Evaluation of Antimicrobial and Antibiofilm Activity of Eucalyptus urograndis (Clone I144) Pyroligneous Extract on Bovine Mastitis Isolate of Multiple-Drug-Resistant Staphylococcus aureus Strains
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Isolates
2.2. Plant Material
2.3. Production of Pyroligneous Extract
2.4. Antimicrobial Activity
2.4.1. Disk Diffusion Method
2.4.2. Minimum Inhibitory Concentration (MIC)
2.4.3. Minimum Bactericidal Concentration (MBC)
2.5. Phenotypic Characterization of Biofilm Formation Using the Congo Red Agar Test
2.6. Biofilm Inhibition
2.7. Statistical Analyses
3. Results and Discussion
3.1. Antimicrobial Sensitivity Profile
3.2. In Vitro Test
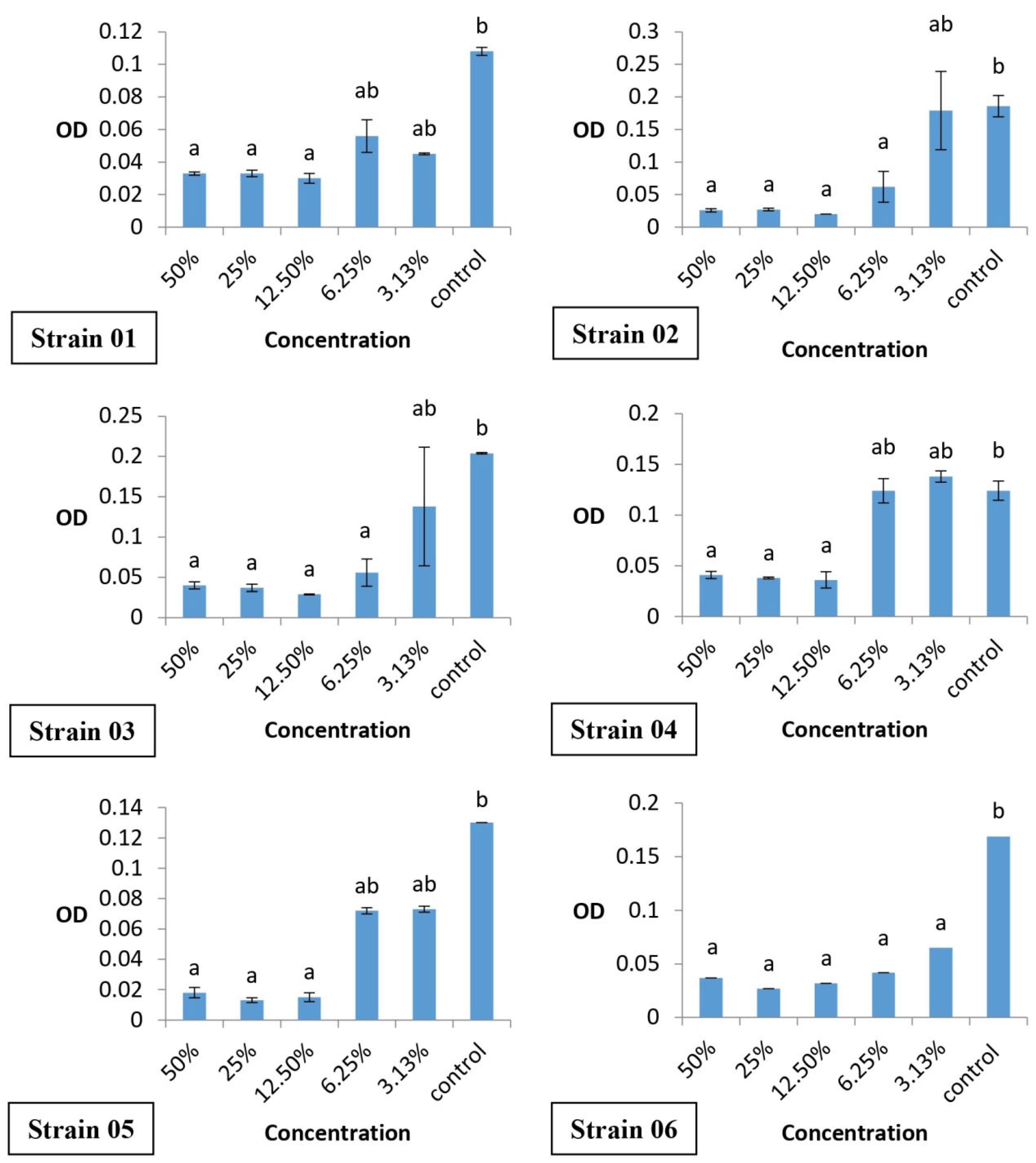
3.3. Biofilm Inhibition by the Crystal Violet Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). Dairy Market Review: Emerging Trends and Outlook in 2023; FAO: Roma, Italy, 2023. [Google Scholar]
- Perkins, N.R.; Petersen, A.; Kristensen, A.R. Pathogen-specific production losses in bovine mastitis. J. Dairy Sci. 2018, 101, 9493–9504. [Google Scholar] [CrossRef]
- Morales-Ubaldo, A.L.; Rivero-Perez, N.; Valladares-Carranza, B.; Velázquez-Ordoñez, V.; Delgadillo-Ruiz, L.; Zaragoza-Bastida, A. Bovine mastitis, a worldwide impact disease: Prevalence, antimicrobial resistance, and viable alternative approaches. Vet. Anim. Sci. 2023, 21, 100306. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Barkema, H.W.; Nobrega, D.B.; Xu, C.; Han, B.; Zhang, C.; Yang, J.; Li, X.; Gao, J. Virulence of Bacteria Causing Mastitis in Dairy Cows: A Literature Review. Microorganisms 2025, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Abed, A.H.; Menshawy, A.M.S.; Zeinhom, M.M.A.; Hossain, D.; Khalifa, E.; Wareth, G.; Awad, M.F. Subclinical Mastitis in Selected Bovine Dairy Herds in North Upper Egypt: Assessment of Prevalence, Causative Bacterial Pathogens, Antimicrobial Resistance and Virulence-Associated Genes. Microorganisms 2021, 9, 1175. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance and Food Safety: The Role of Dairy Production; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Borș, A.; Borș, S.-I.; Floriștean, V.-C. Mastitis impact on high-yielding dairy farm’s reproduction and net present value. Front. Vet. Sci. 2024, 10, 1345782. [Google Scholar] [CrossRef]
- Pérez, V.K.C.; Custódio, D.A.C.; Silva, E.M.M.; Oliveira, J.; Guimarães, A.S.; Brito, M.A.V.P.; Souza-Filho, A.F.; Heinemann, M.B.; Lage, A.P.; Dorneles, E.M.S. Relationship between virulence factors and antimicrobial resistance in Staphylococcus aureus from bovine mastitis. J. Glob. Antimicrob. Resist. 2020, 22, 792–802. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Peng, Q.; Tang, X.; Dong, W.; Sun, N.; Yuan, W. A Review of Biofilm Formation of Staphylococcus aureus and Its Regulation Mechanism. Antibiotics 2022, 12, 12. [Google Scholar] [CrossRef]
