Biotic and Abiotic Factors on Rhizosphere Microorganisms in Grassland Ecosystems
Abstract
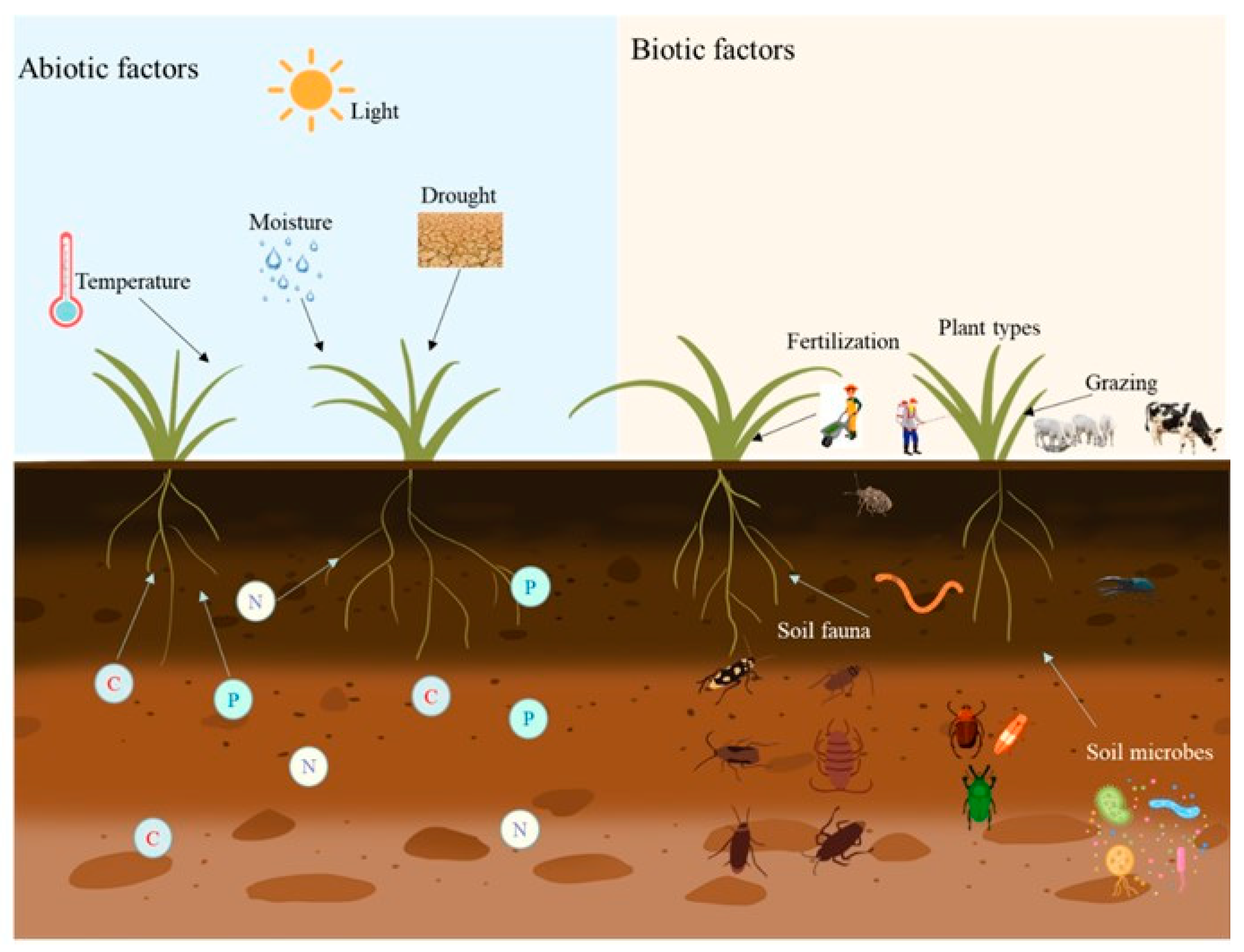
1. Introduction
2. Influence of Abiotic Factors on Rhizosphere Microorganisms
2.1. Effect of Temperature on Rhizosphere Microorganisms
2.2. The Influence of Moisture on Rhizosphere Microbes
2.3. The Influence of Soil Nutrient on Rhizosphere Microbes
2.3.1. The Influence of Soil Carbon on Rhizosphere Microbes
2.3.2. The Influence of Soil Nitrogen on Rhizosphere Microbes
2.3.3. The Influence of Soil Phosphorus on Rhizosphere Microbes
2.3.4. The Influence of Fertilization on Rhizosphere Microbes in Grassland Ecosystems
3. The Influence of Biological Factors on Rhizosphere Microbes
3.1. The Influence of Plant Types on Rhizosphere Microorganisms
3.2. The Influence of Plant Diversity and Community Structure on Rhizosphere Micrbes
3.3. The Influence of Soil Fauna on Rhizosphere Microbes
3.4. The Influence of Grazing on Rhizosphere Microbes
4. Prospects
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartmann, A.; Rothballer, M.; Schmid, M.; Hiltner, L. A pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 2008, 312, 7–14. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef]
- Lange, M.; Azizi-Rad, M.; Dittmann, G.; Lange, D.F.; Orme, A.M.; Schroeter, S.A.; Simon, C.; Gleixner, G. Stability and carbon uptake of the soil microbial community is determined by differences between rhizosphere and bulk soil. Soil Biol. Biochem. 2024, 189, 109280. [Google Scholar] [CrossRef]
- Chen, H.; Jing, Q.; Liu, X.; Zhou, X.; Fang, C.; Li, B.; Zhou, S.; Nie, M. Microbial respiratory thermal adaptation is regulated by r-/K-strategy dominance. Ecol. Lett. 2022, 25, 2489–2499. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zheng, J.; Jia, X.; Bourque, C.P.-A.; Zha, T.; Jin, C.; Xu, M.; Li, X. Biogeographic variations in soil respiration and its basal rate across China suggest thermal adaptation, substrate limitation, and soil moisture constraint. CATENA 2025, 254, 108992. [Google Scholar] [CrossRef]
- Peng, Y.; Chang, J.; Zhao, X.; Shi, Y.; Bai, Y.; Li, Q.; Yang, Y. Grassland carbon sink in China and its promotion strategies. Bull. Natl. Nat. Sci. Found. China 2023, 37, 587–593. [Google Scholar]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, Y.; Janssens, I.A.; Deng, Y.; He, X.; Liu, L.; Yi, Y.; Xiao, N.; Wang, X.; Li, C.; et al. Divergent rhizosphere and non-rhizosphere soil microbial structure and function in long-term warmed steppe due to altered root exudation. Glob. Change Biol. 2024, 30, e17111. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Chen, X.; Meng, Z.; Xu, R.; Duoji, D.; Zhang, J.; He, J.; Wang, Z.; Chen, J.; et al. Soil microbial network complexity predicts ecosystem function along elevation gradients on the Tibetan Plateau. Soil Biol. Biochem. 2022, 172, 108766. [Google Scholar] [CrossRef]
