High-Resolution Wastewater-Based Surveillance of Three Influenza Seasons (2022–2025) Reveals Distinct Seasonal Patterns of Viral Activity in Munich, Germany
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Sampling
2.2. Virus Concentration and Nucleic Acid Extraction
2.3. Quantification of Influenza Viruses with Digital Droplet PCR (ddPCR)
2.4. Quantification of Human Fecal Indicator PMMoV
2.5. Data Sources and Calculation
2.5.1. Notification Data
2.5.2. Primary Care Sentinel Surveillance Data
2.5.3. Normalization of Wastewater Data
2.5.4. Statistical Analysis
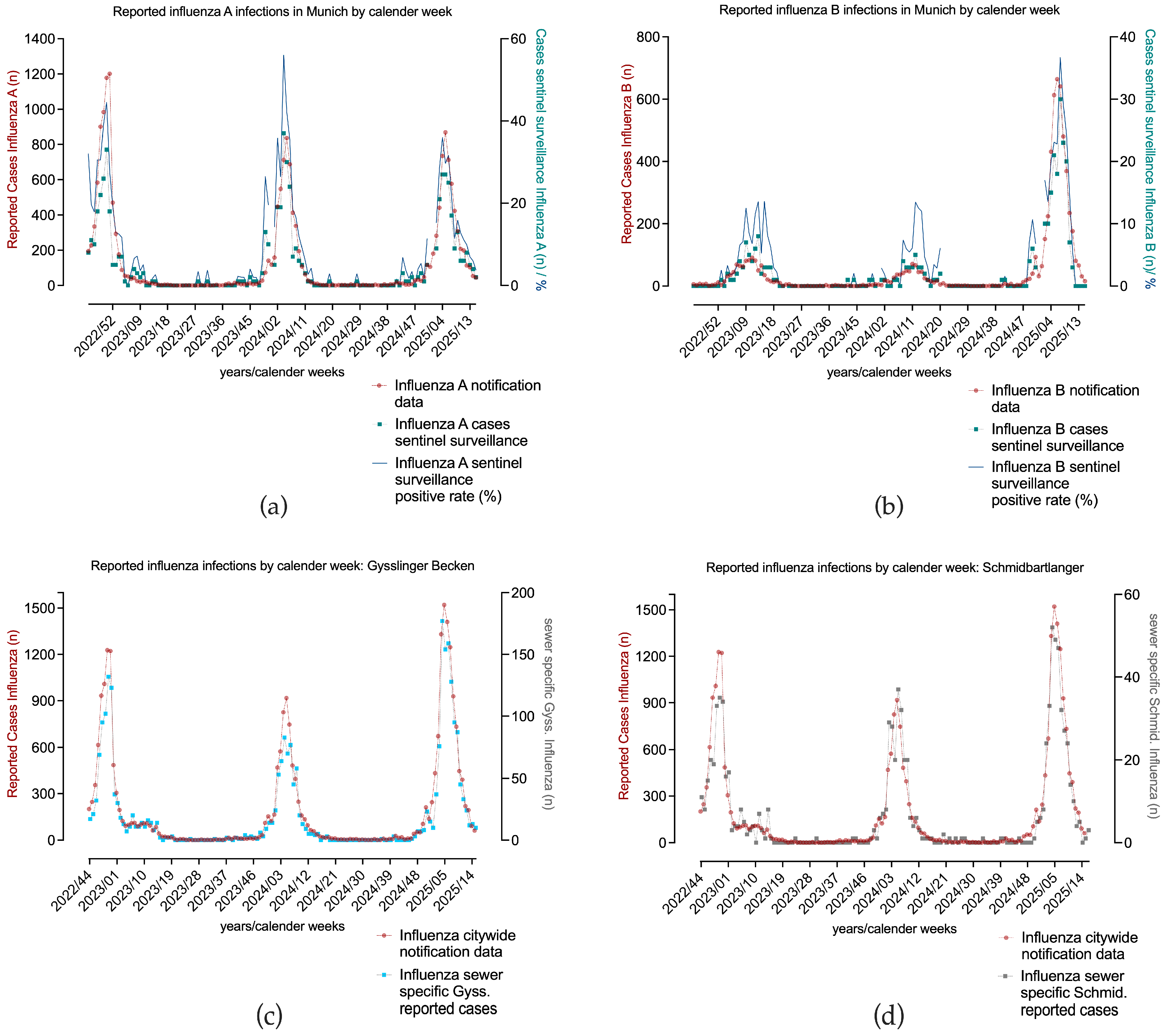
3. Results and Discussion
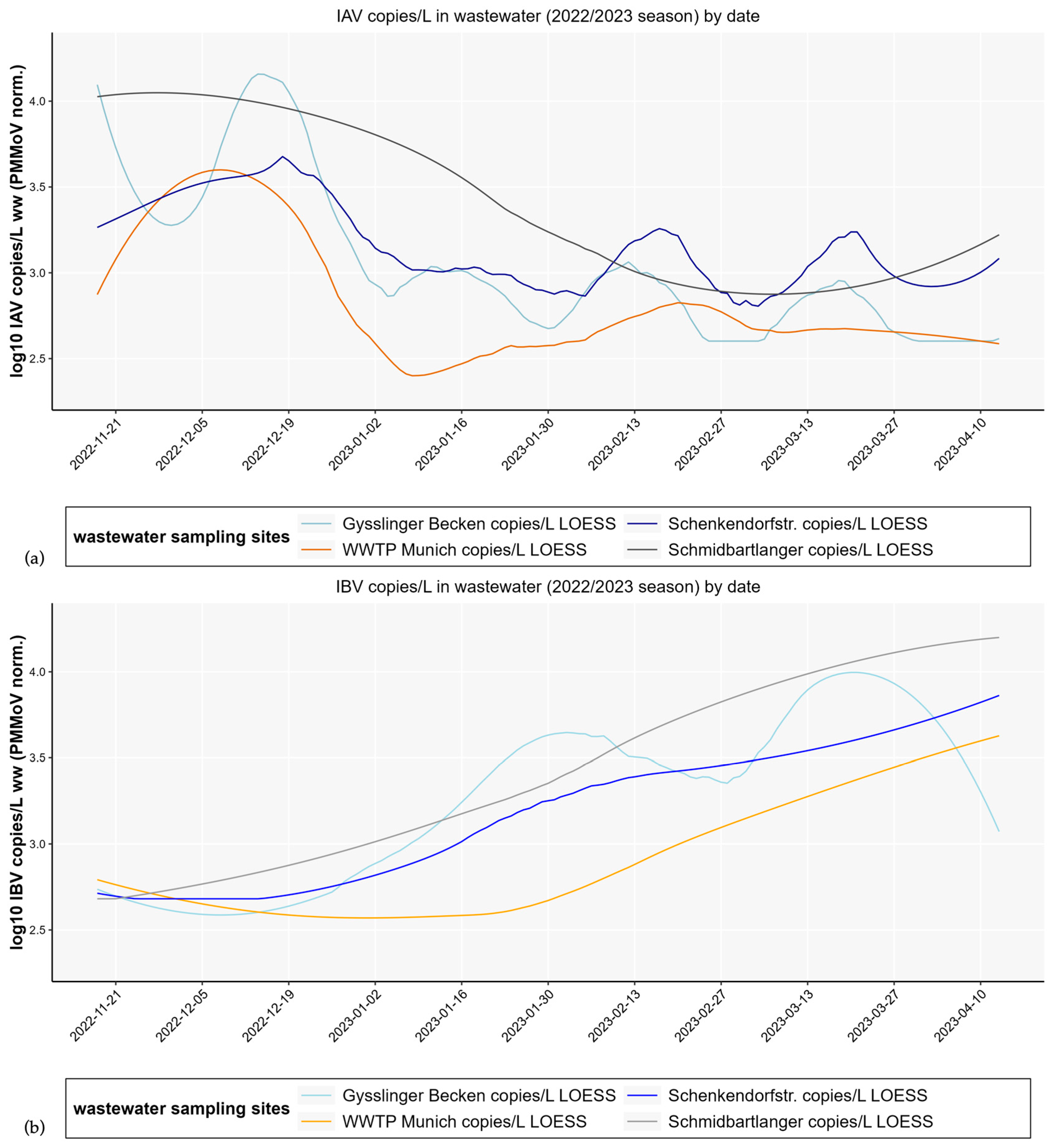
3.1. Differences in Influenza Wastewater Copy Numbers and Development over Time with Respect to Decentralized and Centralized Wastewater Sampling Strategy
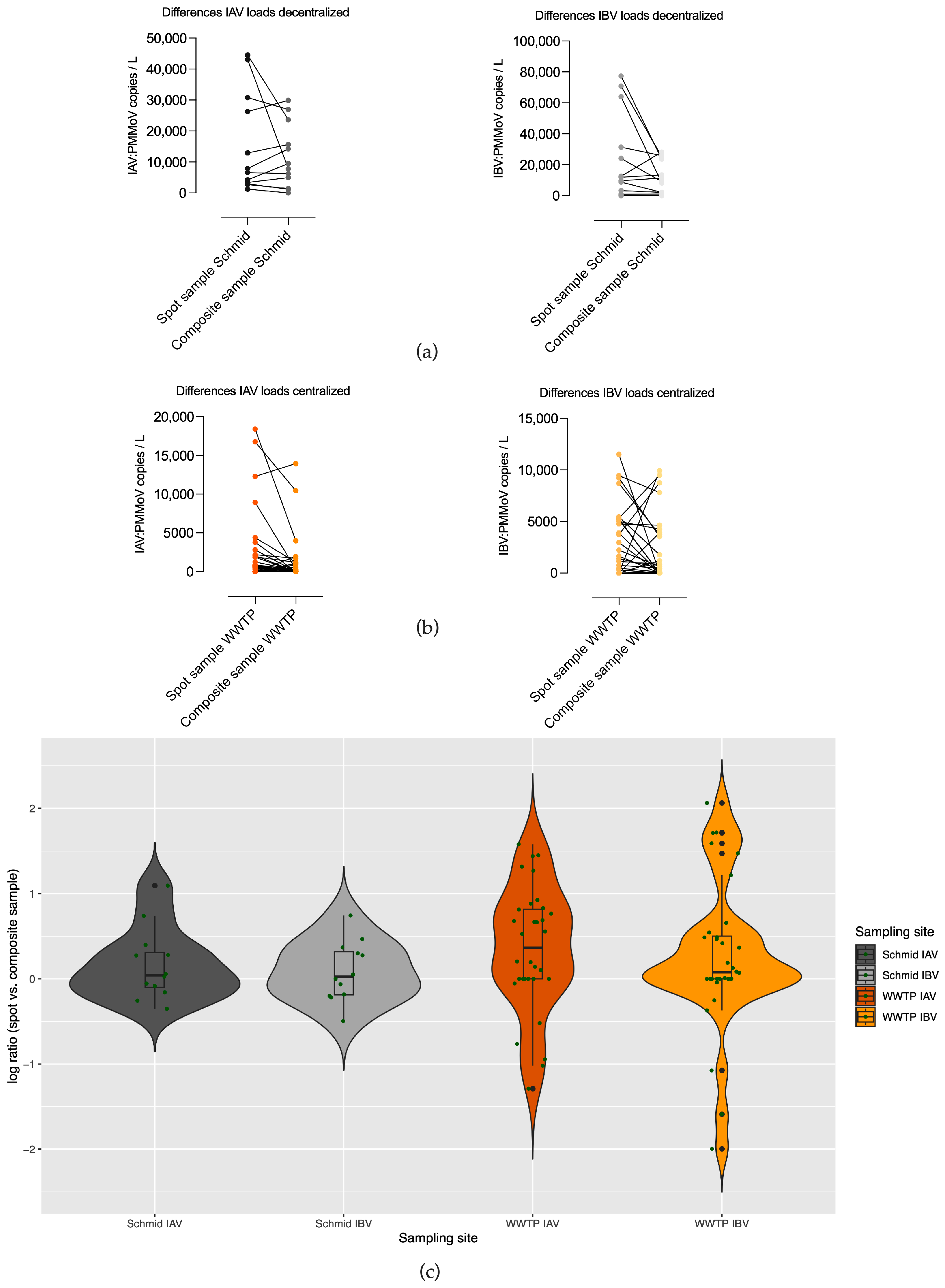
3.2. Differences in Copy Numbers of Qualified Spot Sample and Composite Sample Pairs in Decentralized (Schmidbartlanger Sewer) and Centralized Sampling Site Wastewater Treatment Plant
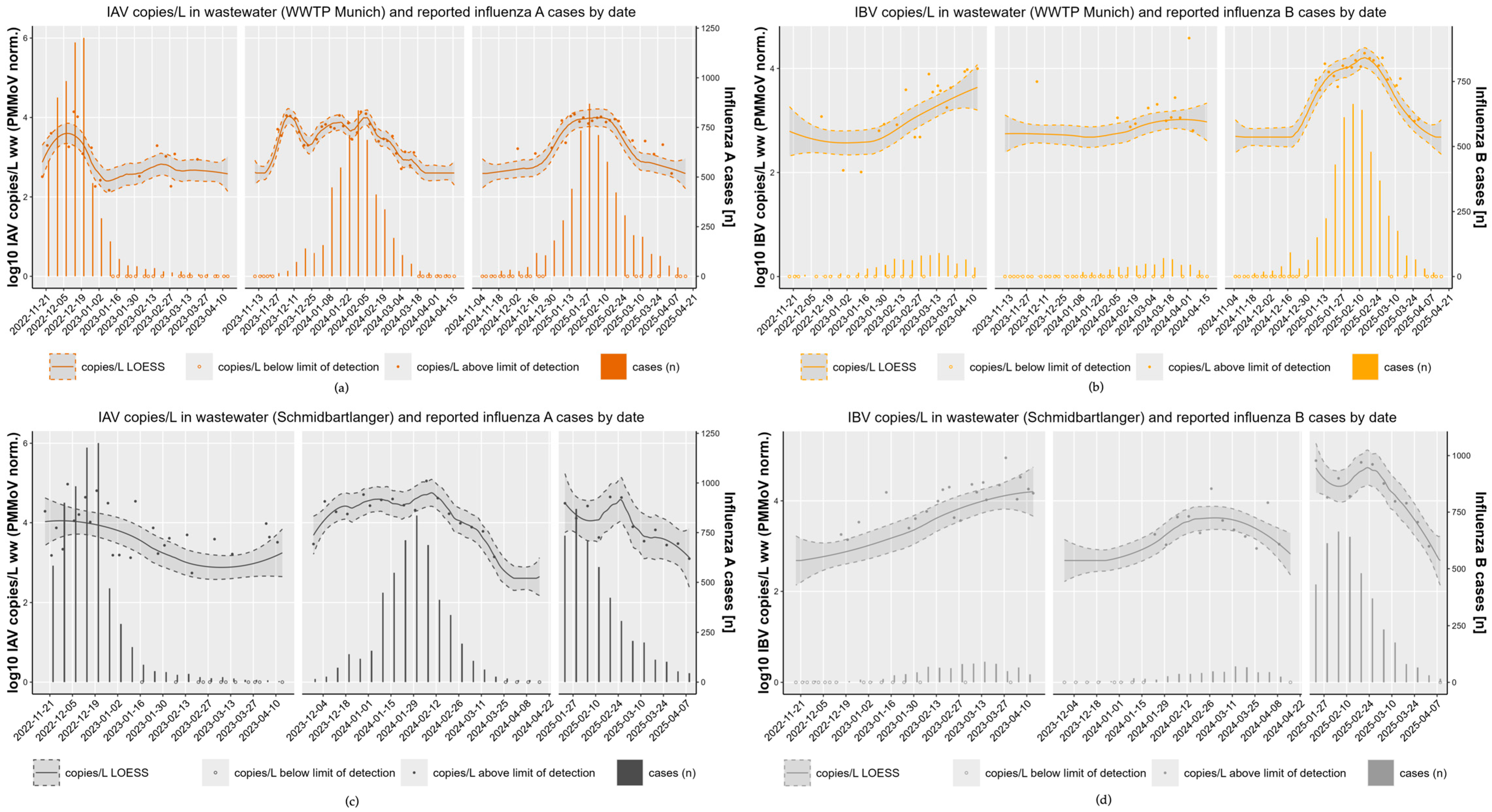
3.3. High Concordance Across Three Consecutive Influenza Seasons (2022/23–2024/25) with Notification Data
3.4. Pronounced Inter-Seasonal Differences
- Season 1–2022/23
- Season 2—2023/24
- Season 3—2024/25
