Abstract
In the fluid (comprising oil and nutrient solution)–microbe–mineral ternary system of oil reservoirs, current microbial enhanced oil recovery (MEOR) technology lacks investigation into the interactions between the latter two components and their application potential in petroleum production. This may explain why MEOR has achieved only partial success while failing to meet full expectations. This review systematically synthesizes the existing fragmented research on reservoirs regarding rock minerals as direct/indirect microbial substrates in MEOR applications. Currently, microbe–mineral interactions enhance oil recovery primarily through the following mechanisms: clay swelling inhibition, induced mineral precipitation, silicate dissolution, wettability alteration, microbial acids etching, and hydrocarbon degradation modulation. Integrating contemporary findings on microbe–mineral interactions, three strategically prioritized MEOR implementation pathways demonstrate particular promise: microbially mediated weathering processes in silicate/carbonate reservoirs, microbial-induced mineral precipitation/dissolution cycles, and microbial leaching-assisted permeability enhancement. Finally, a total of 20 microorganisms potentially applicable for mineral-targeted MEOR were proposed. If MEOR technology could be re-examined from the perspective of microbe–mineral interactions and thoroughly investigated, integrating the knowledge on fluid–microbe binary systems in oil reservoir, this potentially transformative technology may achieve breakthroughs.
1. Introduction
Despite significant efforts in developing and utilizing new energy sources, the world still heavily relies on petroleum resources. As an indispensable strategic resource for modern societal development, crude oil plays a pivotal role in global industrial operations, economic propulsion, and innovation-driven growth. In developed oil reservoirs, approximately two-thirds [1,2,3,4,5] of crude oil remains unrecovered after primary (flowing production [6,7]) and secondary recovery (waterflooding) processes. To access these considerable reserves, almost all available technical methods have been attempted, including physical (ultrasonic [8,9] and electrokinetic [10]); thermal (steam [11,12], hot water [13], in situ combustion [14], and combined thermo-chemical [15,16]); chemical (surfactant [17], polymer [18], alkaline [19], and solvent [20]); gas (CO2 [21] and air [22]); and microbial methods. Most enhanced oil recovery methods that involve energy and mass transfer inevitably imply high costs and substantial pollution. However, microbial enhanced oil recovery (MEOR) technology does not require the continuous input of energy or materials, typically requires minimal surface and downhole equipment support, and causes minimal contamination to both surface and subsurface environments. Consequently, it is widely acknowledged for its dual advantages of economic feasibility and environmental sustainability, garnering significant attention from researchers [3,23].
Traditionally, MEOR has been defined as a tertiary oil recovery method that utilizes microorganisms and their metabolites—such as biomass, biogas [24], biosurfactants [25], microbial acids [26], biosolvents [27,28], and enzymes [29]. Since these metabolic products are typically generated by the microbial metabolism of crude oil [30] or nutrients [31], it is easy to overlook the interactions between the latter two components (microbe and mineral) within the fluid–microbe–mineral (FMM) ternary system in oil reservoirs (Figure 1). This, however, may well explain why despite the substantial potential of MEOR technology being widely acknowledged, its industrial applications have yet to deliver a consistently satisfactory performance [1,27,32].
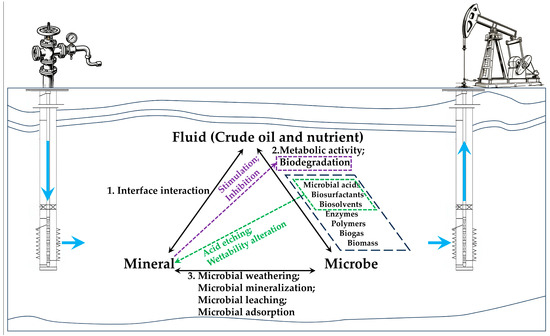
Figure 1.
Schematic diagram of the interactions of the fluid–microbe–mineral (FMM) ternary system in MEOR. Note: The blue arrows indicate the directions of injection and production.
This review provides the first systematic analysis of MEOR mechanisms involving both direct and indirect microbial interactions with rock minerals. Direct microbial actions include microbial biomineralization that inhibits clay swelling [33,34], microbially induced precipitation for plugging high-permeability channels [35,36], and silicate-dissolving microorganisms directly acting on silicate minerals to enhance permeability [37]. Indirect effects refer to the influence of microbial metabolites (biosurfactants [38], biosolvents [39,40,41,42], and microbial acids [27]) generated through fluid–microbe interactions on rock minerals. In reality, microbe–mineral interactions occur more extensively through direct mechanisms than via indirect reactions mediated by metabolic byproducts in most geological contexts [43,44,45]. Furthermore, this review proposes potentially the most promising mineral-targeted MEOR development directions. The proposed research framework focuses on investigations within the FMM system under reservoir conditions (including but not limited to temperature, pressure, and material composition), specifically encompassing the microbial weathering of silicate and carbonate minerals [46], microbial mineralization [47,48], and microbial leaching [49,50,51]. This provides the field with a previously neglected perspective that serves to review and restructure both MEOR research and its applications.
2. MEOR
The earliest conceptualization of MEOR was proposed by Beckmann in 1926 [52]. It was not until the 1940s that researchers like Zobell et al. initiated experimentally validating this concept [53]. The first field-scale implementation of MEOR occurred at the Lisbon Oil Field in Union County, Arkansas, USA [54]. Lazar conducted a systematic review of 30 pre-1991 MEOR field implementations [55], while Maudgalya subsequently documented and analyzed 407 globally distributed MEOR field applications prior to 2007 [1]. In addition, based on summaries of MEOR field tests conducted in China [56], USA, Russia, and other countries [57,58], six primary application types have been identified: microbial flooding recovery [59], cycle microbial recovery [56], microbial selective plugging recovery [54], microbial wax removal [60], genetically engineered microbial enhanced oil recovery [61], and enzyme enhanced oil recovery [29]. The majority of them are generally considered successful. However, few of the tests explain the mechanics of the oil recovery or presented post-treatment analyses or how the results were calculated. This helps explain why MEOR has not gained credibility in the oil industry [1,32].
The mechanisms of MEOR mediated by microbial metabolites can be systematically categorized into four principal components: (1) Biosurfactants predominantly function to diminish oil/water interfacial tension, alter the wettability of porous media, emulsify residual oil, and enhance bacterial migratory capacity [62,63]. (2) Biopolymers, biofilms, and microorganisms selectively occlude high-permeability porous media, wherein biopolymers additionally serve as viscosity modifiers to augment aqueous phase viscosity [56,64]. (3) Biogenic gases, solvents, and acids facilitate the dissolution of carbonate reservoir rocks, thereby enabling aqueous phase penetration into rock pore systems for enhanced residual oil contact. Concurrently, the liberated gases from carbonate dissolution contribute to reservoir pressure augmentation [65]. (4) Reservoir-residing microorganisms utilize crude oil as a carbon substrate, metabolizing long-chain saturated hydrocarbons to reduce crude oil viscosity and improve its mobility [66].
With the rapid advancement of biotechnology, novel functional microbial strains are continuously isolated, identified, and validated for their efficacy in enhancing oil recovery [67,68,69]. The increasing maturity of genetic engineering techniques has enabled the optimization of microbial performance [70,71]. Research on reservoir microorganisms has yielded significant breakthroughs. Comprehensive documentation exists regarding microbial community dynamics across various reservoir types, operational parameters, and temperature–pressure conditions [72,73,74,75].
Nevertheless, it can be empirically concluded that current MEOR research remains fundamentally a black-box paradigm [76,77]. The underlying interaction mechanisms among multifactorial components are yet to be systematically deciphered. Given the substantial body of research on fluid–microbe interactions in MEOR, microbe–mineral interactions under reservoir conditions likely constitute the critical knowledge gap in our understanding.
3. Microbial Inhibition of Clay Swelling
Reservoirs commonly contain clay minerals, among which swelling clays (e.g., montmorillonite, beidellite, and saponite) exhibit dramatic hydration swelling (volumetric increases of 600–1000%) upon water contact (with formation water or injected water) [78]. Clay swelling significantly reduces reservoir porosity, permeability, and oil recovery efficiency [79] (Figure 2), particularly in low-permeability and ultralow-permeability reservoirs [33]. Therefore, effective strategies should be implemented during reservoir development to mitigate clay swelling, thereby enhancing reservoir permeability and hydrocarbon production (Table 1).
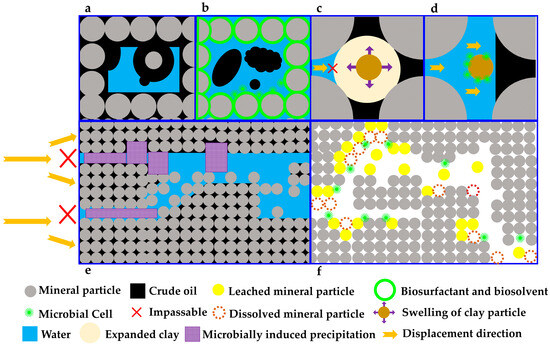
Figure 2.
Schematic diagram of the mechanism for microbial enhanced oil recovery through microbe–mineral interactions in the fluid–microbe–mineral ternary system of oil reservoirs. Note: (a) In the initial state, the rock mineral surfaces in the reservoir are oil-wet, with crude oil trapped on them and predominantly distributed in pore corners, while water exists as a free phase. (b) Under the influence of biosurfactants, biosolvents, and microbial cells, the wettability of the mineral surfaces is altered, leading to a reversal of oil and water distribution compared with (a). (c) Swelling of clay minerals blocks the flow pathways of the displacing fluid (typically water), preventing the crude oil in the pores from being produced. (d) Microbial activity effectively suppresses clay swelling, ensuring unimpeded flow channels and enabling the displacement of crude oil from the pores. (e) Microbially induced precipitation blocks high-permeability water flow channels, diverting the displacing phase into previously unswept areas and mobilizing the crude oil therein. (f) Through microbial weathering—either acid-mediated or non-acidic—mineral particles are dissolved, increasing the population of leached mineral particles and thereby enhancing reservoir porosity and permeability.

Table 1.
Mineral-targeted MEOR studies.
Table 1.