- Qi, H.; Wen, X.; Wang, Z.; Yin, S. Microbial Memory of Drought Reshapes Root-Associated Communities to Enhance Plant Resilience. Plant Cell Environ. 2025. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.W. Evolution of global temperature over the past two million years. Nature 2016, 538, 226–228. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, U.N.; Ball, B.A. Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob. Change Biol. 2015, 21, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Y.; Guo, X.; Ning, D.; Zhou, X.; Feng, J.; Yuan, M.M.; Liu, S.; Guo, J.; Gao, Z.; et al. Reduction of microbial diversity in grassland soil is driven by long-term climate warming. Nat. Microbiol. 2022, 7, 1054–1062. [Google Scholar] [CrossRef]
- Söllinger, A.; Séneca, J.; Dahl, M.B.; Motleleng, L.L.; Prommer, J.; Verbruggen, E.; Sigurdsson, B.D.; Janssens, I.; Peñuelas, J.; Urich, T.; et al. Down-regulation of the bacterial protein biosynthesis machinery in response to weeks, years, and decades of soil warming. Sci. Adv. 2022, 8, eabm3230. [Google Scholar] [CrossRef]
- Bedeke, S.B. Climate change vulnerability and adaptation of crop producers in sub-Saharan Africa: A review on concepts, approaches and methods. Environ. Dev. Sustain. 2023, 25, 1017–1051. [Google Scholar] [CrossRef]
- Furtak, K.; Wolińska, A. The impact of extreme weather events as a consequence of climate change on the soil moisture and on the quality of the soil environment and agriculture—A review. Catena 2023, 231, 107378. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Jiang, L.; Lv, G.; Hu, D.; Wu, D.; Yang, X. Rhizosphere effect and water constraint jointly determined the roles of microorganism in soil phosphorus cycling in arid desert regions. Catena 2023, 222, 106809. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, H.; Zhou, X.; Zhang, L.; Zhou, X.; Zhang, L.; Sha, T.; Zhang, Y. Response characteristics of bulk soil, rhizosphere, and root endophytic microbiota in desert ephemeral plants to increased precipitation. Plant Soil 2025, 513, 3073–3095. [Google Scholar] [CrossRef]
- Allison, S.D. Microbial drought resistance may destabilize soil carbon. Trends Microbiol. 2023, 31, 780–787. [Google Scholar] [CrossRef]
- De Vries, F.T.; Williams, A.; Stringer, F.; Willcocks, R.; McEwing, R.; Langridge, H.; Straathof, A.L. Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 2019, 224, 132–145. [Google Scholar] [CrossRef]
- Dong, L.; Li, M.-X.; Li, S.; Yue, L.-X.; Ali, M.; Han, J.-R.; Lian, W.-H.; Hu, C.-J.; Lin, Z.-L.; Shi, G.-Y.; et al. Aridity drives the variability of desert soil microbiomes across north-western China. Sci. Total Environ. 2024, 907, 168048. [Google Scholar] [CrossRef]
- Baker, N.R.; Zhalnina, K.; Yuan, M.; Herman, D.; Ceja-Navarro, J.A.; Sasse, J.; Jordan, J.S.; Bowen, B.P.; Wu, L.; Fossum, C.; et al. Nutrient and moisture limitations reveal keystone metabolites linking rhizosphere metabolomes and microbiomes. Proc. Natl. Acad. Sci. USA 2024, 121, e2303439121. [Google Scholar] [CrossRef]
