COVID-19 is Associated with a Lipid Storm that Worsens in Cases of Severe Pneumonia
Abstract
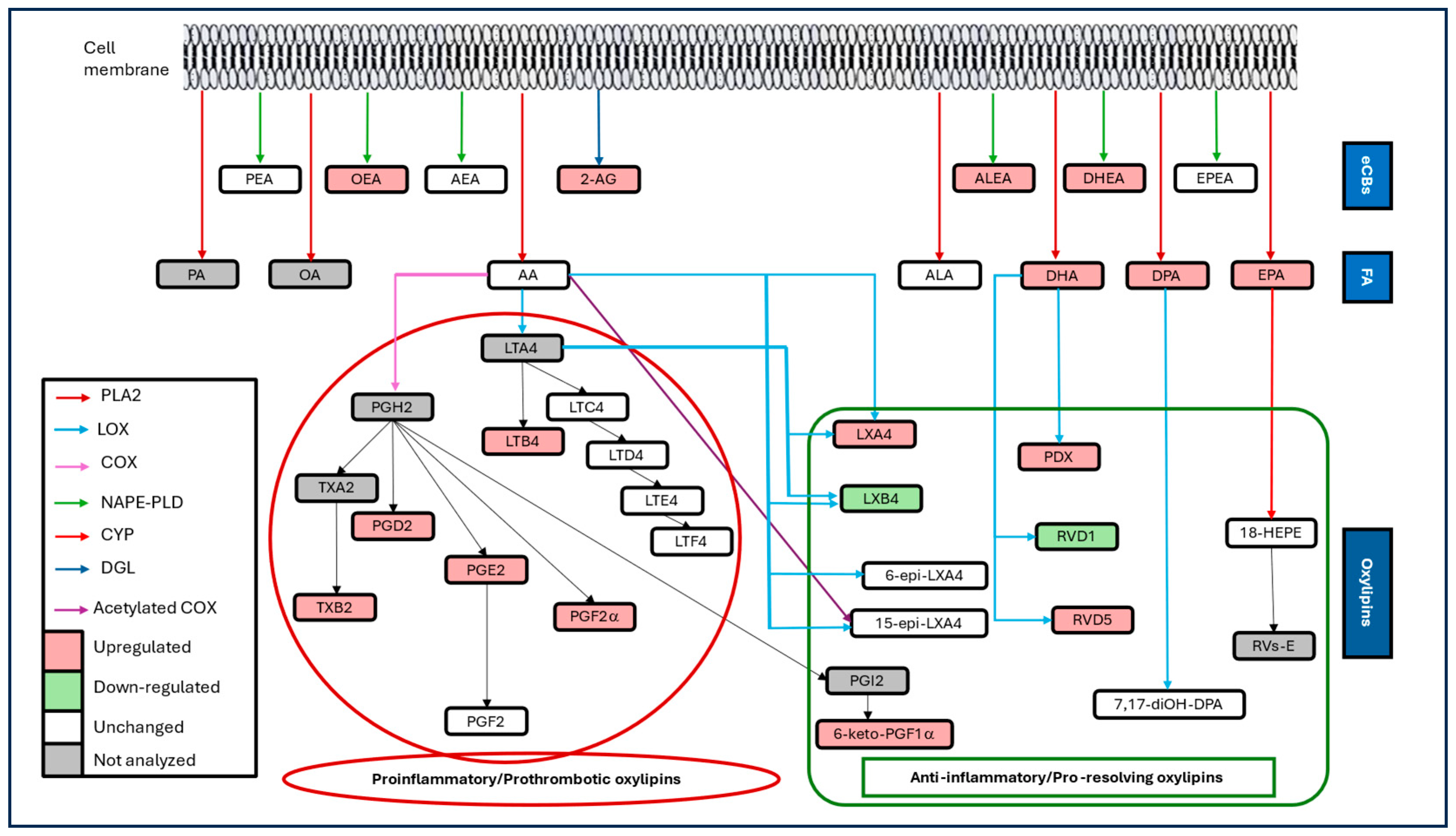
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Collection and Lipid Mediators Profiling
2.3. Statistical Analysis
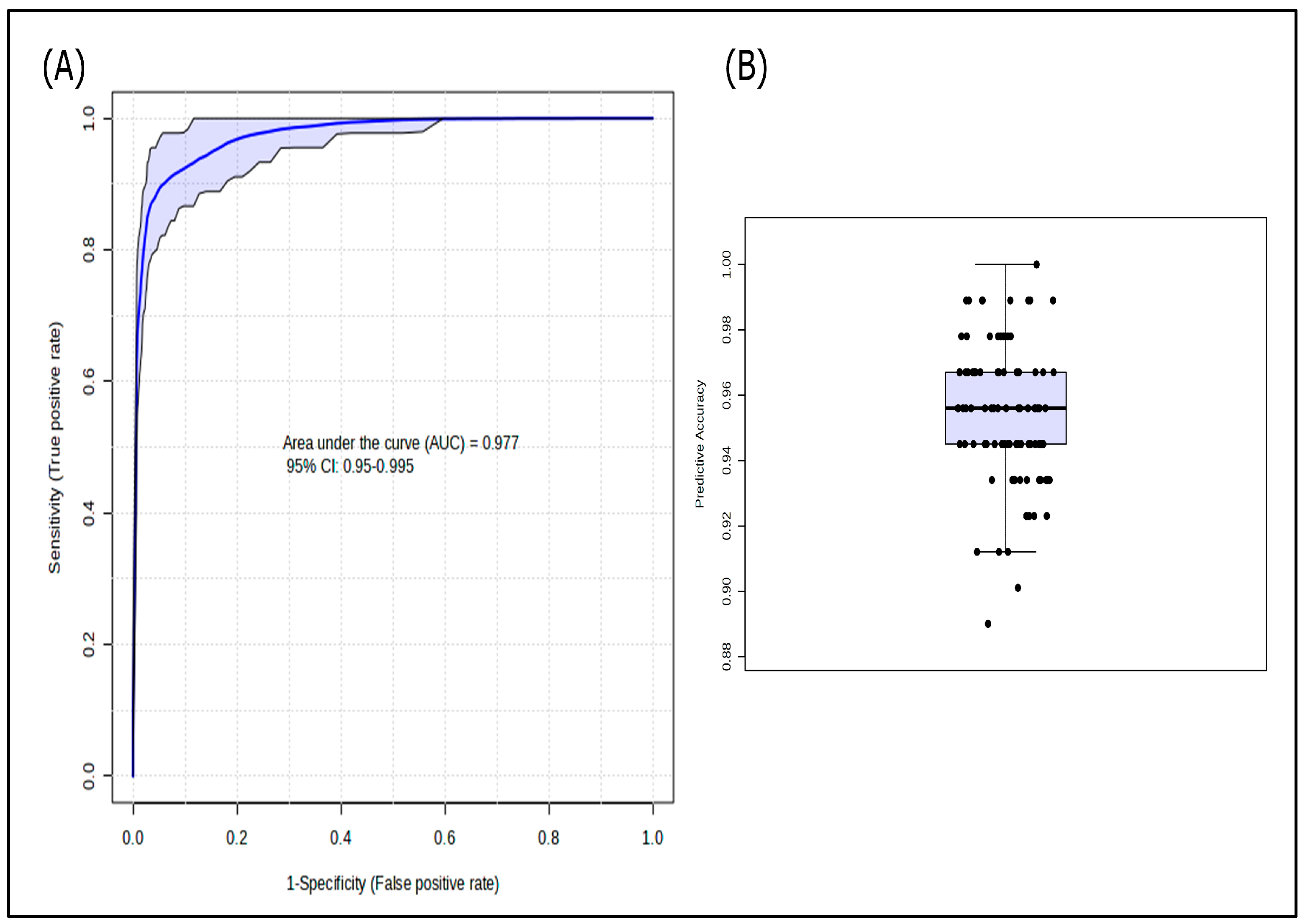
3. Results
3.1. Participants and Disease Characteristics
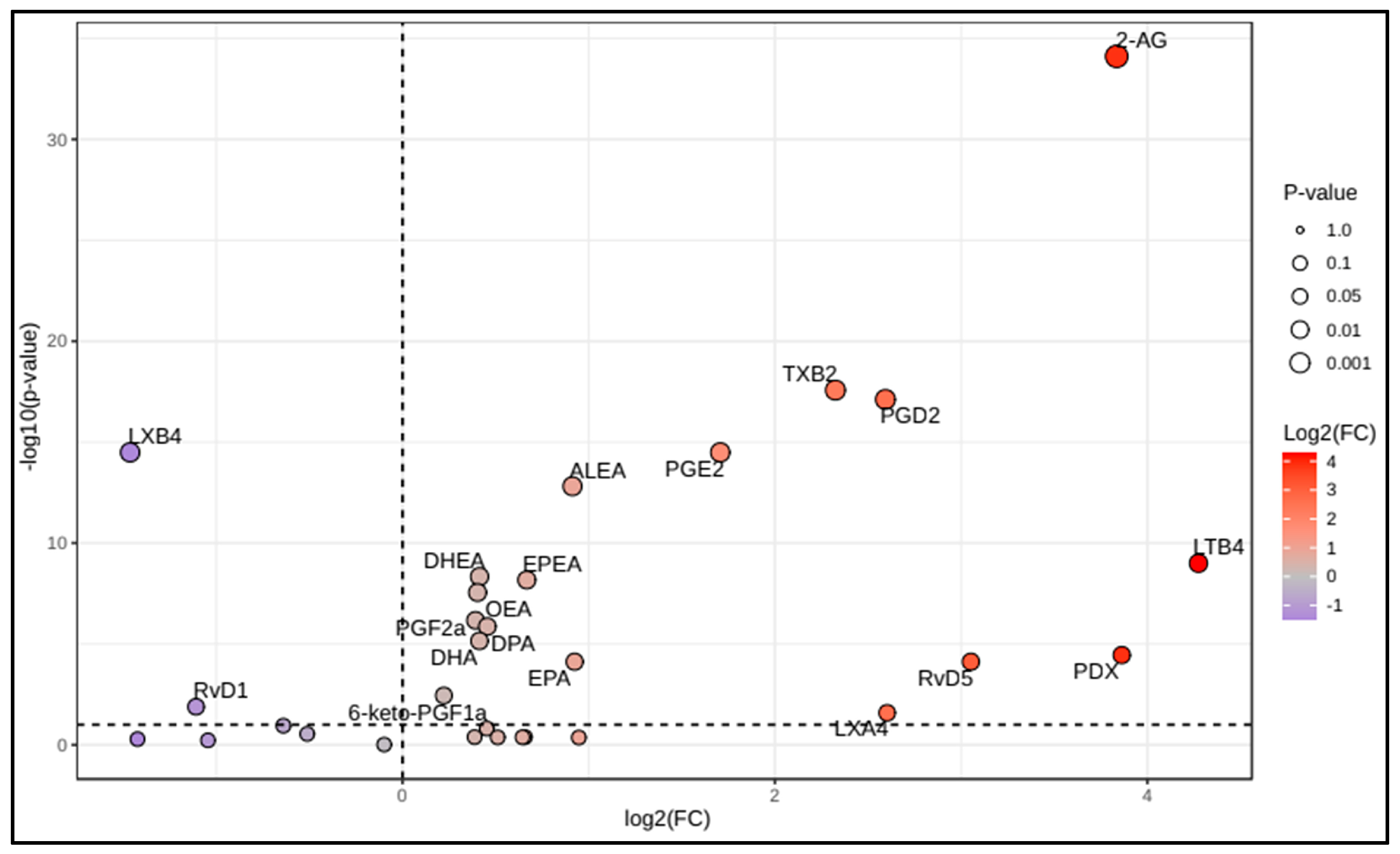
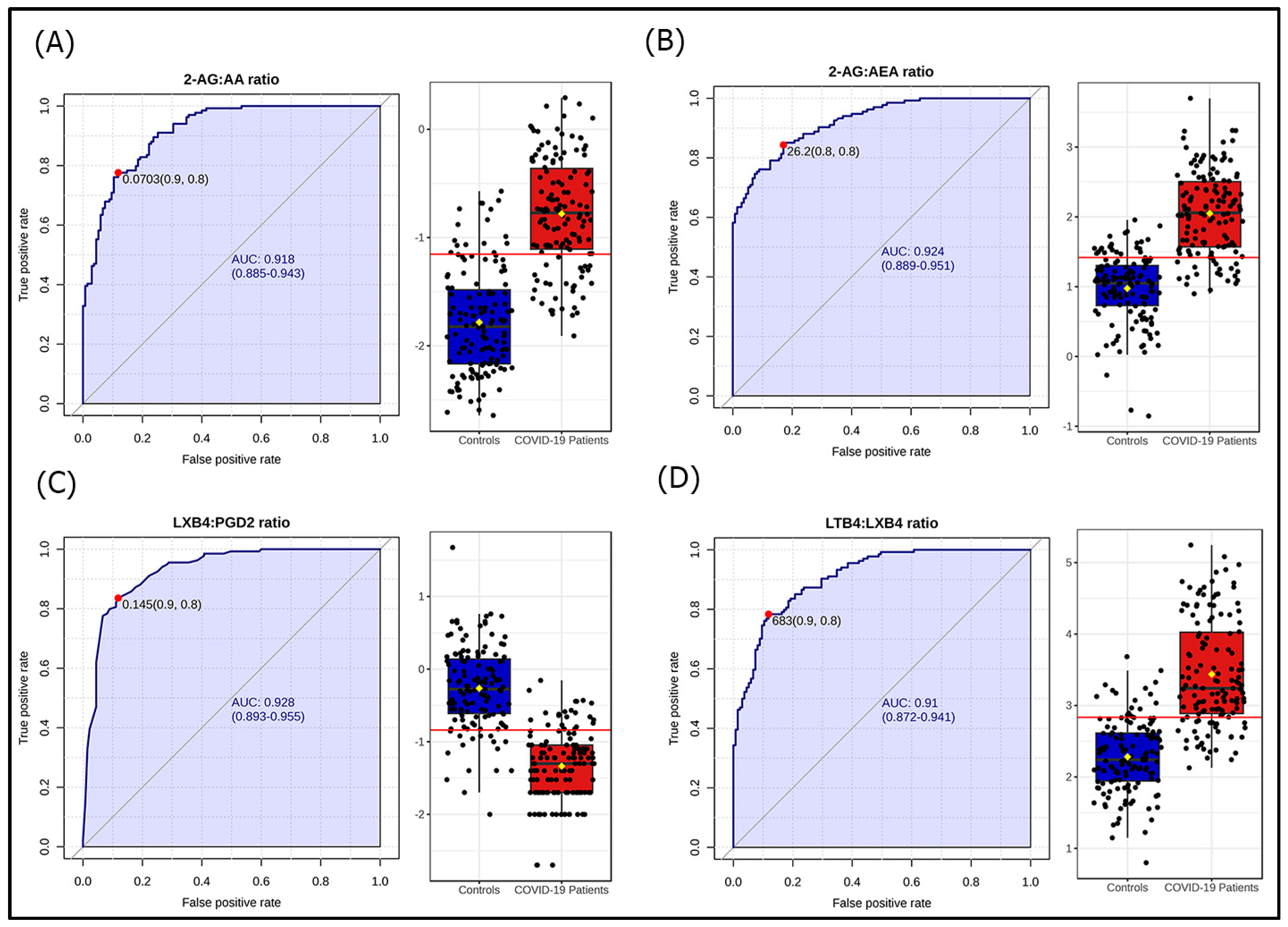
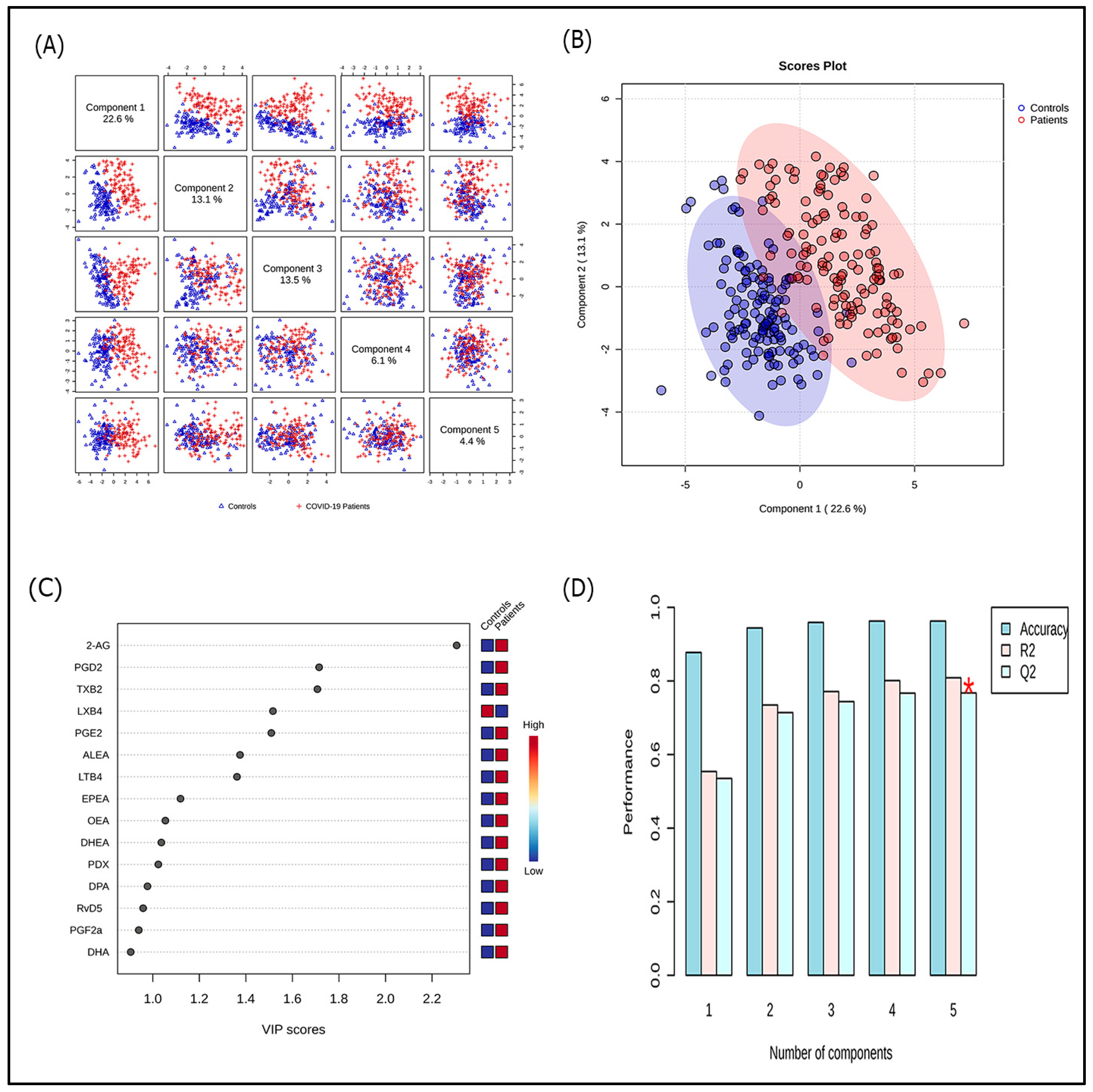
