithelia. J. Anim. Sci. 2000, 78, 1078–1083. [Google Scholar]
- Karnati, S.K.; Sylvester, J.T.; Noftsger, S.M.; Yu, Z.; St-Pierre, N.R.; Firkins, J.L. Assessment of ruminal bacterial populations and protozoal generation time in cows fed different methionine sources. J. Dairy Sci. 2007, 90, 798–809. [Google Scholar] [CrossRef]
- Sun, H.; Wu, Y.M.; Wang, Y.M.; Liu, J.X.; Myung, K.H. Effects of Aspergillus Oryzae Culture and 2-Hydroxy-4-(Methylthio)-Butanoic Acid on In Vitro Rumen Fermentation and Microbial Populations Between Different Roughage Sources. Asian-Australas. J. Anim. Sci. 2014, 27, 1285–1292. [Google Scholar]
- Van Koevering, M.T.; Dolezal, H.G.; Gill, D.R.; Owens, F.N.; Strasia, C.A.; Buchanan, D.S.; Lake, R.; Nissen, S. Effects of beta-hydroxy-beta-methyl butyrate on performance and carcass quality of feedlot steers. J. Anim. Sci. 1994, 72, 1927–1935. [Google Scholar]
- Tang, Z.; Song, B.; Zheng, C.; Zheng, J.; Yin, Y.; Chen, J. Dietary Beta-Hydroxy-Beta-Methyl Butyrate Supplementation Affects Growth, Carcass Characteristics, Meat Quality, and Serum Metabolomics Profile in Broiler Chickens. Front. Physiol. 2021, 12, 633964. [Google Scholar]
- Duan, Y.; Zhong, Y.; Xiao, H.; Zheng, C.; Song, B.; Wang, W.; Guo, Q.; Li, Y.; Han, H.; Gao, J.; et al. Gut microbiota mediates the protective effects of dietary β-hydroxy-β-methylbutyrate (HMB) against obesity induced by high-fat diets. FASEB J. 2019, 33, 10019–10033. [Google Scholar] [PubMed]
- Ji, Q.R.; Zhang, F.S.; Zhang, Y.; Su, Q.Y.M.; He, T.L.; Hou, S.Z.; Gui, L.S. Multi-Omics Revealed Resveratrol and β-Hydroxy-β-methyl Butyric Acid Alone or in Combination Improved the Jejunal Function in Tibetan Sheep. Antioxidants 2024, 13, 892. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.S.; Raza, S.H.A.; Wu, Z.L.; Su, Q.; Ji, Q.R.; He, T.L.; Zhu, K.A.; Zhang, Y.; Hou, S.Z.; et al. Immune, Oxidative, and Morphological Changes in the Livers of Tibetan Sheep after Feeding Resveratrol and β-Hydroxy-β-methyl Butyric Acid: A Transcriptome-Metabolome Integrative Analysis. Int. J. Mol. Sci. 2024, 25, 9865. [Google Scholar]
- Zhang, Y.; Ji, Q.R.; Gui, L.S.; Gebeyew, K.; Hou, S.Z.; Wang, Z.Y.; Han, L.J.; Yang, C. The influence of resveratrol and β-Hydroxy-β-methyl butyric acid supplementation alone or in combination on the development and health of the duodenum in Tibetan sheep. Front. Microbiol. 2025, 16, 1612102. [Google Scholar]
- Chen, X.; Zhang, Y.; Wang, X.H.; Wu, Z.L.; Quyangangmao; Ji, Q.R.; He, T.L.; Zhu, K.N.; Zhang, F.S.; Hou, S.Z.; et al. Transcriptome and lipid metabolome of the subcutaneous fat of Tibetan sheep: A comprehensive analysis of the reduction in fat deposition upon resveratrol and β-hydroxy-β-methyl butyric acid supplementation. Genomics 2025, 117, 111078. [Google Scholar]
- Zhang, X.; Han, L.; Hou, S.; Raza, S.H.A.; Wang, Z.; Yang, B.; Sun, S.; Ding, B.; Gui, L.; Simal-Gandara, J.; et al. Effects of different feeding regimes on muscle metabolism and its association with meat quality of Tibetan sheep. Food Chem. 2022, 374, 131611. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; Hou, S.; Gui, L.; Yuan, Z.; Sun, S.; Wang, Z.; Yang, B. Integrated metabolome and microbiome analysis reveals the effect of rumen-protected sulfur-containing amino acids on the meat quality of Tibetan sheep meat. Front. Microbiol. 2024, 15, 1345388. [Google Scholar]