- Wiesenbauer, J.; Gorka, S.; Jenab, K.; Schuster, R.; Kumar, N.; Rottensteiner, C.; König, A.; Kraemer, S.; Inselsbacher, E.; Kaiser, C. Preferential use of organic acids over sugars by soil microbes in simulated root exudation. Soil Biol. Biochem. 2025, 203, 109738. [Google Scholar] [CrossRef]
- Malik, A.A.; Bouskill, N.J. Drought impacts on microbial trait distribution and feedback to soil carbon cycling. Funct. Ecol. 2022, 36, 1442–1456. [Google Scholar] [CrossRef]
- Martin, F.M.; Öpik, M.; Dickie, I.A. Mycorrhizal research now: From the micro- to the macro-scale. New Phytol. 2024, 242, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Chen, L.; Xun, W.; Shu, X.; Chen, Y.; Sun, X.; Wang, Z.; Ren, Y.; Shen, Q.; et al. Root colonization by beneficial rhizobacteria. FEMS Microbiol. Rev. 2024, 48, fuad066. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root effects on soil organic carbon: A double-edged sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Sun, Z.; Liu, S.; Wang, Q.; Zeng, Z. Rhizosphere effects on soil organic carbon processes in terrestrial ecosystems: A meta-analysis. Geoderma 2022, 412, 115739. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, W.; Nottingham, A.T.; Xiao, D.; Kuzyakov, Y.; Xu, L.; Chen, H.; Xiao, J.; Duan, P.; Tang, T.; et al. Lithological controls on soil aggregates and minerals regulate microbial carbon use efficiency and necromass stability. Environ. Sci. Technol. 2024, 58, 21186–21199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, J.; Zhang, Z.; Liang, C.; Lambers, H.; Zhu, B.; Wang, D.; Wang, J.; Zhang, P.; Li, N.; et al. Rhizosphere as a hotspot for microbial necromass deposition into the soil carbon pool. J. Ecol. 2025, 113, 168–179. [Google Scholar] [CrossRef]
- Favaro, A.; Singh, B.; Warren, C.; Dijkstra, F.A. Differences between priming and rhizosphere priming effects: Concepts and mechanisms. Soil Biol. Biochem. 2025, 205, 109769. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef]
- Fang, J.; Chen, B.; Wang, F.; Li, W.; Zhang, H.; Fang, J.; Liu, S.; Zheng, Z.; Guo, M.; Niu, S. Nitrogen, phosphorus, and potassium co-limitation in terrestrial ecosystems: A global meta-analysis. Plants People Planet 2024, 6, 1329–1340. [Google Scholar] [CrossRef]
- Feng, X.; Dai, G.; Liu, T.; Jia, J.; Zhu, E.; Liu, C.; Zhao, Y.; Wang, Y.; Kang, E.; Xiao, J.; et al. Understanding the mechanisms and potential pathways of soil carbon sequestration from the biogeochemistry perspective. Sci. China Earth Sci. 2024, 67, 3386–3396. [Google Scholar] [CrossRef]
- Lu, J.; Cai, J.; Dijkstra, F.A.; Yin, L.; Wang, P.; Cheng, W. Rhizosphere priming and effects on mobilization and immobilization of multiple soil nutrients. Soil Biol. Biochem. 2024, 199, 109615. [Google Scholar] [CrossRef]