- Kebede, I.A.; Dahesa, G.D. Treatment and control methods of bovine mastitis: A review. Vet. Med. Open J. 2024, 9, 7–14. [Google Scholar] [CrossRef]
- Kirkeby, C.; Halasa, T.; Farre, M.; Chehabi, G.N.; Græsbøll, K. Transmission dynamics of Corynebacterium spp. within two Danish dairy cattle herds. Front. Vet. Sci. 2021, 8, 735345. [Google Scholar] [CrossRef]
- Qaralleh, H.A.; Al-Limoun, M.O.; Khlaifat, A.; Khleifat, K.M.; Al-Tawarah, N.; Alsharafa, K.Y.; Abu-Harirah, H.A. Antibacterial and antibiofilm activities of a traditional herbal formula against respiratory infection causing bacteria. arXiv 2021, arXiv:2102.04301. [Google Scholar] [CrossRef]
- Gama, G.S.P.; Pimenta, A.S.; Feijó, F.; Santos, C.; Fernandes, B.; Oliveira, M.; Souza, E.; Monteiro, T.; Fasciotti, M.; Azevedo, T. Antimicrobial activity and chemical profile of wood vinegar from eucalyptus (Eucalyptus urophylla × Eucalyptus grandis—Clone I144) and bamboo (Bambusa vulgaris). World J. Microbiol. Biotechnol. 2023, 39, 186. [Google Scholar] [CrossRef]
- Gama, G.S.P.; Pimenta, A.S.; Feijó, F.M.C.; Aires, C.A.M.; de Melo, R.R.; dos Santos, C.S.; de Medeiros, L.C.D.; da Costa Monteiro, T.V.; Fasciotti, M.; de Medeiros, P.L.; et al. Antimicrobial Impact of Wood Vinegar Produced Through Co-Pyrolysis of Eucalyptus Wood and Aromatic Herbs. Antibiotics 2024, 13, 1056. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.A.; Feijó, F.M.C.; Alves, N.D.; Pimenta, A.S.; Benicio, L.D.M.; da Silva Júnior, E.C.; Santos, C.S.; Pereira, A.F.; Moura, Y.B.F.; Gama, G.S.P.; et al. Use of a product based on wood vinegar of Eucalyptus clone I144 used in the control of bovine mastitis. Vet. Microbiol. 2023, 279, 109670. [Google Scholar] [CrossRef]
- Salim, A.; Deiana, P.; Fancello, F.; Molinu, M.G.; Santona, M.; Zara, S. Antimicrobial and antibiofilm activities of pomegranate peel phenolic compounds: Varietal screening through a multivariate approach. J. Bioresour. Bioprod. 2023, 8, 146–161. [Google Scholar] [CrossRef]
- Carter, G.R. Fundamentos de Bacteriología y Micología Veterinária; Editorial Acribia: Zaragoza, Spain, 1989. [Google Scholar]
- Fan, H.H.; Kleven, S.H.; Jackwood, M.W. Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum. Avian Dis. 1995, 39, 729–735. [Google Scholar] [CrossRef]
- Brakstad, O.G.; Aasbakk, K.; Maeland, J.A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 1992, 30, 1654–1660. [Google Scholar] [CrossRef]
- Kateete, D.P.; Kimani, C.N.; Katabazi, F.A.; Okeng, A.; Okee, M.S.; Nanteza, A.; Joloba, M.L.; Najjuka, F.C. Identification of Staphylococcus aureus: DNase and mannitol salt agar improve the efficiency of the tube coagulase test. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.A.; Gillespie, B.E.; Oliver, S.P. Antimicrobial susceptibility of coagulase-negative Staphylococcus species isolated from bovine milk. Vet. Microbiol. 2009, 134, 73–81. [Google Scholar] [CrossRef]