- Firth, N.L.; Ross, D.A.; Thonney, M.L. Comparison of ether and chloroform for Soxhlet extraction of freeze-dried animal tissues. J. Assoc. Off. Anal. Chem. 1985, 68, 1228–1231. [Google Scholar]
- Kane, P.F. Comparison of HgO and CuSO4 as digestion catalysts in manual Kjeldahl determination of crude protein in animal feeds: Collaborative study. J. Assoc. Off. Anal. Chem. 1984, 67, 869–877. [Google Scholar]
- Lee, S.H.; Joo, S.T.; Ryu, Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010, 86, 166–170. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, J.; Yu, B.; Zheng, P.; Huang, Z.; Mao, X.; He, J.; Yu, J.; Chen, J.; Chen, D. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef]
- Dong, Z.; Yu, P.; Li, J.; Zhou, H.; Li, R.; Wang, S.; Yang, G.; Nie, Y.; Liu, L.; Bian, X.; et al. Discovery of an ene-reductase initiating resveratrol catabolism in gut microbiota and its application in disease treatment. Cell Rep. 2025, 44, 115517. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, R.; Zhao, W.; Zhao, Y.; Wang, D.; Zhao, S.; Ge, Z.; Ma, Y.; Zhao, X. Gut microbiota-derived 4-hydroxyphenylacetic acid from resveratrol supplementation prevents obesity through SIRT1 signaling activation. Gut Microbes 2025, 17, 2446391. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, H.; Liu, K.; Cao, H.; Mao, H.; Dong, X.; Yin, Z. Analysis of the physical meat quality in partridge (Alectoris chukar) and its relationship with intramuscular fat. Poult. Sci. 2020, 99, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, S.; Bai, Y.; Luo, Z.; Li, Z.; Shi, B.; Shan, A. Effects of dietary resveratrol supplementation in sows on antioxidative status, myofiber characteristic and meat quality of offspring. Meat Sci. 2020, 167, 108176. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Myosin isoforms, muscle fiber types, and transitions. Microsc. Res. Tech. 2000, 50, 500–509. [Google Scholar] [CrossRef]
- Li, J.; Liang, R.; Mao, Y.; Yang, X.; Luo, X.; Qian, Z.; Zhang, Y.; Zhu, L. Effect of dietary resveratrol supplementation on muscle fiber types and meat quality in beef cattle. Meat Sci. 2022, 194, 108986. [Google Scholar] [CrossRef]
- Iwamura, S.; Kaido, T.; Wada, A.; Kido, S.; Harada, D.; Hirata, M.; Miyachi, Y.; Yao, S.; Yagi, S.; Kamo, N.; et al. Perioperative Oral β-Hydroxy-β-Methylbutyrate Supplementation Ameliorates Sarcopenia in Rats Undergoing Major Hepatectomy. J. Nutr. Sci. Vitaminol. 2022, 68, 276–283. [Google Scholar] [CrossRef]
- Cui, Y.; Qi, J.; Li, J.; Zhang, Y.; Yang, X.; Xin, L.; Niu, L.; Xu, B.; Qian, Z.; Zhu, L.; et al. Effects of dietary resveratrol supplementation in cattle on the anti-oxidative capacity and meat quality of beef steaks under high-oxygen packaging. Meat Sci. 2023, 204, 109238. [Google Scholar] [CrossRef]
- He, S.; Yan, X. From resveratrol to its derivatives: New sources of natural antioxidant. Curr. Med. Chem. 2013, 20, 1005–1017. [Google Scholar]