- Henneron, L.; Kardol, P.; Wardle, D.A.; Cros, C.; Fontaine, S. Rhizosphere control of soil nitrogen cycling: A key component of plant economic strategies. New Phytol. 2020, 228, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Beechey-Gradwell, Z.; Mackay, A.; Condron, L.; Bowatte, S.; Agrelo, F.D.L.; Brock, S.; Thompson, D.; Theobald, P.; Lieffering, M.; Shi, S.; et al. Loss of P Fertilizer Effectiveness in Raising Soil P Availability in a Grazed Grassland Enriched With CO2 for 24 Years. Glob. Change Biol. 2025, 31, e70150. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, B.; Li, T.; Dong, Y. Long-term intercropping regulates the community structure of arbuscular mycorrhizal fungi and improves wheat yield. Field Crops Res. 2025, 326, 109874. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Gu, B.; Liu, H.; Dijkstra, F.A.; Han, X.; Jiang, Y. Plant growth strategies and microbial contributions to ecosystem nitrogen retention along a soil acidification gradient. Ecology 2025, 106, e4515. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.Y.; Liu, J.; Wang, S.; Liu, H.; Ding, Y.; Ji, L. Grazing promotes soil phosphorus cycling by enhancing soil microbial functional genes for phosphorus transformation in plant rhizosphere in a semi-arid natural grassland. Geoderma 2023, 430, 116303. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Z.; Chang, S.; Wang, Z.; Li, D.; An, Y.; Hou, F.; Ren, J. Growing season grazing promotes the shallow stratification of soil nutrients while non-growing season grazing sequesters the deep soil nutrients in a typical alpine meadow. Geoderma 2022, 426, 116111. [Google Scholar] [CrossRef]
- Xu, H.; You, C.; Tan, B.; Xu, L.; Liu, Y.; Wang, M.; Xu, Z.; Sardans, J.; Peñuelas, J. Effects of livestock grazing on the relationships between soil microbial community and soil carbon in grassland ecosystems. Sci. Total Environ. 2023, 881, 163416. [Google Scholar] [PubMed]
- Dinneny, J.R. Developmental responses to water and salinity in root systems. Annu. Rev. Cell Dev. Biol. 2019, 35, 239–257. [Google Scholar] [CrossRef]
- Keller, A.B.; Walter, C.A.; Blumenthal, D.M.; Borer, E.T.; Collins, S.L.; DeLancey, L.C.; Fay, P.A.; Hofmockel, K.S.; Knops, J.M.H.; Leakey, A.D.B.; et al. Stronger fertilization effects on aboveground versus belowground plant properties across nine US grasslands. Ecology 2023, 104, e3891. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, S.; Ju, X.; Zhu, Z.; Zhang, Y.; Chen, H.; Jin, K. Soil acidification drives the negative effects of nitrogen enrichment on soil microbial biomass at the global scale. Plant Soil 2024, 503, 517–528. [Google Scholar] [CrossRef]
- Zhao, Z.; Ge, T.; Gunina, A.; Li, Y.; Zhu, Z.; Peng, P.; Wu, J.; Kuzyakov, Y. Carbon and nitrogen availability in paddy soil affects rice photosynthate allocation, microbial community composition, and priming: Combining continuous 13 C labeling with PLFA analysis. Plant Soil 2019, 445, 137–152. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Yin, Q.; Sun, Y.; Li, B.; Feng, Z.; Wu, G. The r/K selection theory and its application in biological wastewater treatment processes. Sci. Total Environ. 2022, 824, 153836. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, G.; Chen, H.; Chen, C.; Wang, J.; Ai, S.; Wei, D.; Li, D.; Ma, B.; Tang, C.; et al. Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 2020, 14, 757–770. [Google Scholar] [CrossRef]