- Nakagawa, S.; Taneike, I.; Mimura, D.; Iwakura, N.; Nakayama, T.; Emura, T.; Kitatsuji, M.; Fujimoto, A.; Yamamoto, T. Gene sequences and specific detection for Panton-Valentine leukocidin. Biochem. Biophys. Res. Commun. 2005, 328, 995–1002. [Google Scholar] [CrossRef]
- Paterson, G.K.; Larsen, A.R.; Robb, A.; Edwards, G.E.; Pennycott, T.W.; Foster, G.; Mot, D.; Hermans, K.; Baert, K.; Peacock, S.J.; et al. The newly described mecA homologue, mecALGA251, is present in methicillin-resistant Staphylococcus aureus isolates from a diverse range of host species. J. Antimicrob. Chemother. 2012, 67, 2809–2813. [Google Scholar] [CrossRef]
- Gama, G.S.P.; Pimenta, A.S.; Feijó, F.M.C.; Santos, C.S.; Castro, R.V.O.; Azevedo, T.K.B.; Medeiros, L.C.D. Effect of pH on the antibacterial and antifungal activity of wood vinegar (pyroligneous extract) from eucalyptus. Rev. Árvore 2023, 47, e4711. [Google Scholar] [CrossRef]
- Carneiro, A.C.O.; Santos, R.C.; Castro, R.V.O.; Castro, A.F.N.M.; Pimenta, A.S.; Pinto, E.M.; Alves, I.C.N. Estudo da decomposição térmica da madeira de oito espécies da região do Seridó. Rio Grande Do Norte. Rev. Árvore 2013, 37, 20–33. [Google Scholar] [CrossRef]
- Pimenta, A.S.; Gama, G.S.P.; Feijó, F.M.C.; Braga, R.M.; de Azevedo, T.K.B.; de Melo, R.R.; de Oliveira Miranda, N.; de Andrade, G.S. Wood Vinegar from Slow Pyrolysis of Eucalyptus Wood: Assessment of Removing Contaminants by Sequential Vacuum Distillation. Forests 2023, 14, 2414. [Google Scholar] [CrossRef]
- CLSI supplement M100; Performance Standards for Antimicrobial Susceptibility Testing, 30th ed. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020.
- Brazilian Committee on Antimicrobial Susceptibility Testing (BRCAST). Documentos Técnicos BRCAST 2025: Padrões Para Testes de Sensibilidade a Antimicrobianos; BRCAST: São Paulo, Brazil, 2025. [Google Scholar]
- Silva, D.D.; Medeiros, E.S.; Rolim, M.B.Q.; Marinho, A.V.; Soares, K.D.A.; Almeida, G.L.P.; Pereira Neto, L.M.; Silva, T.I.B.D. In vitro efficacy of iodine in the pre and post dipping against coagulase negative Staphylococcus isolated in milk of cows with subclinical mastitis. Cienc. Rural 2021, 51, e20200417. [Google Scholar] [CrossRef]
- Freeman, D.J.; Falkiner, F.R.; Keane, C.T. New method for detecting slime production by coagulase-negative staphylococci. J. Clin. Pathol. 1989, 42, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Extremina, C.I.; Costa, L.; Aguiar, A.I.; Peixe, L.; Fonseca, A.P. Optimization of processing conditions for the quantification of enterococci biofilms using microtitre-plates. J. Microbiol. Methods 2011, 84, 167–173. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health; WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/philippines/news/detail-global/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 26 September 2025).