- Chusak, C.; Pasukamonset, P.; Chantarasinlapin, P.; Adisakwattana, S. Postprandial Glycemia, Insulinemia, and Antioxidant Status in Healthy Subjects after Ingestion of Bread made from Anthocyanin-Rich Riceberry Rice. Nutrients 2020, 12, 782. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, P.; Rocchetti, G.; Giuberti, G.; Lucchini, F.; Lucini, L. Phenolic acids, lignans, and low-molecular-weight phenolics exhibit the highest in vitro cellular bioavailability in different digested and faecal-fermented phenolics-rich plant extracts. Food Chem. 2023, 412, 135549. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, G.; Zhang, J.; Zhang, C.; Fan, N.; Xu, Y.; Guo, J.; Yuan, J. Correlation among serum biochemical indices and slaughter traits, texture characteristics and water-holding capacity of Tan sheep. Ital. J. Anim. Sci. 2021, 20, 1781–1790. [Google Scholar] [CrossRef]
- Wang, X.E.; Li, Z.W.; Liu, L.L.; Ren, Q.C. Effects of Supplementing Tributyrin on Meat Quality Characteristics of Foreshank Muscle of Weaned Small-Tailed Han Sheep Lambs. Animals 2024, 14, 1235. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Kent, M.P.; Shackelford, S.D.; Veiseth, E.; Wheeler, T.L. Meat tenderness and muscle growth: Is there any relationship? Meat Sci. 2002, 62, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Ren, C.; Bai, Y.; Ijaz, M.; Hou, C.; Chen, L. Effects of protein posttranslational modifications on meat quality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 289–331. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Utpal, B.K.; Mokhfi, F.Z.; Zehravi, M.; Sweilam, S.H.; Gupta, J.K.; Kareemulla, S.C.R.D.; Rao, A.A.; Kumar, V.V.; Krosuri, P.; Prasad, D.; et al. Resveratrol: A Natural Compound Targeting the PI3K/Akt/mTOR Pathway in Neurological Diseases. Mol. Neurobiol. 2024, 62, 5579–5608. [Google Scholar] [CrossRef]
- Kimura, K.; Cheng, X.W.; Inoue, A.; Hu, L.; Koike, T.; Kuzuya, M. β-Hydroxy-β-methylbutyrate facilitates PI3K/Akt-dependent mammalian target of rapamycin and FoxO1/3a phosphorylations and alleviates tumor necrosis factor α/interferon γ-induced MuRF-1 expression in C2C12 cells. Nutr. Res. 2014, 34, 368–374. [Google Scholar] [CrossRef]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zheng, C.; Song, B.; Guo, Q.; Zhong, Y.; Zhang, S.; Zhang, L.; Duan, G.; Li, F.; Duan, Y. HMB Improves Lipid Metabolism of Bama Xiang Mini-Pigs via Modulating the Bacteroidetes-Acetic Acid-AMPKα Axis. Front. Microbiol. 2021, 12, 736997. [Google Scholar] [CrossRef] [PubMed]
- Bottinelli, R.; Reggiani, C. Human skeletal muscle fibres: Molecular and functional diversity. Prog. Biophys. Mol. Biol. 2000, 73, 195–262. [Google Scholar] [CrossRef]
- Umek, N.; Horvat, S.; Cvetko, E. Skeletal muscle and fiber type-specific intramyocellular lipid accumulation in obese mice. Bosn. J. Basic. Med. Sci. 2021, 21, 730–738. [Google Scholar]
- Lee, M.; Park, S.; Choi, B.; Choi, W.; Lee, H.; Lee, J.M.; Lee, S.T.; Yoo, K.H.; Han, D.; Bang, G.; et al. Cultured meat with enriched organoleptic properties by regulating cell differentiation. Nat. Commun. 2024, 15, 77. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of Amino Acid Taste and Appetite. Adv. Nutr. 2016, 7, 806s–822s. [Google Scholar] [CrossRef]
- Ma, X.; Yu, M.; Liu, Z.; Deng, D.; Cui, Y.; Tian, Z.; Wang, G. Effect of amino acids and their derivatives on meat quality of finishing pigs. J. Food Sci. Technol. 2020, 57, 404–412. [Google Scholar]