3.2. Lipidomic Profile in COVID-19 Patients
3.3. Lipidomic Profile According to Disease Severity and Selected Adverse Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18-HEPE | 18-hydroxyeicosapentaenoic acid |
| 2-AG | 2-arachidonoyl glycerol |
| AA | Arachidonic acid |
| AEA | Anandamide |
| ALA | Alpha-linolenic acid |
| ALEA | Alpha-linolenoylethanolamide |
| AUC | Area Under the Curve |
| COVID-19 | Coronavirus disease 2019 |
| COX | Cyclooxygenase |
| CYP | Cytochrome P450 |
| DHA | Docosahexaenoic acid |
| DHEA | Docosahexaenoylethanolamide |
| DPA | Docosapentaenoic acid |
| eCBs | Endocannabinoids |
| ECS | Endocannabinoid system |
| EPA | Eicosapentaenoic acid |
| EPEA | Eicosapentaenoylethanolamide |
| LC-MS/MS | Liquid chromatography coupled to mass spectrometry in tandem |
| LMs | Lipid mediators |
| LOX | Lipoxygenase |
| LTs | Leukotrienes |
| LXs | Lipoxins |
| OEA | Oleoylethanolamide |
| PDs | Protectins |
| PEA | Palmitoylethanolamide |
| PGs | Prostaglandins |
| PLS-DA | Partial least squares-discriminant analysis |
| PUFAs | Polyunsaturated fatty acids |
| ROC | Receiver Operating Characteristic |
| RVs | Resolvins |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SPMs | Specialized pro-resolving mediators |
| TX | Thromboxane |
| VIP | Variable importance in the projection |
References
- COVID-19 Deaths|WHO COVID-19 Dashboard. Available online: http://data.who.int/dashboards/covid19/cases (accessed on 19 September 2025).