3.5. Limitations of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1 h | 1 hour |
| 24 h | 24 hour(s) |
| CDC | Center for Disease Control |
| Copies/L | Copies per liter |
| COVID-19 | Coronavirus disease 2019 |
| ddPCR | Droplet digital polymerase chain reaction |
| DTT | Dithiothreitol |
| Gyss | Gysslinger Becken sewer |
| IA | Influenza A |
| IAV | Influenza A virus |
| IB | Influenza B |
| IBV | Influenza B virus |
| IfsG | Infektionsschutzgesetz (German infection protection act) |
| LGL | Bavarian Health and Food Safety Authority |
| LoD | Limit of detection |
| MSE | Munich Sewer Authority (Münchner Stadtentwässerung) |
| Norm. | Normalized |
| PMMoV | Pepper mild mottle virus |
| RKI | Robert Koch- Institute |
| RNA | Ribonucleic acid |
| RT-qPCR | Real time quantitative polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus type 2 |
| Schenk | Schenkendorfstr. sewer |
| Schmid | Schmidbartlanger sewer |
| WBE | Wastewater-based epidemiology |
| WBS | Wastewater-based surveillance |
| WWTP | Wastewater treatment plant |
Appendix A
| Target | Description | Oligonucleotide Sequence (5′ to 3′) | Concentration | Ref. |
|---|---|---|---|---|
| Influenza A(M)-gene | Influenza A F Influenza A R Probe HEX/ZEN/3IABkFQ | CAAGACCAATCYTGTCACCTCTGAC GCATTYTGGACAAAVCGTCTACG TGCAGTCCTCGCTCACTGGGCACG | 900 nM 900 nM 250 nM | CDC * |