- He, W.; Posey, E.A.; Steele, C.C.; Savell, J.W.; Bazer, F.W.; Wu, G. Dietary glycine supplementation enhances postweaning growth and meat quality of pigs with intrauterine growth restriction. J. Anim. Sci. 2023, 101, skad354. [Google Scholar] [CrossRef]
- Jiang, S.; Quan, W.; Luo, J.; Lou, A.; Zhou, X.; Li, F.; Shen, Q.W. Low-protein diets supplemented with glycine improves pig growth performance and meat quality: An untargeted metabolomic analysis. Front. Vet. Sci. 2023, 10, 1170573. [Google Scholar] [CrossRef]
- Zhou, M.; Wei, Y.; Feng, Y.; Zhang, S.; Ma, N.; Wang, K.; Tan, P.; Zhao, Y.; Zhao, J.; Ma, X. Arginine Regulates Skeletal Muscle Fiber Type Formation via mTOR Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 6184. [Google Scholar] [CrossRef]
- Dou, L.; Sun, L.; Liu, C.; Su, L.; Chen, X.; Yang, Z.; Hu, G.; Zhang, M.; Zhao, L.; Jin, Y. Effect of dietary arginine supplementation on protein synthesis, meat quality and flavor in growing lambs. Meat Sci. 2023, 204, 109291. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; Zhou, C. l-Arginine and l-lysine can weaken the intermolecular interactions of main myofibrillar proteins: The roles in improving the tenderness of pork Longissimus lumborum muscle. Int. J. Food Sci. Technol. 2023, 58, 3085–3096. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, D.; Chen, Y.; Bao, L.; Fang, P.; Xu, H.; Zhou, B.; Liu, C. Effects of basic amino acid on the tenderness, water binding capacity and texture of cooked marinated chicken breast. LWT-Food Sci. Technol. 2020, 129, 109524. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Xu, P.; Zhu, X.; Zhou, C. l-Lysine and l-arginine inhibit myosin aggregation and interact with acidic amino acid residues of myosin: The role in increasing myosin solubility-ScienceDirect. Food Chem. 2018, 242, 22–28. [Google Scholar] [PubMed]
- Qi, B.; Wang, J.; Hu, M.; Ma, Y.; Wu, S.; Qi, G.; Qiu, K.; Zhang, H. Influences of Beta-Alanine and l-Histidine Supplementation on Growth Performance, Meat Quality, Carnosine Content, and mRNA Expression of Carnosine-Related Enzymes in Broilers. Animals 2021, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Cheng, D.; Chen, S.; Wang, L.; Li, Y.; Ma, X.; Song, X.; Liu, X.; Li, W.; Liang, J.; et al. Genome-wide association analysis of meat quality traits in a porcine Large White × Minzhu intercross population. Int. J. Biol. Sci. 2012, 8, 580–595. [Google Scholar] [CrossRef]
- Wang, C.; Huang, L.; Jin, S.; Hou, R.; Chen, M.; Liu, Y.; Tang, W.; Li, T.; Yin, Y.; He, L. d-Aspartate in Low-Protein Diets Improves the Pork Quality by Regulating Energy and Lipid Metabolism via the Gut Microbes. J. Agric. Food Chem. 2023, 71, 12417–12430. [Google Scholar] [CrossRef]
- Develi-Is, S.; Ozen, G.; Bekpinar, S.; Topal, G.; Unlucerci, Y.; Dogan, B.S.; Uysal, M. Resveratrol improves high-fructose-induced vascular dysfunction in rats. Can. J. Physiol. Pharmacol. 2014, 92, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Dajnowska, A.; Tomaszewska, E.; Świątkiewicz, S.; Arczewska-Włosek, A.; Dobrowolski, P.; Domaradzki, P.; Rudyk, H.; Brezvyn, O.; Muzyka, V.; Kotsyumbas, I.; et al. Yolk Fatty Acid Profile and Amino Acid Composition in Eggs from Hens Supplemented with ß-Hydroxy-ß-Methylbutyrate. Foods 2023, 12, 3733. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Zheng, C.; Zheng, J.; Duan, G.; Yu, J.; Zhang, P.; Yin, Y.; Zhao, X. Different effects of dietary β-hydroxy-β-methylbutyrate on composition of fatty acid and free amino acid, and fatty metabolism in the different muscles of broilers. Poult. Sci. 2023, 102, 103001. [Google Scholar] [CrossRef]
