Microbial Valorization of Agricultural and Agro-Industrial Waste into Bacterial Cellulose: Innovations for Circular Bioeconomy Integration
Abstract
1. Introduction
2. Biosynthesis and Cellulose-Producing Microorganisms
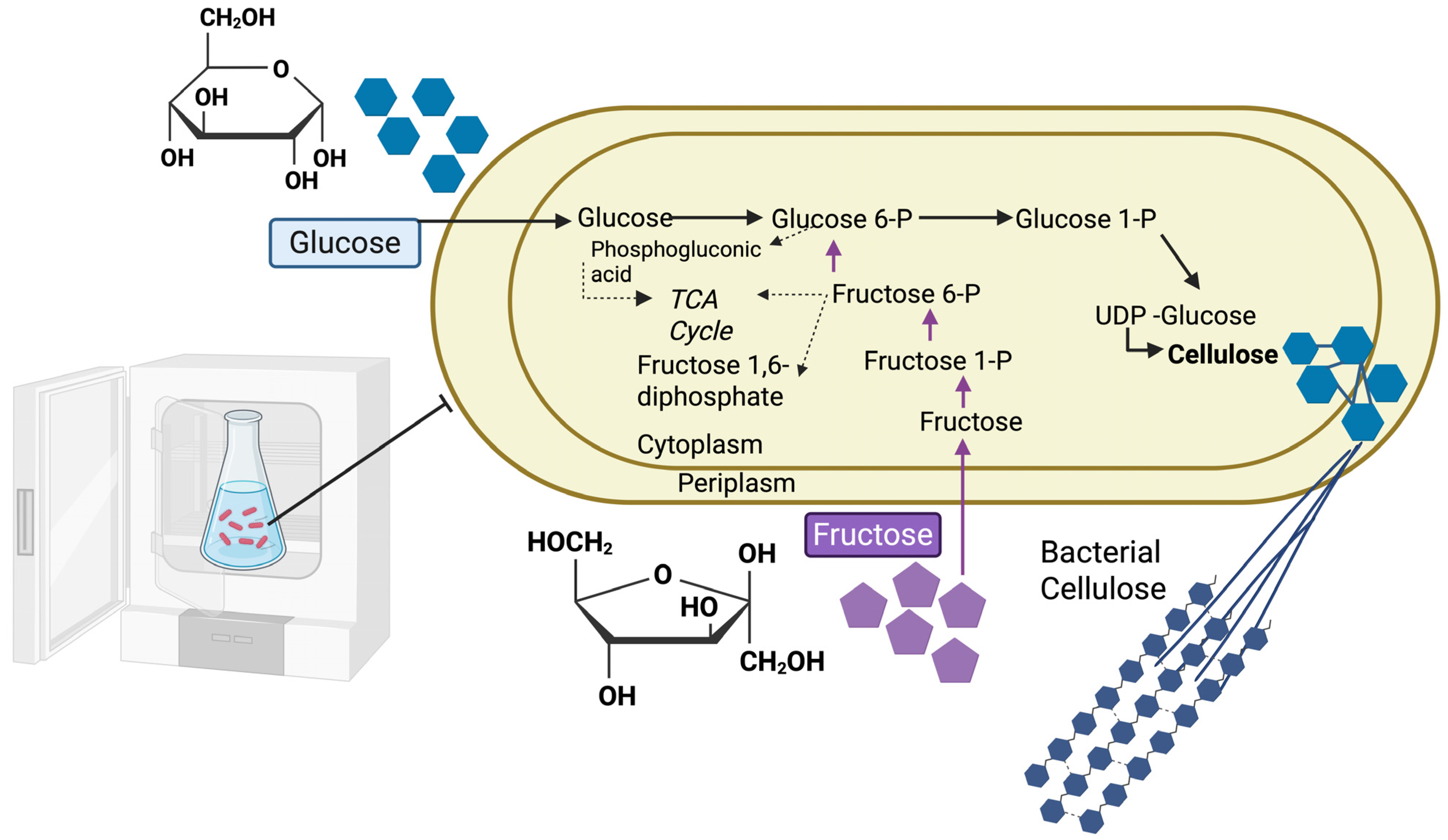
2.1. The Biosynthesis Process of BC
2.2. Microorganisms That Synthesize BC
Synthetic Biology for Functional Modification of BC
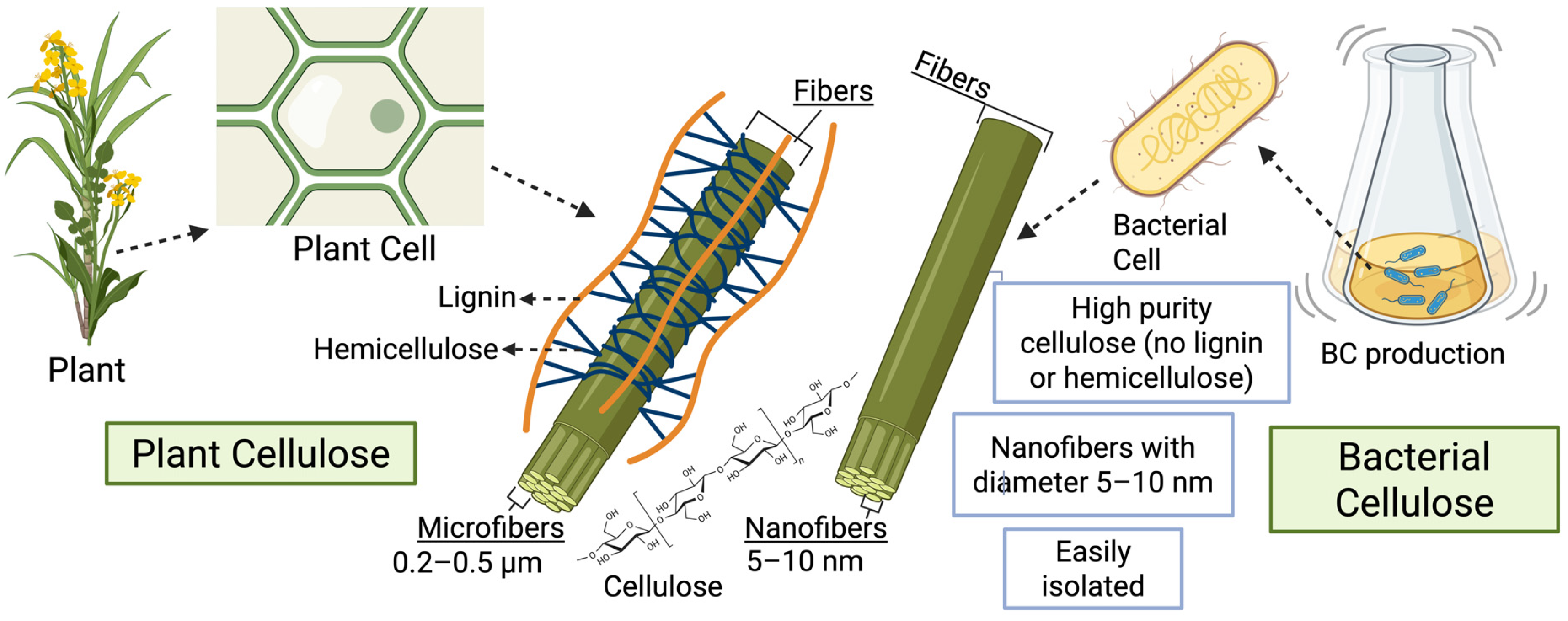
3. Structure and Properties of BC
3.1. Structural Characteristics of BC
3.2. Functional and Physical Properties of BC
3.3. Chemical Properties and Modification Potential of BC
3.4. Biomedical Properties of BC
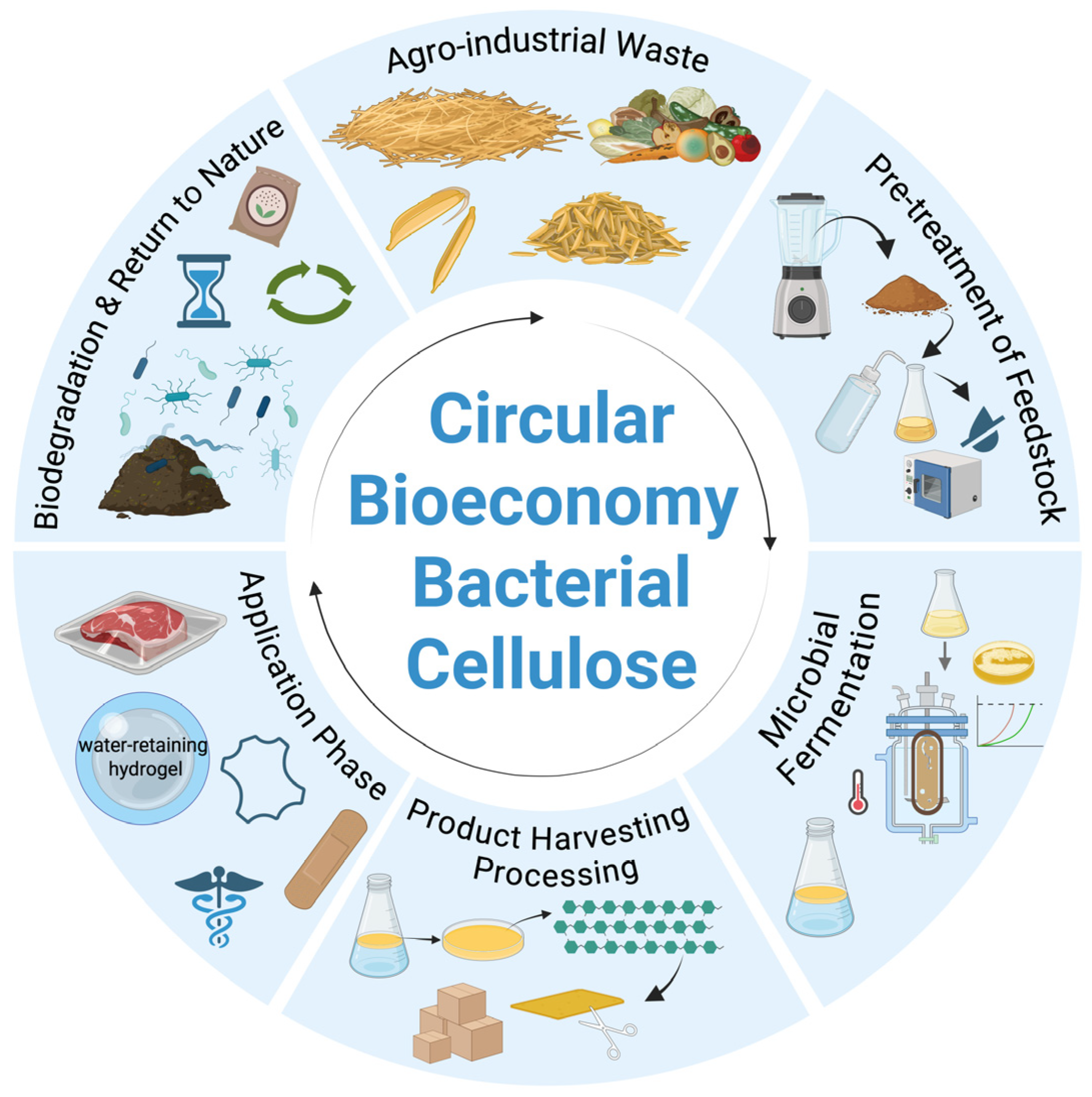
4. Valorization of Agro-Industrial Waste into Bacterial Cellulose
5. Process Optimization Strategies in BC Production
5.1. Genetic Engineering Approaches to Improve Cellulose Yield
5.2. Fermentation Condition Optimization
5.3. Omics Technologies and Advanced Strain Development
5.4. Bioreactor Engineering and Scale-Up Strategies
6. Circular Bioeconomy Applications of BC Production
7. Safety, Regulatory Compliance, and Quality Assurance in Waste-Derived BC Production
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Bacterial cellulose |
| HS | Hestrin–Schramm |
| LCA | Life Cycle Assessment |
| PET | Polyethylene terephthalate |
| PLA | Polylactic acid |
| ALE | Adaptive laboratory evolution |
| SCOBY | Symbiotic culture of bacteria and yeast |
| RDB | Rotating Disk Bioreactor |
References
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-Added Metabolites from Agricultural Waste and Application of Green Extraction Techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, D.; Tsai, M.L.; Ortizo, R.G.G.; Yadav, A.; Nargotra, P.; Chen, C.W.; Sun, P.P.; Dong, C.D. Insights into the Recent Advances of Agro-Industrial Waste Valorization for Sustainable Biogas Production. Bioresour. Technol. 2023, 390, 129829. [Google Scholar] [CrossRef] [PubMed]
- WasteManaged. Agricultural Waste Guide 2025. WasteManaged UK. 2025. Available online: https://www.wastemanaged.co.uk/our-news/agriculture/agricultural-waste-guide/ (accessed on 21 July 2025).
- Camia, A.; Robert, N.; Jonsson, K.; Pilli, R.; García-Condado, S.; López-Lozano, R.; van der Velde, M.; Ronzon, T.; Gurría Albusac, P.; M’Barek, R.; et al. Biomass Production, Supply, Uses and Flows in the European Union: First Results from an Integrated Assessment; JRC Science for Policy Report 2018; Publications Office of the European Union: Luxembourg, 2018; ISBN 9789279894873. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC109869 (accessed on 21 July 2025).
- Jiang, D.; Zhuang, D.; Fu, J.; Huang, Y.; Wen, K. Bioenergy Potential from Crop Residues in China: Availability and Distribution. Renew. Sustain. Energy Rev. 2012, 16, 1377–1382. [Google Scholar] [CrossRef]
- Sileshi, G.W.; Barrios, E.; Lehmann, J.; Tubiello, F.N. An Organic Matter Database (OMD): Consolidating Global Residue Data from Agriculture, Fisheries, Forestry and Related Industries. Earth Syst. Sci. Data 2025, 17, 369–391. [Google Scholar] [CrossRef]
- Silva, S.O.; Mafra, A.K.C.; Pelissari, F.M.; Rodrigues de Lemos, L.; Molina, G. Biotechnology in Agro-Industry: Valorization of Agricultural Wastes, By-Products and Sustainable Practices. Microorganisms 2025, 13, 1789. [Google Scholar] [CrossRef]
- Pandey, A.K.; Thakur, S.; Mehra, R.; Kaler, R.S.S.; Paul, M.; Kumar, A. Transforming Agri-Food Waste: Innovative Pathways toward a Zero-Waste Circular Economy. Food Chem. X 2025, 28, 102604. [Google Scholar] [CrossRef]
- Chen, S.C.-I.; Dang, X.; Xu, Q.-Q.; Own, C.-M. Transforming Waste into Value: Sustainable Recycling of Agricultural Resources under the ‘Carbon Peak and Carbon Neutrality’ Vision. Sustainability 2025, 17, 55. [Google Scholar] [CrossRef]
- Sundaram, T.; Govindarajan, R.K.; Vinayagam, S.; Krishnan, V.; Nagarajan, S.; Gnanasekaran, G.R.; Baek, K.-H.; Sekar, S.K.R. Advancements in Biosurfactant Production Using Agro-Industrial Waste for Industrial and Environmental Applications. Front. Microbiol. 2024, 15, 1357302. [Google Scholar] [CrossRef]
- Kazimierczuk, K.; Barrows, S.E.; Olarte, M.V.; Qafoku, N.P. Decarbonization of Agriculture: The Greenhouse Gas Impacts and Economics of Existing and Emerging Climate-Smart Practices. ACS Eng. Au 2023, 3, 426–442. [Google Scholar] [CrossRef]
- Martirani-VonAbercron, S.M.; Pacheco-Sánchez, D. Bacterial Cellulose: A Highly Versatile Nanomaterial. Microb. Biotechnol. 2023, 16, 1174–1178. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Comparative Study of Plant and Bacterial Cellulose Pellicles Regenerated from Dissolved States. Int. J. Biol. Macromol. 2019, 137, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Singh, P.K.; Pattnaik, R.; Kumar, S.; Ojha, S.K.; Srichandan, H.; Parhi, P.K.; Jyothi, R.K. Biochemistry, Synthesis, and Applications of Bacterial Cellulose: A Review. Front. Bioeng. Biotechnol. 2022, 10, 780409. [Google Scholar] [CrossRef] [PubMed]
- Thongwai, N.; Futui, W.; Ladpala, N.; Sirichai, B.; Weechan, A.; Kanklai, J.; Rungsirivanich, P. Characterization of Bacterial Cellulose Produced by Komagataeibacter maltaceti P285 Isolated from Contaminated Honey Wine. Microorganisms 2022, 10, 528. [Google Scholar] [CrossRef]