| Influenza B NS-gene | Influenza B F Influenza B R Probe FAM/ZEN/3IABkFQ | TCCTCAAYTCACTCTTCGAGCG CGGTGCTCTTGACCAAATTGG CCAATTCGAGCAGCTGAAACTGCGGTG | 900 nM 900 nM 250 nM | CDC * |
| Sampling Site | Median of IAV PMMoV Norm. Copies/L Wastewater | Median of IBV PMMoV Norm. Copies/L Wastewater |
|---|---|---|
| WWTP Munich | 374 | 551 |
| Schmidbartlanger | 2418 | 3381 |
| Gysslinger Becken | 447 | 1081 |
| Schenkendorfstr. | 1091 | 1370 |
| Description | Pearson r, 95% CI | p-Value |
|---|---|---|
| Schmid.-Gyss. IAV 22/23 Schmid.-Gyss. IBV 22/23 | 0.72 [0.63; 0.79] 0.78 [0.70; 0.83] | <0.0001 <0.0001 |
| Schmid.-Schenk. IAV 22/23 Schmid.-Schenk. IBV 22/23 | 0.67 [0.57; 0.75] 0.98 [0.97; 0.98] | <0.0001 <0.0001 |
| Schmid.-WWTP IAV 22/23 Schmid.-WWTP IBV 22/23 Schmid.-WWTP IAV 23/24 Schmid.-WWTP IBV 23/24 Schmid.-WWTP IAV 24/25 Schmid.-WWTP IBV 24/25 | 0.67 [0.57; 0.75] 0.90 [0.87; 0.93] 0.89 [0.85; 0.92] 0.59 [0.47; 0.69] 0.84 [0.76; 0.90] 0.90 [0.85; 0.94] | <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 <0.0001 |
| Gyss.-Schenk. IAV 22/23 Gyss.-Schenk. IBV 22/23 | 0.83 [0.77; 0.87] 0.84 [0.79; 0.88] | <0.0001 <0.0001 |
| Gyss.-WWTP IAV 22/23 Gyss.-WWTP IBV 22/23 | 0.75 [0.67; 0.81] 0.60 [0.49; 0.69] | <0.0001 <0.0001 |