Mineral-targeted MEOR studies.
| No. | Year | Type | Microorganism | Mechanism | Result | Ref. |
|---|---|---|---|---|---|---|
| 1 | 2013 | Laboratory experiment | Enterobacter cloacae | Biosurfactants alter mineral wettability | Increased crude oil recovery by 5% to 10% | [41] |
| 2 | 2016 | Field test | Sporosarcina pasteurii | Microbially induced calcium carbonate precipitation | Pressure rose, injection rate dropped (from 1.9 to 0.47 L/min) | [80] |
| 3 | 2017 | Laboratory experiment | Microbial communities | Microbial acids-induced carbonate dissolution | Porosity increased by 14.89–68.29%, and permeability improved by 35.77–137.83% | [81] |
| 4 | 2018 | Field test | Sporosarcina pasteurii | Microbially induced calcium carbonate precipitation | Injection rate dropped (1.28 gallons per minute (gpm) to less than 0.05 gpm) | [82] |
| 5 | 2018 | Laboratory experiment | 4 Fe(III)-reducing microbial strains | Inhibition of montmorillonite hydro-swelling | Inhibition of Ca-montmorillonite swelling at a rate of 48.9% | [33] |
| 6 | 2018 | Laboratory experiment | Alcaligenes faecalis | Biosurfactants alter mineral wettability | Contact angle decreased from 156° to 86°, shifting from oil-wet to intermediate-wet, enhancing oil recovery by 5.2–8.2% | [42] |
| 7 | 2019 | Laboratory experiment | Bacillus subtilis | Biosurfactants alter mineral wettability | The wettability was modified from the values indicating an intermediate water-wet condition to a strong water-wet condition | [39] |
| 8 | 2020 | Laboratory experiment | Sporosarcina pasteurii | Microbially induced calcium carbonate precipitation | The permeability of large-, medium-, and small-aperture core samples declined to 47%, 32%, and 16% of their initial values, respectively | [83] |
| 9 | 2022 | Laboratory experiment | Proteus hauseri | Inhibition of montmorillonite hydro-swelling | The waterflooding injection pressure was reduced by 61.1%, while the core permeability and oil recovery were enhanced by 49.6% and 8.1%, respectively | [84] |
| 10 | 2023 | Laboratory experiment | Flaviflexus huanghaiensis, Shewanella chilikensis | Inhibition of hydro-swelling and prevention of plugging-related damage | The relative anti-swelling rate of montmorillonite in water improved by 46.2%, 39.7%, 36.6%, 38.4%, and 34.6% under different pressures | [34] |
| 11 | 2023 | Laboratory experiment | Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans, Sulfobacillus thermosulfidooxidans | Biosurfactants alter mineral wettability | Microorganisms promoted a highly water-wet condition but enhanced asphaltene adsorption | [40,85] |
| 12 | 2025 | Laboratory experiment | Paenibacillus mucilaginosus | Microbial-mediated crystallization | Core permeability decreased by 66.67%, the porosity dropped to 8.32%, the plugging rate reached 63.08% | [35] |
| 13 | 2025 | Laboratory experiment | Paenibacillus mucilaginosus | Dissolution of silicate minerals under neutral conditions | The porosity increased by 1.4% and permeability increased by 12.3 mD of low-permeability cores | [37] |
| 14 | 2025 | Laboratory experiment | Bacillus subtilis | Prevention of asphaltene adsorption on carbonate minerals | The bioproducts reduced the asphaltene adsorption by up to 75% | [86] |
Research has demonstrated that indigenous Fe(III)-reducing microbes [84], such as Shewanella [87], Bacillus [88], Deferribacter thermophilus [89], and Geoalkalibacter subterraneus [90], exhibit high environmental adaptability to oil reservoirs and can mediate structural transformation. These microbes facilitate the transformation of swelling montmorillonite into non-swelling illite or other secondary minerals, thereby effectively suppressing clay swelling oil reservoirs.
Dissimilatory iron-reducing bacteria (DIRB)-mediated bioreduction can also significantly alter the physicochemical properties of clay minerals, thereby accelerating the illitization of montmorillonite [34,91]. The structural destabilization of montmorillonite induced by DIRB-driven bioreduction leads to the degradation of its crystalline framework, accompanied by the release of structural and interlayer water molecules. Consequently, this process markedly diminishes both interlayer and external swelling capacity, while facilitating the contraction of montmorillonite [92].
Water intrusion into hydrocarbon reservoirs containing swelling clays appears inevitable during both drilling and development phases, making induced water-sensitive formation damage one of the key factors contributing to productivity decline. The early intervention of microbial clay swelling prevention techniques, as early as the initial stages of hydrocarbon development or even during drilling operations, represents a highly promising solution strategy. Building upon preventive measures, research on microbial-induced clay contraction holds even greater value for reservoirs already affected by water-sensitive damage.
4. Microbially Induced Precipitation
Reservoir heterogeneity adversely impacts sweep efficiency during waterflooding, as injected fluids preferentially migrate through high-permeability “thief zones”, bypassing oil-saturated low-permeability regions. The selective plugging of these “thief zones” forcibly redirects displacement fluids into previously unswept, oil-bearing zones [83] (Figure 2). This flow redistribution equilibrates volumetric flux between high- and low-permeability strata, resulting in a more uniform flood front advancement and significantly improved macroscopic sweep efficiency. Consequently, utilizing MEOR for targeted “thief zones” conformance control emerges as the most technically viable, operationally practical, and economically feasible method to optimize sweep efficiency and enhance ultimate hydrocarbon recovery.
One of the most prevalent occurrences involves microbially induced carbonate precipitation (MICP) [83]. Sporosarcina pasteurii, the most widely utilized microorganism in MICP applications, secretes highly active urease during its metabolic processes [93,94] (Table 1). This enzyme catalyzes the hydrolysis of urea into ammonia (NH3) and carbon dioxide (CO2) (Figure S1). The resultant hydrolytic products subsequently undergo diffusional transport across the cell envelope into the bulk aqueous phase, where rapid secondary hydrolysis generates ammonium cations (NH4+) and carbonate anions (CO32−). Under conditions of local calcium ion (Ca2+) supersaturation, these carbonate moieties participate in heterogeneous nucleation via ionic association, ultimately precipitating as crystalline calcium carbonate (CaCO3) [95,96] (Figure S1). A recent study has demonstrated that Paenibacillus mucilaginosus can mediate CO2 fixation into amorphous and crystalline carbonate minerals. This process not only contributes to MEOR but also provides novel insights for carbon sequestration strategies [35]. Furthermore, the plugging capacity of Bacillus subtilis-mediated MICP has been extensively characterized [97]. Beyond single microbial strains, studies have demonstrated that microbial consortia-mediated MICP exhibits a superior performance—a finding that warrants greater attention in MEOR applications [98].
Of note, any form of controlled microbial mineralization (microbially induced crystallization phenomenon) in reservoir formations holds potential for MEOR applications. This encompasses the precipitation of calcite, aragonite, vaterite, and dolomite [99,100,101]. Particularly, inorganic phosphate precipitation may demonstrate the most significant potential for MEOR implementation. Microorganisms facilitate inorganic phosphate precipitation either by promoting direct sedimentation or through cellular assimilation into organic components [102]. Their primary roles involve supplying reactive phosphate/calcium phosphate, while maintaining pH and redox conditions conducive to phosphate precipitation. By modulating the precipitation–dissolution dynamics of these minerals, this approach not only accomplishes MEOR’s primary goal of microbial conformance control in high-permeability zones, but also delivers auxiliary benefits: the released mineral ions stimulate metabolic activity in other MEOR functional microbial communities. Notably, the petroleum industry exercises stringent control over the formation of all sulfur-bearing minerals that may ultimately convert to hydrogen sulfide, given its severe threats to operational safety.
5. Microbial Weathering of Silicates and Carbonate Minerals
Globally, carbonate reservoirs have emerged as primary hydrocarbon production resources owing to their widespread distribution, consistent thickness, and extensive scale. The Middle East contributes nearly two-thirds of global oil output, with 80% of its oil-bearing formations comprising carbonate rocks. In North America, approximately half of the total oil production is derived from carbonate reservoirs. China hosts nearly 3 × 106 km2 of carbonate rocks, covering roughly one-third of its terrestrial area [103]. Silicate minerals such as feldspar, clay, quartz, and mica are also widely present in oil reservoirs [104]. Among these, clay minerals often act as cementing agents and interstitial fillings between mineral grains [105,106], and their disruption may lead to particle detachment (Figure 2). As the two dominant rock mineral types in reservoirs, research findings on their microbial weathering could likely enhance the permeability of rock media in reservoirs, ultimately achieving the goal of improved oil recovery.
Silicate-dissolving bacteria demonstrate robust capabilities in decomposing silicate minerals under neutral pH conditions [107,108,109,110]. Traditionally, these bacteria have been extensively utilized in agricultural biofertilizers, where they mobilize potassium ions from silicate minerals for crop nutrition [111]. In a groundbreaking application, researchers have pioneered their use in MEOR [37] (Table 1). Comparative core flooding experiments revealed that Paenibacillus mucilaginosus significantly enhances pore network connectivity (porosity increase by 1.4%) and fluid transport capacity (permeability improvement by 12.3 mD) under neutral pH conditions, ultimately achieving a 6.9% incremental oil recovery factor [37] (Table 1 and Figure 2). It is foreseeable that other microorganisms co-applied with silicate-dissolving bacteria in MEOR may utilize their silicate-derived metabolic byproducts as nutrients, creating a synergistic promotion effect.
Although the role of microbial acids etching in enhancing reservoir rock permeability is widely acknowledged, dedicated studies on this topic remain scarce (Table 1). Most research only peripherally mentions this potential MEOR mechanism during efficacy analysis [112,113,114], likely because microbial acids corrosion is generally presumed to have a limited impact on substantial oil recovery improvement. This explains why reports on acid-producing microorganisms, biogenic acid characteristics, and their effects on the porosity and permeability of oil reservoir remain scarce [115]. A comparative investigation has revealed that acid-producing bacterium (Bacillus licheniformis) alone exhibits inferior oil recovery enhancement compared with biosurfactant-producing bacterium (Pseudomonas aeruginosa) or even silicate-dissolving bacterium (Paenibacillus mucilaginosus) [37]. Another separate study, directly relating rock type to MEOR and pH buffering, reports the result of an experimental study conducted using microbial communities from an oil reservoir with low-permeability (<40 mD) limestone rock samples [81]. The post-MEOR treatment analysis of four replicate core samples revealed an average increase in porosity and permeability. Notably, the study recorded a significant pH decrease from neutral (7.0) to acidic conditions (5.2 ± 0.5) [81] (Table 1).
The microbial weathering of minerals is a synergistic effect driven by multiple factors, including but not limited to organic acid dissolution [116,117], redox reactions [118,119], chelation [120,121], and biomechanical processes [122]. Microbially produced organic acids comprise bacterial acids (formic, acetic, lactic, pyruvic, succinic) and fungal acids (gluconic, oxalic, citric). These organic acids not only significantly reduce the pH of local microenvironments but also chelate metal cations in minerals. For example, dihydroxybenzoic acid and salicylic acid can chelate aluminum, iron, and calcium ions [123]. Chelation occurs between microbial-derived biomacromolecules/polymers and mineral elements [124], which is essentially a mineral solubilization process [125]. As microorganisms can use insoluble minerals as electron acceptors [126] to drive redox reactions, the redox transformation of compounds within mineral structures destabilizes the crystal lattice. Consequently, the mineral structure is disrupted, potentially leading to dissolution [127]. Studies have also shown that quinones, cysteine, and melanin-like heteropolymers may directly participate in electron transfer with minerals [128,129] and potentially contribute to microbial mineral weathering.
If relying solely on microbial weathering alone, its relatively low reaction rate prevents the immediate enhancement of petroleum production efficiency and crude oil recovery. However, over longer time scales, its impact may become non-negligible. For instance, applying such microbes in production reservoirs scheduled for long-term shutdown—where no additional operations are conducted during the closure period—could yield gradual positive effects. Unfortunately, no studies have yet reported on such subtle yet beneficial measures.
6. Wettability Alteration
Wettability analysis serves as a fundamental parameter in reservoir engineering, governing capillary pressure dynamics, irreducible water saturation, residual oil saturation, and ultimate oil recovery rates [130,131]. Research has demonstrated that the majority of carbonate reservoirs display oil-wet behavior, owing to the chemisorption of polar organic compounds (particularly asphaltenes and naphthenic acids) onto the carbonate mineral surfaces [132,133] (Figure 2). Surfactant adsorption efficiency in subsurface formations is predominantly controlled by rock mineralogy, which ultimately dictates the degree of wettability modification [134].