- Cielecka, I.; Ryngajłło, M.; Maniukiewicz, W.; Bielecki, S. Highly Stretchable Bacterial Cellulose Produced by Komagataeibacter hansenii SI1. Polymers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, M.K.; Singh, A. Low-Cost Bacterial Cellulose Production from Agricultural Waste for Antibacterial Applications. J. Bioact. Compat. Polym. 2024, 40, 94–116. [Google Scholar] [CrossRef]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Toplicean, I.-M.; Datcu, A.-D. An Overview on Bioeconomy in Agricultural Sector, Biomass Production, Recycling Methods, and Circular Economy Considerations. Agriculture 2024, 14, 1143. [Google Scholar] [CrossRef]
- Ufitikirezi, J.d.D.M.; Filip, M.; Ghorbani, M.; Zoubek, T.; Olšan, P.; Bumbálek, R.; Strob, M.; Bartoš, P.; Umurungi, S.N.; Murindangabo, Y.T.; et al. Agricultural Waste Valorization: Exploring Environmentally Friendly Approaches to Bioenergy Conversion. Sustainability 2024, 16, 3617. [Google Scholar] [CrossRef]
- Cano-Gómez, C.I.; Wong-Arguelles, C.; Hinojosa-López, J.I.; Muñiz-Márquez, D.B.; Wong-Paz, J.E. Novel Insights into Agro-Industrial Waste: Exploring Techno-Economic Viability as an Alternative Source of Water Recovery. Waste 2025, 3, 15. [Google Scholar] [CrossRef]
- Cruz, M.A.; Flor-Unda, O.; Avila, A.; Garcia, M.D.; Cerda-Mejía, L. Advances in Bacterial Cellulose Production: A Scoping Review. Coatings 2024, 14, 1401. [Google Scholar] [CrossRef]
- Moreno-Díaz, C.; González-Arranz, S.; Martínez-Cerezo, C. Bacterial Cellulose Production within a Circular Economy Framework: Utilizing Organic Waste. Polymers 2024, 16, 2735. [Google Scholar] [CrossRef] [PubMed]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Singh, A.; Walker, K.T.; Ledesma-Amaro, R.; Ellis, T. Engineering Bacterial Cellulose by Synthetic Biology. Int. J. Mol. Sci. 2020, 21, 9185. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Nguyen, L.M.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Synthesis Strategies, Regeneration, Cost Analysis, Challenges and Future Prospects of Bacterial Cellulose-Based Aerogels for Water Treatment: A Review. Chemosphere 2024, 362, 142654. [Google Scholar] [CrossRef]
- Perwez, M.; Al Asheh, S. Valorization of Agro-Industrial Waste through Solid-State Fermentation: Mini Review. Biotechnol. Rep. 2024, 45, e00873. [Google Scholar] [CrossRef]
- Santos, M.R.D.; Durval, I.J.B.; Medeiros, A.D.M.; Silva Júnior, C.J.G.D.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Biotechnology in Food Packaging Using Bacterial Cellulose. Foods 2024, 13, 3327. [Google Scholar] [CrossRef]
- Ibrahim, I.D.; Hamam, Y.; Sadiku, E.R.; Ndambuki, J.M.; Kupolati, W.K.; Jamiru, T.; Eze, A.A.; Snyman, J. Need for Sustainable Packaging: An Overview. Polymers 2022, 14, 4430. [Google Scholar] [CrossRef]
- Choi, S.M.; Rao, K.M.; Zo, S.M.; Shin, E.J.; Han, S.S. Bacterial Cellulose and Its Applications. Polymers 2022, 14, 1080. [Google Scholar] [CrossRef]
- Pal, P.; Singh, A.K.; Srivastava, R.K.; Rathore, S.S.; Sahoo, U.K.; Subudhi, S.; Sarangi, P.K.; Prus, P. Circular Bioeconomy in Action: Transforming Food Wastes into Renewable Food Resources. Foods 2024, 13, 3007. [Google Scholar] [CrossRef]
- Carvalho, A.C.F.; Ghosh, S.; Hoffmann, T.G.; Prudêncio, E.S.; de Souza, C.K.; Roy, S. Valuing Agro-Industrial Waste in the Development of Sustainable Food Packaging Based on the System of a Circular Bioeconomy: A Review. Clean. Waste Syst. 2025, 11, 100275. [Google Scholar] [CrossRef]
- Marchetti, A.; Palhas, M.; Villano, M.; Reis, M.A.M.; Fradinho, J. Unlocking the Potential of Food Industry By-Products: Sustainable Volatile Fatty Acids Production via Mixed Culture Acidogenic Fermentation of Reground Pasta. J. Clean. Prod. 2025, 526, 146633. [Google Scholar] [CrossRef]
- Marchetti, A.; Palhas, M.; Villano, M.; Reis, M.A.M.; Fradinho, J. Valorization of Reground Pasta By-Product through PHA Production with Phototrophic Purple Bacteria. Catalysts 2024, 14, 239. [Google Scholar] [CrossRef]
- Rezić Meštrović, I.; Somogyi Škoc, M.; Dragun, D.D.; Glagolić, P.; Meštrović, E. Sustainable Solutions for Producing Advanced Biopolymer Membranes—From Net-Zero Technology to Zero Waste. Polymers 2025, 17, 1432. [Google Scholar] [CrossRef] [PubMed]
- Barja, F. Bacterial Nanocellulose Production and Biomedical Applications. J. Biomed. Res. 2021, 35, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Knöller, A.; Widenmeyer, M.; Bill, J.; Burghard, Z. Fast-Growing Bacterial Cellulose with Outstanding Mechanical Properties via Cross-Linking by Multivalent Ions. Materials 2020, 13, 2838. [Google Scholar] [CrossRef]
- Usvalampi, A.; Li, H.; Frey, A.D. Production of Glucose 6-Phosphate from a Cellulosic Feedstock in a One-Pot Multi-Enzyme Synthesis. Front. Bioeng. Biotechnol. 2021, 9, 678038. [Google Scholar] [CrossRef]
- Saleh, A.K.; El-Gendi, H.; Soliman, N.A.; El-Zawawy, W.K.; Abdel-Fattah, Y.R. Bioprocess Development for Bacterial Cellulose Biosynthesis by Novel Lactiplantibacillus plantarum Isolate along with Characterization and Antimicrobial Assessment of Fabricated Membrane. Sci. Rep. 2022, 12, 2181. [Google Scholar] [CrossRef]