- Lopes-Pacheco, M.; Silva, P.L.; Cruz, F.F.; Battaglini, D.; Robba, C.; Pelosi, P.; Morales, M.M.; Neves, C.C.; Rocco, P.R.M. Pathogenesis of Multiple Organ Injury in COVID-19 and Potential Therapeutic Strategies. Front. Physiol. 2021, 12, 593223. [Google Scholar] [CrossRef]
- Silva, M.J.A.; Ribeiro, L.R.; Gouveia, M.I.M.; Marcelino, B.d.R.; Santos, C.S.d.; Lima, K.V.B.; Lima, L.N.G.C. Hyperinflammatory Response in COVID-19: A Systematic Review. Viruses 2023, 15, 553. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated Fatty Acids and Fatty Acid-Derived Lipid Mediators: Recent Advances in the Understanding of Their Biosynthesis, Structures, and Functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and Pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Novel Pro-Resolving Lipid Mediators in Inflammation Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Novel Lipid Mediators and Resolution Mechanisms in Acute Inflammation: To Resolve or Not? Am. J. Pathol. 2010, 177, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Marzo, V.D.; Bifulco, M.; Petrocellis, L.D. The Endocannabinoid System and Its Therapeutic Exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/Chemokine-Receptor System. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Schwarz, B.; Sharma, L.; Roberts, L.; Peng, X.; Bermejo, S.; Leighton, I.; Casanovas-Massana, A.; Minasyan, M.; Farhadian, S.; Ko, A.I.; et al. Cutting Edge: Severe SARS-CoV-2 Infection in Humans Is Defined by a Shift in the Serum Lipidome, Resulting in Dysregulation of Eicosanoid Immune Mediators. J. Immunol. 2021, 206, 329–334. [Google Scholar] [CrossRef]
- Koenis, D.S.; Beegun, I.; Jouvene, C.C.; Aguirre, G.A.; Souza, P.R.; Gonzalez-Nunez, M.; Ly, L.; Pistorius, K.; Kocher, H.M.; Ricketts, W.; et al. Disrupted Resolution Mechanisms Favor Altered Phagocyte Responses in COVID-19. Circ. Res. 2021, 129, e54–e71. [Google Scholar] [CrossRef]
- Regidor, P.-A.; De La Rosa, X.; Santos, F.G.; Rizo, J.M.; Gracia Banzo, R.; Silva, R.S. Acute Severe SARS COVID-19 Patients Produce pro-Resolving Lipids Mediators and Eicosanoids. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6782–6796. [Google Scholar] [CrossRef]
- McReynolds, C.B.; Cortes-Puch, I.; Ravindran, R.; Khan, I.H.; Hammock, B.G.; Shih, P.-A.B.; Hammock, B.D.; Yang, J. Plasma Linoleate Diols Are Potential Biomarkers for Severe COVID-19 Infections. Front. Physiol. 2021, 12, 663869. [Google Scholar] [CrossRef]
- Palmas, F.; Clarke, J.; Colas, R.A.; Gomez, E.A.; Keogh, A.; Boylan, M.; McEvoy, N.; McElvaney, O.J.; McElvaney, O.; Alalqam, R.; et al. Dysregulated Plasma Lipid Mediator Profiles in Critically Ill COVID-19 Patients. PLoS ONE 2021, 16, e0256226. [Google Scholar] [CrossRef]
- Pérez, M.M.; Pimentel, V.E.; Fuzo, C.A.; da Silva-Neto, P.V.; Toro, D.M.; Fraga-Silva, T.F.C.; Gardinassi, L.G.; Oliveira, C.N.S.; Souza, C.O.S.; Torre-Neto, N.T.; et al. Acetylcholine, Fatty Acids, and Lipid Mediators re Linked to COVID-19 Severity. J. Immunol. 2022, 209, 250–261. [Google Scholar] [CrossRef]
- Castañé, H.; Iftimie, S.; Baiges-Gaya, G.; Rodríguez-Tomàs, E.; Jiménez-Franco, A.; López-Azcona, A.F.; Garrido, P.; Castro, A.; Camps, J.; Joven, J. Machine Learning and Semi-Targeted Lipidomics Identify Distinct Serum Lipid Signatures in Hospitalized COVID-19-Positive and COVID-19-Negative Patients. Metabolism 2022, 131, 155197. [Google Scholar] [CrossRef]
- Karu, N.; Kindt, A.; Lamont, L.; van Gammeren, A.J.; Ermens, A.A.M.; Harms, A.C.; Portengen, L.; Vermeulen, R.C.H.; Dik, W.A.; Langerak, A.W.; et al. Plasma Oxylipins and Their Precursors Are Strongly Associated with COVID-19 Severity and with Immune Response Markers. Metabolites 2022, 12, 619. [Google Scholar] [CrossRef]
- Borras, E.; McCartney, M.M.; Rojas, D.E.; Hicks, T.L.; Tran, N.K.; Tham, T.; Juarez, M.M.; Franzi, L.; Harper, R.W.; E Davis, C.; et al. Oxylipin Concentration Shift in Exhaled Breath Condensate (EBC) of SARS-CoV-2 Infected Patients. J. Breath Res. 2023, 17, 047103. [Google Scholar] [CrossRef]