- Zhan, Y.; Xu, S.; Hou, Z.; Gao, X.; Su, J.; Peng, B.; Zhao, J.; Wang, Z.; Cheng, M.; Zhang, A.; et al. Co-inoculation of phosphate-solubilizing bacteria and phosphate accumulating bacteria in phosphorus-enriched composting regulates phosphorus transformation by facilitating polyphosphate formation. Bioresour. Technol. 2023, 390, 129870. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; Ding, W.; Cao, S.; Fang, L.; Zhou, J.; Zhang, Y. Arbuscular mycorrhizal fungi: Effects on secondary metabolite accumulation of traditional Chinese medicines. Plant Biol. 2022, 24, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Müller, D.B.; Srinivas, G.; Garrido-Oter, R.; Potthoff, E.; Rott, M.; Dombrowski, N.; Münch, P.C.; Spaepen, S.; Remus-Emsermann, M.; et al. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2022, 110, 21–33. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621, Correction in Nat. Rev. Microbiol. 2020, 19, 72. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Morales, G.G.; Pérez, O.J.; Yañes, J.M.S.; Vázquez, P.Á.; Castillo, F.C. The sweet clover-Sinorhizobium meliloti system as a useful interaction for nitrogen fixation and as a soil improver. Review. Rev. Mex. De Cienc. Pecu. 2024, 15, 208–229. [Google Scholar]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Schultes, S.R.; Rüger, L.; Niedeggen, D.; Freudenthal, J.; Frindte, K.; Becker, M.F.; Metzner, R.; Pflugfelder, D.; Chlubek, A.; Hinz, C.; et al. Photosynthate distribution determines spatial patterns in the rhizosphere microbiota of the maize root system. Nat. Commun. 2025, 16, 7286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Zhang, Y.; Liao, J.; Guan, K.; Zhai, J.; Meng, P.; Tang, X.; Dong, T.; Song, Y. Root hair developmental regulators orchestrate drought triggered microbiome changes and the interaction with beneficial Rhizobiaceae. Nat. Commun. 2024, 15, 10068. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-G.; Gao, Z.; Fu, X.-X.; Chen, X.-M.; Shen, S.; Zhou, S.-L. Coordination of carbon assimilation, allocation, and utilization for systemic improvement of cereal yield. Front. Plant Sci. 2023, 14, 1206829. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.; Pieterse, C.M.; de Jonge, R.; Berendsen, R.L. The soil-borne legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef]
- Barnes, A.D.; Scherber, C.; Brose, U.; Borer, E.T.; Ebeling, A.; Gauzens, B.; Giling, D.P.; Hines, J.; Isbell, F.; Ristok, C.; et al. Biodiversity enhances the multitrophic control of arthropod herbivory. Sci. Adv. 2020, 6, eabb6603. [Google Scholar] [CrossRef]
