Chlamydia pecorum Infection Associated with Ocular Disease in Goats in Alabama, USA
Abstract
1. Introduction
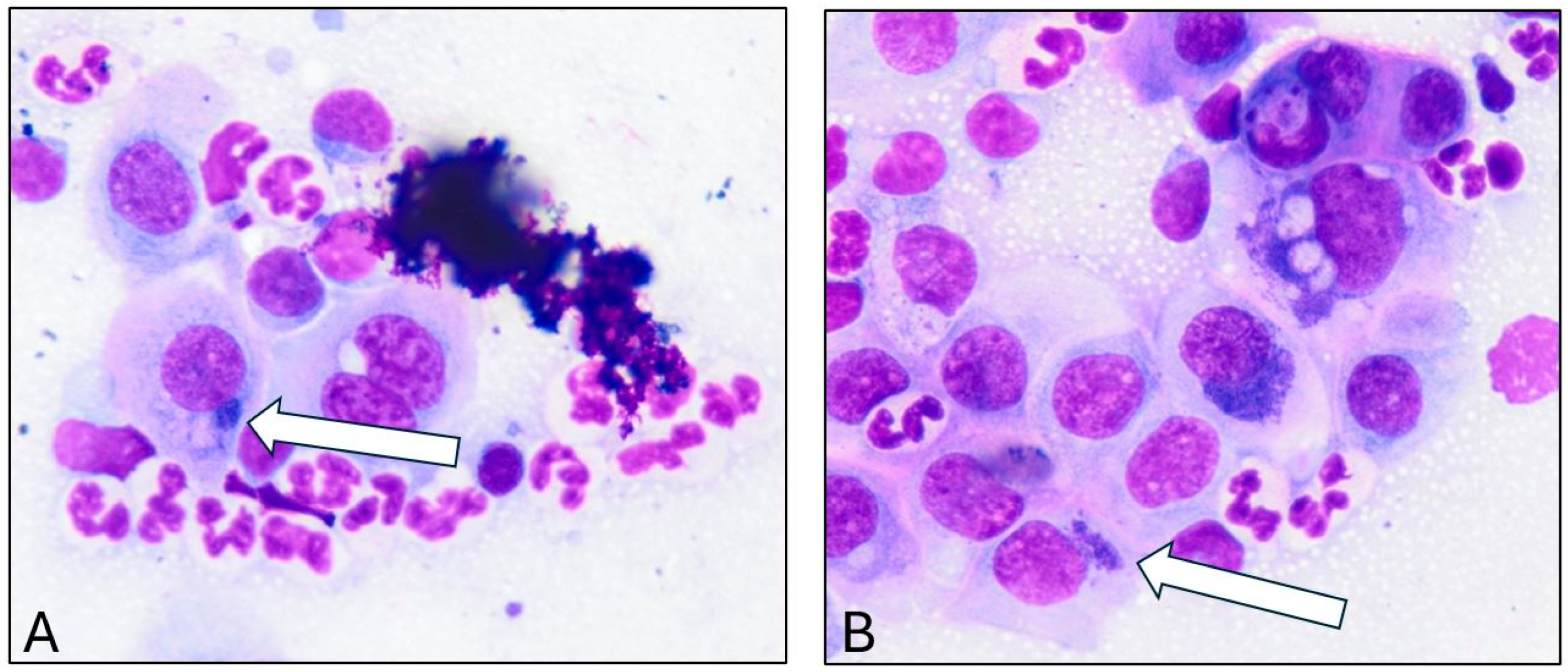
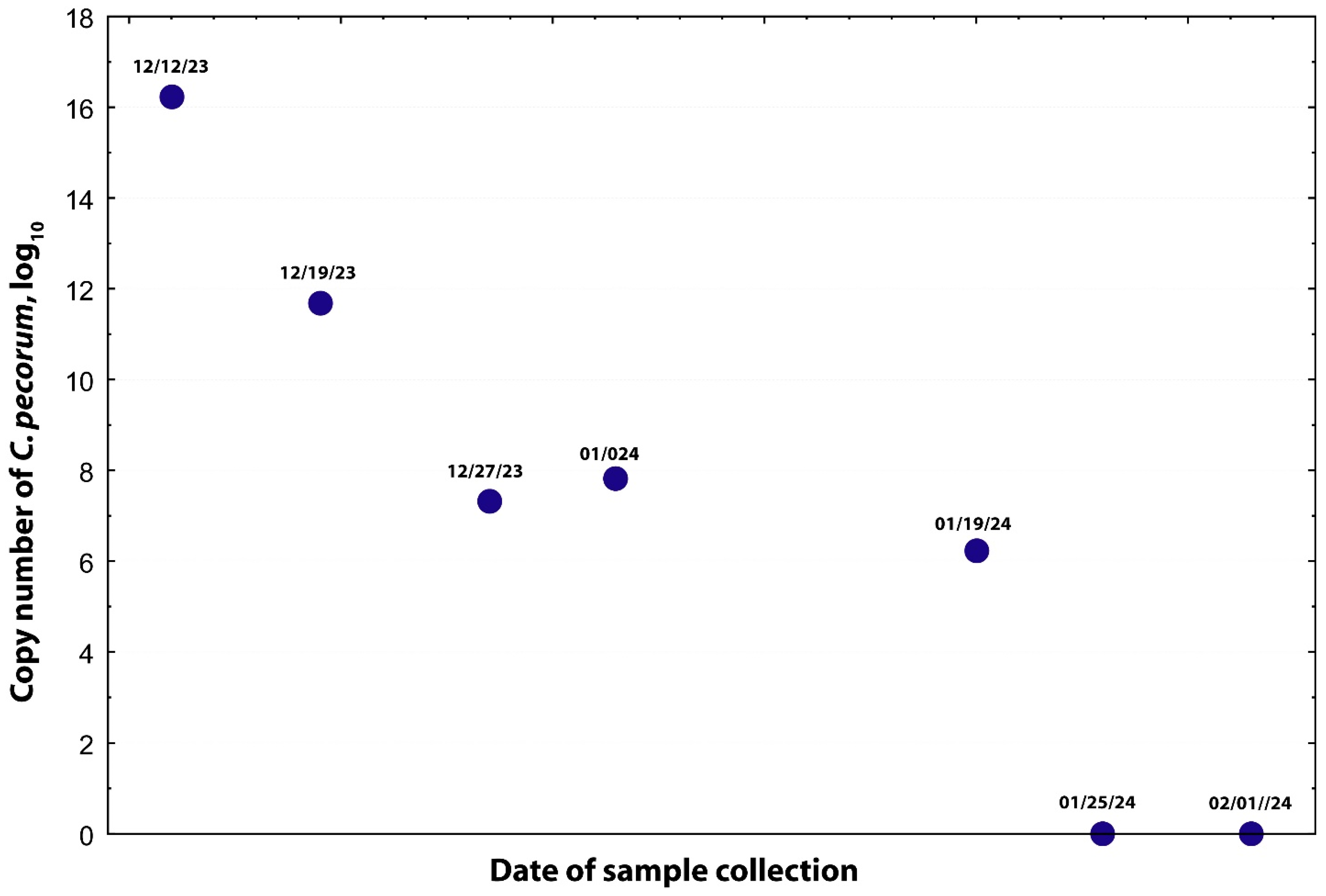
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nietfeld, J.C. Chlamydial infections in small ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Bommana, S.; Walker, E.; Desclozeaux, M.; Jelocnik, M.; Timms, P.; Polkinghorne, A.; Carver, S. Molecular and serological dynamics of Chlamydia pecorum infection in a longitudinal study of prime lamb production. PeerJ 2018, 6, e4296. [Google Scholar] [CrossRef] [PubMed]
- Giannitti, F.; Anderson, M.; Miller, M.; Rowe, J.; Sverlow, K.; Vasquez, M.; Canton, G. Chlamydia pecorum: Fetal and placental lesions in sporadic caprine abortion. J. Vet. Diagn. Investig. 2016, 28, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Dias-Alves, A.; Cabezon, O.; Borel, N.; Lopez-Olvera, J.R.; Mentaberre, G.; Lavin, S.; Fernandez Aguilar, X. Molecular Detection and Identification of Chlamydiaceae in the Eyes of Wild and Domestic Ruminant Hosts from Northern Spain. Pathogens 2021, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aguilar, X.; Rossi, L.; Cabezon, O.; Giorgino, A.; Victoriano Llopis, I.; Frey, J.; Lopez-Olvera, J.R. Infectious keratoconjunctivitis and occurrence of Mycoplasma conjunctivae and Chlamydiaceae in small domestic ruminants from Central Karakoram, Pakistan. Vet. Rec. 2017, 181, 237. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, P.P.; Jenkins, C.; Gerber, P.F.; Olmo, L.; Xaikhue, T.; Theppangna, W.; Walkden-Brown, S.W. Case-control study to identify the causative agents of ophthalmia and conjunctivitis in goats in Savannakhet province of Lao PDR. Vet. Microbiol. 2024, 296, 110195. [Google Scholar] [CrossRef] [PubMed]