- Biagini, D.; Franzini, M.; Oliveri, P.; Lomonaco, T.; Ghimenti, S.; Bonini, A.; Vivaldi, F.; Macera, L.; Balas, L.; Durand, T.; et al. MS-Based Targeted Profiling of Oxylipins in COVID-19: A New Insight into Inflammation Regulation. Free Radic. Biol. Med. 2022, 180, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.; Jha, R.R.; Ortori, C.A.; Lunt, E.; Tighe, P.J.; Irving, W.L.; Gohir, S.A.; Kim, D.-H.; Valdes, A.M.; Tarr, A.W.; et al. Serum Levels of Proinflammatory Lipid Mediators and Specialized Proresolving Molecules Are Increased in Patients with Severe Acute Respiratory Syndrome Coronavirus 2 and Correlate with Markers of the Adaptive Immune Response. J. Infect. Dis. 2022, 225, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Irún, P.; Gracia, R.; Piazuelo, E.; Pardo, J.; Morte, E.; Paño, J.R.; Boza, J.; Carrera-Lasfuentes, P.; Higuera, G.A.; Lanas, A. Serum Lipid Mediator Profiles in COVID-19 Patients and Lung Disease Severity: A Pilot Study. Sci. Rep. 2023, 13, 6497. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.C.S.; da Silva-Neto, P.V.; Toro, D.M.; Fuzo, C.A.; Nardini, V.; Pimentel, V.E.; Pérez, M.M.; Fraga-Silva, T.F.C.; Oliveira, C.N.S.; Degiovani, A.M.; et al. The Interplay among Glucocorticoid Therapy, Platelet-Activating Factor and Endocannabinoid Release Influences the Inflammatory Response to COVID-19. Viruses 2023, 15, 573. [Google Scholar] [CrossRef]
- Velasco, M.; Posada-Ayala, M.; Pérez-Fernández, E.; Loria, F.; Amores, M.; Ramos, J.M.; Jaime, E.; Guijarro, C.; Romero, J.; Pazos, M.R. Circulating Endocannabinoid Levels in SARS-CoV-2 Infection and Their Potential Role in the Inflammatory Response. Sci. Rep. 2024, 14, 19558. [Google Scholar] [CrossRef]
- Basu, A.; Pommerolle, L.; Arif, M.; Meliton, A.Y.; Udofia, I.; Wu, D.; Mutlu, G.M.; Gochuico, B.R.; Summer, R.; Adegunsoye, A.; et al. Elevated Blood Anandamide Levels in Acute COVID-19 Pneumonia with Respiratory Failure. Am. J. Med. Sci. 2025, 370, 271–277. [Google Scholar] [CrossRef]
- Parchem, K.; Letsiou, S.; Petan, T.; Oskolkova, O.; Medina, I.; Kuda, O.; O'DOnnell, V.B.; Nicolaou, A.; Fedorova, M.; Bochkov, V.; et al. Oxylipin Profiling for Clinical Research: Current Status and Future Perspectives. Prog. Lipid Res. 2024, 95, 101276. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of Tunisia. [Situation Update in Tunisia—Coronavirus]. Point de Situation en Tunisie—Coronavirus. Available online: http://coronavirus.rns.tn/point-de-situation-en-tunisie/ (accessed on 10 August 2025).
- Farhan, A.; Ayed, K.; Zayati, S.; Akrout, R.; Dlala, A.; Abouda, A.; Zoghlami, N.; Mahjoubi, H.; Labbane, I.; Gati, A. Optimizing Clinical Management of COVID-19: A Predictive Model for Unvaccinated Patients Admitted to ICU. Pathogens 2025, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, F.; Taktak, A.; Gargouri, S.; Chtourou, A.; Kharrat, R.; Rebai, A.; Feki-Berrajah, L.; Karray-Hakim, H. Impact of the COVID-19 Pandemic on the Molecular Epidemiology of Respiratory Rhinoviruses and Enteroviruses in Tunisia. Virology 2025, 610, 110624. [Google Scholar] [CrossRef]
- Guideline Clinical Management of COVID-19 Patients: Living Guideline. 18 November 2021. Available online: https://iris.who.int/server/api/core/bitstreams/670e3b89-b4ae-429b-abee-7b0d3d54af49/content (accessed on 18 September 2025).
- Ben-Mustapha, Y.; Ben-Fradj, M.K.; Hadj-Taieb, S.; Serghini, M.; Ben Ahmed, M.; Boubaker, J.; Feki, M. Altered Mucosal and Plasma Polyunsaturated Fatty Acids, Oxylipins, and Endocannabinoids Profiles in Crohn’s Disease. Prostaglandins Other Lipid Mediat. 2023, 168, 106741. [Google Scholar] [CrossRef]