Biosurfactants and biosolvents derived from microbial metabolism actively modify the wettability of rock minerals during MEOR processes [39] (Table 1 and Figure 2). Multiple microbial strains, particularly those belonging to the genera Bacillus, Rhodococcus, Acinetobacter, Enterobacter, Alcaligenes, and Pseudomonas, exhibit efficient biosurfactant production in MEOR [39,41,42,135,136,137] (Table 1). Bacterial adhesion and biofilm formation constitute another key wettability alteration mechanism (Figure 2). Cells preferentially colonize surfaces rather than proliferating planktonically in aqueous media [138]. Biofilms show significantly higher antimicrobial resistance than planktonic cells. Bacterial adhesion and biofilm formation induce physicochemical modifications at rock surfaces. While the exact wettability alteration mechanisms require further elucidation, the predominant hypotheses (that include bacterial surface adhesion, biofilm formation, the adsorption of bacterial metabolites, and biosurfactant activity [138]) indicate that the shift from oil-wet to water-wet rock surfaces facilitates the displacement of crude oil from reservoir pores.
Biosurfactants and biosolvents capable of altering the wettability of mineral surfaces, though originating from fluid–microbe interactions, ultimately target minerals and thus qualify as indirect microbial interactions with rock minerals. In contrast, the direct adhesion of bacterial cells to mineral surfaces constitutes direct interactions. In reality, both microorganisms and their metabolic products (e.g., surfactants and organic solvents) tend to accumulate predominantly at the oil–water interface rather than the oil–rock interface. This preference arises because the oil–water interface offers richer nutrients, gases, and aqueous environments, whereas penetrating the viscous crude oil to reach the oil–rock interface requires greater propulsion capacity and leads to inferior metabolic conditions for most microorganisms. Only specific microbes—such as lithophilic bacteria, endolithic bacteria, and mineral-colonizing bacteria—possess the capability to effectively colonize mineral surfaces, thereby enabling the more efficient alteration of mineral surface wettability.
7. Impact of Minerals on Microbial Hydrocarbon Degradation
The minerals present in oil reservoirs (feldspar, quartz, calcite, kaolinite, illite, smectite, and chlorite) influence crude oil degradation. Although the underlying mechanisms have rarely been studied in detail [139,140], this mineral-driven effect likely serves as a pivotal mechanism in MEOR processes.
The research findings in this field demonstrate that different minerals exert distinct effects on microbial hydrocarbon degradation. A comparative study on four iron-bearing mineral phases demonstrated that magnetite, hematite, and ferrihydrite significantly enhance hydrocarbon biodegradation, whereas Fe3+ exhibited inhibitory effects [141]. These were mainly attributed to the reinforced interspecific relationships induced by special species and the synergistic effects of substance conversion under the biocurrent stimulation [141].
The influence of clay minerals on microbial hydrocarbon degradation is highly complex (Table 2). For example, although illite exerted a negative effect on Pseudomonas stutzeri degrading heavy oil by inhibiting the biodegradation of 64 saturated hydrocarbons and 50 aromatic hydrocarbons, it selectively stimulated the biodegradation of 45 aromatic hydrocarbons with a specific structure [142]. As another example, despite reports of kaolinite’s inhibitory effects on microbial hydrocarbon degradation, a number of studies have documented its capacity to stimulate the process (Table 2). As a third example, saponite enhances microbial hydrocarbon degradation in some studies, but shows no effects (either positive or negative) in others (Table 2).
In addition, most clay minerals such as montmorillonite, palygorskite, vermiculite, bentonite [143], and nontronite [144] consistently demonstrate stimulatory effects on microbial hydrocarbon degradation (Table 2). The observed variations may result from differences in microbial conditions (species or consortia), crude oil composition (saturated hydrocarbons, aromatic hydrocarbons, resins, and asphaltenes; SARA fractions), compound-specific responses (phenanthrene, naphthalene, and anthracene), or the source of clay minerals [142] (Table 2).
The microbial degradation of crude oil generates metabolites utilized in MEOR, and the influence of minerals on this process undoubtedly exerts indirect effects on ultimate recovery efficiency. However, current research findings predominantly focus on the microbial remediation of petroleum contamination, representing typical surface environments (e.g., soil and water bodies). Studies examining mineral impacts on microbial crude oil degradation under authentic reservoir conditions (e.g., temperature, pressure) remain relatively limited.

Table 2.
Effect of minerals on the microbial degradation of petroleum hydrocarbons.
Table 2.
Effect of minerals on the microbial degradation of petroleum hydrocarbons.
| No. | Mineral | Substrate | Effect | Mechanism of Influence | Degrader | Ref. |
|---|---|---|---|---|---|---|
| 1 | Mix clay | Crude oil | Stimulation for saturated hydrocarbons, neutral for aromatic hydrocarbons | Increases biological accessibility | Microbial community | [145] |
| 2 | Kaolinite | Heavy oil in the environment | Stimulation | C-O-Na-Si stimulates metabolism | Microbial community | [146] |
| 3 | Montmorillonite | Heavy oil in the environment | Stimulation | Stimulates growth and buffer pH | Pseudomonas aeruginosa + Microbial community | [147] |
| Kaolinite | ||||||
| 4 | Montmorillonite | Heavy oil in the environment | Stimulation | Stimulates growth and buffer pH, C-O-Na-Si stimulates metabolism | Microbial community | [148] |
| Montmorillonite | Stimulation | |||||
| 5 | Vermiculite | Naphthalene, Anthracene | Stimulation | Protects from toxicity | Microbial community | [149] |
| 6 | Montmorillonite | Crude oil | Stimulation | Adsorbent | Microbial community | [150] |
| 7 | Montmorillonite | Saturated hydrocarbons in crude oil | Stimulation | High specific surface area | Microbial community | [151] |
| Palygorskite | Stimulation | High specific surface area | ||||
| Saponite | Neutral | / | ||||
| Kaolinite | Inhibition | No local bridging effect, low specific surface area | ||||
| 8 | Saponite | Crude oil | Stimulation | High specific surface area and cation exchange capacity | Microbial community | [152] |
| 9 | Kaolinite | Crude oil | Inhibition | Low specific surface area and cation exchange capacity | Microbial community | [153] |
| Palygorskite | Stimulation | High specific surface area and cation exchange capacity | ||||
| Saponite | Neutral | / | ||||
| Montmorillonite | Stimulation | High specific surface area and cation exchange capacity | ||||
| 10 | Montmorillonite | Crude oil | Stimulation | / | Microbial community | [154] |
| 11 | Montmorillonite | Phenanthrene and dibenzothiophene compounds | Stimulation | / | Microbial community | [155] |
| 12 | Calcium bentonite | Crude oil in the environment | Stimulation | High specific surface area | Microbial community | [156] |
| Fuller soil | Stimulation | |||||
| Kaolinite | Stimulation | |||||
| 13 | Palygorskite | Phenanthrene(C14) | Stimulation | Stimulate biofilm formation and accommodate extracellular enzymes | Burkholderia sartisoli | [157] |
| 14 | Montmorillonite | Phenanthrene(C14) | Stimulation | High specific surface area and cation exchange capacity | Burkholderia sartisoli + Microbial community | [158] |
| Palygorskite | ||||||
| 15 | Montmorillonite | Crude oil | Stimulation | Stimulates contact with nutrients | Microbial community | [159] |
| Saponite | Stimulation | Increases nutrients utilization | ||||
| 16 | Montmorillonite | Aromatic hydrocarbons in crude oil | Stimulation | High specific surface area and cation exchange capacity | Microbial community | [160] |
| Saponite | Stimulation | |||||
| Palygorskite | Stimulation | Channel structure | ||||
| Kaolinite | Inhibition | Influence of impurities | ||||
| 17 | Kaolinite | Phenanthrene | Stimulation | Silicon/oxygen atoms stimulate biological effects | Sphingomonas sp. GY2B | [161] |
| Quartz | Stimulation | |||||
| 18 | Nontronite | Crude oil | Stimulation | Stimulates ion exchange and nutrient absorption | Alcanivorax borkumensis | [144] |
| 19 | Bentonite | Aromatic hydrocarbons and cadmium contaminated soil | Stimulation | Adsorption of heavy metals | Microbial community | [162] |
| 20 | Palygorskite | Crude oil contaminated soil | Neutral | / | Microbial community | [163] |
| 21 | Illite | Heavy oil | Inhibition for all saturated hydrocarbons and 50 aromatic hydrocarbons, stimulation for 45 aromatic hydrocarbons | Adsorption and cation-π | Pseudomonas stutzeri | [142] |
Note: / indicates that there is no relevant information in the literature.
8. Promising Mineral-Targeting Microbes for MEOR
Although the current knowledge of microbe–mineral interplay within the FMM ternary system in MEOR remains incomplete, existing studies have confirmed their considerable potential for MEOR applications. Building upon the research findings on microbe–mineral interactions under non-reservoir conditions (non-MEOR targets), this review proposes that the microorganisms most likely to achieve breakthroughs in mineral-targeted MEOR in the future include lithophilic bacteria, endolithic bacteria, and mineral-colonizing bacteria, which demonstrate multifunctional capabilities encompassing, but not limited to, microbial weathering, mineralization, and leaching activities (Table 3).

Table 3.
Promising mineral-targeting microbes for MEOR.
Table 3.
Promising mineral-targeting microbes for MEOR.
| No. | Microorganism | Function | Ref. |
|---|---|---|---|
| 1 | Acidithiobacillus ferrooxidans | Microbial leaching of copper | [164] |
| 2 | Acidithiobacillus thiooxidans | Microbial leaching of chalcopyrite | [165] |
| 3 | Arthrobacter sp. | Accelerating the release of Fe from hornblende | [166] |
| 4 | Bacillus cereus | Dissolution of manganese | [167] |
| 5 | Cupriavidus metallidurans | Microbial leaching of copper | [168] |
| 6 | Delftia acidovorans | Formation of gold nanoparticles | [169] |
| 7 | Ferroplasma acidarmanus | Microbial leaching of pyrite, marcasite, and arsenopyrite | [170] |
| 8 | Gallionella ferruginea | Its organic molecules retard mineral growth | [171] |
| 9 | Geobacter sulfurreducens | Formation of Cr(III) crystals | [172] |
| 10 | Leptospirillum ferrooxidans | Microbial leaching of copper | [173] |
| 11 | Leptothrix discophora | Formation of ferromanganese nodules | [174] |
| 12 | Mariprofundus ferrooxydans | Co-precipitation with iron | [175] |
| 13 | Methanocaldococcus jannaschii | Metal ion binding-mediated silicification | [176] |
| 14 | Nitrobacter winogradskyi | Microbial weathering of serpentinized ultrabasites | [177] |
| 15 | Pseudomonas fluorescens | Microbial leaching of Fe, Ni, and Co | [178] |
| 16 | Pseudomonas putida | Dissolution of aluminum from metakaolin | [179] |
| 17 | Rhodococcus spp. | Microbial leaching of sulfur, iron, and silica | [180] |
| 18 | Shewanella piezotolerans | Reduction and biomineralization of iron | [181] |
| 19 | Sporosarcina pasteurii | Induced calcium carbonate mineralization | [182] |
| 20 | Sulfolobus metallicus | Copper leaching | [183] |
Microbial leaching, also known as hydrometallurgical technology, utilizes the metabolic characteristics of specific microorganisms to oxidize or reduce target metal components in ores, enabling their separation in ionic or precipitated forms. This process aims to enrich valuable components or remove harmful elements. Currently, extensive research focuses on Acidithiobacillus ferrooxidans, which are proven to leach metals such as copper [164], zinc [184], cadmium, nickel [185], vanadium [186], uranium [187], lithium [188], phosphorus [189], iron [190], cobalt [191], molybdenum [192], and tellurium [193]. Additionally, Paenibacillus mucilaginosus, as a silicate-dissolving bacterium demonstrates significant desilication capabilities [37]. Ammonia-producing bacteria (e.g., Providencia) [194] can also leach copper. Moreover, certain substances can enhance the bioleaching process, such as surfactants [195], o-phenylenediamine [196], silver nitrate [197], and some metal ions [198]. Microbial leaching induces crystalline lattice disintegration, resulting in macroscopic mineral dissolution. This process enhances the permeability of the reservoir rock matrix while reducing flow resistance, thereby facilitating MEOR. Furthermore, ions leached from minerals stimulate the metabolic activity of other MEOR-functional microorganisms (e.g., hydrocarbon-degrading microbes and biosurfactant-producing bacteria). This auxiliary effect enables synergistic cooperation between mineral-leaching microorganisms and other MEOR-functional microorganisms.