- Skiba, E.A.; Shavyrkina, N.A.; Budaeva, V.V.; Sitnikova, A.E.; Korchagina, A.A.; Bychin, N.V.; Gladysheva, E.K.; Pavlov, I.N.; Zharikov, A.N.; Lubyansky, V.G.; et al. Biosynthesis of Bacterial Cellulose by Extended Cultivation with Multiple Removal of BC Pellicles. Polymers 2021, 13, 2118. [Google Scholar] [CrossRef]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Saad, W.Z.; Navaderi, M.; Mohamad, R. Production and Status of Bacterial Cellulose in Biomedical Engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Buldum, G.; Mantalaris, A. Systematic Understanding of Recent Developments in Bacterial Cellulose Biosynthesis at Genetic, Bioprocess and Product Levels. Int. J. Mol. Sci. 2021, 22, 7192. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, K.; Bian, L.; Li, G.; Zhang, C. High-Yield Bacterial Cellulose Production from Rice Bran Using a Genetically Characterized Komagataeibacter europaeus Strain. Int. J. Biol. Macromol. 2025, 310, 143201. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Gauba, P.; Mathur, G. Exploring the Biosynthesis, Production, and Functional Properties of Bacterial Cellulose. In Innovative Advancements in Biotechnology. Advances in Science, Technology & Innovation; Rani, V., Jain, C.K., Gauba, P., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar]
- Omadjela, O.; Narahari, A.; Strumillo, J.; Mélida, H.; Mazur, O.; Bulone, V.; Zimmer, J. BcsA and BcsB Form the Catalytically Active Core of Bacterial Cellulose Synthase Sufficient for In Vitro Cellulose Synthesis. Proc. Natl. Acad. Sci. USA 2013, 110, 17856–17861. [Google Scholar] [CrossRef]
- Morgan, J.L.; McNamara, J.T.; Zimmer, J. Mechanism of Activation of Bacterial Cellulose Synthase by Cyclic di-GMP. Nat. Struct. Mol. Biol. 2014, 21, 489–496. [Google Scholar] [CrossRef]
- Acheson, J.F.; Derewenda, Z.S.; Zimmer, J. Architecture of the Cellulose Synthase Outer Membrane Channel and Its Association with the Periplasmic TPR Domain. Structure 2019, 27, 1855–1861.e3. [Google Scholar] [CrossRef]
- Abidi, W.; Decossas, M.; Torres-Sánchez, L.; Puygrenier, L.; Létoffé, S.; Ghigo, J.M.; Krasteva, P.V. Bacterial Crystalline Cellulose Secretion via a Supramolecular BcsHD Scaffold. Sci. Adv. 2022, 8, eadd1170. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y. Bacterial Cellulose Biosynthesis: Diversity of Operons, Subunits, Products, and Functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef]
- Mehta, K.; Pfeffer, S.; Brown, R.M. Characterization of an acsD Disruption Mutant Provides Additional Evidence for the Hierarchical Cell-Directed Self-Assembly of Cellulose in Gluconacetobacter xylinus. Cellulose 2015, 22, 119–137. [Google Scholar] [CrossRef]
- Kondo, T.; Nakamura, Y.; Nojima, S.; Yao, M.; Imai, T. The BcsD Subunit of Type I Bacterial Cellulose Synthase Interacts Dynamically with the BcsAB Catalytic Core Complex. FEBS Lett. 2022, 596, 3069–3086. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial Cellulose—A Masterpiece of Nature’s Arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Yue, C.; Ding, C.; Xu, M.; Hu, M.; Zhang, R. Self-Assembly Behavior of Collagen and Its Composite Materials: Preparation, Characterizations, and Biomedical Engineering and Allied Applications. Gels 2024, 10, 642. [Google Scholar] [CrossRef]
- Vasil’kov, A.; Budnikov, A.; Gromovykh, T.; Pigaleva, M.; Sadykova, V.; Arkharova, N.; Naumkin, A. Effect of Bacterial Cellulose Plasma Treatment on the Biological Activity of Ag Nanoparticles Deposited Using Magnetron Deposition. Polymers 2022, 14, 3907. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Osmen, S.; Reimhult, E. Cellulosic Biofilm Formation of Komagataeibacter in Kombucha at Oil–Water Interfaces. Biofilm 2022, 4, 100071. [Google Scholar] [CrossRef]
- Augimeri, R.V.; Varley, A.J.; Strap, J.L. Establishing a Role for Bacterial Cellulose in Environmental Interactions: Lessons Learned from Diverse Biofilm-Producing Proteobacteria. Front. Microbiol. 2015, 6, 1282. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, R.; Saba, T.; Khaliq, H.; Mitchell, J.; Do, L.; Chambi, L.; Diaz, K.; Kennedy, T.; Alkassab, K.; Huynh, T.; et al. Biofilms: Formation, Drug Resistance and Alternatives to Conventional Approaches. AIMS Microbiol. 2022, 8, 239–277. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Prudnikova, S.V.; Kiselev, E.G.; Nemtsev, I.V.; Vasiliev, A.D.; Kuzmin, A.P.; Shishatskaya, E.I. Bacterial Cellulose (BC) and BC Composites: Production and Properties. Nanomaterials 2022, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Rühs, P.A.; Storz, F.; López Gómez, Y.A.; Haug, M.; Fischer, P. 3D Bacterial Cellulose Biofilms Formed by Foam Templating. NPJ Biofilms Microbiomes 2018, 4, 21. [Google Scholar] [CrossRef]
- Gomes, R.J.; Ida, E.I.; Spinosa, W.A. Bacterial Cellulose Production by Komagataeibacter hansenii Can Be Improved by Successive Batch Culture. Braz. J. Microbiol. 2023, 54, 703–713. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Almihyawi, R.A.H.; Musazade, E.; Alhussany, N.; Zhang, S.; Chen, H. Production and Characterization of Bacterial Cellulose by Rhizobium sp. Isolated from Bean Root. Sci. Rep. 2024, 14, 10848. [Google Scholar] [CrossRef] [PubMed]
- Ryngajłło, M.; Jędrzejczak-Krzepkowska, M.; Kubiak, K.; Ludwicka, K.; Bielecki, S. Towards Control of Cellulose Biosynthesis by Komagataeibacter Using Systems-Level and Strain Engineering Strategies: Current Progress and Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 6565–6585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Siddique, F.; Wei, Y.; Haque, M.A.; Na, L.; Yang, X.; Lin, C.S.K. Efficient Production of Bacterial Cellulose Using Komagataeibacter sucrofermentans on Sustainable Feedstocks. ChemSusChem 2025, 18, e202401578. [Google Scholar] [CrossRef] [PubMed]
- Matthysse, A.G.; Thomas, D.L.; White, A.R. Mechanism of Cellulose Synthesis in Agrobacterium tumefaciens. J. Bacteriol. 1995, 177, 1076–1081. [Google Scholar] [CrossRef]