| Schenk.-WWTP IAV 22/23 Schenk.-WWTP IBV 22/23 | 0.86 [0.81; 0.90] 0.84 [0.78; 0.88] | <0.0001 <0.0001 |
| Description | Pearson r, 95% CI | p-Value |
|---|---|---|
| City notification-sentinel IA City notification-sentinel IB | 0.90 [0.86; 0.93] 0.61 [0.49; 0.71] | <0.0001 <0.0001 |
| City notification-Gyss. sewer specific notification IA + IB | 0.99 [0.98; 0.99] | <0.0001 |
| City notification-Schmid. sewer specific notification IA + IB | 0.96 [0.94; 0.97] | <0.0001 |
| Description | Pearson r, 95% CI | p-Value |
|---|---|---|
| Schmid r 22/23 IAV Schmid r 22/23 IBV | 0.87 [0.70; 0.95] 0.86 [0.69; 0.94] | <0.0001 <0.0001 |
| Schmid r 23/24 IAV Schmid r 23/24 IBV | 0.64 [0.28; 0.84] 0.83 [0.61; 0.93] | 0.0023 <0.0001 |
| Schmid r 24/25 IAV Schmid r 24/25 IBV | 0.71 [0.23; 0.91] 0.75 [0.31; 0.93] | 0.01 0.005 |
| WWTP r 22/23 IAV WWTP r 22/23 IBV | 0.90 [0.76; 0.96] 0.75 [0.47; 0.89] | <0.0001 <0.0001 |
| WWTP r 23/24 IAV WWTP r 23/24 IBV | 0.64 [0.30; 0.84] 0.83 [0.62; 0.93] | 0.0013 <0.0001 |
| WWTP r 24/25 IAV WWTP r 24/25 IBV | 0.93 [0.84; 0.97] 0.89 [0.75; 0.95] | <0.0001 <0.0001 |
References
- European Centre for Disease Prevention and Control. Factsheet About Seasonal Influenza. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet (accessed on 1 September 2025).