9. Concluding Remarks
Although research on MEOR remains active, studies targeting minerals—both in the laboratory and field trials—are relatively inadequate (Table 1). This may not stem from a lack of insight into the full complexity of the interacting systems but rather reflect a pragmatic trade-off due to the significant mismatch between the reaction rates of microbe–mineral interactions and the scale required for oil and gas production. The cases and mechanisms reviewed here suggest that, despite its current lack of attention, mineral-targeted MEOR holds considerable promise.
Overall, the microbial inhibition of clay swelling does not reverse already-swollen clay structures, limiting its applicability in reservoirs requiring enhanced oil recovery after water flooding. This mechanism is more suitable during early-stage water injection. Both acid-producing and non-acid-producing microbes (e.g., silicate-weathering microorganisms) can significantly enhance reservoir porosity and permeability through microbial weathering. However, these mechanisms still require validation via field tests. To date, microbially induced precipitation is the only mechanism that has been field-tested—Sporosarcina pasteurii has successfully induced precipitation to block high-permeability channels, reduce ineffective water cycling, and improve sweep efficiency. Meanwhile, microbes that produce biosurfactants or biosolvents primarily function by reducing interfacial tension and improving displacement efficiency, which clearly falls under fluid–microbe interactions. Altering mineral surface wettability is only a secondary effect. Moreover, research on microorganisms that preferentially colonize the oil–rock interface rather than the oil–water interface remains insufficient.
Significant knowledge gaps persist in the field of mineral-targeted MEOR. Laboratory studies on microbe–mineral interactions under MEOR conditions are limited, not to mention field applications. The current understanding of such interactions is largely based on near-surface environmental conditions; their stability and effectiveness under reservoir conditions—high-temperature, high-pressure, insufficient oxygen, and hydrocarbon-rich settings—remain largely unknown.
Looking forward, microorganisms capable of efficient mineral interactions—whether through microbial mineralization, weathering, or other mechanisms—should be actively investigated for MEOR applications. Examples include silicate-degrading microbes, acid-producing microorganisms, those used in bioleaching, and those efficient at forming biominerals. With advances in petroleum engineering and genetic engineering, previous constraints on MEOR implementation—such as reservoir redox conditions, temperature, and pressure tolerance—have become less restrictive, opening new pathways for the microbial enhancement of oil recovery. We posit that incorporating microbe–mineral interaction studies into the FMM ternary system will propel MEOR into a transformative new phase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms13122706/s1, Figure S1: Mechanism of urease-induced carbonate precipitation for plugging.
Author Contributions
Methodology, formal analysis, investigation, writing—original draft, writing—review and editing, L.L.; Writing—review and editing, project administration, funding acquisition, C.Z.; Writing—review and editing, resources, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 52170096), the Erdos City Science and Technology Cooperation Major Project (No. 2022EEDSKJZDZX015–2), and the Fundamental Research Funds for the Central Universities (Top Innovative Talents Fund of CUMTB) (No. BBJ2024051).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The content of this review initially originated from the doctoral dissertation of the corresponding author, Li Lei, completed at the China University of Petroleum (Beijing), under the supervision of Wan Yunyang. We acknowledge Zhang Yue for her contribution to Figure 2.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MEOR | Microbial Enhanced Oil Recovery |
| FMM | Fluid–Microbe–Mineral |
| DIRB | Dissimilatory Iron-Reducing Bacteria |
| MICP | Microbially Induced Carbonate Precipitation |
| SARA | Saturated hydrocarbons, Aromatic hydrocarbons, Resins, and Asphaltenes |
References
- Brown, L.R. Microbial enhanced oil recovery (MEOR). Curr. Opin. Microbiol. 2010, 13, 316–320. [Google Scholar] [CrossRef]
- Al-Marzouqi, A.H.; Zekri, A.Y.; Azzam, A.A.; Dowaidar, A. Hydrocarbon recovery from porous media using supercritical fluid extraction. J. Porous Media. 2009, 12, 489–500. [Google Scholar] [CrossRef]
- Sen, R. Biotechnology in petroleum recovery: The microbial EOR. Prog. Energy Combust. Sci. 2008, 34, 714–724. [Google Scholar] [CrossRef]
- Suthar, H.; Hingurao, K.; Desai, A.; Nerurkar, A. Evaluation of bioemulsifier mediated Microbial Enhanced Oil Recovery using sand pack column. J. Microbiol. Methods 2008, 75, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.; Simpson, D.R.; Duncan, K.E.; McInerney, M.J.; Folmsbee, M.; Fincher, T.; Knapp, R.M. In situ biosurfactant production by Bacillus strains injected into a limestone petroleum reservoir. Appl. Environ. Microbiol. 2007, 73, 1239–1247. [Google Scholar] [CrossRef]
- Belyaev, S.S.; Borzenkov, I.A.; Nazina, T.N.; Rozanova, E.P.; Glumov, I.F.; Ibatullin, R.R.; Ivanov, M.V. Use of microorganisms in the biotechnology for the enhancement of oil recovery. Microbiology 2004, 73, 590–598. [Google Scholar] [CrossRef]
- Mu, B.; Nazina, T.N. Recent advances in petroleum microbiology. Microorganisms 2022, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Agi, A.; Junin, R.; Chong, A.S. Intermittent ultrasonic wave to improve oil recovery. J. Petrol. Sci. Eng. 2018, 166, 577–591. [Google Scholar] [CrossRef]
- Lim, M.W.; Von Lau, E.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil-present works and future directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vardhan, K.H.; Jeevanantham, S.; Karishma, S.B.; Yaashikaa, P.R.; Vellaichamy, P. A review on systematic approach for microbial enhanced oil recovery technologies: Opportunities and challenges. J. Clean. Prod. 2020, 258, 120777. [Google Scholar] [CrossRef]
- Huang, S.; Chen, X.; Liu, H.; Jiang, J.; Cao, M.; Xia, Y. Experimental and numerical study of solvent optimization during horizontal-well solvent-enhanced steam flooding in thin heavy-oil reservoirs. Fuel 2018, 228, 379–389. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, H.; Huang, S.; Wu, K.; Chen, X.; Wang, D.; Xiong, H. Environmental and economic benefits of Solvent-Assisted Steam-Gravity Drainage for bitumen through horizontal well: A comprehensive modeling analysis. Energy 2018, 164, 418–431. [Google Scholar] [CrossRef]
- Han, B.; Cheng, W.; Nian, Y. Experimental study on effect of temperature field on recovery of reservoir using hot water flooding. Energy Procedia 2017, 142, 3759–3765. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Kim, M.; Abedini, A.; Lele, P.; Guerrero, A.; Sinton, D. Microfluidic pore-scale comparison of alcohol- and alkaline-based SAGD processes. J. Petrol. Sci. Eng. 2017, 154, 139–149. [Google Scholar] [CrossRef]
- Saboorian-Jooybari, H.; Dejam, M.; Chen, Z. Heavy oil polymer flooding from laboratory core floods to pilot tests and field applications: Half-century studies. J. Petrol. Sci. Eng. 2016, 142, 85–100. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Shadizadeh, S.R. Experimental investigation of a natural surfactant adsorption on shale-sandstone reservoir rocks: Static and dynamic conditions. Fuel 2015, 159, 15–26. [Google Scholar] [CrossRef]
- Sheng, J.J. A comprehensive review of alkaline–surfactant–polymer (ASP) flooding. Asia-Pac. J. Chem. Eng. 2014, 9, 471–489. [Google Scholar] [CrossRef]
- Khlaifat, A.L.; Dakhlallah, D.; Sufyan, F. A critical review of alkaline flooding: Mechanism, hybrid flooding methods, laboratory work, pilot projects, and field applications. Energies 2022, 15, 3820. [Google Scholar] [CrossRef]
- Dang, F.; Li, S.; Feng, S. Greening strategy for heavy oil thermal recovery assisted by environmental-friendly solvent dimethyl ether. Geoen. Sci. Eng. 2025, 251, 213889. [Google Scholar] [CrossRef]
- Esene, C.; Rezaei, N.; Aborig, A.; Zendehboudi, S. Comprehensive review of carbonated water injection for enhanced oil recovery. Fuel 2019, 237, 1086–1107. [Google Scholar] [CrossRef]
- Jia, H.; Sheng, J.J. Discussion of the feasibility of air injection for enhanced oil recovery in shale oil reservoirs. Petroleum 2017, 3, 249–257. [Google Scholar] [CrossRef]
- Maleki, M.; Kazemzadeh, Y.; Dehghan Monfared, A.; Hasan-Zadeh, A.; Abbasi, S. Bio-enhanced oil recovery (BEOR) methods: All-important review of the occasions and challenges. Can. J. Chem. Eng. 2024, 102, 2364–2390. [Google Scholar] [CrossRef]
- Zhu, W.; Zhao, J.; Han, H.; Sun, G.; Song, Z. High-pressure microscopic investigation on the oil recovery mechanism by in situ biogases in petroleum reservoirs. Energy Fuels 2015, 29, 7866–7874. [Google Scholar] [CrossRef]
- Banat, I.M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: A review. Bioresour. Technol. 1995, 51, 1–12. [Google Scholar] [CrossRef]
- Lourdes, R.S.; Cheng, S.Y.; Chew, K.W.; Ma, Z.; Show, P.L. Prospects of microbial enhanced oil recovery: Mechanisms and environmental sustainability. Sustain. Energy Technol. Assess 2022, 53, 102527. [Google Scholar] [CrossRef]
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent developments in microbial enhanced oil recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Almeida, P.E.; Moreira, R.S.; Almeida, R.; Guimaraes, A.K.; Carvalho, A.S.; Quintella, C.; Esperidia, M.; Taft, C. Selection and application of microorganisms to improve oil recovery. Eng. Life Sci. 2004, 4, 319–325. [Google Scholar] [CrossRef]