- Jones, C.M.; Athanasiou, T. Summary Receiver Operating Characteristic Curve Analysis Techniques in the Evaluation of Diagnostic Tests. Ann. Thorac. Surg. 2005, 79, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Gilligan, M.M.; Huang, S.; Gartung, A.; Cortés-Puch, I.; Sime, P.J.; Phipps, R.P.; Serhan, C.N.; Hammock, B.D. Inflammation Resolution: A Dual-Pronged Approach to Averting Cytokine Storms in COVID-19? Cancer Metastasis Rev. 2020, 39, 337–340. [Google Scholar] [CrossRef]
- Das, U.N. Bioactive Lipid-Based Therapeutic Approach to COVID-19 and Other Similar Infections. Arch. Med. Sci. AMS 2021, 19, 1327–1359. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid Storm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523, Erratum in Nat. Rev. Immunol. 2015, 15, 724. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Gosselin, D.; Reichart, D.; Glass, C.K.; Dennis, E.A. Phospholipase A2 Regulates Eicosanoid Class Switching During Inflammasome Activation. Proc. Natl. Acad. Sci. USA 2014, 111, 12746–12751. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Calder, P.C. Bioactive Omega-3 Fatty Acids are Associated with Reduced Risk and Severity of SARS-CoV-2 Infection. Am. J. Clin. Nutr. 2023, 117, 213–215. [Google Scholar] [CrossRef]
- Mbow, M.; Hoving, D.; Cisse, M.; Diallo, I.; Honkpehedji, Y.J.; Huisman, W.; Pothast, C.R.; Jongsma, M.L.M.; König, M.H.; de Kroon, A.C.; et al. Immune Responses to SARS-CoV-2 in Sub-Saharan Africa and Western Europe: A Retrospective, Population-Based, Cross-Sectional Study. Lancet Microbe 2025, 6, 100942. [Google Scholar] [CrossRef]
- Yue, H.-Y.; Zeng, J.; Wang, Y.; Deng, M.-J.; Peng, W.; Tan, X.; Jiang, H. Efficacy of Omega-3 Fatty Acids for Hospitalized COVID-19 Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Asia Pac. J. Clin. Nutr. 2023, 32, 308–320. [Google Scholar] [CrossRef] [PubMed]
| COVID-19 Form | |||
|---|---|---|---|
| All Forms (n = 134) | Non-Severe (n = 49) | Severe (n = 85) | |
| General characteristics | |||
| Female | 53 (39.6%) | 16 (32.7%) | 37 (43.5%) |
| Age, year | 63.3 ± 14.1 | 61.5 ± 14.3 | 64.4 ± 13.7 |
| Age ≥ 65 years | 66 (49.3%) | 22 (44.9%) | 44 (51.8%) |
| Body mass index, kg/m2 | 28.3 ± 5.09 | 27.3 ± 4.37 | 28.9 ± 5.40 |
| Tobacco smoking | 49 (36,6%) | 15 (30.6%) | 34 (40.0%) |
| Statins use | 16 (11.9%) | 5 (10.2%) | 11 (12.9%) |
| Aspirin use | 12 (9.0%) | 5 (10.2%) | 7 (8.20%) |
| Anticoagulation therapy | 6 (4.5%) | 3 (6.10%) | 3 (3.50%) |
| Comorbidities | |||
| Diabetes | 70 (52.2%) | 22 (44.9%) | 48 (56.5%) |
| Hypertension | 80 (59.7%) | 28 (57.1%) | 52 (61.2%) |
| Obesity | 39 (29.1%) | 13 (26.5%) | 26 (30.6%) |
| Cardiac disease | 24 (17.9%) | 8 (16.3%) | 15 (17.6%) |
| Respiratory disease | 12 (9.0%) | 5 (10.20%) | 7 (8.20%) |
| Thyroid disorder | 8 (6.0%) | 4 (8.20%) | 4 (4.70%) |
| Symptoms | |||
| Days since symptoms onset | 10 ± 3.70 | 11.2 ± 3.54 | 11.1 ± 3.74 |
| Fatigue | 113 (84.3%) | 36 (73.5%) | 77 (90.6%) ** |
| Cough | 96 (71.6%) | 34 (69.4%) | 62 (72.9%) |
| Fever | 93 (69.4%) | 38 (77.6%) | 55 (64.7%) |
| Dyspnoea | 78 (58.2%) | 25 (51.0%) | 53 (62.4%) |
| Myalgia | 73 (54.4%) | 28 (57.1%) | 45 (52.9%) |
| Arthralgia | 53 (39.6%) | 19 (38.8%) | 34 (40.0%) |
| Chills | 54 (40.3%) | 20 (40.8%) | 34 (40.0%) |
| Headache | 48 (35.8%) | 15 (30.6%) | 33 (38.8%) |
| Anorexia | 42 (31.3%) | 15 (30.6%) | 27 (31.8%) |
| Diarrhoea | 38 (28.4%) | 9 (18.4%) | 29 (34.1%) * |
| Sweating | 35 (26.1%) | 14 (28.6%) | 21 (24.7%) |
| Chest pain | 24 (17.9%) | 5 (10.2%) | 19 (22.4%) |
| Nausea/vomiting | 23 (17.2%) | 6 (12.2%) | 17 (20.0%) |
| Anosmia | 26 (19.4%) | 12 (24.5%) | 14 (16.5%) |
| Ageusia | 21 (15.7%) | 7 (14.3%) | 14 (16.5%) |
| Vertigo | 16 (11.9%) | 5 (10.2%) | 11 (12.9%) |
| Initial examination | |||
| Temperature, °C | 37.2 ± 0.82 | 37.1 ± 0.95 | 37.3 ± 0.78 |
| Fever | 19 (14.2%) | 6 (12.2%) | 13 (15.3%) |
| Respiratory rate, cpm | 27.1 ± 6.04 | 22.9 ± 4.43 | 28.9 ± 5.30 *** |
| Respiratory rate > 30 cpm | 27 (20.1%) | 0 | 27 (31.8%) *** |
| SpO2 in room air 1, % | 88 ± 5.14 | 92.7 ± 2.34 | 85.7 ± 4.75 |
| SpO2 < 88% in room air | 41 (35.7%) | 0 | 41 (58.6%) *** |
