Chemical Inactivation of Bacillus subtilis Endospores Preserves Recombinant Protein Antigenic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Recombinant B. subtilis Spores
2.2. Preparation of the Sporicidal Solutions
2.3. Recombinant Bacillus subtilis Spore Inactivation
2.4. Electron Microscopy of Inactivated Recombinant Bacillus subtilis Spores
2.5. Immunoblot and Immunofluorescence of Inactivated Recombinant Bacillus subtilis Spores
2.6. Immunogenicity of Inactivated B. subtilis Spores Expressing A. baumannii TBDR
2.7. Recovery of Inactivated Recombinant Bacillus subtilis Spores in BALB/c Mice Feces
2.8. Statistical Analysis
3. Results
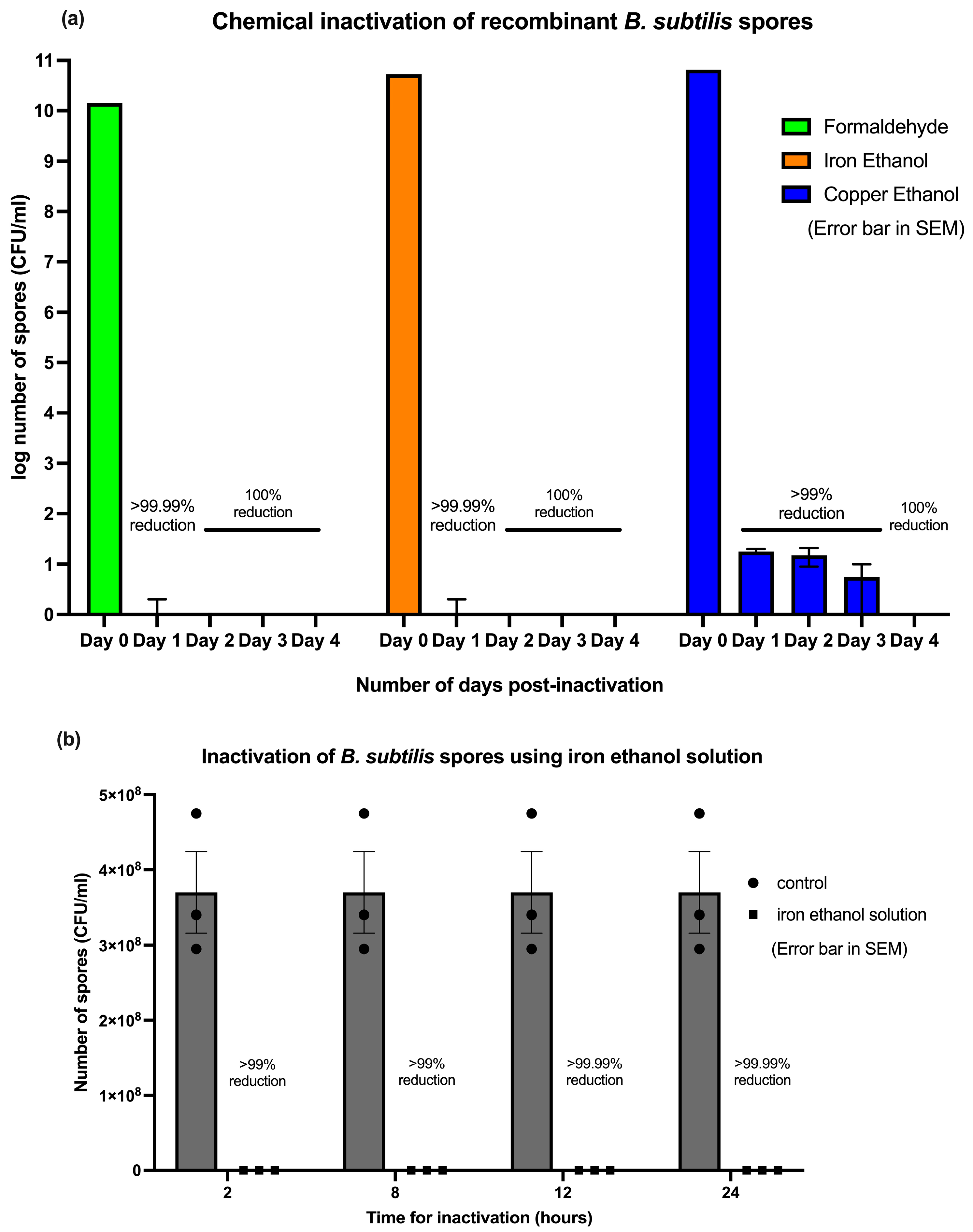
3.1. Inactivation of the B. subtilis Spores Expressing A. baumannii TBDR
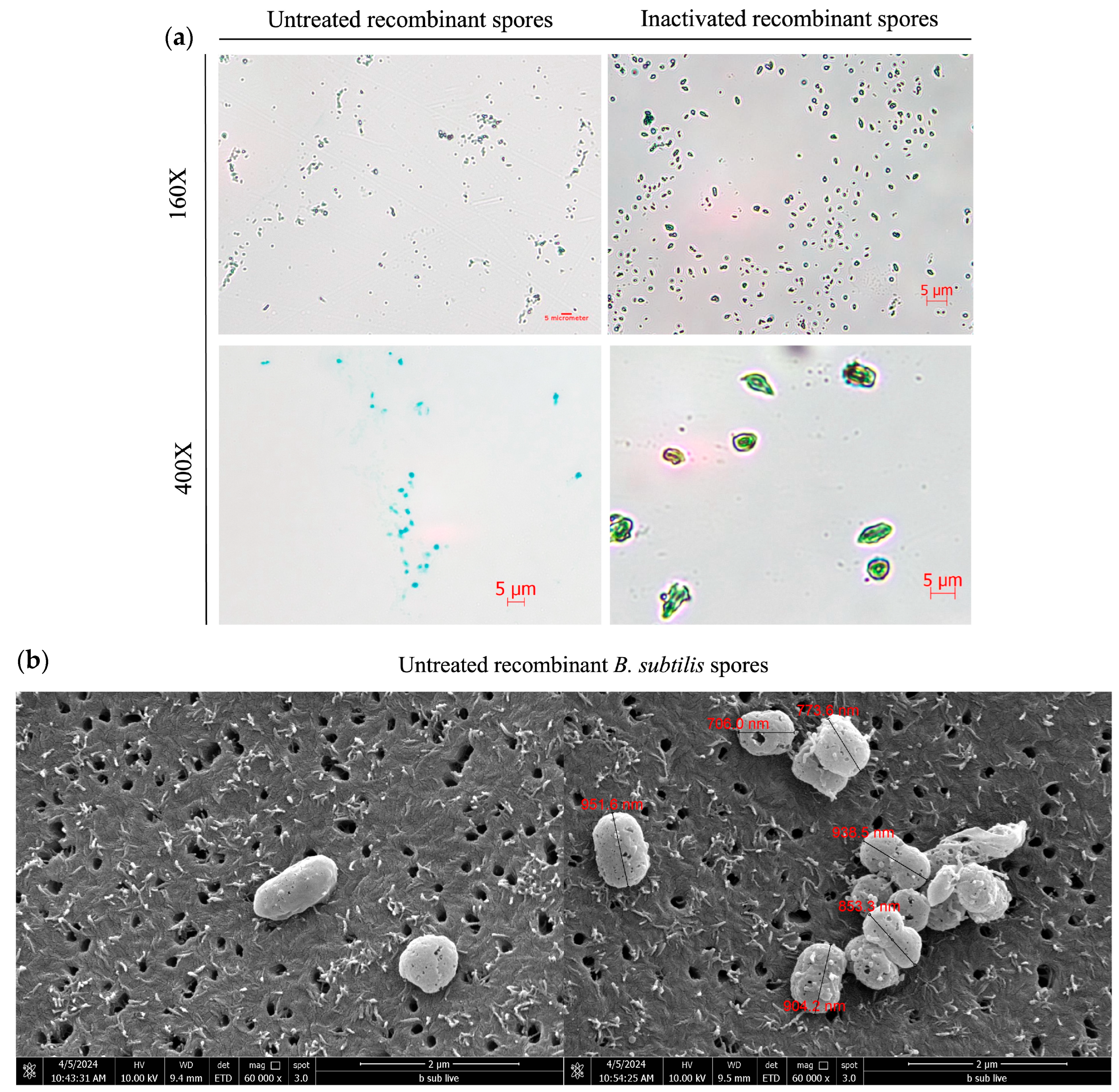
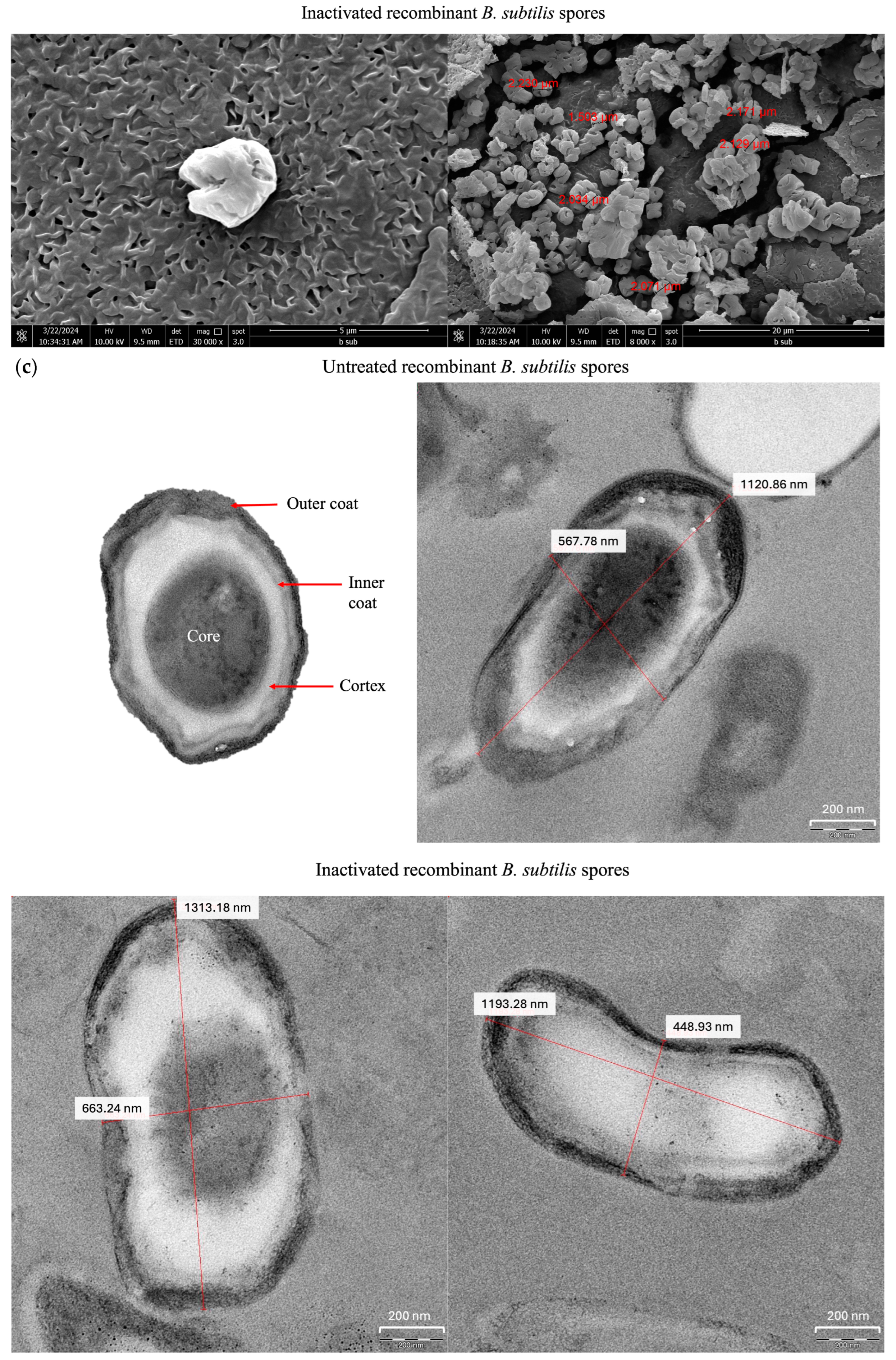
3.2. Morphological Characterization of Inactivated B. subtilis Spores Expressing A. baumannii TBDR
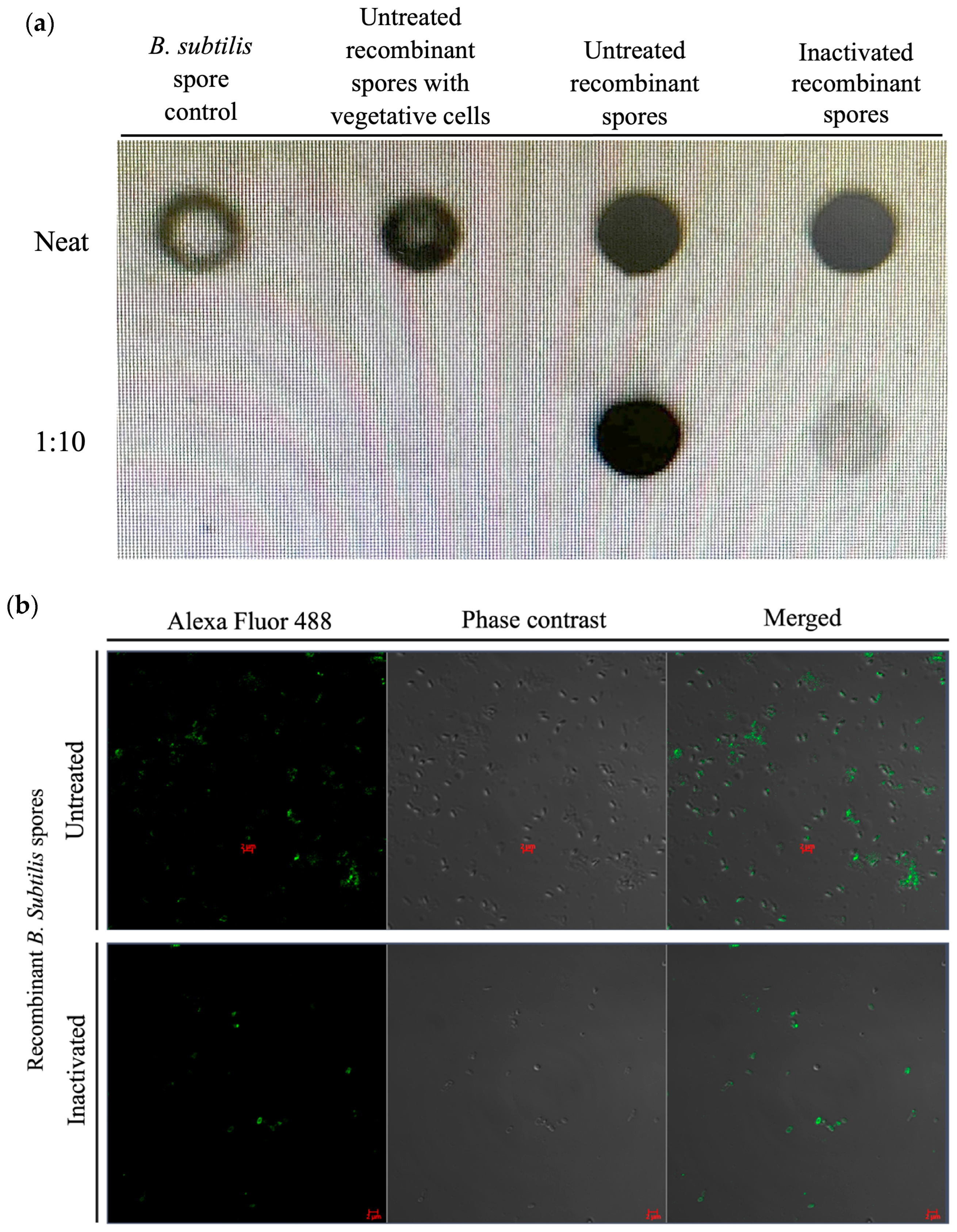
3.3. Surface Protein Detection of Inactivated B. subtilis Spores Expressing A. baumannii TBDR
3.4. Immunogenicity of Inactivated B. subtilis Spores Expressing A. baumannii TBDR
3.5. Recovery of Inactivated Recombinant Bacillus subtilis Spores in BALB/c Mice Feces
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. baumannii | Acinetobacter baumannii |
| B. subtilis | Bacillus subtilis |
| BAL | Broncheoalveolar Lavage |
| BSA | Bovine Serum Albumin |
| CFU | Colony Forming Unit |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked Immunosorbent Assay |