- Aurepatipan, N.; Champreda, V.; Kanokratana, P.; Chitov, T.; Bovonsombut, S. Assessment of bacterial communities and activities of thermotolerant enzymes produced by bacteria indigenous to oil-bearing sandstone cores for potential application in Enhanced Oil Recovery. J. Petrol. Sci. Eng. 2018, 163, 295–302. [Google Scholar] [CrossRef]
- Zheng, C.; Yu, L.; Huang, L.; Xiu, J.; Huang, Z. Investigation of a hydrocarbon-degrading strain, Rhodococcus ruber Z25, for the potential of microbial enhanced oil recovery. J. Petrol. Sci. Eng. 2012, 81, 49–56. [Google Scholar] [CrossRef]
- Astuti, D.I.; Purwasena, I.A.; Priharto, N.; Ariadji, T.; Afifah, L.N.; Saputro, R.B.; Aditiawati, P.; Persada, G.P.; Ananggadipa, A.A.; Abqory, M.H.; et al. Bacterial community dynamics during MEOR biostimulation of an oil reservoir in sumatera Indonesia. J. Petrol. Sci. Eng. 2022, 208, 109558. [Google Scholar] [CrossRef]
- Quraishi, M.; Bhatia, S.K.; Pandit, S.; Gupta, P.K.; Rangarajan, V.; Lahiri, D.; Varjani, S.; Mehariya, S.; Yang, Y.H. Exploiting microbes in the petroleum field: Analyzing the credibility of microbial enhanced oil recovery (MEOR). Energies 2021, 14, 4684. [Google Scholar] [CrossRef]
- Cui, K.; Sun, S.; Xiao, M.; Liu, T.; Xu, Q.; Dong, H.; Wang, D.; Gong, Y.; Sha, T.; Hou, J.; et al. Microbial mineralization of montmorillonite in low-permeability oil reservoirs for microbial enhanced oil recovery. Appl. Environ. Microbiol. 2018, 84, e118–e176. [Google Scholar] [CrossRef]
- Dong, H.; Yu, L.; Xu, T.; Liu, Y.; Fu, J.; He, Y.; Gao, J.; Wang, J.; Sun, S.; She, Y.; et al. Cultivation and biogeochemical analyses reveal insights into biomineralization caused by piezotolerant iron-reducing bacteria from petroleum reservoirs and their application in MEOR. Sci. Total Environ. 2023, 903, 166465. [Google Scholar] [CrossRef]
- Huang, M.; Zheng, X.; He, X.; Lin, X.; Ye, J. Paenibacillus mucilaginosus-induced CO2 fixation into amorphous and crystalline carbonate minerals. Process Biochem. 2025, 157, 242–255. [Google Scholar] [CrossRef]
- Zhu, H.; Carlson, H.K.; Coates, J.D. Applicability of anaerobic nitrate-dependent Fe(II) oxidation to microbial enhanced oil recovery (MEOR). Environ. Sci. Technol. 2013, 47, 8970–8977. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.; Su, P.; Mu, H. Lab-scale experimental study of microbial enhanced oil recovery on low-permeability cores using the silicate bacterium Paenibacillus mucilaginosus. Microorganisms 2025, 13, 738. [Google Scholar] [CrossRef] [PubMed]
- Khajepour, H.; Mahmoodi, M.; Biria, D.; Ayatollahi, S. Investigation of wettability alteration through relative permeability measurement during MEOR process: A micromodel study. J. Petrol. Sci. Eng. 2014, 120, 10–17. [Google Scholar] [CrossRef]
- Park, T.; Jeon, M.; Yoon, S.; Lee, K.S.; Kwon, T. Modification of interfacial tension and wettability in oil-brine-quartz system by in situ bacterial biosurfactant production at reservoir conditions: Implications for microbial enhanced oil recovery. Energy Fuels 2019, 33, 4909–4920. [Google Scholar] [CrossRef]
- Soleimani, Y.; Mohammadi, M.; Schaffie, M.; Zabihi, R.; Ranjbar, M. An experimental study of the effects of bacteria on asphaltene adsorption and wettability alteration of dolomite and quartz. Sci. Rep. 2023, 13, 21497. [Google Scholar] [CrossRef]
- Sarafzadeh, P.; Hezave, A.Z.; Ravanbakhsh, M.; Niazi, A.; Ayatollahi, S. Enterobacter cloacae as biosurfactant producing bacterium: Differentiating its effects on interfacial tension and wettability alteration Mechanisms for oil recovery during MEOR process. Colloid Surf. B-Biointerfaces 2013, 105, 223–229. [Google Scholar] [CrossRef]
- Najafi-Marghmaleki, A.; Kord, S.; Hashemi, A.; Motamedi, H. Experimental investigation of efficiency of MEOR process in a carbonate oil reservoir using Alcaligenes faecalis: Impact of interfacial tension reduction and wettability alteration mechanisms. Fuel 2018, 232, 27–35. [Google Scholar] [CrossRef]
- Ng, D.H.P.; Kumar, A.; Cao, B. Microorganisms meet solid minerals: Interactions and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 6935–6946. [Google Scholar] [CrossRef]
- Li, G.L.; Zhou, C.H.; Fiore, S.; Yu, W.H. Interactions between microorganisms and clay minerals: New insights and broader applications. Appl. Clay Sci. 2019, 177, 91–113. [Google Scholar] [CrossRef]
- Jarwar, M.A.; Dumontet, S.; Pasquale, V.; Chen, C. Microbial induced carbonate precipitation: Environments, applications, and mechanisms. Geomicrobiol. J. 2022, 39, 833–851. [Google Scholar] [CrossRef]
- Fang, Q.; Lu, A.; Hong, H.; Kuzyakov, Y.; Algeo, T.J.J.; Zhao, L.; Olshansky, Y.; Moravec, B.; Barrientes, D.M.M.; Chorover, J. Mineral weathering is linked to microbial priming in the critical zone. Nat. Commun. 2023, 14, 345. [Google Scholar] [CrossRef]
- Kumar, A.; Song, H.; Mishra, S.; Zhang, W.; Zhang, Y.L.; Zhang, Q.; Yu, Z. Application of microbial-induced carbonate precipitation (MICP) techniques to remove heavy metal in the natural environment: A critical review. Chemosphere 2023, 318, 137894. [Google Scholar] [CrossRef]
- Taharia, M.; Dey, D.; Das, K.; Sukul, U.; Chen, J.; Banerjee, P.; Dey, G.; Sharma, R.K.; Lin, P.; Chen, C.Y. Microbial induced carbonate precipitation for remediation of heavy metals, ions and radioactive elements: A comprehensive exploration of prospective applications in water and soil treatment. Ecotoxicol. Environ. Saf. 2024, 271, 115990. [Google Scholar] [CrossRef]
- Hutchins, S.R.; Davidson, M.S.; Brierley, J.A.; Brierley, C.L. Microorganisms in reclamation of metals. Annu. Rev. Microbiol. 1986, 40, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I. Microbial leaching of metals from sulfide minerals. Biotechnol. Adv. 2001, 19, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Falagan, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Beckman, J.W. Action of bacteria on mineral oil. J. Ind. Eng. Chem. 1926, 4, 23–26. [Google Scholar]
- Zobell, C.E. Action of microorganisms on hydrocarbons. Bacteriol. Rev. 1946, 10, 1–49. [Google Scholar] [CrossRef]
- Wu, B.; Xiu, J.; Yu, L.; Huang, L.; Yi, L.; Ma, Y. Research advances of microbial enhanced oil recovery. Heliyon 2022, 8, e11424. [Google Scholar] [CrossRef]
- Lazar, I. Ch. A-1 MEOR Field Trials Carried out over the World During the Last 35 Years. In Developments in Petroleum Science; Donaldson, E.C., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 31, pp. 485–530. [Google Scholar]
- Gao, C. Experiences of microbial enhanced oil recovery in Chinese oil fields. J. Petrol. Sci. Eng. 2018, 166, 55–62. [Google Scholar] [CrossRef]
- Safdel, M.; Anbaz, M.A.; Daryasafar, A.; Jamialahmadi, M. Microbial enhanced oil recovery, a critical review on worldwide implemented field trials in different countries. Renew. Sust. Energ. Rev. 2017, 74, 159–172. [Google Scholar] [CrossRef]
- Yernazarova, A.; Shaimerdenova, U.; Akimbekov, N.; Kaiyrmanova, G.; Shaken, M.; Izmailova, A. Exploring the use of microbial enhanced oil recovery in Kazakhstan: A review. Front. Microbiol. 2024, 15, 1394838. [Google Scholar] [CrossRef]
- Liu, J.F.; Ma, L.J.; Mu, B.Z.; Liu, R.L.; Ni, F.T.; Zhou, J.X. The field pilot of microbial enhanced oil recovery in a high temperature petroleum reservoir. J. Pet. Sci. Eng. 2005, 48, 265–271. [Google Scholar] [CrossRef]
- She, H.; Kong, D.; Li, Y.; Hu, Z.; Guo, H. Recent advance of microbial enhanced oil recovery (MEOR) in China. Geofluids 2019, 2019, 1871392. [Google Scholar] [CrossRef]
- Niu, J.; Liu, Q.; Lv, J.; Peng, B. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J. Petrol. Sci. Eng. 2020, 192, 107350. [Google Scholar] [CrossRef]
- Hajibagheri, F.; Lashkarbolooki, M.; Ayatollahi, S.; Hashemi, A. The synergic effects of anionic and cationic chemical surfactants, and bacterial solution on wettability alteration of carbonate rock: An experimental investigation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 422–429. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, W.; Xue, Q. Biosurfactant production and oil degradation by Bacillus siamensis and its potential applications in enhanced heavy oil recovery. Int. Biodeterior. Biodegrad. 2022, 169, 105388. [Google Scholar] [CrossRef]
- Qi, Y.; Zheng, C.; Lv, C.; Lun, Z.; Ma, T. Compatibility between weak gel and microorganisms in weak gel-assisted microbial enhanced oil recovery. J. Biosci. Bioeng. 2018, 126, 235–240. [Google Scholar] [CrossRef]
- Rathi, R.; Lavania, M.; Kukreti, V.; Lal, B. Evaluating the potential of indigenous methanogenic consortium for enhanced oil and gas recovery from high temperature depleted oil reservoir. J. Biotechnol. 2018, 283, 43–50. [Google Scholar] [CrossRef]
- Tao, K.; Liu, X.; Chen, X.; Hu, X.; Cao, L.; Yuan, X. Biodegradation of crude oil by a defined co-culture of indigenous bacterial consortium and exogenous Bacillus subtilis. Bioresour. Technol. 2017, 224, 327–332. [Google Scholar] [CrossRef]
- Ashish; Debnath Das, M. Application of biosurfactant produced by an adaptive strain of C-tropicalis MTCC230 in microbial enhanced oil recovery (MEOR) and removal of motor oil from contaminated sand and water. J. Petrol. Sci. Eng. 2018, 170, 40–48. [Google Scholar] [CrossRef]
- Zhao, X.; Liao, Z.; Liu, T.; Cheng, W.; Gao, G.; Yang, M.; Ma, T.; Li, G. Investigation of the transport and metabolic patterns of oil-displacing bacterium FY-07-G in the microcosm model using X-CT technology. J. Appl. Microbiol. 2023, 134, lxad281. [Google Scholar] [CrossRef]