- Campbell, J.W.; Morgan-Kiss, R.M.; Cronan, J.E. A new Escherichia coli metabolic competency: Growth on fatty acids by a novel anaerobic β-oxidation pathway. Mol. Microbiol. 2003, 47, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated Fatty acids. J. Nutr. Metab. 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Pewan, S.B.; Otto, J.R.; Huerlimann, R.; Budd, A.M.; Mwangi, F.W.; Edmunds, R.C.; Holman, B.W.B.; Henry, M.L.E.; Kinobe, R.T.; Adegboye, O.A.; et al. Genetics of Omega-3 Long-Chain Polyunsaturated Fatty Acid Metabolism and Meat Eating Quality in Tattykeel Australian White Lambs. Genes 2020, 11, 587. [Google Scholar] [CrossRef]
- Şahin, A.; Aksoy, Y.; Uğurlutepe, E.; Kul, E.; Ulutaş, Z. Fatty acid profilies and some meat quality traits at different slaughter weights of Brown Swiss bulls. Trop. Anim. Health Prod. 2021, 53, 380. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Gong, H.; Cui, L.; Zhang, W.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Huang, L.; et al. Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on GWAS data in six pig populations. Meat Sci. 2019, 150, 47–55. [Google Scholar] [CrossRef]
- Astrup, A.; Teicholz, N.; Magkos, F.; Bier, D.M.; Brenna, J.T.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; Yusuf, S.; et al. Dietary Saturated Fats and Health: Are the U.S. Guidelines Evidence-Based? Nutrients 2021, 13, 3305. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, Cd011737. [Google Scholar] [CrossRef] [PubMed]
- Kouba, M.; Mourot, J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie 2011, 93, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Han, L.; Hou, S.; Gui, L.; Yuan, Z.; Sun, S.; Wang, Z.; Yang, B.; Yang, C. Insights into the effects of saline forage on the meat quality of Tibetan sheep by metabolome and multivariate analysis. Food Chem. X 2024, 22, 101411. [Google Scholar] [CrossRef]
- Repossi, G.; Das, U.N.; Eynard, A.R. Molecular Basis of the Beneficial Actions of Resveratrol. Arch. Med. Res. 2020, 51, 105–114. [Google Scholar] [CrossRef]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef]
- Watanabe, N.; Komiya, Y.; Sato, Y.; Watanabe, Y.; Suzuki, T.; Arihara, K. Oleic acid up-regulates myosin heavy chain (MyHC) 1 expression and increases mitochondrial mass and maximum respiration in C2C12 myoblasts. Biochem. Biophys. Res. Commun. 2020, 525, 406–411. [Google Scholar] [CrossRef]
- Lv, W.; Liu, X.; Sha, Y.; Shi, H.; Wei, H.; Luo, Y.; Wang, J.; Li, S.; Hu, J.; Guo, X.; et al. Rumen Fermentation-Microbiota-Host Gene Expression Interactions to Reveal the Adaptability of Tibetan Sheep in Different Periods. Animals 2021, 11, 3529. [Google Scholar]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the rumen microbiota and its relationship with residual feed intake in sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef]