- Robledo, M.; Rivera, L.; Jiménez-Zurdo, J.I.; Rivas, R.; Dazzo, F.; Velázquez, E.; Martínez-Molina, E.; Hirsch, A.M.; Mateos, P.F. Role of Rhizobium Endoglucanase CelC2 in Cellulose Biosynthesis and Biofilm Formation on Plant Roots and Abiotic Surfaces. Microb. Cell Fact. 2012, 11, 125. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Shah, N.; Park, J.K. Recent Advancements in Bioreactions of Cellular and Cell-Free Systems: A Study of Bacterial Cellulose as a Model. Korean J. Chem. Eng. 2017, 34, 1591–1599. [Google Scholar] [CrossRef]
- Quijano, L.; Rodrigues, R.; Fischer, D.; Tovar-Castro, J.D.; Payne, A.; Navone, L.; Hu, Y.; Yan, H.; Pinmanee, P.; Poon, E.; et al. Bacterial Cellulose Cookbook: A Systematic Review on Sustainable and Cost-Effective Substrates. J. Bioresour. Bioprod. 2024, 9, 379–409. [Google Scholar] [CrossRef]
- Khiabani, A.; Sarabi-Jamab, M.; Shakeri, M.S.; Pahlevanlo, A.; Emadzadeh, B. Exploring the Acetobacteraceae Family Isolated from Kombucha SCOBYs Worldwide and Comparing Yield and Characteristics of Biocellulose under Various Fermentation Conditions. Sci. Rep. 2024, 14, 26616. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Wang, J.; Mu, J.; Ma, Q.; Lu, X. Symbiosis of Acetic Acid Bacteria and Yeast Isolated from Black Tea Fungus Mimicking the Kombucha Environment in Bacterial Cellulose Synthesis. Int. Food Res. J. 2023, 30, 1504–1518. [Google Scholar] [CrossRef]
- Mehta, A.; Serventi, L.; Kumar, L.; Torrico, D.D. The Scoop on SCOBY (Symbiotic Culture of Bacteria and Yeast): Exploring Consumer Behaviours towards a Novel Ice Cream. Foods 2023, 12, 3152. [Google Scholar] [CrossRef]
- Venturelli, G.; Villa, F.; Petraretti, M.; Guagliano, G.; Levi, M.; Petrini, P. Bacterial Cellulose for Scalable and Sustainable Bio-Gels in the Circular Economy. Gels 2025, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.X.; Mutukumira, A.N. Kombucha: Production and Microbiological Research. Foods 2022, 11, 3456. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Li, J.; Bao, Z.; Hu, M.; Nian, R.; Feng, D.; An, D.; Li, X.; Xian, M.; Zhang, H. A Natural In Situ Fabrication Method of Functional Bacterial Cellulose Using a Microorganism. Nat. Commun. 2019, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Reyes, J.M.; Rodríguez-Quiroz, R.E.; Hernández-Rodríguez, J.P.; Rodríguez-Romero, B.A.; Flores-Breceda, H.; Napoles-Armenta, J.; Romero-Soto, I.C.; Galindo-Rodríguez, S.A.; Báez-González, J.G.; Treviño-Garza, M.Z.; et al. Production and Characterization of Biocomposite Films of Bacterial Cellulose from Kombucha and Coated with Chitosan. Polymers 2022, 14, 3632. [Google Scholar] [CrossRef]
- Walker, K.T.; Li, I.S.; Keane, J.; Goosens, V.J.; Song, W.; Lee, K.-Y.; Ellis, T. Self-Pigmenting Textiles Grown from Cellulose-Producing Bacteria with Engineered Tyrosinase Expression. Nat. Biotechnol. 2025, 43, 345–354. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, Y.; Cui, Y.; Huang, Y.; Chen, Y. Enhanced Production of Bacterial Cellulose by Co-Culturing Komagataeibacter xylinus and Lactobacillus plantarum. Prep. Biochem. Biotechnol. 2025, 55, 1–9. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Torres, C.A.V.; Freitas, F.; Reis, M.A.M.; Crespo, M.T.B. Functional and Genomic Characterization of Komagataeibacter uvaceti FXV3, a Multiple Stress-Resistant Bacterium Producing Increased Levels of Cellulose. Biotechnol. Rep. 2021, 30, e00606. [Google Scholar] [CrossRef]
- Pogorelova, N.; Parshin, D.; Lipovka, A.; Besov, A.; Digel, I.; Larionov, P. Structural and Viscoelastic Properties of Bacterial Cellulose Composites: Implications for Prosthetics. Polymers 2024, 16, 3200. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The Nanofication and Functionalization of Bacterial Cellulose and Its Applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Song, B.; Zhao, S.; Shen, W.; Collings, C.; Ding, S.-Y. Direct Measurement of Plant Cellulose Microfibril and Bundles in Native Cell Walls. Front. Plant Sci. 2020, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Białkowska, A.M. Enzymatic Functionalization of Bacterial Nanocellulose: Current Approaches and Future Prospects. J. Nanobiotechnol. 2025, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Buldum, G.; Bismarck, A.; Mantalaris, A. Recombinant Biosynthesis of Bacterial Cellulose in Genetically Modified Escherichia coli. Bioprocess Biosyst. Eng. 2018, 41, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Zuppolini, S.; Salama, A.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Cellulose Amphiphilic Materials: Chemistry, Process and Applications. Pharmaceutics 2022, 14, 386. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- French, A.D. Combining Computational Chemistry and Crystallography for a Better Understanding of the Structure of Cellulose. Adv. Carbohydr. Chem. Biochem. 2021, 80, 15–93. [Google Scholar]
- Horikawa, Y. Structural Diversity of Natural Cellulose and Related Applications Using Delignified Wood. J. Wood Sci. 2022, 68, 54. [Google Scholar] [CrossRef]
- Machado, B.; Costa, S.M.; Costa, I.; Fangueiro, R.; Ferreira, D.P. The Potential of Algae as a Source of Cellulose and Its Derivatives for Biomedical Applications. Cellulose 2024, 31, 3353–3376. [Google Scholar] [CrossRef]
- Nicolas, W.J.; Ghosal, D.; Tocheva, E.I.; Meyerowitz, E.M.; Jensen, G.J. Structure of the Bacterial Cellulose Ribbon and Its Assembly-Guiding Cytoskeleton by Electron Cryotomography. J. Bacteriol. 2021, 203, e00371-20. [Google Scholar] [CrossRef]