- European Centre for Disease Prevention and Control. Seasonal Influenza 2022−2023 Annual Epidemiological Report for 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/seasonal-influenza-annual-epidemiological-report-20222023 (accessed on 1 September 2025).
- Kiefer, A.; Kabesch, M.; Ambrosch, A. The Frequency of Hospitalizations for RSV and Influenza Among Children and Adults: An Analysis from the Years 2016–2022. Dtsch. Arztebl. Int. 2023, 120, 534–535. [Google Scholar] [CrossRef] [PubMed]
- Terliesner, N.; Unterwalder, N.; Edelmann, A.; Corman, V.; Knaust, A.; Rosenfeld, L.; Gratopp, A.; Ringe, H.; Martin, L.; von Bernuth, H.; et al. Viral infections in hospitalized children in Germany during the COVID-19 pandemic: Association with non-pharmaceutical interventions. Front. Pediatr. 2022, 10, 935483. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Reingold, A. The Burden of Influenza: A Complex Problem. Curr. Epidemiol. Rep. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Robert Koch-Institute. Influenza (Part 1): Diseases Caused by Seasonal Influenza Viruses. Available online: https://www.rki.de/DE/Aktuelles/Publikationen/RKI-Ratgeber/Ratgeber/Ratgeber_Influenza_saisonal.html (accessed on 1 September 2025).
- Rubio-Acero, R.; Beyerl, J.; Muenchhoff, M.; Roth, M.S.; Castelletti, N.; Paunovic, I.; Radon, K.; Springer, B.; Nagel, C.; Boehm, B.; et al. Spatially resolved qualified sewage spot sampling to track SARS-CoV-2 dynamics in Munich—One year of experience. Sci. Total Environ. 2021, 797, 149031. [Google Scholar] [CrossRef]
- Lowry, S.A.; Wolfe, M.K.; Boehm, A.B. Respiratory virus concentrations in human excretions that contribute to wastewater: A systematic review and meta-analysis. J. Water Health 2023, 21, 831–848. [Google Scholar] [CrossRef]
- Boehm, A.B.; Wolfe, M.K.; White, B.J.; Hughes, B.; Duong, D.; Bidwell, A. More than a Tripledemic: Influenza A Virus, Respiratory Syncytial Virus, SARS-CoV-2, and Human Metapneumovirus in Wastewater during Winter 2022–2023. Environ. Sci. Technol. Lett. 2023, 10, 622. [Google Scholar] [CrossRef]
- Mercier, E.; D’Aoust, P.M.; Thakali, O.; Hegazy, N.; Jia, J.J.; Zhang, Z.; Eid, W.; Plaza-Diaz, J.; Kabir, M.P.; Fang, W.; et al. Municipal and Neighbourhood Level Wastewater Surveillance and Subtyping of an Influenza Virus Outbreak. Sci. Rep. 2022, 12, 15777. [Google Scholar] [CrossRef]
- Toribio-Avedillo, D.; Gómez-Gómez, C.; Sala-Comorera, L.; Galofré, B.; Muniesa, M. Adapted methods for monitoring influenza virus and respiratory syncytial virus in sludge and wastewater. Sci. Total Environ. 2024, 918, 170636. [Google Scholar] [CrossRef] [PubMed]
- Dumke, R.; Geissler, M.; Skupin, A.; Helm, B.; Mayer, R.; Schubert, S.; Oertel, R.; Renner, B.; Dalpke, A.H. Simultaneous Detection of SARS-CoV-2 and Influenza Virus in Wastewater of Two Cities in Southeastern Germany, January to May 2022. Int. J. Environ. Res. Public Health 2022, 19, 13374. [Google Scholar] [CrossRef]
- Germano Emma, R.; Flores, T.; Freed Grace, S.; Kim, K.; Tulinsky Grace, H.; Yang, A.; Rose Oliver, J.; Ray Caroline, A.; Autry, A.; Catallozzi, M.; et al. Building-level wastewater surveillance localizes interseasonal influenza variation. mSphere 2024, 9, e00600–e00623. [Google Scholar] [CrossRef]
- Heijnen, L.; Medema, G. Surveillance of Influenza A and the pandemic influenza A (H1N1) 2009 in sewage and surface water in the Netherlands. J. Water Health 2011, 9, 434–442. [Google Scholar] [CrossRef]
- Markt, R.; Stillebacher, F.; Nägele, F.; Kammerer, A.; Peer, N.; Payr, M.; Scheffknecht, C.; Dria, S.; Draxl-Weiskopf, S.; Mayr, M.; et al. Expanding the Pathogen Panel in Wastewater Epidemiology to Influenza and Norovirus. Viruses 2023, 15, 263. [Google Scholar] [CrossRef]
- Vo, V.; Harrington, A.; Chang, C.-L.; Baker, H.; Moshi, M.A.; Ghani, N.; Itorralba, J.Y.; Tillett, R.L.; Dahlmann, E.; Basazinew, N.; et al. Identification and genome sequencing of an influenza H3N2 variant in wastewater from elementary schools during a surge of influenza A cases in Las Vegas, Nevada. Sci. Total Environ. 2023, 872, 162058. [Google Scholar] [CrossRef]
- Ahmed, W.; Bivins, A.; Stephens, M.; Metcalfe, S.; Smith, W.J.M.; Sirikanchana, K.; Kitajima, M.; Simpson, S.L. Occurrence of multiple respiratory viruses in wastewater in Queensland, Australia: Potential for community disease surveillance. Sci. Total Environ. 2023, 864, 161023. [Google Scholar] [CrossRef]