| GRAS | Generally Regarded as Safe |
| IgG | Immunoglobulin G |
| MDR | Multidrug Resistant |
| PEG | Polyethylene Glycol |
| TBDR | TonB Dependent Receptor |
References
- MatRahim, N.A.; Jones, K.M.; Keegan, B.P.; Strych, U.; Zhan, B.; Lee, H.Y.; AbuBakar, S. TonB-Dependent Receptor Protein Displayed on Spores of Bacillus subtilis Stimulates Protective Immune Responses against Acinetobacter baumannii. Vaccines 2023, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Saggese, A.; Baccigalupi, L.; Donadio, G.; Ricca, E.; Isticato, R. The Bacterial Spore as a Mucosal Vaccine Delivery System. Int. J. Mol. Sci. 2023, 24, 10880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; An, Y.; Zabed, H.M.; Guo, Q.; Yang, M.; Yuan, J.; Li, W.; Sun, W.; Qi, X. Bacillus subtilis Spore Surface Display Technology: A Review of Its Development and Applications. J. Microbiol. Biotechnol. 2019, 29, 179–190. [Google Scholar] [CrossRef]
- Williams, N.; Weir, T.L. Spore-Based Probiotic Bacillus subtilis: Current Applications in Humans and Future Perspectives. Fermentation 2024, 10, 78. [Google Scholar] [CrossRef]
- Romero-Rodríguez, A.; Ruiz-Villafán, B.; Martínez-de la Peña, C.F.; Sánchez, S. Targeting the Impossible: A Review of New Strategies against Endospores. Antibiotics 2023, 12, 248. [Google Scholar] [CrossRef]
- Cho, W.I.; Chung, M.S. Bacillus Spores: A Review of Their Properties and Inactivation Processing Technologies. Food Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef]