- Thulluri, C.; Balasubramaniam, R.; Velankar, H.R. Generation of Highly Amenable Cellulose-Iβ via Selective Delignification of Rice Straw Using a Reusable Cyclic Ether-Assisted Deep Eutectic Solvent System. Sci. Rep. 2021, 11, 1591. [Google Scholar] [CrossRef]
- Al-Hasabe, A.S.H.; Abdull Razis, A.F.B.; Baharum, N.A.B.; Yu, C.Y.; Mat Isa, N. Production and Characterization of Bacterial Cellulose Synthesized by Enterobacter chuandaensis Strain AEC Using Phoenix dactylifera and Musa acuminata. Arch. Microbiol. 2024, 206, 447. [Google Scholar] [CrossRef]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An Integrated Approach to Optimizing Cellulose Mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Horue, M.; Silva, J.M.; Berti, I.R.; Brandão, L.R.; Barud, H.D.S.; Castro, G.R. Bacterial Cellulose-Based Materials as Dressings for Wound Healing. Pharmaceutics 2023, 15, 424. [Google Scholar] [CrossRef] [PubMed]
- Caro-Astorga, J.; Walker, K.T.; Herrera, N.; Lee, K.Y.; Ellis, T. Bacterial Cellulose Spheroids as Building Blocks for 3D and Patterned Living Materials and for Regeneration. Nat. Commun. 2021, 12, 5027. [Google Scholar] [CrossRef] [PubMed]
- Tofanica, B.M.; Mikhailidi, A.; Samuil, C.; Ungureanu, O.C.; Fortună, M.E.; Ungureanu, E. Advances in Cellulose-Based Hydrogels: Current Trends and Challenges. Gels 2024, 10, 842. [Google Scholar] [CrossRef]
- van Zyl, E.M.; Kennedy, M.A.; Nason, W.; Fenlon, S.J.; Young, E.M.; Smith, L.J.; Bhatia, S.R.; Coburn, J.M. Structural Properties of Optically Clear Bacterial Cellulose Produced by Komagataeibacter hansenii Using Arabitol. Biomater. Adv. 2023, 148, 213345. [Google Scholar] [CrossRef]
- Suryanto, H.; Muhajir, M.; Sutrisno, T.A.; Zakia, N.; Yanuhar, U. The Mechanical Strength and Morphology of Bacterial Cellulose Films: The Effect of NaOH Concentration. Mater. Sci. Eng. 2019, 515, 012053. [Google Scholar] [CrossRef]
- Gibson, L.J. The Hierarchical Structure and Mechanics of Plant Materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Pogorelova, N.; Rogachev, E.; Digel, I.; Chernigova, S.; Nardin, D. Bacterial Cellulose Nanocomposites: Morphology and Mechanical Properties. Materials 2020, 13, 2849. [Google Scholar] [CrossRef]
- Utoiu, E.; Manoiu, V.S.; Oprita, E.I.; Craciunescu, O. Bacterial Cellulose: A Sustainable Source for Hydrogels and 3D-Printed Scaffolds for Tissue Engineering. Gels 2024, 10, 387. [Google Scholar] [CrossRef]
- Lupașcu, R.E.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Popa, L.; Velescu, B.Ș.; Arsene, A.L. An Overview Regarding Microbial Aspects of Production and Applications of Bacterial Cellulose. Materials 2022, 15, 676. [Google Scholar] [CrossRef] [PubMed]
- Gelin, K.; Bodin, A.; Gatenholm, P.; Mihranyan, A.; Edwards, K.; Strømme, M. Characterization of Water in Bacterial Cellulose Using Dielectric Spectroscopy and Electron Microscopy. Polymer 2007, 48, 7623–7631. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial Cellulose: A Versatile Biopolymer for Wound Dressing Applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Lan, L.; Ping, J.; Xiong, J.; Ying, Y. Sustainable Natural Bio-Origin Materials for Future Flexible Devices. Adv. Sci. 2022, 9, 2200560. [Google Scholar] [CrossRef]
- He, W.; Wu, J.; Xu, J.; Mosselhy, D.A.; Zheng, Y.; Yang, S. Bacterial Cellulose: Functional Modification and Wound Healing Applications. Adv. Wound Care 2021, 10, 623–640. [Google Scholar] [CrossRef]
- Khan, R.; Shah, M.D.; Shah, L.; Lee, P.C.; Khan, I. Bacterial Polysaccharides—A Big Source for Prebiotics and Therapeutics. Front. Nutr. 2022, 9, 1031935. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Smola-Dmochowska, A.; Lewicka, K.; Macyk, A.; Rychter, P.; Pamuła, E.; Dobrzyński, P. Biodegradable Polymers and Polymer Composites with Antibacterial Properties. Int. J. Mol. Sci. 2023, 24, 7473. [Google Scholar] [CrossRef]
- Mauro, F.; Corrado, B.; De Gregorio, V.; Lagreca, E.; Di Natale, C.; Vecchione, R.; Netti, P.A. Exploring the Evolution of Bacterial Cellulose Precursors and Their Potential Use as Cellulose-Based Building Blocks. Sci. Rep. 2024, 14, 11613. [Google Scholar] [CrossRef]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.H.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Anwar, B.; Bundjali, B.; Sunarya, Y.; Arcana, I.M. Properties of Bacterial Cellulose and Its Nanocrystalline Obtained from Pineapple Peel Waste Juice. Fibers Polym. 2021, 22, 1228–1236. [Google Scholar] [CrossRef]
- Behera, B.C.; Sethi, B.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Microbial Cellulases—Diversity and Biotechnology with Reference to Mangrove Environment: A Review. J. Genet. Eng. Biotechnol. 2017, 15, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.; Li, D.; Cheng, F. The Recent Progress of the Cellulose-Based Antibacterial Hydrogel. Gels 2024, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Huang, L.H.; Zhao, X.Q.; Li, W.C.; Wang, Y.Y.; Jia, S.R.; Zhong, C. Bacterial Cellulose and Its Potential for Biomedical Applications. Biotechnol. Adv. 2021, 53, 107856. [Google Scholar] [CrossRef]