- Faherty, E.A.G.; Yuce, D.; Korban, C.; Bemis, K.; Kowalski, R.; Gretsch, S.; Ramirez, E.; Poretsky, R.; Packman, A.; Leisman, K.P.; et al. Correlation of wastewater surveillance data with traditional influenza surveillance measures in Cook County, Illinois, October 2022–April 2023. Sci. Total Environ. 2024, 912, 169551. [Google Scholar] [CrossRef]
- Lehto, K.-M.; Länsivaara, A.; Hyder, R.; Luomala, O.; Lipponen, A.; Hokajärvi, A.-M.; Heikinheimo, A.; Pitkänen, T.; Oikarinen, S. Wastewater-based surveillance is an efficient monitoring tool for tracking influenza A in the community. Water Res. 2024, 257, 121650. [Google Scholar] [CrossRef]
- Saldana, M.A.; Geng, J.; Shen, L.; Ghanem-Uzqueda, A.; Van Orman, S.; Tilley, K.B.; Sun, D.; Willes, D.; Smith, A.L. Amplitude multiplexed wastewater surveillance for campus health: Tracking SARS-CoV-2, influenza A, and norovirus. Environ. Sci. Water Res. Technol. 2025, 11, 77–87. [Google Scholar] [CrossRef]
- Wolfe, M.K.; Duong, D.; Bakker, K.M.; Ammerman, M.; Mortenson, L.; Hughes, B.; Arts, P.; Lauring, A.S.; Fitzsimmons, W.J.; Bendall, E.; et al. Wastewater-Based Detection of Two Influenza Outbreaks. Environ. Sci. Technol. Lett. 2022, 9, 687–692. [Google Scholar] [CrossRef]
- Wolken, M.; Sun, T.; McCall, C.; Schneider, R.; Caton, K.; Hundley, C.; Hopkins, L.; Ensor, K.; Domakonda, K.; Kalvapalle, P.; et al. Wastewater surveillance of SARS-CoV-2 and influenza in preK-12 schools shows school, community, and citywide infections. Water Res. 2023, 231, 119648. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Maswanganye, T.N.; Jeje, O.; Winston, D.; Lamssali, M.; Deng, D.; Blakley, I.; Fodor, A.A.; Jeffers-Francis, L. Wastewater Speaks: Evaluating SARS-CoV-2 Surveillance, Sampling Methods, and Seasonal Infection Trends on a University Campus. Microorganisms 2025, 13, 924. [Google Scholar] [CrossRef]
- Lu, E.; Ai, Y.; Davis, A.; Straathof, J.; Halloran, K.; Hull, N.; Winston, R.; Weir, M.H.; Soller, J.; Bohrerova, Z.; et al. Wastewater surveillance of SARS-CoV-2 in dormitories as a part of comprehensive university campus COVID-19 monitoring. Environ. Res. 2022, 212, 113580. [Google Scholar] [CrossRef]
- Scott, L.C.; Aubee, A.; Babahaji, L.; Vigil, K.; Tims, S.; Aw, T.G. Targeted wastewater surveillance of SARS-CoV-2 on a university campus for COVID-19 outbreak detection and mitigation. Environ. Res. 2021, 200, 111374. [Google Scholar] [CrossRef]
- Sellers, S.C.; Gosnell, E.; Bryant, D.; Belmonte, S.; Self, S.; McCarter, M.S.J.; Kennedy, K.; Norman, R.S. Building-level wastewater surveillance of SARS-CoV-2 is associated with transmission and variant trends in a university setting. Environ. Res. 2022, 215, 114277. [Google Scholar] [CrossRef]
- Augusto, M.R.; Claro, I.C.M.; Siqueira, A.K.; Sousa, G.S.; Caldereiro, C.R.; Duran, A.F.A.; de Miranda, T.B.; Bomediano Camillo, L.d.M.; Cabral, A.D.; de Freitas Bueno, R. Sampling strategies for wastewater surveillance: Evaluating the variability of SARS-CoV-2 RNA concentration in composite and grab samples. J. Environ. Chem. Eng. 2022, 10, 107478. [Google Scholar] [CrossRef]
- George, A.D.; Kaya, D.; Layton, B.A.; Bailey, K.; Mansell, S.; Kelly, C.; Williamson, K.J.; Radniecki, T.S. Impact of Sampling Type, Frequency, and Scale of the Collection System on SARS-CoV-2 Quantification Fidelity. Environ. Sci. Technol. Lett. 2022, 9, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kmush, B.L.; Monk, D.; Green, H.; Sachs†, D.A.; Zeng, T.; Larsen, D.A. Comparability of 24-hour composite and grab samples for detection of SARS-2-CoV RNA in wastewater. FEMS Microbes 2022, 3, xtac017. [Google Scholar] [CrossRef]
- Länsivaara, A.; Palmroth, M.; Kaarela, O.; Hyöty, H.; Oikarinen, S.; Lehto, K.-M. Virus detection in influent, activated sludge, and effluent from municipal wastewater treatment plants using composite and grab samples in Finland. Environ. Res. 2025, 279, 121776. [Google Scholar] [CrossRef]
- U.S. Centers for Disease Control and Prevention (CDC). CDC’s Influenza SARS-CoV-2 Multiplex Assay. Available online: https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/lab/multiplex.html (accessed on 13 November 2025).
- Robert Koch-Institute and Federal Environment Agency. Wastewater Surveillance AMELAG Data Set. Available online: https://zenodo.org/records/16840446 (accessed on 1 September 2025).