- Sweeney, D.A.; Kalil, A.C. Didn’t inhale? Time to reconsider aerosolized antibiotics in the treatment of ventilator-associated pneumonia. Crit. Care 2018, 22, 333. [Google Scholar] [CrossRef]
- Goltermann, L.; Haurat, M.F.; Zeng, X.; Hoiby, N.; Douthwaite, S. Macrolide resistance through uL4 and uL22 ribosomal mutations in Pseudomonas aeruginosa. Nat. Commun. 2024, 15, 63329. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Hackbarth, C.J.; Chansky, K.M.; Chambers, H.F. A Proteolytic Transmembrane Signaling Pathway and Resistance to β-Lactams in Staphylococci. Science 2001, 291, 1962–1965. [Google Scholar] [CrossRef]
- Urushibara, N.; Aung, M.S.; Kawaguchiya, M.; Kobayashi, N. Novel staphylococcal cassette chromosome mec (SCCmec) type XIV (5A) and a truncated SCCmec element in SCC composite islands carrying speG in ST5 MRSA in Japan. J. Antimicrob. Chemother. 2020, 75, 46–50. [Google Scholar] [CrossRef]
- Rey Pérez, J.; Zálama Rosa, L.; García Sánchez, A.; Hermoso de Mendoza Salcedo, J.; Alonso Rodríguez, J.M.; Cerrato Horrillo, R.; Zurita, S.G.; Gil Molino, M. Multiple Antimicrobial Resistance in Methicillin-Resistant Staphylococcus sciuri Group Isolates from Wild Ungulates in Spain. Antibiotics 2021, 10, 920. [Google Scholar] [CrossRef] [PubMed]
- Roshan, M.; Parmanand, A.D.; Behera, M.; Vats, A.; Gautam, D.; Deb, R.; Parkunan, T.; Sachinandan, D. Virulence and enterotoxin gene profile of methicillin-resistant Staphylococcus aureus isolates from bovine mastitis. Comp. Immunol. Microbiol. Infect. Dis. 2022, 80, 101724. [Google Scholar] [CrossRef]
- Nor Amdan, N.A.; Shahrulzamri, N.A.; Hashim, R.; Mohamad Jamil, N. Understanding the evolution of macrolides resistance: A mini review. J. Glob. Antimicrob. Resist. 2024, 38, 368–375. [Google Scholar] [CrossRef]
- Abrantes, J.A.; Silva, J.C.; Santos, L.M.; Oliveira, P.R. Bacterial resistance to antimicrobials: A review of the main species involved in infectious processes. Rev. Bras. Análises Clínicas 2021, 53, 202–210. [Google Scholar] [CrossRef]
- Lade, H.; Kim, J.S. Molecular determinants of β-lactam resistance in methicillin-resistant Staphylococcus aureus (MRSA): An updated review. Antibiotics 2023, 12, 1362. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of antibacterial resistance, strategies and next-generation antimicrobials to contain antimicrobial resistance: A review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef] [PubMed]
- Lang, M.; Carvalho, A.; Baharoglu, Z.; Mazel, D. Aminoglycoside uptake, stress, and potentiation in Gram-negative bacteria: New therapies with old molecules. Microbiol. Mol. Biol. Rev. 2023, 87, e00036-22. [Google Scholar] [CrossRef] [PubMed]
- Hushyar, S.; Doghaheh, H.P.; Arzanlou, M. Evaluation of aminoglycoside- and methicillin-resistant Staphylococcus aureus: Phenotypic and genotypic insights from clinical specimens in Ardabil, Iran. BMC Infect. Dis. 2025, 25, 285. [Google Scholar] [CrossRef]