- Girard, V.D.; Chaussé, J.; Borduas, M.; Dubuc, É.; Iorio-Morin, C.; Brisebois, S.; Vermette, P. In Vitro and In Vivo Biocompatibility of Bacterial Cellulose. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35488. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; da Silva Junior, C.J.G.; de Medeiros, A.D.M.; do Nascimento, H.A.; Sarubbo, M.; de Medeiros, T.P.M.; Costa, A.F.S.; Sarubbo, L.A. Bacterial Cellulose as a Versatile Biomaterial for Wound Dressing Application. Molecules 2022, 27, 5580. [Google Scholar] [CrossRef]
- Joseph, A.; Umamaheswari, S.; Vassou, M.C. Bacterial Cellulose: A Versatile Biomaterial for Biomedical Application. Carbohydr. Res. 2025, 552, 109350. [Google Scholar] [CrossRef]
- Hou, S.; Xia, Z.; Pan, J.; Wang, N.; Gao, H.; Ren, J.; Xia, X. Bacterial Cellulose Applied in Wound Dressing Materials: Production and Functional Modification—A Review. Macromol. Biosci. 2024, 24, e2300333. [Google Scholar] [CrossRef]
- Wang, B.T.; Hu, S.; Yu, X.Y.; Jin, L.; Zhu, Y.J.; Jin, F.J. Studies of Cellulose and Starch Utilization and the Regulatory Mechanisms of Related Enzymes in Fungi. Polymers 2020, 12, 530. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.B.; Hakkarainen, M. Degradation of Cellulose Derivatives in Laboratory, Man-Made, and Natural Environments. Biomacromolecules 2022, 23, 2713–2729. [Google Scholar] [CrossRef] [PubMed]
- Mubayi, V.; Ahern, C.B.; Calusinska, M.; O’Malley, M.A. Toward a Circular Bioeconomy: Designing Microbes and Polymers for Biodegradation. ACS Synth. Biol. 2024, 13, 1978–1993, Erratum in ACS Synth Biol. 2024, 14, 304–306. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.M.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.M.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.N.; et al. Use of Industrial Wastes as Sustainable Nutrient Sources for Bacterial Cellulose (BC) Production: Mechanism, Advances, and Future Perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of Nano Bacterial Cellulose from Beverage Industrial Waste of Citrus Peel and Pomace Using Komagataeibacter xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable Bacterial Cellulose Production by Achromobacter Using Mango Peel Waste. Microb. Cell Fact. 2023, 22, 24. [Google Scholar] [CrossRef]
- Yilmaz, M.; Goksungur, Y. Optimization of Bacterial Cellulose Production from Waste Figs by Komagataeibacter xylinus. Fermentation 2024, 10, 466. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from Agricultural Residues by Two Komagataeibacter sp. Strains. Bioengineered 2022, 13, 10010–10025. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, R.; Yadav, A.; Aggarwal, N.K. Sustainable and Cost-Effective Production of Bacterial Cellulose by Using Rice Straw Hydrolysate: A Response Surface Approach. Braz. J. Microbiol. 2025, 56, 1543–1553. [Google Scholar] [CrossRef]
- Chen, L.; Hong, F.; Yang, X.X.; Han, S.F. Biotransformation of Wheat Straw to Bacterial Cellulose and Its Mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef]
- Quiñones-Cerna, C.; Rodríguez-Soto, J.C.; Barraza-Jáuregui, G.; Huanes-Carranza, J.; Cruz-Monzón, J.A.; Ugarte-López, W.; Hurtado-Butrón, F.; Samanamud-Moreno, F.; Haro-Carranza, D.; Valdivieso-Moreno, S.; et al. Bioconversion of Agroindustrial Asparagus Waste into Bacterial Cellulose by Komagataeibacter rhaeticus. Sustainability 2024, 16, 736. [Google Scholar] [CrossRef]
- Yin, N.; Santos, T.M.; Auer, G.K.; Crooks, J.A.; Oliver, P.M.; Weibel, D.B. Bacterial Cellulose as a Substrate for Microbial Cell Culture. Appl. Environ. Microbiol. 2014, 80, 1926–1932. [Google Scholar] [CrossRef] [PubMed]
- Anguluri, K.; La China, S.; Brugnoli, M.; Cassanelli, S.; Gullo, M. Better under Stress: Improving Bacterial Cellulose Production by Komagataeibacter xylinus K2G30 (UMCC 2756) Using Adaptive Laboratory Evolution. Front. Microbiol. 2022, 13, 994097. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Azin, M.; Zare, D. Enhanced Bacterial Cellulose Production by Indigenous Isolates: Insights from Mutagenesis and Evolutionary Techniques. Int. J. Biol. Macromol. 2025, 293, 139934. [Google Scholar] [CrossRef]
- Montenegro-Silva, P.; Ellis, T.; Dourado, F.; Gama, M.; Domingues, L. Enhanced Bacterial Cellulose Production in Komagataeibacter sucrofermentans: Impact of Different PQQ-Dependent Dehydrogenase Knockouts and Ethanol Supplementation. Biotechnol. Biofuels 2024, 17, 35. [Google Scholar] [CrossRef]
- Deng, S.; Wang, L.; Chen, G.; Qin, Q.; Dong, S.; Zhang, H. Complete Genome Analysis of the Cellulose Producing Strain Komagataeibacter sucrofermentans SMEG01. Sci. Rep. 2025, 15, 23102. [Google Scholar] [CrossRef]
- Cannazza, P.; Rissanen, A.J.; Sarlin, E.; Guizelini, D.; Minardi, C.; Losoi, P.; Molinari, F.; Romano, D.; Mangayil, R. Characterization, Genome Analysis and Genetic Tractability Studies of a New Nanocellulose Producing Komagataeibacter intermedius Isolate. Sci. Rep. 2022, 12, 20520. [Google Scholar] [CrossRef]
- Jacek, P.; Ryngajłło, M.; Bielecki, S. Structural Changes of Bacterial Nanocellulose Pellicles Induced by Genetic Modification of Komagataeibacter hansenii ATCC 23769. Appl. Microbiol. Biotechnol. 2019, 103, 5339–5353. [Google Scholar] [CrossRef]
- Raiszadeh-Jahromi, Y.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization of Bacterial Cellulose Production by Komagataeibacter xylinus PTCC 1734 in a Low-Cost Medium Using Optimal Combined Design. J. Food Sci. Technol. 2020, 57, 2524–2533. [Google Scholar] [CrossRef]
- Mangayil, R.; Sarlin, E.; Ellis, T.; Santala, V. Modulating Bacterial Nanocellulose Crystallinity through Post-Transcriptional Repression in Komagataeibacter xylinus. Carbohydr. Polym. Technol. Appl. 2025, 9, 100734. [Google Scholar] [CrossRef]