- Ye, Y.; Ellenberg, R.M.; Graham, K.E.; Wigginton, K.R. Survivability, Partitioning, and Recovery of Enveloped Viruses in Untreated Municipal Wastewater. Environ. Sci. Technol. 2016, 50, 5077–5085. [Google Scholar] [CrossRef] [PubMed]
- Mac Mahon, J.; Criado Monleon, A.J.; Gill, L.W.; O’Sullivan, J.J.; Meijer, W.G. Wastewater-based epidemiology (WBE) for SARS-CoV-2—A review focussing on the significance of the sewer network using a Dublin city catchment case study. Water Sci. Technol. 2022, 86, 1402–1425. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, S.; Manayan Parambil, A.; Jayaram, A.; Varamballi, P.; Mukhopadhyay, C.; Jagadesh, A. Surveillance of influenza A and B viruses from community and hospital wastewater treatment plants. Environ. Microbiol. Rep. 2024, 16, e13317. [Google Scholar] [CrossRef]
- Toribio-Avedillo, D.; Gómez-Gómez, C.; Sala-Comorera, L.; Rodríguez-Rubio, L.; Carcereny, A.; García-Pedemonte, D.; Pintó, R.M.; Guix, S.; Galofré, B.; Bosch, A.; et al. Monitoring influenza and respiratory syncytial virus in wastewater. Beyond COVID-19. Sci. Total Environ. 2023, 892, 164495. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chaves, S.S.; Perez, A.; D’Mello, T.; Kirley, P.D.; Yousey-Hindes, K.; Farley, M.M.; Harris, M.; Sharangpani, R.; Lynfield, R.; et al. Comparing Clinical Characteristics Between Hospitalized Adults with Laboratory-Confirmed Influenza A and B Virus Infection. Clin. Infect. Dis. 2014, 59, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Buda, S.; Dürrwald, R.; Biere, B.; Reiche, J.; Buchholz, U.; Tolksdorf, K.; Schilling, J.; Goerlitz, L.; Streib, V.; Preuß, U.; et al. Robert Koch-Institute Influenza Weekly Report Week 20/2021; Publication Server of Robert Koch Institute: Berlin, Germany, 2021. [Google Scholar] [CrossRef]
- Buda, S.; Dürrwald, R.; Biere, B.; Reiche, J.; Buchholz, U.; Tolksdorf, K.; Schilling, J.; Goerlitz, L.; Streib, V.; Preuß, U.; et al. Robert Koch-Institute ARE (Acute Respiratory Diseases) Weekly Report Week 20/2022; Publication Server of Robert Koch Institute: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Buda, S.; Dürrwald, R.; Biere, B.; Reiche, J.; Buchholz, U.; Tolksdorf, K.; Schilling, J.; Goerlitz, L.; Streib, V.; Preuß, U.; et al. Robert Koch-Institute ARE (Acute Respiratory Diseases) Weekly Report Week 14/2023; Publication Server of Robert Koch Institute: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- Buda, S.; Dürrwald, R.; Biere, B.; Reiche, J.; Buchholz, U.; Tolksdorf, K.; Schilling, J.; Goerlitz, L.; Streib, V.; Preuß, U.; et al. Robert Koch-Institute ARE (Acute Respiratory Diseases) Weekly Report Week 49/2023; Publication Server of Robert Koch Institute: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- Buda, S.; Dürrwald, R.; Biere, B.; Reiche, J.; Buchholz, U.; Tolksdorf, K.; Gvaladze, T.; Schilling, J.; Goerlitz, L.; Streib, V.; et al. Robert Koch-Institute ARE (Acute Respiratory Diseases) Weekly Report Week 05/2024; Publication Server of Robert Koch Institute: Berlin, Germany, 2024. [Google Scholar] [CrossRef]
- Buda, S.; Dürrwald, R.; Biere, B.; Reiche, J.; Buchholz, U.; Tolksdorf, K.; Gvaladze, T.; Schilling, J.; Lehfeld, A.-S.; Cai, W.; et al. Robert Koch-Institute ARE (Acute Respiratory Diseases) Weekly Report Week 08/2025; Publication Server of Robert Koch Institute: Berlin, Germany, 2025. [Google Scholar] [CrossRef]
- Robert Koch-Institute. Virological Analyses at the National Reference Center for Influenza Viruses 2024/25 Season. Available online: https://www.rki.de/DE/Themen/Forschung-und-Forschungsdaten/Nationale-Referenzzentren-und-Konsiliarlabore/Influenza/zirkulierende/VirolAnalysen_2024_25.html (accessed on 1 September 2025).
- Robert Koch-Institute. VacMap-Dashboard Vaccination Events in Germany. Available online: https://www.rki.de/vacmap (accessed on 1 September 2025).