- Costa, S.S.; Junqueira, E.; Palma, C.; Viveiros, M.; Melo-Cristino, J.; Amaral, L.; Couto, I. Resistance to Antimicrobials Mediated by Efflux Pumps in Staphylococcus aureus. Antibiotics 2013, 2, 83–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, X.; Li, J.; Liu, Y.; Li, Y. Role of parC mutations at position 84 on high-level delafloxacin resistance in Staphylococcus aureus. Antibiotics 2024, 13, 641. [Google Scholar] [CrossRef]
- Huynh, T.Q.; Tran, V.N.; Thai, V.C.; Nguyen, H.A.; Nguyen, N.T.G.; Tran, M.K.; Nguyen, T.P.T.; Le, C.A.; Ho, L.T.N.; Surian, N.U.; et al. Genomic alterations involved in fluoroquinolone resistance development in Staphylococcus aureus. PLoS ONE 2023, 18, e0287973. [Google Scholar] [CrossRef]
- Ferrero, L.; Cameron, B.; Manse, B.; Lagneaux, D.; Crouzet, J.; Famechon, A.; Blanche, F. Cloning and primary structure of Staphylococcus aureus DNA topoisomerase IV: A primary target of fluoroquinolones. Mol. Microbiol. 1994, 13, 641–653. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic action and resistance: Updated review of mechanisms, spread, influencing factors, and alternative approaches for combating resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef]
- Terreni, M.; Taccani, M.; Pregnolato, M. New antibiotics for multidrug-resistant bacterial strains: Latest research developments and future perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Mlynarczyk-Bonikowska, B.; Kowalewski, C.; Krolak-Ulinska, A.; Marusza, W. Molecular Mechanisms of Drug Resistance in Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 8088. [Google Scholar] [CrossRef]
- van Duijkeren, E.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of Bacterial Resistance to Antimicrobial Agents. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Fesler, A.T.; Loncaric, I.; Wu, C.; Kadlec, K.; Wang, Y.; Shen, J. Antimicrobial resistance among staphylococci of animal origin. Microbiol. Spectr. 2018, 6, 1–29. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, L.S.; Sharkey, L.K.; Lee, Y.H.J.; Hachani, A.; Monk, R.I.; Stinear, P.T. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef]
- McMillan, K.; Moore, S.C.; McAuley, C.M.; Fegan, N.; Fox, E.M. Characterization of Staphylococcus aureus isolates from raw milk sources in Victoria, Australia. BMC Microbiol. 2016, 16, 169. [Google Scholar] [CrossRef]
- Teo, C.L. Antimicrobial study of pyroligneous extract from Rhizophora apiculata against urinary tract pathogens. J. Teknol. Sci. Eng. 2021, 84, 49–55. [Google Scholar] [CrossRef]
- Souza, J.L.S.; Guimarães, V.B.D.S.; Campos, A.D.; Lund, R.G. Antimicrobial potential of pyroligneous extracts—A systematic review and technological prospecting. Braz. J. Microbiol. 2018, 49 (Suppl. 1), 128–139. [Google Scholar] [CrossRef]