- Jelocnik, M.; Forshaw, D.; Cotter, J.; Roberts, D.; Timms, P.; Polkinghorne, A. Molecular and pathological insights into Chlamydia pecorum-associated sporadic bovine encephalomyelitis (SBE) in Western Australia. BMC Vet. Res. 2014, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Jelocnik, M.; Self, R.; Timms, P.; Borel, N.; Polkinghorne, A. Novel sequence types of Chlamydia pecorum infect free-ranging Alpine ibex (Capra ibex) and red deer (Cervus elaphus) in Switzerland. J. Wildl. Dis. 2015, 51, 479–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Guo, W.; Kaltenboeck, B.; Sachse, K.; Yang, Y.; Lu, G.; Zhang, J.; Luan, L.; You, J.; Huang, K.; et al. Chlamydia pecorum is the endemic intestinal species in cattle while C. gallinacea, C. psittaci and C. pneumoniae associate with sporadic systemic infection. Vet. Microbiol. 2016, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wilsmore, A.J.; Dagnall, G.J.; Woodland, R.M. Experimental conjunctival infection of lambs with a strain of Chlamydia psittaci isolated from the eyes of a sheep naturally affected with keratoconjunctivitis. Vet. Rec. 1990, 127, 229–231. [Google Scholar] [PubMed]
- Hopkins, J.B.; Stephenson, E.H.; Storz, J.; Pierson, R.E. Conjunctivitis associated with chlamydial polyarthritis in lambs. J. Am. Vet. Med. Assoc. 1973, 163, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Gulaydin, A.; Gulaydin, O.; Akgul, M.B.; Sindak, N.; Yildirim, O. Investigation of the presence of Chlamydia spp., Mycoplasma spp. and Moraxella ovis in infectious keratoconjunctivitis cases in sheep and goats in Siirt province and evaluation of clinical findings. Pol. J. Vet. Sci. 2024, 27, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, J.; Kaltenboeck, B.; Gong, J.; Fan, W.; Wang, C. Chlamydia gallinacea, not C. psittaci, is the endemic chlamydial species in chicken (Gallus gallus). Sci. Rep. 2016, 6, 19638. [Google Scholar] [CrossRef] [PubMed]