- Lin, X.; Zheng, X.; Liu, R.; Wen, Y.; Huang, M.; Gou, R.; Yan, Y.; Shi, Y.; Tang, J. Extracellular polymeric substances production by ZL-02 for microbial enhanced oil recovery. Ind. Eng. Chem. Res. 2021, 60, 842–850. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, B.H.; Cui, Q.F.; Wu, Y.T. Genetically modified indigenous Pseudomonas aeruginosa drove bacterial community to change positively toward microbial enhanced oil recovery applications. J. Appl. Microbiol. 2024, 135, lxae168. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Z.; Luo, Y.; Zhong, W.; Xiao, M.; Yi, W.; Yu, L.; Fu, P. Exopolysaccharide production by a genetically engineered Enterobacter cloacae strain for microbial enhanced oil recovery. Bioresour. Technol. 2011, 102, 6153–6158. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wu, G.; Ren, F.; Chang, Q.; Yu, B.; Xue, Y.; Mu, B. Microbial community dynamics in Baolige oilfield during MEOR treatment, revealed by Illumina MiSeq sequencing. Appl. Microbiol. Biotechnol. 2016, 100, 1469–1478. [Google Scholar] [CrossRef]
- Zhang, F.; She, Y.H.; Li, H.M.; Zhang, X.T.; Shu, F.C.; Wang, Z.L.; Yu, L.J.; Hou, D.J. Impact of an indigenous microbial enhanced oil recovery field trial on microbial community structure in a high pour-point oil reservoir. Appl. Microbiol. Biotechnol. 2012, 95, 811–821. [Google Scholar] [CrossRef]
- Cheng, W.; Fan, H.; Yun, Y.; Zhao, X.; Su, Z.; Tian, X.; Liu, D.; Ma, T.; Li, G. Effects of nutrient injection on the Xinjiang oil field microbial community studied in a long core flooding simulation device. Front. Microbiol. 2023, 14, 1230274. [Google Scholar] [CrossRef]
- Pang, J.; Wu, T.; Yu, X.; Zhou, C.; Gao, J.; Chen, H. Microbial community distribution in low permeability reservoirs and their positive impact on enhanced oil recovery. Microorganisms 2025, 13, 1230. [Google Scholar] [CrossRef] [PubMed]
- Noorman, H.J.; Heijnen, J.J.; Luyben, K.C.-A.M. Linear relations in microbial reaction systems: A general overview of their origin, form, and use. Biotechnol. Bioeng. 1991, 38, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Golan, O.; Gampp, O.; Eckert, L.; Sauer, U. Overall biomass yield on multiple nutrient sources. NPJ Syst. Biol. Appl. 2025, 11, 17. [Google Scholar] [CrossRef]
- Yuan, Z.; Liao, X.; Zhang, K.; Zhao, X.; Chen, Z. The effect of inorganic salt precipitation on oil recovery during CO2 flooding: A case study of Chang 8 block in Changqing oilfield, NW China. Petrol. Explor. Dev. 2021, 48, 442–449. [Google Scholar] [CrossRef]
- Sharifipour, M.; Nakhaee, A.; Pourafshary, P. Model development of permeability impairment due to clay swelling in porous media using micromodels. J. Petrol. Sci. Eng. 2019, 175, 728–742. [Google Scholar] [CrossRef]
- Phillips, A.J.; Cunningham, A.B.; Gerlach, R.; Hiebert, R.; Hwang, C.C.; Lomans, B.P.; Westrich, J.; Mantilla, C.; Kirksey, J.; Esposito, R.; et al. Fracture sealing with microbially-induced calcium carbonate precipitation: A field study. Environ. Sci. Technol. 2016, 50, 4111–4117. [Google Scholar] [CrossRef] [PubMed]
- Rinanti, A. The potential of indigenous bacteria to increase porosity-permeability of reservoir rock: A preliminary study. Int. J. Geomate. 2017, 12, 71–75. [Google Scholar] [CrossRef]
- Phillips, A.J.; Troyer, E.; Hiebert, R.; Kirkland, C.; Gerlach, R.; Cunningham, A.B.; Spangler, L.; Kirksey, J.; Rowe, W.; Esposito, R. Enhancing wellbore cement integrity with microbially induced calcite precipitation (MICP): A field scale demonstration. J. Petrol. Sci. Eng. 2018, 171, 1141–1148. [Google Scholar] [CrossRef]
- Song, C.P.; Elsworth, D. Microbially induced calcium carbonate plugging for enhanced oil recovery. Geofluids 2020, 2020, 5921789. [Google Scholar] [CrossRef]
- Cui, K.; Wang, C.; Li, L.; Zou, J.; Huang, W.; Zhang, Z.; Wang, H.; Guo, K. Controlling the hydro-swelling of smectite clay minerals by Fe(III) reducing bacteria for enhanced oil recovery from low-permeability reservoirs. Energies 2022, 15, 4393. [Google Scholar] [CrossRef]
- Kelly, D.P.; Wood, A.P. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, N.; Schaffie, M.; Ranjbar, M.; Zabihi, R. Investigating the impact of Bacillus subtilis bioproducts on static adsorption of asphaltene on dolomite and calcite. Fuel 2025, 397, 135240. [Google Scholar] [CrossRef]
- Kostka, J.E.; Haefele, E.; Viehweger, R.; Stucki, J.W. Respiration and dissolution of iron(III)-containing clay minerals by bacteria. Environ. Sci. Technol. 1999, 33, 3127–3133. [Google Scholar] [CrossRef]
- Boone, D.R.; Liu, Y.; Zhao, Z.J.; Balkwill, D.L.; Drake, G.R.; Stevens, T.O.; Aldrich, H.C. Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int. J. Syst. Bacteriol. 1995, 45, 441–448. [Google Scholar] [CrossRef]
- Greene, A.C.; Patel, B.K.; Sheehy, A.J. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int. J. Syst. Bacteriol. 1997, 47, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.C.; Patel, B.; Yacob, S. Geoalkalibacter subterraneus sp nov., an anaerobic Fe(III)- and Mn(IV)-reducing bacterium from a petroleum reservoir, and emended descriptions of the family Desulfuromonadaceae and the genus Geoalkalibacter. Int. J. Syst. Evol. Microbiol. 2009, 59, 781–785. [Google Scholar] [CrossRef]
- Kim, J.; Dong, H.L.; Yang, K.; Park, H.; Elliott, W.C.; Spivack, A.; Koo, T.H.; Kim, G.; Morono, Y.; Henkel, S.; et al. Naturally occurring, microbially induced smectite-to-illite reaction. Geology 2019, 47, 535–539. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, C.; Lu, G.; Yi, X.; Wang, H.; Dang, Z. Role of microbial activity in Fe(III) hydroxysulfate mineral transformations in an acid mine drainage-impacted site from the Dabaoshan Mine. Sci. Total Environ. 2018, 616, 647–657. [Google Scholar] [CrossRef]
- Song, C.P.; Elsworth, D.; Zhi, S.; Wang, C.Y. The influence of particle morphology on microbially induced CaCO3 clogging in granular media. Mar. Geores. Geotechnol. 2021, 39, 74–81. [Google Scholar] [CrossRef]
- Song, C.P.; Chen, Y.L.; Wang, J.H. Plugging high-permeability zones of oil reservoirs by microbially mediated calcium carbonate precipitation. ACS Omega 2020, 5, 14376–14383. [Google Scholar] [CrossRef]
- DeJong, J.T.; Mortensen, B.M.; Martinez, B.C.; Nelson, D.C. Bio-mediated soil improvement. Ecol. Eng. 2010, 36, 197–210. [Google Scholar] [CrossRef]
- Rebata-Landa, V.; Santamarina, J.C. Mechanical limits to microbial activity in deep sediments. Geochem. Geophys. Geosyst. 2006, 7, Q11006. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, B.; Chen, J.; Yan, G. Research on plugging characteristics of microorganism induced calcite precipitation in sandstone environment. J. Petrol. Sci. Eng. 2022, 218, 111040. [Google Scholar] [CrossRef]
- Su, F.; Yang, Y.Y. Microbially induced carbonate precipitation via methanogenesis pathway by a microbial consortium enriched from activated anaerobic sludge. J. Appl. Microbiol. 2021, 131, 236–256. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.; Grujic, D.; Tiens, A.J. Microbial mediation as a possible mechanism for natural dolomite formation at low temperatures. Nature 1995, 377, 220–222. [Google Scholar] [CrossRef]
- Sondi, I.; Juracic, M. Whiting events and the formation of aragonite in Mediterranean Karstic Marine Lakes: New evidence on its biologically induced inorganic origin. Sedimentology 2010, 57, 85–95. [Google Scholar] [CrossRef]
- Nonoyama, T.; Kinoshita, T.; Higuchi, M.; Nagata, K.; Tanaka, M.; Sato, K.; Kato, K. Multistep growth mechanism of calcium phosphate in the earliest stage of morphology-controlled biomineralization. Langmuir 2011, 27, 7077–7083. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.X.; Li, S.Y.; Li, B.F.; Chen, D.Q.; Liu, Z.Y.; Li, Z.M. A review of development methods and EOR technologies for carbonate reservoirs. Petrol. Sci. 2020, 17, 990–1013. [Google Scholar] [CrossRef]
- Capelli, I.A.; Scasso, R.A.; Spangenberg, J.E.; Kietzmann, D.A.; Cravero, F.; Duperron, M.; Adatte, T. Mineralogy and geochemistry of deeply-buried marine sediments of the Vaca Muerta-Quintuco system in the Neuquen Basin (Chacay Melehue section), Argentina: Paleoclimatic and paleoenvironmental implications for the global Tithonian-Valanginian reconstructions. J. S. Am. Earth Sci. 2021, 107, 103103. [Google Scholar]
- Worden, R.H.; Griffiths, J.; Wooldridge, L.J.; Utley, J.; Lawan, A.Y.; Muhammed, D.D.; Simon, N.; Armitage, P.J. Chlorite in sandstones. Earth-Sci. Rev. 2020, 204, 103105. [Google Scholar] [CrossRef]
- Lai, J.; Wang, G.; Ran, Y.; Zhou, Z.; Cui, Y. Impact of diagenesis on the reservoir quality of tight oil sandstones: The case of Upper Triassic Yanchang Formation Chang 7 oil layers in Ordos Basin, China. J. Petrol. Sci. Eng. 2016, 145, 54–65. [Google Scholar] [CrossRef]
- Osman, A.G. Study of some characteristics of silicate bacteria. J. Sci. Technol. 2009, 10, 24–31. [Google Scholar]
- Chiang, Y.W.; Santos, R.M.; Van Audenaerde, A.; Monballiu, A.; Van Gerven, T.; Meesschaert, B. Chemoorganotrophic bioleaching of olivine for nickel recovery. Minerals 2014, 4, 553–564. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Ye, H.; Du, D.; Sun, P.; Ma, M.; Zhang, T.C. Bioleaching of silicon in electrolytic manganese residue (EMR) by Paenibacillus mucilaginosus: Impact of silicate mineral structures. Chemosphere 2020, 256, 127043. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Ye, H.; Du, D.; Gan, C.; Wuri, L.; Sun, P.; Wen, J. Bioleaching of silicon in electrolytic manganese residue using single and mixed silicate bacteria. Bioprocess. Biosyst. Eng. 2019, 42, 1819–1828. [Google Scholar] [CrossRef]