- Soares, W.N.C.; Lira, G.P.O.; Santos, C.S.; Dias, G.N.; Pimenta, A.S.; Pereira, A.F.; Benício, L.D.M.; Rodrigues, G.S.O.; Amora, S.S.A.; Alves, N.D.; et al. Pyroligneous acid from Mimosa tenuiflora and Eucalyptus urograndis as an antimicrobial in dairy goats. J. Appl. Microbiol. 2021, 131, 604–614. [Google Scholar] [CrossRef]
- Feijo, F.M.C.; Fernandes, F.D.C.; Alves, N.D.; Pimenta, A.S.; Santos, C.S.; Rodrigues, G.S.D.O.; Pereira, A.F.; Benicio, L.D.M.; Moura, Y.B.F. Efficiency of pyroligneous extract from Jurema Preta (Mimosa tenuiflora [Willd.] Poiret) as an antiseptic in cats (Felis catus) subjected to ovariosalpingohysterectomy. Animals 2022, 12, 2325. [Google Scholar] [CrossRef] [PubMed]
- Wald-Dickler, N.; Holtom, P.; Spellberg, B. Busting the Myth of “Static vs Cidal”: A Systemic Literature Review. Clin. Infect. Dis. 2018, 66, 1470–1474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batista, S.; Fernández-Pittol, M.; San Nicolás, L.; Martínez, D.; Narváez, S.; Espasa, M.; Garcia Losilla, E.; Rubio, M.; Garrigo, M.; Tudó, G.; et al. Design and Validation of a Simplified Method to Determine Minimum Bactericidal Concentration in Nontuberculous Mycobacteria. Antibiotics 2025, 14, 381. [Google Scholar] [CrossRef]
- de Souza, J.L.S.; Alves, T.; Camerini, L.; Nedel, F.; Campos, A.D.; Lund, R.G. Antimicrobial and cytotoxic capacity of pyroligneous extracts films of Eucalyptus grandis and chitosan for oral applications. Sci. Rep. 2021, 11, 21531. [Google Scholar] [CrossRef] [PubMed]
- de Souza Araújo, E.; Pimenta, A.S.; Feijó, F.M.C.; Castro, R.V.O.; Fasciotti, M.; Monteiro, T.V.C.; de Lima, K.M.G. Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. J. Appl. Microbiol. 2018, 124, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Gama, G.S.P.; Pimenta, A.S.; Feijó, F.M.C.; dos Santos, C.S.; Castro, R.V.O.; de Azevedo, T.K.B.; de Medeiros, L.C.D. Effect of pH on the antimicrobial activity of wood vinegar (pyroligneous extract) from Eucalyptus. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Mirani, Z.A.; Fatima, A.; Urooj, S.; Aziz, M.; Khan, M.N.; Abbas, T. Relationship of cell surface hydrophobicity with biofilm formation and growth rate: A study on Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. Iran. J. Basic. Med. Sci. 2018, 21, 760. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Akinduti, P.A.; Osunlola, O.L.; Adebekun, F.A.; Viavonu, D.T.; Elughi, G.N.; Popoola, O.; Abdulsalami, S.A. Antibacterial activity of Ocimum sanctum L. essential oil against multidrug resistance bacteria vaginosis. Med. Microecol. 2024, 22, 100115. [Google Scholar] [CrossRef]