| PaO2/FiO2, mm Hg | 230 ± 82.0 | 284 ± 80.2 | 201 ± 67.6 *** |
| PaO2/FiO2 < 300 mm Hg | 106 (80.3%) | 26 (55.3%) | 80 (94.1%) *** |
| C-reactive protein, mg/L | 170 (66–164) | 93 (43–126) | 126 (79–201) *** |
| D-dimers, ng/mL | 662 (475–1283) | 577 (360–909) | 742 (530–1493) ** |
| Controls (n = 135) | Patients (n = 134) | p-Value | |
|---|---|---|---|
| Polyunsaturated fatty acids, ng/mL | |||
| Alpha-linolenic acid | 877 (497–1683) | 973 (500–1579) | 0.969 |
| Arachidonic acid | 613 (296–1450) | 547 (343–898) | 0.210 |
| Eicosapentaenoic acid | 74 (39–185) | 147 (61–389) | <0.001 |
| Docosapentaenoic acid | 188 (101–378) | 321 (203–513) | <0.001 |
| Docosahexaenoic acid | 476 (268–926) | 780 (474–1130) | <0.001 |
| Oxylipins, pg/mL | |||
| Proinflammatory oxylipins | |||
| 6-keto-prostaglandin F1a | 5.76 (3.38–9.94) | 7.65 (4.58–13) | 0.002 |
| Prostaglandin F2a | 18.0 (10.7–28.3) | 27.9 (17.8–40.8) | <0.001 |
| Prostaglandin E2 | 7.86 (4.41–13.1) | 20.2 (10.3–47.2) | <0.001 |
| Prostaglandin D2 | 8.21 (4.66–12.4) | 35.4 (11.1–90.5) | <0.001 |
| Thromboxane B2 | 49.6 (28.6–112) | 264 (82.5–556) | <0.001 |
| Leukotriene B4 | 838 (344–1878) | 2850 (762–19,560) | <0.001 |
| Leukotriene D4 | 8.48 (8.48–8.48) | 7.12 (3.38–13.1) | 0.328 |
| Leukotriene E4 | 10.6 (5.25–10.6) | 7.14 (2.03–13.1) | 0.109 |
| Leukotriene F4 | 2.79 (2.79–6.59) | 4.05 (1.3–8.92) | 0.324 |
| ∑ proinflammatory oxylipins | 995 (516–2047) | 3292 (1286–20,983) | <0.001 |
| Pro-resolving oxylipins (SPMs) | |||
| 6-epi-lipoxin A4 | 2.76 (1.35–4.43) | 3.63 (0.82–25.1) | 0.348 |
| Lipoxin A4 | 2.35 (1.36–4.34) | 4.07 (0.99–25.8) | 0.016 |
| 15-epi-lipoxin A4 | 0.47 (0.26–1.22) | 0.43 (0.21–1.02) | 0.075 |
| Lipoxin B4 | 4.53 (2.4–7.66) | 1.48 (0.74–3.03) | <0.001 |
| Resolvin D1 | 3.72 (1.57–14.3) | 2.72 (1.21–5.78) | 0.008 |
| Resolvin D5 | 4.33 (2.22–8.12) | 8.15 (2.51–35.9) | <0.001 |
| 7,17-di-OH-DPA | 9.41 (5.28–18.5) | 11.1 (4.02–32.5) | 0.389 |
| 18-HEPE | 18.2 (12.9–33.4) | 24.9 (7.55–69.9) | 0.367 |
| Protectin DX | 2.72 (1.71–6.07) | 8.35 (1.92–59.4) | <0.001 |
| ∑ pro-resolving oxylipins | 54.4 (40.1–105) | 87.2 (31.2–280) | 0.033 |
| Endocannabinoids, ng/mL | |||
| 2-arachidonoylglycerol | 9.02 (5.90–16.6) | 91.6 (32.5–258) | <0.001 |
| Palmitoylethanolamide | 2.47 (1.84–10.8) | 2.92 (2.21–4.03) | 0.484 |
| Oleoylethanolamide | 2.19 (1.57–2.88) | 3.05 (2.24–3.57) | <0.001 |
| Anandamide | 0.76 (0.55–1.87) | 0.85 (0.56–1.14) | 0.575 |
| Alpha-linolenoylethanolamide | 0.042 (0.030–0.065) | 0.076 (0.055–0.109) | <0.001 |
| Eicosapentaenoylethanolamide | 0.044 (0.027–0.067) | 0.072 (0.049–0.109) | <0.001 |
| Docosahexaenoylethanolamide | 0.76 (0.60–0.98) | 1.08 (0.84–1.36) | <0.001 |
| ∑ endocannabinoids | 20.7 (12.2–34.4) | 99.1 (41.4–262) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouabdallah, A.; Ben-Fradj, M.K.; Hammami, M.B.; Abdelmalek, R.; Sanhaji, H.; Klopfenstein, T.; Feki, M. COVID-19 is Associated with a Lipid Storm that Worsens in Cases of Severe Pneumonia. Microorganisms 2025, 13, 2622. https://doi.org/10.3390/microorganisms13112622
Bouabdallah A, Ben-Fradj MK, Hammami MB, Abdelmalek R, Sanhaji H, Klopfenstein T, Feki M. COVID-19 is Associated with a Lipid Storm that Worsens in Cases of Severe Pneumonia. Microorganisms. 2025; 13(11):2622. https://doi.org/10.3390/microorganisms13112622
Chicago/Turabian StyleBouabdallah, Amani, Mohamed Kacem Ben-Fradj, Mohamed Bessem Hammami, Rim Abdelmalek, Haifa Sanhaji, Timothée Klopfenstein, and Moncef Feki. 2025. "COVID-19 is Associated with a Lipid Storm that Worsens in Cases of Severe Pneumonia" Microorganisms 13, no. 11: 2622. https://doi.org/10.3390/microorganisms13112622
APA StyleBouabdallah, A., Ben-Fradj, M. K., Hammami, M. B., Abdelmalek, R., Sanhaji, H., Klopfenstein, T., & Feki, M. (2025). COVID-19 is Associated with a Lipid Storm that Worsens in Cases of Severe Pneumonia. Microorganisms, 13(11), 2622. https://doi.org/10.3390/microorganisms13112622