- Shukla, K.; Negi, R.; Kaur, T.; Devi, R.; Kour, D.; Yadav, A.N. First report on rhizospheric silicate mineral weathering bacteria from Indian Himalayas and their roles for plant growth promotion of tomato (Solanum lycopersium L.). Natl. Acad. Sci. Lett.-India. 2023, 46, 435–438. [Google Scholar] [CrossRef]
- Heenan, J.; Porter, A.; Ntarlagiannis, D.; Young, L.Y.; Werkema, D.D.; Slater, L.D. Sensitivity of the spectral induced polarization method to microbial enhanced oil recovery processes. Geophysics 2013, 78, E261–E269. [Google Scholar] [CrossRef]
- Kapse, N.; Dagar, S.S.; Dhakephalkar, P.K. Appropriate characterization of reservoir properties and investigation of their effect on microbial enhanced oil recovery through simulated laboratory studies. Sci. Rep. 2024, 14, 15401. [Google Scholar] [CrossRef]
- Sokolova, D.S.; Babich, T.L.; Semenova, E.M.; Khisametdinov, M.R.; Mardanov, A.V.; Minikh, A.A.; Nazina, T.N. Microbiological characteristics of sapropel and the possibility of its application for enhanced oil recovery from carbonate reservoirs. Microbiology 2024, 93, S15–S20. [Google Scholar] [CrossRef]
- Alkan, H.; Mukherjee, S.; Kögler, F. Reservoir engineering of in-situ MEOR; impact of microbial community. J. Petrol. Sci. Eng. 2020, 195, 107928. [Google Scholar] [CrossRef]
- McMaster, T.J. Atomic Force Microscopy of the fungi-mineral interface: Applications in mineral dissolution, weathering and biogeochemistry. Curr. Opin. Biotechnol. 2012, 23, 562–569. [Google Scholar] [CrossRef]
- Valix, M.; Usai, F.; Malik, R. Fungal bio-leaching of low grade laterite ores. Miner. Eng. 2001, 14, 197–203. [Google Scholar] [CrossRef]
- Brown, D.J.; Helmke, P.A.; Clayton, M.K. Robust geochemical indices for redox and weathering on a granitic laterite landscape in central Uganda. Geochim. Cosmochim. Acta 2003, 67, 2711–2723. [Google Scholar] [CrossRef]
- Shelobolina, E.; Xu, H.F.; Konishi, H.; Kukkadapu, R.; Wu, T.; Blöthe, M.; Roden, E. Microbial lithotrophic oxidation of structural Fe(II) in biotite. Appl. Environ. Microbiol. 2012, 78, 5746–5752. [Google Scholar] [CrossRef]
- Koretsky, C. The significance of surface complexation reactions in hydrologic systems: A geochemist’s perspective. J. Hydrol. 2000, 230, 127–171. [Google Scholar] [CrossRef]
- Lian, B.; Chen, Y.; Zhao, J.; Teng, H.H.; Zhu, L.; Yuan, S. Microbial flocculation by Bacillus mucilaginosus: Applications and mechanisms. Bioresour. Technol. 2008, 99, 4825–4831. [Google Scholar] [CrossRef]
- Bonneville, S.; Morgan, D.J.; Schmalenberger, A.; Bray, A.; Brown, A.; Banwart, S.A.; Benning, L.G. Tree-mycorrhiza symbiosis accelerate mineral weathering: Evidences from nanometer-scale elemental fluxes at the hypha-mineral interface. Geochim. Cosmochim. Acta 2011, 75, 6988–7005. [Google Scholar] [CrossRef]
- Adamo, P.; Violante, P. Weathering of rocks and neogenesis of minerals associated with lichen activity. Appl. Clay Sci. 2000, 16, 229–256. [Google Scholar] [CrossRef]
- Lian, B.; Wang, B.; Pan, M.; Liu, C.; Teng, H.H. Microbial release of potassium from K-bearing minerals by thermophilic fungus Aspergillus fumigatus. Geochim. Cosmochim. Acta 2008, 72, 87–98. [Google Scholar] [CrossRef]
- Liu, W.X.; Xu, X.S.; Wu, X.H.; Yang, Q.Y.; Luo, Y.M.; Christie, P. Decomposition of silicate minerals by Bacillus mucilaginosus in liquid culture. Environ. Geochem. Health 2006, 28, 133–140. [Google Scholar] [CrossRef]
- Newman, D.K. Microbiology-How bacteria respire minerals. Science 2001, 292, 1312–1313. [Google Scholar] [CrossRef]
- Uroz, S.; Calvaruso, C.; Turpault, M.P.; Frey-Klett, P. Mineral weathering by bacteria: Ecology, actors and mechanisms. Trends Microbiol. 2009, 17, 378–387. [Google Scholar] [CrossRef]
- Newman, D.K.; Kolter, R. A role for excreted quinones in extracellular electron transfer. Nature 2000, 405, 94–97. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Kappler, A.; Newman, D.K. Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 2004, 70, 921–928. [Google Scholar] [CrossRef]
- Amott, E. Observations relating to the wettability of porous rock. Trans. AIME 1959, 216, 156–162. [Google Scholar] [CrossRef]
- Esfandyari, H.; Hoseini, A.H.; Shadizadeh, S.R.; Davarpanah, A. Simultaneous evaluation of capillary pressure and wettability alteration based on the USBM and imbibition tests on carbonate minerals. J. Petrol. Sci. Eng. 2021, 200, 108285. [Google Scholar] [CrossRef]
- Davarpanah, A.; Mirshekari, B. Numerical simulation and laboratory evaluation of alkali-surfactant-polymer and foam flooding. Int. J. Environ. Sci. Technol. 2020, 17, 1123–1136. [Google Scholar] [CrossRef]
- Jadhunandan, P.P.; Morrow, N.R. Effect of wettability on waterflood recovery for crude-oil/brine/rock systems. SPE Reserv. Eng. 1995, 10, 40–46. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, Z.; Zhang, X.; Davarpanah, A. Impact of anionic and cationic surfactants interfacial tension on the oil recovery enhancement. Powder Technol. 2020, 373, 93–98. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Selvarajan, R.; Chikere, C.B. Microbial surfactants: The next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms 2019, 7, 581. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, R.T.; Johnson, A.C.; Edyvean, R. Biotechnology in the petroleum industry: An overview. Int. Biodeterior. Biodegrad. 2014, 86, 225–237. [Google Scholar] [CrossRef]
- Nikolova, C.; Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: Current state of knowledge and future perspectives. Front. Bioeng. Biotechnol. 2021, 9, 626639. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Mahmoodi, M.; Niazi, A.; Al-Wahaibi, Y.; Ayatollahi, S. Investigating wettability alteration during MEOR process, a micro/macro scale analysis. Colloid Surf. B-Biointerfaces 2012, 95, 129–136. [Google Scholar] [CrossRef]
- Seewald, J.S. Aqueous geochemistry of low molecular weight hydrocarbons at elevated temperatures and pressures: Constraints from mineral buffered laboratory experiments. Geochim. Cosmochim. Acta 2001, 65, 1641–1664. [Google Scholar] [CrossRef]
- van Berk, W.; Schulz, H.M.; Fu, Y.J. Controls on CO2 fate and behavior in the Gullfaks oil field (Norway): How hydrogeochemical modeling can help decipher organic-inorganic interactions. AAPG Bull 2013, 97, 2233–2255. [Google Scholar] [CrossRef]
- Chen, X.; Han, T.; Miao, X.; Zhang, X.; Zhao, L.; Sun, Y.; Ye, H.; Li, X.; Li, Y. Ferrihydrite enhanced the electrogenic hydrocarbon degradation in soil microbial electrochemical remediation. Chem. Eng. J. 2022, 446, 136901. [Google Scholar] [CrossRef]
- Li, L.; Wan, Y.Y.; Mu, H.M.; Shi, S.B.; Chen, J.F. Interaction between illite and a Pseudomonas stutzeri-heavy oil biodegradation complex. Microorganisms 2023, 11, 330. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Faustorilla, M.V.; Naidu, R. Effect of surface-tailored biocompatible organoclay on the bioavailability and mineralization of polycyclic aromatic hydrocarbons in long-term contaminated soil. Environ. Technol. Innov. 2018, 10, 152–161. [Google Scholar] [CrossRef]
- Warr, L.N.; Schlüter, M.; Schauer, F.; Olson, G.M.; Basirico, L.M.; Portier, R.J. Nontronite-enhanced biodegradation of Deepwater Horizon crude oil by Alcanivorax borkumensis. Appl. Clay Sci. 2018, 158, 11–20. [Google Scholar] [CrossRef]
- Weise, A.M.; Lee, K. The effect of clay-oil flocculation on natural oil degradation. Int. Oil Spill Conf. Proc. 1997, 1997, 955–956. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Tazaki, K. How kaolinite plays an essential role in remediating oil-polluted seawater. Clay Miner. 2005, 40, 481–491. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Tazaki, K.; Asada, R.; Kogure, K. Interaction between clay minerals and hydrocarbon-utilizing indigenous microorganisms in high concentrations of heavy oil: Implications for bioremediation. Clay Miner. 2005, 40, 105–114. [Google Scholar] [CrossRef]
- Warr, L.N.; Perdrial, J.N.; Lett, M.; Heinrich-Salmeron, A.; Khodja, M. Clay mineral-enhanced bioremediation of marine oil pollution. Appl. Clay Sci. 2009, 46, 337–345. [Google Scholar] [CrossRef]
- Froehner, S.; Luz, E.; Maceno, M. Enhanced biodegradation of naphthalene and anthracene by modified vermiculite mixed with soil. Water Air Soil Pollut. 2009, 202, 169–177. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Head, I.M.; Manning, D.A.C. Effect of modified montmorillonites on the biodegradation and adsorption of biomarkers such as hopanes, steranes and diasteranes. Environ. Sci. Pollut. Res. 2013, 20, 8881–8889. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.C.; Fialips, C.I. Biodegradation of crude oil saturated fraction supported on clays. Biodegradation 2014, 25, 153–165. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Manning, D.A.C.; Fialips, C.I. Microbial degradation of crude oil hydrocarbons on organoclay minerals. J. Environ. Manag. 2014, 144, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.C.; Fialips, C.I. Effect of acid activated clay minerals on biodegradation of crude oil hydrocarbons. Int. Biodeterior. Biodegrad. 2014, 88, 185–191. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Jones, M.D.; Head, I.M.; Manning, D.A.C.; Fialips, C.I. Biodegradation and adsorption of crude oil hydrocarbons supported on homoionic montmorillonite clay minerals. Appl. Clay Sci. 2014, 87, 81–86. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Head, I.M.; Manning, D.A.C. Biodegradation and adsorption of C1- and C2-phenanthrenes and C1- and C2-dibenzothiophenes in the presence of clay minerals: Effect on forensic diagnostic ratios. Biodegradation 2014, 25, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Warr, L.N.; Friese, A.; Schwarz, F.; Schauer, F.; Portier, R.J.; Basirico, L.M.; Olson, G.M. Experimental study of clay-hydrocarbon interactions relevant to the biodegradation of the Deepwater Horizon oil from the Gulf of Mexico. Chemosphere 2016, 162, 208–221. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Naidu, R. Bacterial mineralization of phenanthrene on thermally activated palygorskite: A 14C radiotracer study. Sci. Total Environ. 2017, 579, 709–717. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Mild acid and alkali treated clay minerals enhance bioremediation of polycyclic aromatic hydrocarbons in long-term contaminated soil: A 14C-tracer study. Environ. Pollut. 2017, 223, 255–265. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Fialips, C.I. Removal of crude oil polycyclic aromatic hydrocarbons via organoclay-microbe-oil interactions. Chemosphere 2017, 174, 28–38. [Google Scholar] [CrossRef]