- Mazzantini, D.; Massimino, M.; Calvigioni, M.; Rossi, V.; Celandroni, F.; Lupetti, A.; Batoni, G.; Ghelardi, E. Anti-Staphylococcal Biofilm Effects of a Liposome-Based Formulation Containing Citrus Polyphenols. Antibiotics 2024, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Suwal, N.; Subba, R.K.; Paudyal, P.; Khanal, D.P.; Panthi, M.; Suwal, N.; Nassan, M.A.; Alqarni, M.; Batiha, G.E.; Koirala, N. Antimicrobial and antibiofilm potential of Curcuma longa Linn. Rhizome extract against biofilm producing Staphylococcus aureus and Pseudomonas aeruginosa isolates. Cell Mol. Biol. 2021, 67, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Er-Rahmani, S.; Errabiti, B.; Matencio, A.; Trotta, F.; Latrache, H.; Koraichi, S.I.; Elabed, S. Plant-derived bioactive compounds for the inhibition of biofilm formation: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2024, 31, 34859–34880. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Wang, M.; Zhang, Z.; Guo, Q.; Yao, J. Mechanistic investigation of the pyrolysis temperature of reed wood vinegar for maximising the antibacterial activity of Escherichia coli and its inhibitory activity. Biology 2024, 13, 912. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, X.; Liu, M.; Yu, C. Biofilm formation: Mechanistic insights and therapeutic targets. Mol. Biomed. 2023, 4, 49. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2021, 10, 3. [Google Scholar] [CrossRef]
- Žiemytė, M.; Carda-Diéguez, M.; Rodríguez-Díaz, J.C.; Ventero, M.P.; Mira, A.; Ferrer, M.D. Real-time monitoring of Pseudomonas aeruginosa biofilm growth dynamics and persister cells’ eradication. Emerg. Microbes Infect. 2021, 10, 2062–2075. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. IJMM 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister Cells and the Paradox of Chronic Infections: Dormant persister cells are tolerant to antibiotics and are largely responsible for recalcitrance of chronic infections. Microbe 2010, 5, 429–437. [Google Scholar] [CrossRef]
- Neca, C.S.M.; Oliveira, H.S.; Severino, J.X.; Barbosa, L.F.P.; Dias, T.V.I. Use of herbal medicines: A literature review. Res. Soc. Dev. 2022, 11, e564111537333. [Google Scholar] [CrossRef]
| Gene | Primer Sequence | Fragment Size (pb) | Reference |
|---|---|---|---|
| nuc | R: AGCCAAGCCTTGACGAACTAAGC | 270 | Kateete et al. (2010) [21] |
| F: GCGATTGATGGTGATACGGTT | |||
| blaZ | F: AAGAGATTTGCCTATGCTTC R: GGCAATATGATCAAGATAC | 517 | Sawant et al. (2009) [22] |
| mecA | F: TGGTATGTGGAAGTTAGATTGGGAT R: CTAATCTCATATGTGTTCCTGTATTGGC | 155 | Nakagawa et al. (2005) [23] |
| mecC | F: CATTAAAATCAGAGCGAGGC R: TGGCTGAACCCATTTTTGAT | 188 | Paterson et al. (2012) [24] |
| Major Compounds | Relative Peak Areas (%) |
|---|---|
| Furfural | 17.2 |
| 2-Methoxy-phenol | 9.4 |
| Phenol | 8.5 |
| 5-Methyl-2-Furancarboxaldehyde | 4.4 |
| 2-Methoxy-3-methyl-Phenol | 4.4 |
| 1-(2-Furanyl)-ethanone | 1.5 |
| 2,3-Dimethyl-2-cyclopenten-1-one | 1.4 |
| 2,5-Dihydro-3,5-dimethyl-2-furanone | 1.0 |
| 3-Methyl-2-Cyclopenten-1-one | 2.4 |
| 1,2,3-Trimethoxy-5-methyl-benzene | 1.9 |
| 3-Methyl-Phenol, | 3.3 |