- Dai, X.; Tian, Y.; Li, J.; Luo, Y.; Liu, D.; Zheng, H.; Wang, J.; Dong, Z.; Hu, S.; Huang, L. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386. [Google Scholar] [CrossRef]
- Presley, L.L.; Wei, B.; Braun, J.; Borneman, J. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 2010, 76, 936–941. [Google Scholar] [CrossRef]
- Zuo, Z.; Pei, L.; Liu, T.; Liu, X.; Chen, Y.; Hu, Z. Investigation of Gut Microbiota Disorders in Sepsis and Sepsis Complicated with Acute Gastrointestinal Injury Based on 16S rRNA Genes Illumina Sequencing. Infect. Drug Resist. 2023, 16, 7389–7403. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Chen, W.; Liu, C.; Meng, Q.; Zhou, Z. Rumen Microbiome and Metabolome of High and Low Residual Feed Intake Angus Heifers. Front. Vet. Sci. 2022, 9, 812861. [Google Scholar] [CrossRef]
- Markande, A.R.; Vemuluri, V.R.; Shouche, Y.S.; Nerurkar, A.S. Characterization of Solibacillus silvestris strain AM1 that produces amyloid bioemulsifier. J. Basic. Microbiol. 2018, 58, 523–531. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, X.; Tao, G.; Hao, W.; Wang, L.; Lan, Z.; Song, Y.; Wu, M.; Huang, J.Q. Ferulic acid and feruloylated oligosaccharides alleviate anxiety and depression symptom via regulating gut microbiome and microbial metabolism. Food Res. Int. 2022, 162, 111887. [Google Scholar] [CrossRef]
- Jiao, A.; Diao, H.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Wang, Q.; Wang, H.; et al. Infusion of short chain fatty acids in the ileum improves the carcass traits, meat quality and lipid metabolism of growing pigs. Anim. Nutr. 2021, 7, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Khas, E.; Ao, C.; Bai, C. Effects of Allium mongolicum Regel ethanol extract on three flavor-related rumen branched-chain fatty acids, rumen fermentation and rumen bacteria in lambs. Front. Microbiol. 2022, 13, 978057. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Guo, H.H.; Huang, S.; Feng, C.L.; Han, Y.X.; Jiang, J.D. Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J. Chromatogr. B 2017, 1057, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.; Sutren, M.; Lepercq, P.; Juste, C.; Rigottier-Gois, L.; Lhoste, E.; Lemée, R.; Le Ruyet, P.; Doré, J.; Andrieux, C. Influence of Camembert consumption on the composition and metabolism of intestinal microbiota: A study in human microbiota-associated rats. Br. J. Nutr. 2004, 92, 429–438. [Google Scholar] [CrossRef]


| Items | Content (%) | |
|---|---|---|
| Ingredient | Corn | 51.50 |
| Soybean meal | 2.00 | |
| Rapeseed meal | 12.80 | |
| Cottonseed meal | 2.00 | |
| Palm meal | 25.00 | |
| NaCl | 1.00 | |
| Limestone | 1.00 | |
| Baking soda | 0.10 | |
| Premix 1 | 4.60 | |
| Total | 100.00 | |
| Nutrient levels | Digestible energy (MJ/kg) 2 | 12.71 |
| Crude protein | 14.27 | |
| Ether extract | 3.29 | |
| Neutral detergent fiber | 26.70 | |
| Acid detergent fiber | 19.97 | |
| Ca | 0.86 | |
| P | 0.40 |
| Items | Group | p-Value | |||
|---|---|---|---|---|---|
| C | RES | HMB | RES-HMB | ||
| Eye muscle area | 24.00 ± 1.10 | 25.60 ± 1.36 | 22.87 ± 1.55 | 25.40 ± 1.28 | 0.445 |
| pH | 6.46 ± 0.07 | 6.51 ± 0.11 | 6.43 ± 0.07 | 6.59 ± 0.05 | 0.520 |