- Meekins, J.M.; Apley, M.D.; Lubbers, B.; Peddireddi, L.; Rankin, A.J. Evaluation of conjunctival bacterial flora in a herd of goats in the Midwestern United States. Vet. Ophthalmol. 2017, 20, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olvera, J.R.; Ramirez, E.; Martinez-Carrasco, C.; Granados, J.E. Wildlife-Livestock Host Community Maintains Simultaneous Epidemiologic Cycles of Mycoplasma conjunctivae in a Mountain Ecosystem. Vet. Sci. 2024, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Unterweger, C.; Schwarz, L.; Jelocnik, M.; Borel, N.; Brunthaler, R.; Inic-Kanada, A.; Marti, H. Isolation of Tetracycline-Resistant Chlamydia suis from a Pig Herd Affected by Reproductive Disorders and Conjunctivitis. Antibiotics 2020, 9, 187. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stockler, J.W.; Passler, T.; Bowden, A.-C.; Barua, S.; Chenoweth, K.; Wang, C. Chlamydia pecorum Infection Associated with Ocular Disease in Goats in Alabama, USA. Microorganisms 2025, 13, 2715. https://doi.org/10.3390/microorganisms13122715
Stockler JW, Passler T, Bowden A-C, Barua S, Chenoweth K, Wang C. Chlamydia pecorum Infection Associated with Ocular Disease in Goats in Alabama, USA. Microorganisms. 2025; 13(12):2715. https://doi.org/10.3390/microorganisms13122715
Chicago/Turabian StyleStockler, Jenna Workman, Thomas Passler, Anna-Catherine Bowden, Subarna Barua, Kelly Chenoweth, and Chengming Wang. 2025. "Chlamydia pecorum Infection Associated with Ocular Disease in Goats in Alabama, USA" Microorganisms 13, no. 12: 2715. https://doi.org/10.3390/microorganisms13122715
APA StyleStockler, J. W., Passler, T., Bowden, A.-C., Barua, S., Chenoweth, K., & Wang, C. (2025). Chlamydia pecorum Infection Associated with Ocular Disease in Goats in Alabama, USA. Microorganisms, 13(12), 2715. https://doi.org/10.3390/microorganisms13122715