- Cannazza, P.; Rissanen, A.J.; Guizelini, D.; Losoi, P.; Sarlin, E.; Romano, D.; Santala, V.; Mangayil, R. Characterization of Komagataeibacter Isolate Reveals New Prospects in Waste Stream Valorization for Bacterial Cellulose Production. Microorganisms 2021, 9, 2230. [Google Scholar] [CrossRef] [PubMed]
- Fei, S.; Fu, M.; Kang, J.; Luo, J.; Wang, Y.; Jia, J.; Li, C. Enhancing Bacterial Cellulose Production of Komagataeibacter nataicola through Fermented Coconut Water by Saccharomyces cerevisiae: A Metabonomics Approach. Curr. Res. Food Sci. 2024, 8, 100761. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, Y.; Feng, Y.; Jia, S.; Wang, S.; Zhong, C. Tailoring Bacterial Cellulose through the CRISPR/Cas9-Mediated Gene Editing Tool in Komagataeibacter xylinus. ACS Synth. Biol. 2025, 14, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Liu, Q.J.; Sun, X.W.; Li, X.J.; Liu, M.; Jia, S.R.; Xie, Y.Y.; Zhong, C. Tailoring Bacterial Cellulose Structure through CRISPR Interference-Mediated Downregulation of galU in Komagataeibacter xylinus CGMCC 2955. Biotechnol. Bioeng. 2020, 117, 2165–2176. [Google Scholar] [CrossRef]
- Jang, W.D.; Kim, T.Y.; Kim, H.U.; Shim, W.Y.; Ryu, J.Y.; Park, J.H.; Lee, S.Y. Genomic and Metabolic Analysis of Komagataeibacter xylinus DSM 2325 Producing Bacterial Cellulose Nanofiber. Biotechnol. Bioeng. 2019, 116, 3372–3381. [Google Scholar] [CrossRef]
- Florea, M.; Hagemann, H.; Santosa, G.; Abbott, J.; Micklem, C.N.; Spencer-Milnes, X.; Ellis, T. Engineering Control of Bacterial Cellulose Production Using a Genetic Toolkit and a New Cellulose-Producing Strain. Proc. Natl. Acad. Sci. USA 2016, 113, E3431–E3440. [Google Scholar] [CrossRef]
- Schiros, T.N.; Antrobus, R.; Farías, D.; Chiu, Y.T.; Joseph, C.T.; Esdaille, S.; Sanchirico, G.K.; Miquelon, G.; An, D.; Russell, S.T.; et al. Microbial Nanocellulose Biotextiles for a Circular Materials Economy. Environ. Sci. Adv. 2022, 1, 276–284. [Google Scholar] [CrossRef]
- Krusong, W.; Pothimon, R.; China, S.; Thompson, A.K. Consecutive Bacterial Cellulose Production by Luffa Sponge Enmeshed with Cellulose Microfibrils of Acetobacter xylinum under Continuous Aeration. 3 Biotech 2021, 11, 6. [Google Scholar] [CrossRef]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized Culture Conditions for Bacterial Cellulose Production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Hur, D.H.; Choi, W.S.; Kim, T.Y.; Lee, S.Y.; Park, J.H.; Jeong, K.J. Enhanced Production of Bacterial Cellulose in Komagataeibacter xylinus via Tuning of Biosynthesis Genes with Synthetic RBS. J. Microbiol. Biotechnol. 2020, 30, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Akhoon, B.A.; Qiao, Q.; Stewart, A.; Chen, J.; Rodriguez Lopez, C.M.; Corbin, K.R. Pangenomic Analysis of the Bacterial Cellulose-Producing Genera Komagataeibacter and Novacetimonas. Int. J. Biol. Macromol. 2025, 298, 139980. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Liu, J.; Li, J.; Peng, Z.; Gan, X.; Zhang, Y.; Zhong, C. CRISPR-Guided Base Editor Enables Efficient and Multiplex Genome Editing in Bacterial Cellulose-Producing Komagataeibacter Species. Appl. Environ. Microbiol. 2025, 91, e02455-24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Enhanced Production of Bacterial Cellulose by Using a Biofilm Reactor and Its Material Property Analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef]
- Cáceres, R.; Oyarzún, P.; Vargas, J.P.; Cuevas, F.; Torres, K.; Elgueta, E.; Martínez, I.; Núñez, D. Sustainable Production of Bacterial Cellulose in a Rotary Disk Bioreactor: Grape Pomace as a Replacement for the Carbon Source. Fermentation 2025, 11, 441. [Google Scholar] [CrossRef]
- Almeida-Naranjo, C.E.; Aguilar, A.D.; Valle, V.; Bastidas-Caldes, C.; Debut, A.; Sinchiguano, B. A Circular Bioeconomy Approach to Using Post-Bioadsorbent Materials Intended for the Removal of Domestic Wastewater Contaminants as Potential Reinforcements. Polymers 2024, 16, 1822. [Google Scholar] [CrossRef]
- Henry, S.; Dhital, S.; Sumer, H.; Butardo, V., Jr. Solid-State Fermentation of Cereal Waste Improves the Bioavailability and Yield of Bacterial Cellulose Production by a Novacetimonas sp. Isolate. Foods 2024, 13, 3052. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Abdelmoneim, T.S.; Ahmed, A.I.; Awad, M.F.; El-Demerdash, A. Recent Advances in Bacterial Cellulose: A Low-Cost Effective Production Media, Optimization Strategies and Applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Islam, M.U.; Ullah, M.W.; Khan, S.; Shah, N.; Park, J.K. Strategies for Cost-Effective and Enhanced Production of Bacterial Cellulose. Int. J. Biol. Macromol. 2017, 102, 1166–1173. [Google Scholar] [CrossRef]
- Forte, F.; Moresi, M. Environmental Footprint of Bacterial Cellulose Produced by Gluconacetobacter xylinus Using Different Media: A Cradle-to-Gate LCA Approach. Fermentation 2024, 10, 316. [Google Scholar]
- Saravanan, C.; Kumar, P.S.; Show, P.L.; Nguyen, V.K.; Kamyab, H.; Tran, T.K. A Comprehensive Techno-Economic Review on Sustainable Production of Bacterial Cellulose from Agri-Food Residues. Bioresour. Technol. 2023, 388, 129743. [Google Scholar]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope. Plastics—The Facts 2024: An Analysis of European Plastics Production, Demand and Waste Data; PlasticsEurope: Brussels, Belgium, 2024; Available online: https://plasticseurope.org (accessed on 15 September 2025).