- Taylor, W.; Camilleri, E.; Craft, D.L.; Korza, G.; Granados, M.R.; Peterson, J.; Szczpaniak, R.; Weller, S.K.; Moeller, R.; Douki, T.; et al. DNA Damage Kills Bacterial Spores and Cells Exposed to 222-Nanometer UV Radiation. Appl. Environ. Microbiol. 2020, 86, e03039-19. [Google Scholar] [CrossRef]
- Helfinstine, S.L.; Vargas-Aburto, C.; Uribe, R.M.; Woolverton, C.J. Inactivation of Bacillus Endospores in Envelopes by Electron Beam Irradiation. Appl. Environ. Microbiol. 2005, 71, 7029–7032. [Google Scholar] [CrossRef]
- Amorim, E.O.C.; Tribst, A.A.L.; Augusto, P.E.D.; Cristianini, M. Inactivation of E. coli and B. subtilis Spores in Ozonized Cassava Starch. Food Sci. Technol. 2013, 33, 289–294. [Google Scholar] [CrossRef]
- Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. [Google Scholar] [CrossRef]
- Billeaud, C. High Hydrostatic Pressure Treatment Ensures the Microbiological Safety of Human Milk Including Bacillus cereus and Preservation of Bioactive Proteins Including Lipase and Immuno-Proteins: A Narrative Review. Foods 2021, 10, 1327. [Google Scholar] [CrossRef]
- Woldemariam, H.W.; Emire, S.A. High Pressure Processing of Foods for Microbial and Mycotoxins Control: Current Trends and Future Prospects. Cogent Food Agric. 2019, 5, 1622184. [Google Scholar] [CrossRef]
- Pedersen, M.R.; Hansen, E.W. Research and Reports Inactivation of B. subtilis Spores and E. coli Endotoxin by Ethylene Oxide. J. Clin. Pharm. Ther. 1989, 14, 373–380. [Google Scholar]