- Li, G.; Wang, K.; Qin, Q.; Li, Q.; Mo, F.; Nangia, V.; Liu, Y. Integrated microbiome and metabolomic analysis reveal responses of rhizosphere bacterial communities and root exudate composition to drought and genotype in rice (Oryza sativa L.). Rice 2023, 16, 19. [Google Scholar] [CrossRef]
- Luo, W.; Ni, M.; Wang, Y.; Lan, R.; Eissenstat, D.M.; Cahill, J.F.; Li, B.; Chu, C. Limited evidence of vertical fine-root segregation in a subtropical forest. New Phytol. 2021, 231, 2308–2318. [Google Scholar] [CrossRef]
- Yuan, L.; Xie, X.; Zhang, Y.; Li, J.; van Kleunen, M. The soil microbial community and nitrogen availability affect the growth, biochemistry and potential allelopathic effects of the invasive plant Solidago canadensis. Plant Soil 2024, 510, 491–505. [Google Scholar] [CrossRef]
- Zeng, Q.; Hu, H.; Ge, A.; Xiong, C.; Zhai, C.; Duan, G.; Han, L.; Huang, S.; Zhang, L. Plant-microbiome interactions and their impacts on plant adaptation to climate change. J. Integr. Plant Biol. 2025, 67, 826–844. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Yue, S.; Tian, J.; Chen, H.; Jiang, H.; Siddique, K.H.; Zhan, A.; Fang, Q.; Yu, Q. Soil microbial community and network changes after long-term use of plastic mulch and nitrogen fertilization on semiarid farmland. Geoderma 2021, 396, 115086. [Google Scholar] [CrossRef]
- Liu, P.; Sun, M.; Xia, S.; Ju, J.; Mao, W.; Zhao, H.; Hao, Y. Earthworms and lactic acid bacteria (LAB) cooperate to promote the biodegradation of tetracycline residues in livestock manure. Waste Manag. 2024, 186, 166–175. [Google Scholar] [CrossRef]
- Edwards, C.A.; Arancon, N.Q. Biology and Ecology of Earthworms; Springer: Berlin/Heidelberg, Germany, 2022; pp. 275–301. [Google Scholar]
- Wu, D.; Du, E.; Eisenhauer, N.; Mathieu, J.; Chu, C. Global engineering effects of soil invertebrates on ecosystem functions. Nature 2025, 640, 120–129. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Zhao, W.; Yin, H. The rhizosphere and hyphosphere differentially regulate microbiomes and nutrient cycling within soil aggregates in an ectomycorrhiza-dominated forest. Geoderma 2025, 463, 117570. [Google Scholar] [CrossRef]
- Slonka, M.; Vosteen, I.; Mendoza-Mendoza, A.; Rostás, M. Ecological functions of fungal sesquiterpenes in the food preference and fitness of soil Collembola. R. Soc. Open Sci. 2024, 11, 231549. [Google Scholar] [CrossRef]
- Yang, X.; Shang, G.; Wang, X. Biochemical, transcriptomic, gut microbiome responses and defense mechanisms of the earthworm Eisenia fetida to salt stress. Ecotoxicol. Environ. Saf. 2022, 239, 113684. [Google Scholar] [CrossRef]
- Sellegounder, D.; Liu, Y.; Wibisono, P.; Chen, C.H.; Leap, D.; Sun, J. Neuronal GPCR NPR-8 regulates C. elegans defense against pathogen infection. Sci. Adv. 2019, 5, eaaw4717. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Hu, H.-W.; Zhu, D.; Zhu, Y.-G.; He, J.-Z. Calling for comprehensive explorations between soil invertebrates and arbuscular mycorrhizas. Trends Plant Sci. 2022, 27, 793–801. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S. Nematodes as drivers of plant performance in natural systems. Trends Plant Sci. 2021, 26, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, Y.; Wang, C. Rhizosphere interactions between earthworms (Eisenia fetida) and arbuscular mycorrhizal fungus (Funneliformis mosseae) promote utilization efficiency of phytate phosphorus in maize. Appl. Soil Ecol. 2015, 94, 30–39. [Google Scholar] [CrossRef]