| L* | 32.48 ± 1.51 | 35.19 ± 1.82 | 34.09 ± 0.60 | 33.32 ± 2.83 | 0.769 |
| a* | 21.72 ± 1.73 b | 22.28 ± 1.38 b | 26.89 ± 1.62 a | 27.00 ± 1.75 a | 0.050 |
| b* | 18.75 ± 0.53 a | 17.06 ± 0.73 ab | 14.77 ± 1.19 b | 10.18 ± 0.63 c | <0.001 |
| Thawing loss rate (%) | 1.99 ± 0.32 | 1.63 ± 0.32 | 1.81 ± 0.45 | 1.47 ± 0.30 | 0.751 |
| Cooking loss rate (%) | 54.98 ± 1.66 a | 35.84 ± 1.32 bc | 53.09 ± 1.06 a | 33.95 ± 0.92 b | <0.001 |
| Shear force (N) | 56.74 ± 3.10 a | 41.64 ± 1.72 b | 43.52 ± 2.01 b | 38.71 ± 1.76 b | 0.001 |
| Water holding capacity (N) | 10.95 ± 0.64 b | 15.08 ± 0.91 b | 20.54 ± 1.39 a | 23.09 ± 1.92 a | <0.001 |
| Hardness (N) | 22.46 ± 1.20 a | 19.97 ± 0.39 b | 14.66 ± 0.34 c | 18.36 ± 0.68 b | <0.001 |
| Stickness (mj) | 0.77 ± 0.05 a | 0.55 ± 0.04 b | 0.55 ± 0.08 b | 0.53 ± 0.03 b | 0.015 |
| Cohesion (mj) | 0.39 ± 0.03 | 0.44 ± 0.03 | 0.49 ± 0.06 | 0.48 ± 0.03 | 0.277 |
| Elasticity (mm) | 3.15 ± 0.06 | 3.38 ± 0.15 | 3.21 ± 0.13 | 3.49 ± 0.10 | 0.174 |
| Chewiness (mj) | 42.30 ± 0.98 | 37.93 ± 1.57 | 38.27 ± 0.82 | 37.20 ± 2.63 | 0.160 |
| Adhesiveness (N) | 11.19 ± 0.90 a | 9.03 ± 0.68 ab | 8.48 ± 0.95 b | 7.61 ± 0.68 b | 0.034 |
| Protein (%) | 20.93 ± 0.17 b | 20.33 ± 0.38 b | 21.07 ± 0.21 a | 21.87 ± 0.31 a | 0.008 |
| Moisture (%) | 74.10 ± 0.15 | 75.67 ± 0.68 | 74.13 ± 0.45 | 74.53 ± 0.12 | 0.052 |
| Fat (%) | 2.23 ± 0.01 a | 1.20 ± 0.03 b | 1.20 ± 0.00 b | 1.13 ± 0.03 c | <0.001 |
| Ash (%) | 0.80 ± 0.03 | 0.63 ± 0.11 | 0.73 ± 0.04 | 0.77 ± 0.06 | 0.340 |
| Items | Group | p-Value | |||
|---|---|---|---|---|---|
| C | RES | HMB | RES-HMB | ||
| EAA | |||||
| Lysine (µmol/kg) | 19.70 ± 2.74 | 34.20 ± 6.19 | 20.81 ± 0.52 | 23.73 ± 2.42 | 0.076 |
| Methionine (µmol/kg) | 9.67 ± 2.74 | 14.43 ± 2.97 | 10.07 ± 0.95 | 7.55 ± 1.20 | 0.227 |
| Leucine (µmol/kg) | 24.20 ± 3.94 | 32.18 ± 3.34 | 25.09 ± 0.96 | 22.70 ± 2.07 | 0.164 |
| Isoleucine (µmol/kg) | 17.29 ± 3.66 | 23.81 ± 1.73 | 18.55 ± 1.27 | 19.78 ± 1.73 | 0.282 |
| Valine (µmol/kg) | 20.34 ± 2.02 | 28.13 ± 3.58 | 19.92 ± 0.55 | 23.92 ± 1.81 | 0.105 |
| Threonine (µmol/kg) | 30.92 ± 1.44 c | 46.72 ± 2.17 b | 56.29 ± 1.65 a | 60.90 ± 4.58 a | 0.001 |
| Asparagine (µmol/kg) | 0.49 ± 0.03 | 0.47 ± 0.09 | 0.39 ± 0.02 | 0.66 ± 0.14 | 0.221 |
| Glutamine (µmol/kg) | 0.62 ± 0.05 | 0.67 ± 0.12 | 0.62 ± 0.02 | 0.53 ± 0.08 | 0.644 |
| Cysteine (µmol/kg) | 0.03 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.865 |
| Tryptophan (µmol/kg) | 0.20 ± 0.04 | 0.29 ± 0.06 | 0.23 ± 0.01 | 0.14 ± 0.01 | 0.137 |
| NEAA | |||||
| Glutamate (µmol/kg) | 6.21 ± 1.50 | 4.08 ± 0.96 | 4.51 ± 0.54 | 6.34 ± 0.71 | 0.488 |
| Glycine (µmol/kg) | 93.33 ± 1.70 b | 126.09 ± 10.47 b | 103.31 ± 4.78 b | 181.84 ± 11.10 a | 0.003 |
| Aspartate (µmol/kg) | 8.20 ± 1.11 b | 36.17 ± 0.45 a | 24.45 ± 1.22 a | 16.92 ± 0.46 a | 0.001 |
| Arginine (µmol/kg) | 16.10 ± 1.27 b | 27.87 ± 1.36 a | 17.75 ± 0.84 b | 25.06 ± 1.56 a | 0.001 |
| Serine (µmol/kg) | 31.66 ± 3.71 b | 54.38 ± 7.95 a | 36.16 ± 2.62 b | 42.19 ± 1.61 a | 0.040 |
| Tyrosine (µmol/kg) | 20.12 ± 3.22 | 30.21 ± 6.87 | 18.47 ± 1.32 | 20.24 ± 2.60 | 0.236 |
| Histidine (µmol/kg) | 79.09 ± 1.38 b | 131.00 ± 4.86 a | 107.91 ± 3.06 a | 113.16 ± 3.29 a | 0.001 |
| Proline (µmol/kg) | 13.76 ± 0.85 | 15.81 ± 1.72 | 13.20 ± 0.11 | 16.02 ± 1.05 | 0.243 |