- Penloglou, G.; Basna, A.; Pavlou, A.; Kiparissides, C. Techno-Economic Considerations on Nanocellulose’s Future Progress: A Short Review. Processes 2023, 11, 2312. [Google Scholar] [CrossRef]
- Uma Shankar, D.D.; Mani, D.; Sudarsan, S.; Sukumara, S. Sustainable Microbial Cellulose Production Using Ocean Water. Cell Rep. Sustain. 2025, 2, 100427. [Google Scholar] [CrossRef]
- Absharina, D.; Putra, F.J.N.; Ogino, C.; Kocsubé, S.; Veres, C.; Vágvölgyi, C. Bacterial Cellulose Production in Co-Culture Systems: Opportunities, Challenges, and Future Directions. Appl. Microbiol. 2025, 5, 92. [Google Scholar] [CrossRef]
- Katyal, M.; Singh, R.; Mahajan, R.; Sharma, A.; Gupta, R.; Aggarwal, N.K.; Yadav, A. A Novel Cost-Effective Methodology for the Screening of Nanocellulose Producing Micro-Organisms. Bioproc. Biosyst. Eng. 2024, 47, 1595–1603. [Google Scholar] [CrossRef]
- Mouro, C.; Gomes, A.; Gomes, A.P.; Gouveia, I.C. Sustainable Bacterial Cellulose Production Using Low-Cost Fruit Wastewater Feedstocks. Nanomaterials 2025, 15, 271. [Google Scholar] [CrossRef]
- Bryszewska, M.A.; Pareja, D.G.; Kaczmarek, L.; Sobczyk-Guzenda, A.; Piotrowska, M.; Batory, D. SCOBY Cellulose-Based Materials Hydrophobized Using Stearic Acid and Apple Powder. Int. J. Mol. Sci. 2024, 25, 13746. [Google Scholar] [CrossRef]
- Jose, S.A.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A Comprehensive Review on Cellulose Nanofibers, Nanomaterials, and Composites: Manufacturing, Properties, and Applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef]
- Xing, Q.; Qian, Z.; Tahtinen, M.; Yap, A.H.; Yates, K.; Zhao, F. Aligned Nanofibrous Cell-Derived Extracellular Matrix for Anisotropic Vascular Graft Construction. Adv. Healthc. Mater. 2017, 6, 1601333. [Google Scholar] [CrossRef]
- Rackov, N.; Janßen, N.; Akkache, A.; Drotleff, B.; Beyer, B.; Scoppola, E.; Vrana, N.E.; Hengge, R.; Bidan, C.M.; Hathroubi, S. Bacterial Cellulose: Enhancing Productivity and Material Properties through Repeated Harvest. Biofilm 2025, 9, 100276. [Google Scholar] [CrossRef] [PubMed]
- Yanti, N.A.; Ahmad, S.W.; Ramadhan, O.A.N.; Jamili; Muzuni; Walhidayah, T.; Mamangkey, J. Properties and Application of Edible Modified Bacterial Cellulose Film Based on Sago Liquid Waste as Food Packaging. Polymers 2021, 13, 3570. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. [Google Scholar] [CrossRef] [PubMed]
- Datta, R. Enzymatic Degradation of Cellulose in Soil: A Review. Heliyon 2024, 10, e24022. [Google Scholar] [CrossRef]
- Avcioglu, N.H. Bacterial Cellulose: Recent Progress in Production and Industrial Applications. World J. Microbiol. Biotechnol. 2022, 38, 86. [Google Scholar] [CrossRef]
- Tsouko, E.; Pilafidis, S.; Kourmentza, K.; Gomes, H.I.; Sarris, G.; Koralli, P.; Papagiannopoulos, A.; Pispas, S.; Sarris, D. A Sustainable Bioprocess to Produce Bacterial Cellulose Using Waste Streams from Wine Distilleries and the Biodiesel Industry: Evaluation of BC for Adsorption of Phenolic Compounds, Dyes and Metals. Biotechnol. Biofuels 2024, 17, 40. [Google Scholar] [CrossRef]
- Marestoni, L.D.; Barud, H.S.; Gomes, R.J.; Catarino, R.P.F.; Hata, N.N.Y.; Ressutte, J.B.; Spinosa, W.A. Commercial and Potential Applications of Bacterial Cellulose in Brazil: Ten Years Review. Polímeros 2020, 30, e2020047. [Google Scholar] [CrossRef]
- ISO 10993-1; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process. International Organization for Standardization: Geneva, Switzerland, 2018.
- Popa, L.; Ghica, M.V.; Tudoroiu, E.E.; Ionescu, D.G. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Thomas, D.; Madhavan, A.; Sindhu, R.; Binod, P.; Varjani, S.; Awasthi, M.K.; Pandey, A. Bacterial Nanocellulose: Engineering, Production, and Applications. Bioengineered 2021, 12, 11463–11483. [Google Scholar] [CrossRef]
- Shahaban, O.P.S.; Khasherao, B.Y.; Shams, R.; Dar, A.H.; Dash, K.K. Recent Advancements in Development and Application of Microbial Cellulose in Food and Non-Food Systems. Food Sci. Biotechnol. 2024, 33, 1529–1540. [Google Scholar] [CrossRef]
- Ujjwal, R.R.; Slaughter, G. Advances in Bacterial Cellulose-Based Scaffolds for Tissue Engineering: Review. J. Biomed. Mater. Res. A 2025, 113, e37912. [Google Scholar] [CrossRef]
- Taokaew, S. Bacterial Nanocellulose Produced by Cost-Effective and Sustainable Methods and Its Applications: A Review. Fermentation 2024, 10, 316. [Google Scholar] [CrossRef]
- Soares da Silva, F.; Branco, S.; Dourado, F.O.Q.; Neto, B. Life Cycle Assessment of Bacterial Cellulose and Comparison to Other Cellulosic Sources. J. Clean. Prod. 2025, 493, 144876. [Google Scholar] [CrossRef]
- Catarino, R.P.F.; Mascareli, V.A.B.; Leite da Costa, V.L.; Pavanello, A.C.L.; Spinosa, W.A. Sustainability and influencing factors in bacterial cellulose production: A review of the impact of microorganisms, culture media and cultivation methods. Food Technol. Biotechnol. 2025, 63, 332–350. [Google Scholar] [CrossRef]
- Yeo, Y.T.; Lim, C.M.; Huaco, A.I.V.; Chen, W.N. Food circular economy and safety considerations in waste management of urban manufacturing side streams. NPJ Sci. Food 2024, 8, 65. [Google Scholar] [CrossRef]
| Waste Substrate | Pretreatment | Microbial Strain | BC Yield | Fermentation Time | Productivity (g/L·h) | Pretreatment Complexity | Application | Ref. |
|---|---|---|---|---|---|---|---|---|
| Mango peel waste | Nitric acid hydrolysis | Achromobacter sp. S3 | 1.22 g/L (optimized) | ~7 d (168 h) | 0.0073 | Medium | Low-cost BC films | [130] |
| Orange peel & pomace | Enzymatic hydrolysis (yeast extract | K. xylinus CICC 10529 | 5.7 ± 0.7 g/L | ~12 d (288 h) | 0.0198 | Medium | Food packaging, hydrogels | [129] |
| Waste figs (discard) | Blended (natural sugars) | K. xylinus (RSM optimized) | 8.45 g/L | ~14 d (336 h) | 0.0251 | Low | High-strength BC membranes | [131] |
| Sugarcane bagasse | Enzymatic hydrolysate | Komagataeibacter sp. | 1.2 g/L (agitated) | ~10 d (240 h) | 0.0050 | Medium | Biorefinery integration | [132] |
| Corncob (corn stover) | Enzymatic hydrolysate | Komagataeibacter sp. | 1.6 g/L (agitated) | ~10 d (240 h) | 0.0067 | Medium | Biofilms, composites | [132] |
| Rice straw | 2% NaOH + enzymatic hydrolysis | K. xylinus (optimized) | 7.17 ± 0.05 g/L | ~12 d (288 h) | 0.0249 | High | Biomedical-grade BC | [133] |
| Rice bran (scale-up) | Autoclave (nutrient-rich) | K. europaeus (15 L) | 20.7 g/L (15 d) | 15 d (360 h) | 0.0575 | Low | Industrial BC production | [79] |
| Asparagus peel | Acid/enzyme hydrolysate | K. rhaeticus QK23 | 2.57 g/L (25 d) | 25 d (600 h) | 0.0043 | High | Composite applications | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkozhayev, A.M.; Abaildayev, A.; Kossalbayev, B.D.; Tastambek, K.T.; Kadirshe, D.K.; Toleutay, G. Microbial Valorization of Agricultural and Agro-Industrial Waste into Bacterial Cellulose: Innovations for Circular Bioeconomy Integration. Microorganisms 2025, 13, 2686. https://doi.org/10.3390/microorganisms13122686
Belkozhayev AM, Abaildayev A, Kossalbayev BD, Tastambek KT, Kadirshe DK, Toleutay G. Microbial Valorization of Agricultural and Agro-Industrial Waste into Bacterial Cellulose: Innovations for Circular Bioeconomy Integration. Microorganisms. 2025; 13(12):2686. https://doi.org/10.3390/microorganisms13122686
Chicago/Turabian StyleBelkozhayev, Ayaz M., Arman Abaildayev, Bekzhan D. Kossalbayev, Kuanysh T. Tastambek, Danara K. Kadirshe, and Gaukhar Toleutay. 2025. "Microbial Valorization of Agricultural and Agro-Industrial Waste into Bacterial Cellulose: Innovations for Circular Bioeconomy Integration" Microorganisms 13, no. 12: 2686. https://doi.org/10.3390/microorganisms13122686
APA StyleBelkozhayev, A. M., Abaildayev, A., Kossalbayev, B. D., Tastambek, K. T., Kadirshe, D. K., & Toleutay, G. (2025). Microbial Valorization of Agricultural and Agro-Industrial Waste into Bacterial Cellulose: Innovations for Circular Bioeconomy Integration. Microorganisms, 13(12), 2686. https://doi.org/10.3390/microorganisms13122686