- Setlow, P. Spores of Bacillus subtilis: Their Resistance to and Killing by Radiation, Heat and Chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Rogers, J.V.; Choi, Y.W.; Richter, W.R.; Rudnicki, D.C.; Joseph, D.W.; Sabourin, C.L.K.; Taylor, M.L.; Chang, J.C.S. Formaldehyde Gas Inactivation of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus Spores on Indoor Surface Materials. J. Appl. Microbiol. 2007, 103, 1104–1112. [Google Scholar] [CrossRef]
- Kida, N.; Mochizuki, Y.; Taguchi, F. An Effective Sporicidal Reagent against Bacillus subtilis Spores. Microbiol. Immunol. 2003, 47, 279–283. [Google Scholar] [CrossRef]
- Pan, J.G.; Choi, S.K.; Jung, H.C.; Kim, E.J. Display of Native Proteins on Bacillus subtilis Spores. FEMS Microbiol. Lett. 2014, 358, 209–217. [Google Scholar] [CrossRef]
- Saperi, A.A.; Hazan, A.; Zulkifli, N.; Lee, H.-Y.; MatRahim, N.-A.; AbuBakar, S. Immunization with Inactivated Bacillus subtilis Spores Expressing TonB-Dependent Receptor (TBDR) Protects Against Multidrug-Resistant Acinetobacter baumannii Infection. Vaccines 2025, 13, 616. [Google Scholar] [CrossRef]
- Harrold, Z.R.; Hertel, M.R.; Gorman-Lewis, D. Optimizing Bacillus subtilis Spore Isolation and Quantifying Spore Harvest Purity. J. Microbiol. Methods 2011, 87, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.D.