| Sarcosine (µmol/kg) | 33.63 ± 0.30 | 35.97 ± 6.02 | 31.39 ± 0.49 | 30.91 ± 0.14 | 0.639 |
| Cystine (µmol/kg) | 14.43 ± 1.42 | 19.89 ± 2.22 | 15.71 ± 1.39 | 17.15 ± 2.20 | 0.264 |
| Items | Group | p-Value | |||
|---|---|---|---|---|---|
| C | RES | HMB | RES-HMB | ||
| C8:0 (µg/g) | 1.72 ± 0.11 | 1.56 ± 0.26 | 1.87 ± 0.22 | 1.36 ± 0.18 | 0.354 |
| C11:0 (µg/g) | 0.51 ± 0.36 | 0.23 ± 0.13 | 0.23 ± 0.04 | 0.53 ± 0.04 | 0.563 |
| C21:0 (µg/g) | 6.64 ± 0.70 | 8.10 ± 1.00 | 6.97 ± 0.92 | 7.50 ± 0.76 | 0.655 |
| C22:0 (µg/g) | 287.61 ± 6.26 a | 261.59 ± 3.18 b | 265.02 ± 4.29 ab | 246.61 ± 12.40 bc | 0.028 |
| C23:0 (µg/g) | 198.47 ± 3.70 | 195.23 ± 13.43 | 192.39 ± 14.45 | 173.67 ± 8.09 | 0.419 |
| C24:0 (µg/g) | 772.17 ± 14.43 a | 761.16 ± 23.38 a | 750.19 ± 39.70 a | 628.12 ± 26.89 b | 0.020 |
| C15:1N5 (µg/g) | 267.17 ± 2.15 | 244.15 ± 11.22 | 252.72 ± 17.77 | 237.01 ± 7.92 | 0.333 |
| C18:3N3 (µg/g) | 113.99 ± 9.70 b | 165.81 ± 19.17 a | 143.33 ± 7.03 a | 168.91 ± 2.67 a | 0.031 |
| C20:3N6 (µg/g) | 70.61 ± 4.91 b | 89.57 ± 2.12 a | 72.06 ± 4.27 b | 91.02 ± 4.14 a | 0.010 |
| C22:5N3 (µg/g) | 8.50 ± 0.29 | 8.26 ± 0.60 | 8.72 ± 1.13 | 9.16 ± 0.66 | 0.843 |
| C22:4N6 (µg/g) | 11.75 ± 0.40 b | 12.22 ± 0.61 b | 12.03 ± 1.04 b | 22.47 ± 2.12 a | 0.001 |
| C22:5N6 (µg/g) | 42.58 ± 3.85 | 41.36 ± 0.61 | 42.58 ± 1.41 | 43.93 ± 3.04 | 0.916 |
| SFA | 1267.11 ± 9.64 a | 1227.86 ± 16.85 a | 1216.67 ± 26.11 b | 1057.79 ± 10.34 b | <0.001 |
| UFA | 514.60 ± 8.40 b | 561.36 ± 2.09 a | 531.19 ± 12.88 b | 572.50 ± 2.10 a | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, J.; Ji, Q.; Zhu, K.; Wu, Z.; Chen, X.; Hou, S.; Gui, L.; Yang, C. Metabolome Combined with 16S rDNA Sequencing Reveals a Novel Mechanistic Insight into the Collaboration of Resveratrol and β-Hydroxy-β-Methylbutyric Acid in Regulating the Meat Quality of Tibetan Sheep Through Altering Rumen Microbiota. Microorganisms 2025, 13, 2845. https://doi.org/10.3390/microorganisms13122845
Gan J, Ji Q, Zhu K, Wu Z, Chen X, Hou S, Gui L, Yang C. Metabolome Combined with 16S rDNA Sequencing Reveals a Novel Mechanistic Insight into the Collaboration of Resveratrol and β-Hydroxy-β-Methylbutyric Acid in Regulating the Meat Quality of Tibetan Sheep Through Altering Rumen Microbiota. Microorganisms. 2025; 13(12):2845. https://doi.org/10.3390/microorganisms13122845
Chicago/Turabian StyleGan, Jiacheng, Qiurong Ji, Kaina Zhu, Zhenling Wu, Xuan Chen, Shengzhen Hou, Linsheng Gui, and Chao Yang. 2025. "Metabolome Combined with 16S rDNA Sequencing Reveals a Novel Mechanistic Insight into the Collaboration of Resveratrol and β-Hydroxy-β-Methylbutyric Acid in Regulating the Meat Quality of Tibetan Sheep Through Altering Rumen Microbiota" Microorganisms 13, no. 12: 2845. https://doi.org/10.3390/microorganisms13122845
APA StyleGan, J., Ji, Q., Zhu, K., Wu, Z., Chen, X., Hou, S., Gui, L., & Yang, C. (2025). Metabolome Combined with 16S rDNA Sequencing Reveals a Novel Mechanistic Insight into the Collaboration of Resveratrol and β-Hydroxy-β-Methylbutyric Acid in Regulating the Meat Quality of Tibetan Sheep Through Altering Rumen Microbiota. Microorganisms, 13(12), 2845. https://doi.org/10.3390/microorganisms13122845