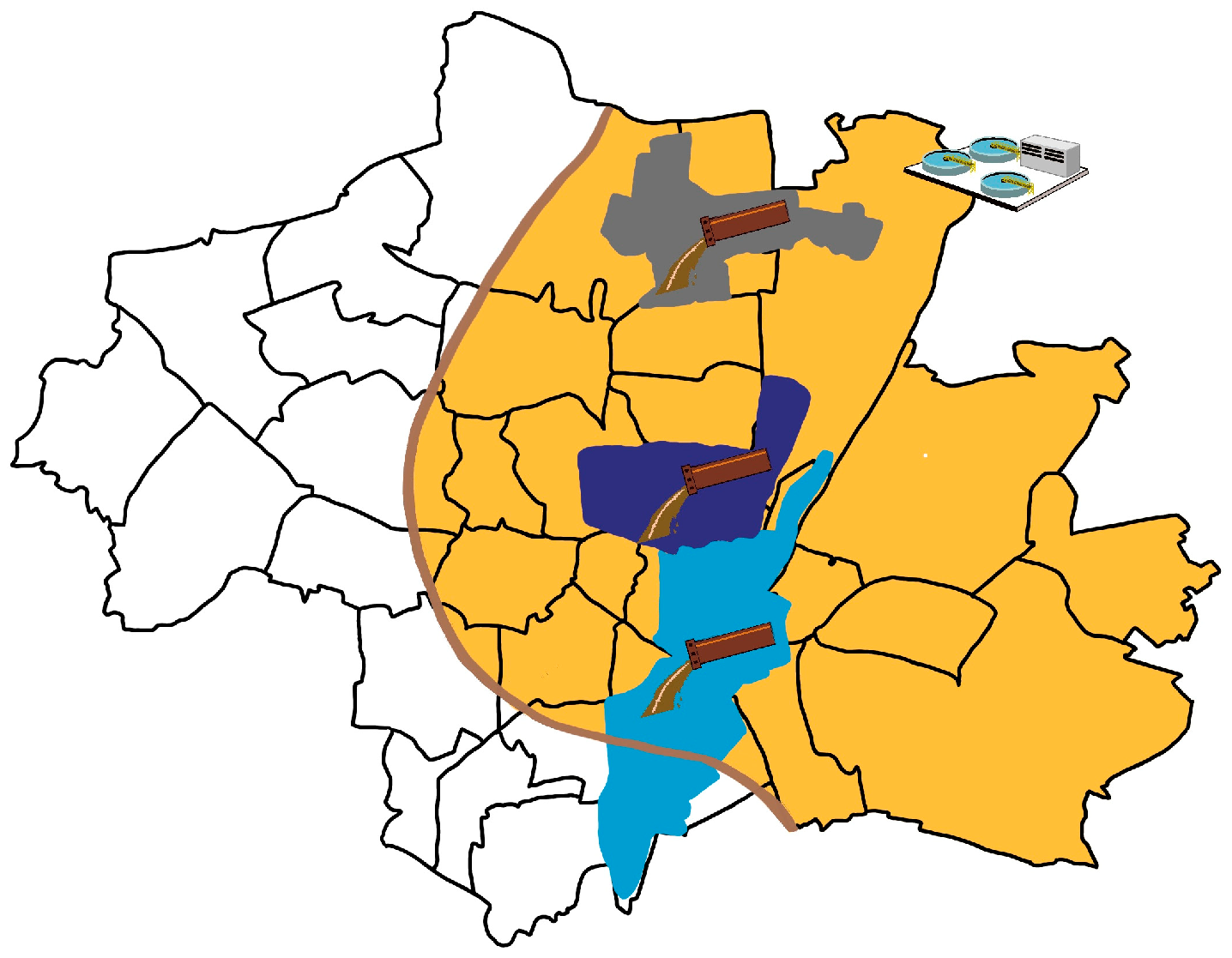
| Catchment Area | Sampling Period | Connected Inhabitants | Maximum Flow [L/s] | Catchment Area Size [ha] | Max. Time Sink to Sampling [h] |
|---|---|---|---|---|---|
| Schmidbartlanger | 22/23, 23/24, 24/25 | 67,000 | 180 | 670 | 5 |
| Schenkendorfstr. | 22/23 | 118,000 | 580 | 1050 | 5 |
| Gysslinger Becken | 22/23 | 158,000 | 630 | 1150 | 5 |
| WWTP Gut Grosslappen | 22/23, 23/24, 24/25 | 961,000 | 9000 | 20,000 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neusser, J.; Zierer, A.; Riedl, A.; Javanmardi, J.; Rubio-Acero, R.; Waldeck, E.; Kletke, T.; Bschorer, A.; Huber, S.; Dudler, P.; et al. High-Resolution Wastewater-Based Surveillance of Three Influenza Seasons (2022–2025) Reveals Distinct Seasonal Patterns of Viral Activity in Munich, Germany. Microorganisms 2025, 13, 2630. https://doi.org/10.3390/microorganisms13112630
Neusser J, Zierer A, Riedl A, Javanmardi J, Rubio-Acero R, Waldeck E, Kletke T, Bschorer A, Huber S, Dudler P, et al. High-Resolution Wastewater-Based Surveillance of Three Influenza Seasons (2022–2025) Reveals Distinct Seasonal Patterns of Viral Activity in Munich, Germany. Microorganisms. 2025; 13(11):2630. https://doi.org/10.3390/microorganisms13112630
Chicago/Turabian StyleNeusser, Jessica, Astrid Zierer, Anna Riedl, Jasmin Javanmardi, Raquel Rubio-Acero, Elisabeth Waldeck, Thomas Kletke, Annemarie Bschorer, Stefanie Huber, Patrick Dudler, and et al. 2025. "High-Resolution Wastewater-Based Surveillance of Three Influenza Seasons (2022–2025) Reveals Distinct Seasonal Patterns of Viral Activity in Munich, Germany" Microorganisms 13, no. 11: 2630. https://doi.org/10.3390/microorganisms13112630
APA StyleNeusser, J., Zierer, A., Riedl, A., Javanmardi, J., Rubio-Acero, R., Waldeck, E., Kletke, T., Bschorer, A., Huber, S., Dudler, P., Hoch, M., Böhmer, M. M., Herr, C., Eberle, U., Sing, A., Ackermann, N., Hoelscher, M., Springer, K., & Wieser, A. (2025). High-Resolution Wastewater-Based Surveillance of Three Influenza Seasons (2022–2025) Reveals Distinct Seasonal Patterns of Viral Activity in Munich, Germany. Microorganisms, 13(11), 2630. https://doi.org/10.3390/microorganisms13112630