- Breitkreuz, C.; Reitz, T.; Schulz, E.; Tarkka, M.T. Drought and plant community composition affect the metabolic and genotypic diversity of pseudomonas strains in grassland soils. Microorganisms 2021, 9, 1677. [Google Scholar] [CrossRef]
- Liu, N.; Kan, H.; Yang, G.; Zhang, Y. Changes in plant, soil, and microbes in a typical steppe from simulated grazing: Explaining potential change in soil C. Ecol. Monogr. 2015, 85, 269–286. [Google Scholar] [CrossRef]
- Soussana, J.-F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 190, 9–17. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Niu, S.; Tian, D.; Wu, Q.; Gao, X.; Schellenberg, M.P.; Han, G. Diversity of plant and soil microbes mediates the response of ecosystem multifunctionality to grazing disturbance. Sci. Total Environ. 2021, 776, 145730. [Google Scholar] [CrossRef] [PubMed]
- Zhai, C.; Han, L.; Xiong, C.; Ge, A.; Yue, X.; Li, Y.; Zhou, Z.; Feng, J.; Ru, J.; Song, J.; et al. Soil microbial diversity and network complexity drive the ecosystem multifunctionality of temperate grasslands under changing precipitation. Sci. Total Environ. 2024, 906, 167217. [Google Scholar] [CrossRef]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ke, X.; Dai, L.; Cao, G.; Zhou, H.; Guo, X. Moderate grazing increased alpine meadow soils bacterial abundance and diversity index on the Tibetan Plateau. Ecol. Evol. 2020, 10, 8681–8687. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiqige, B.; Liu, Y.; Tian, Y.; Liu, L.; Guo, W.; Wang, P.; Zhou, D.; Wen, H.; Zhi, Q.; Wu, Y.; et al. Biotic and Abiotic Factors on Rhizosphere Microorganisms in Grassland Ecosystems. Microorganisms 2025, 13, 2645. https://doi.org/10.3390/microorganisms13122645
Qiqige B, Liu Y, Tian Y, Liu L, Guo W, Wang P, Zhou D, Wen H, Zhi Q, Wu Y, et al. Biotic and Abiotic Factors on Rhizosphere Microorganisms in Grassland Ecosystems. Microorganisms. 2025; 13(12):2645. https://doi.org/10.3390/microorganisms13122645
Chicago/Turabian StyleQiqige, Bademu, Yuzhen Liu, Yu Tian, Li Liu, Weiwei Guo, Ping Wang, Dayou Zhou, Hui Wen, Qiuying Zhi, Yuxuan Wu, and et al. 2025. "Biotic and Abiotic Factors on Rhizosphere Microorganisms in Grassland Ecosystems" Microorganisms 13, no. 12: 2645. https://doi.org/10.3390/microorganisms13122645
APA StyleQiqige, B., Liu, Y., Tian, Y., Liu, L., Guo, W., Wang, P., Zhou, D., Wen, H., Zhi, Q., Wu, Y., Hu, X., Li, M., & Li, J. (2025). Biotic and Abiotic Factors on Rhizosphere Microorganisms in Grassland Ecosystems. Microorganisms, 13(12), 2645. https://doi.org/10.3390/microorganisms13122645