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial Approaches in Tissue Engineering Using Metal Ions and Nanoparticles: From Mechanisms to Applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Govindaraju, M.; Shekar, H.S.; Sateesha, S.B.; Vasudeva Raju, P.; Sambasiva Rao, K.R.; Rao, K.S.J.; Rajamma, A.J. Copper Interactions with DNA of Chromatin and Its Role in Neurodegenerative Disorders. J. Pharm. Anal. 2013, 3, 354–359. [Google Scholar] [CrossRef]
- Morris, D.L. DNA-Bound Metal Ions: Recent Developments. Biomol. Concepts 2014, 5, 397–407. [Google Scholar] [CrossRef]
- Golin, A.P.; Choi, D.; Ghahary, A. Hand Sanitizers: A Review of Ingredients, Mechanisms of Action, Modes of Delivery, and Efficacy against Coronaviruses. Am. J. Infect. Control 2020, 48, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Coleman, W.H.; Chen, D.; Li, Y.Q.; Cowan, A.E.; Setlow, P. How Moist Heat Kills Spores of Bacillus subtilis. J. Bacteriol. 2007, 189, 8458. [Google Scholar] [CrossRef]
- Zhou, W.; Orr, M.W.; Jian, G.; Watt, S.K.; Lee, V.T.; Zachariah, M.R. Inactivation of Bacterial Spores Subjected to Sub-Second Thermal Stress. Chem. Eng. J. 2015, 279, 578–588. [Google Scholar] [CrossRef]
- Klein, V.J.; Irla, M.; López, M.G.; Brautaset, T.; Brito, L.F. Unravelling Formaldehyde Metabolism in Bacteria: Road towards Synthetic Methylotrophy. Microorganisms 2022, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.C.C.; Lai, N.C.Y.; Wu, K.C.; Choi, M.C.; Ma, C.H.Y.; Lin, J.; Kuok, C.N.; Leong, W.L.; Lam, W.K.; Hamied, Y.K.; et al. Safety and Immunogenicity of Inactivated Bacillus subtilis Spores as a Heterologous Antibody Booster for COVID-19 Vaccines. Vaccines 2022, 10, 1014. [Google Scholar] [CrossRef] [PubMed]