| 1,2,5-Trimethoxybenzene | 3.5 |
| 4-Methyl-Phenol | 2.3 |
| 1,2-Cyclopentanedione-3-methyl | 3.3 |
| 2,6-Dimethoxy-Phenol, | 10.7 |
| 3-ethyl-2-hydroxy-2-cyclopenten-1-one | 1.0 |
| 4-Ethyl-2-methoxy-phenol | 1.8 |
| Strains/Antimicrobials | PEN | CEF | AMI | FLU | MAC | SUL | Resistance Pattern | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | AMP | CFO | AMI | GEN | NOR | OFX | ERI | SUT | N | % | |
| SA1 | R | R | S | S | S | S | S | R | R | 4/9 | 44.44% |
| SA2 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
| SA3 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
| SA4 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
| SA5 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
| SA6 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
| SA7 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
| SA8 | R | R | R | R | R | R | R | R | R | 9/9 | 100% |
| SA9 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
| SA10 | R | R | R | R | R | S | S | R | R | 7/9 | 77.77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Melo, I.K.; Santos, C.S.; Alves, N.D.; Araujo, G.L.; Clarindo, A.M.; Pimenta, A.S.; Barreto Maia Leite, D.P.d.S.; Mota, R.A.; Carneiro Feijó, F.M. Evaluation of Antimicrobial and Antibiofilm Activity of Eucalyptus urograndis (Clone I144) Pyroligneous Extract on Bovine Mastitis Isolate of Multiple-Drug-Resistant Staphylococcus aureus Strains. Microorganisms 2025, 13, 2771. https://doi.org/10.3390/microorganisms13122771
de Melo IK, Santos CS, Alves ND, Araujo GL, Clarindo AM, Pimenta AS, Barreto Maia Leite DPdS, Mota RA, Carneiro Feijó FM. Evaluation of Antimicrobial and Antibiofilm Activity of Eucalyptus urograndis (Clone I144) Pyroligneous Extract on Bovine Mastitis Isolate of Multiple-Drug-Resistant Staphylococcus aureus Strains. Microorganisms. 2025; 13(12):2771. https://doi.org/10.3390/microorganisms13122771
Chicago/Turabian Stylede Melo, Isadora Karoline, Caio Sergio Santos, Nilza Dutra Alves, Gustavo Lopes Araujo, Aline Maciel Clarindo, Alexandre Santos Pimenta, Denny Parente de Sá Barreto Maia Leite, Rinaldo Aparecido Mota, and Francisco Marlon Carneiro Feijó. 2025. "Evaluation of Antimicrobial and Antibiofilm Activity of Eucalyptus urograndis (Clone I144) Pyroligneous Extract on Bovine Mastitis Isolate of Multiple-Drug-Resistant Staphylococcus aureus Strains" Microorganisms 13, no. 12: 2771. https://doi.org/10.3390/microorganisms13122771
APA Stylede Melo, I. K., Santos, C. S., Alves, N. D., Araujo, G. L., Clarindo, A. M., Pimenta, A. S., Barreto Maia Leite, D. P. d. S., Mota, R. A., & Carneiro Feijó, F. M. (2025). Evaluation of Antimicrobial and Antibiofilm Activity of Eucalyptus urograndis (Clone I144) Pyroligneous Extract on Bovine Mastitis Isolate of Multiple-Drug-Resistant Staphylococcus aureus Strains. Microorganisms, 13(12), 2771. https://doi.org/10.3390/microorganisms13122771