- Ugochukwu, U.C.; Fialips, C.I. Crude oil polycyclic aromatic hydrocarbons removal via clay-microbe-oil interactions: Effect of acid activated clay minerals. Chemosphere 2017, 178, 65–72. [Google Scholar] [CrossRef]
- Gong, B.; Wu, P.; Ruan, B.; Zhang, Y.; Lai, X.; Yu, L.; Li, Y.; Dang, Z. Differential regulation of phenanthrene biodegradation process by kaolinite and quartz and the underlying mechanism. J. Hazard. Mater. 2018, 349, 51–59. [Google Scholar] [CrossRef]
- Biswas, B.; Sarkar, B.; Mandal, A.; Naidu, R. Heavy metal-immobilizing organoclay facilitates polycyclic aromatic hydrocarbon biodegradation in mixed-contaminated soil. J. Hazard. Mater. 2015, 298, 129–137. [Google Scholar] [CrossRef]
- Tolpeshta, I.I.; Erkenova, M.I. Effect of palygorskite clay, fertilizers, and lime on the degradation of oil products in oligotrophic peat soil under laboratory experimental conditions. Eurasian Soil Sci. 2018, 51, 229–240. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Xu, J.; Liang, B. Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J. Hazard. Mater. 2009, 172, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.Q.; Xiong, S.Y.; Zhang, W.M.; Wang, G.X. A comparison of bioleaching of chalcopyrite using pure culture or a mixed culture. Miner. Eng. 2005, 18, 987–990. [Google Scholar] [CrossRef]
- Kalinowski, B.E.; Liermann, L.J.; Brantley, S.L.; Barnes, A.; Pantano, C.G. X-ray photoelectron evidence for bacteria-enhanced dissolution of hornblende. Geochim. Cosmochim. Acta 2000, 64, 1331–1343. [Google Scholar] [CrossRef]
- Sanket, A.S.; Ghosh, S.; Sahoo, R.; Nayak, S.; Das, A.P. Molecular identification of acidophilic manganese (Mn)-solubilizing bacteria from mining effluents and their application in mineral beneficiation. Geomicrobiol. J. 2017, 34, 71–80. [Google Scholar] [CrossRef]
- Byloos, B.; Coninx, I.; Van Hoey, O.; Cockell, C.; Nicholson, N.; Ilyin, V.; Van Houdt, R.; Boon, N.; Leys, N. The impact of space flight on survival and interaction of Cupriavidus metallidurans CH34 with basalt, a volcanic moon analog rock. Front. Microbiol. 2017, 8, 671. [Google Scholar] [CrossRef]
- Wyatt, M.A.; Johnston, C.W.; Magarvey, N.A. Gold nanoparticle formation via microbial metallophore chemistries. J. Nanopart. Res. 2014, 16, 2212. [Google Scholar] [CrossRef]
- Edwards, K.J.; Hu, B.; Hamers, R.J.; Banfield, J.F. A new look at microbial leaching patterns on sulfide minerals. FEMS Microbiol. Ecol. 2001, 34, 197–206. [Google Scholar] [CrossRef]
- Chan, C.S.; Fakra, S.C.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: Implications for biosignature formation. ISME J. 2011, 5, 717–727. [Google Scholar] [CrossRef]
- Dou, X.; Su, H.; Xu, D.; Liu, C.; Meng, H.; Li, H.; Zhang, J.; Dang, Y.; Feng, L.; Zhang, L.; et al. Enhancement effects of dissolved organic matter leached from sewage sludge on microbial reduction and immobilization of Cr(VI) by Geobacter sulfurreducens. Sci. Total Environ. 2022, 835, 155301. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Qin, W.Q.; Wang, J.; Zhen, S.J.; Yang, C.R.; Zhang, J.W.; Nai, S.S.; Qiu, G.Z. Bioleaching of chalcopyrite by pure and mixed culture. Trans. Nonferrous Met. Soc. China 2008, 18, 1491–1496. [Google Scholar] [CrossRef]
- Ali, D.; Koner, S.; Hussain, A.; Hsu, B.M. Molecular mechanisms and biomineralization processes of ferromanganese nodule formation: Insights its effect on nutrient imbalance and heavy metal immobilization in native soil profiles. Earth-Sci. Rev. 2025, 261, 105029. [Google Scholar] [CrossRef]
- Bennett, S.A.; Toner, B.M.; Barco, R.; Edwards, K.J. Carbon adsorption onto Fe oxyhydroxide stalks produced by a lithotrophic iron-oxidizing bacteria. Geobiology 2014, 12, 146–156. [Google Scholar] [CrossRef]
- Orange, F.; Disnar, J.R.; Westall, F.; Prieur, D.; Baillif, P. Metal cation binding by the hyperthermophilic microorganism, Archaea Methanocaldococcus jannaschii, and its effects on silicification. Palaeontology 2011, 54, 953–964. [Google Scholar] [CrossRef]
- Lebedeva, E.V.; Lialikova, N.N.; Bugel’skii, I.I. Participation of nitrifying bacteria in weathering of serpentinized ultrabasites. Mikrobiologiya 1978, 47, 1101–1107. [Google Scholar]
- Edberg, F.; Kalinowski, B.E.; Holmström, S.; Holm, K. Mobilization of metals from uranium mine waste: The role of pyoverdines produced by Pseudomonas fluorescens. Geobiology 2010, 8, 278–292. [Google Scholar] [CrossRef]
- Saleh, D.K.; Abdollahi, H.; Noaparast, M.; Nosratabad, A.F.; Tuovinen, O.H. Dissolution of Al from metakaolin with carboxylic acids produced by Aspergillus niger, Penicillium bilaji, Pseudomonas putida, and Pseudomonas koreensis. Hydrometallurgy 2019, 186, 235–243. [Google Scholar] [CrossRef]
- Etemadifar, Z.; Etemadzadeh, S.S.; Emtiazi, G. A novel approach for bioleaching of sulfur, iron, and silica impurities from coal by growing and resting cells of Rhodococcus spp. Geomicrobiol. J. 2019, 36, 123–129. [Google Scholar] [CrossRef]
- Wu, W.F.; Wang, F.P.; Li, J.H.; Yang, X.W.; Xiao, X.; Pan, Y.X. Iron reduction and mineralization of deep-sea iron reducing bacterium Shewanella piezotolerans WP3 at elevated hydrostatic pressures. Geobiology 2013, 11, 593–601. [Google Scholar] [CrossRef]
- Pei, D.; Liu, Z.M.; Hu, B.R.; Wu, W.J. Progress on mineralization mechanism and application research of Sporosarcina pasteurii. Prog. Biochem. Biophys. 2020, 47, 467–482. [Google Scholar]
- Liang, Y.; Zhu, S.; Wang, J.; Ai, C.; Qin, W. Adsorption and leaching of chalcopyrite by Sulfolobus metallicus YN24 cultured in the distinct energy sources. Int. J. Miner. Metall. Mater. 2015, 22, 549–552. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, G.Z.; Qin, W.Q.; Zhang, Y.S. Microbial leaching of mannatite by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Trans. Nonferrous Met. Soc. China 2006, 16, 937–942. [Google Scholar] [CrossRef]
- Kim, S.; Bae, J.; Park, H.; Cha, D. Bioleaching of cadmium and nickel from synthetic sediments by Acidithiobacillus ferrooxidans. Environ. Geochem. Health 2005, 27, 229–235. [Google Scholar] [CrossRef]
- Bredberg, K.; Karlsson, H.T.; Holst, O. Reduction of vanadium(V) with Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Bioresour. Technol. 2004, 92, 93–96. [Google Scholar] [CrossRef]
- Abhilash; Pandey, B.D. Microbial processing of apatite rich low grade Indian uranium ore in bioreactor. Bioresour. Technol. 2013, 128, 619–623. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Gu, W.; Bai, J. Ultrasound enhances the recycling process and mechanism of lithium from spent LiFePO4 batteries by Acidithiobacillus ferrooxidans. Sci. Rep. 2025, 15, 24490. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, T.M.; Yawar, W. Bacterial solubilization of phosphorus from phosphate rock containing sulfur-mud. Hydrometallurgy 2010, 103, 54–59. [Google Scholar] [CrossRef]
- Bhaskar, S.; Manu, B.; Sreenivasa, M.Y. Bioleaching of iron from fly ash using a novel isolated Acidithiobacillus ferrooxidans strain and evaluation of catalytic role of leached iron in the Fenton’s oxidation of Cephelaxin. J. Indian Chem. Soc. 2020, 97, 360–367. [Google Scholar]
- Marrero, J.; Coto, O.; Goldmann, S.; Graupner, T.; Schippers, A. Recovery of nickel and cobalt from laterite tailings by reductive dissolution under aerobic conditions using Acidithiobacillus species. Environ. Sci. Technol. 2015, 49, 6674–6682. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.P. Sequential biological process for molybdenum extraction from hydrodesulphurization spent catalyst. Chemosphere 2016, 160, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, A.J.; Vaughan, J.; Southam, G.; Van der Ent, A.; Nkrumah, P.N.; Ma, X.D.; Parbhakar-Fox, A. Review on metal extraction technologies suitable for critical metal recovery from mining and processing wastes. Miner. Eng. 2022, 182, 107537. [Google Scholar] [CrossRef]
- Wang, H.; Wu, A.; Xiong, Y.; Hu, K.; Huang, M. Effect of bioleaching on alkaline copper oxide ores with ammonia producing strain. J. Pure Appl. Microbiol. 2013, 7, 255–261. [Google Scholar]
- Kaplan, D.L.; Kaplan, A.M. 2,4,6-Trinitrotoluene-surfactant complexes: Decomposition, mutagenicity and soil leaching studies. Environ. Sci. Technol. 1982, 16, 566–571. [Google Scholar] [CrossRef]
- Lan, Z.; Hu, Y.; Qin, W. Effect of surfactant OPD on the bioleaching of marmatite. Miner. Eng. 2009, 22, 10–13. [Google Scholar] [CrossRef]
- Abdollahi, H.; Noaparast, M.; Shafaei, S.Z.; Manafi, Z.; Muñoz, J.A.; Tuovinen, O.H. Silver-catalyzed bioleaching of copper, molybdenum from a chalcopyrite-molybdenite concentrate. Int. Biodeterior. Biodegrad. 2015, 104, 194–200. [Google Scholar] [CrossRef]
- Pathak, A.; Morrison, L.; Healy, M.G. Catalytic potential of selected metal ions for bioleaching, and potential techno-economic and environmental issues: A critical review. Bioresour. Technol. 2017, 229, 211–221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).