| Groups | Number of Recombinant B. subtilis Recovered (CFU/mL) | |||
|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | |
| Diluent control | 0 | 0 | 0 | 0 |
| B. subtilis spores control | 0 | 0 | 0 | 0 |
| Untreated recombinant B. subtilis spores | TNTC | TNTC | 2.2 × 102 | 0 |
| Inactivated recombinant B. subtilis spores | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saperi, A.A.; Hazan, A.; Zulkifli, N.; Lee, H.Y.; AbuBakar, S. Chemical Inactivation of Bacillus subtilis Endospores Preserves Recombinant Protein Antigenic Properties. Microorganisms 2025, 13, 2629. https://doi.org/10.3390/microorganisms13112629
Saperi AA, Hazan A, Zulkifli N, Lee HY, AbuBakar S. Chemical Inactivation of Bacillus subtilis Endospores Preserves Recombinant Protein Antigenic Properties. Microorganisms. 2025; 13(11):2629. https://doi.org/10.3390/microorganisms13112629
Chicago/Turabian StyleSaperi, Amalia A., Atiqah Hazan, Nurfatihah Zulkifli, Hai Yen Lee, and Sazaly AbuBakar. 2025. "Chemical Inactivation of Bacillus subtilis Endospores Preserves Recombinant Protein Antigenic Properties" Microorganisms 13, no. 11: 2629. https://doi.org/10.3390/microorganisms13112629
APA StyleSaperi, A. A., Hazan, A., Zulkifli, N., Lee, H. Y., & AbuBakar, S. (2025). Chemical Inactivation of Bacillus subtilis Endospores Preserves Recombinant Protein Antigenic Properties. Microorganisms, 13(11), 2629. https://doi.org/10.3390/microorganisms13112629







