Soil-like Substrate Technology Improves Soil Nutrient Content and Enzyme Activity, Enhancing Soil Microbial Community Structure and Restoring Soils in Ecologically Sensitive Areas of the Loess Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
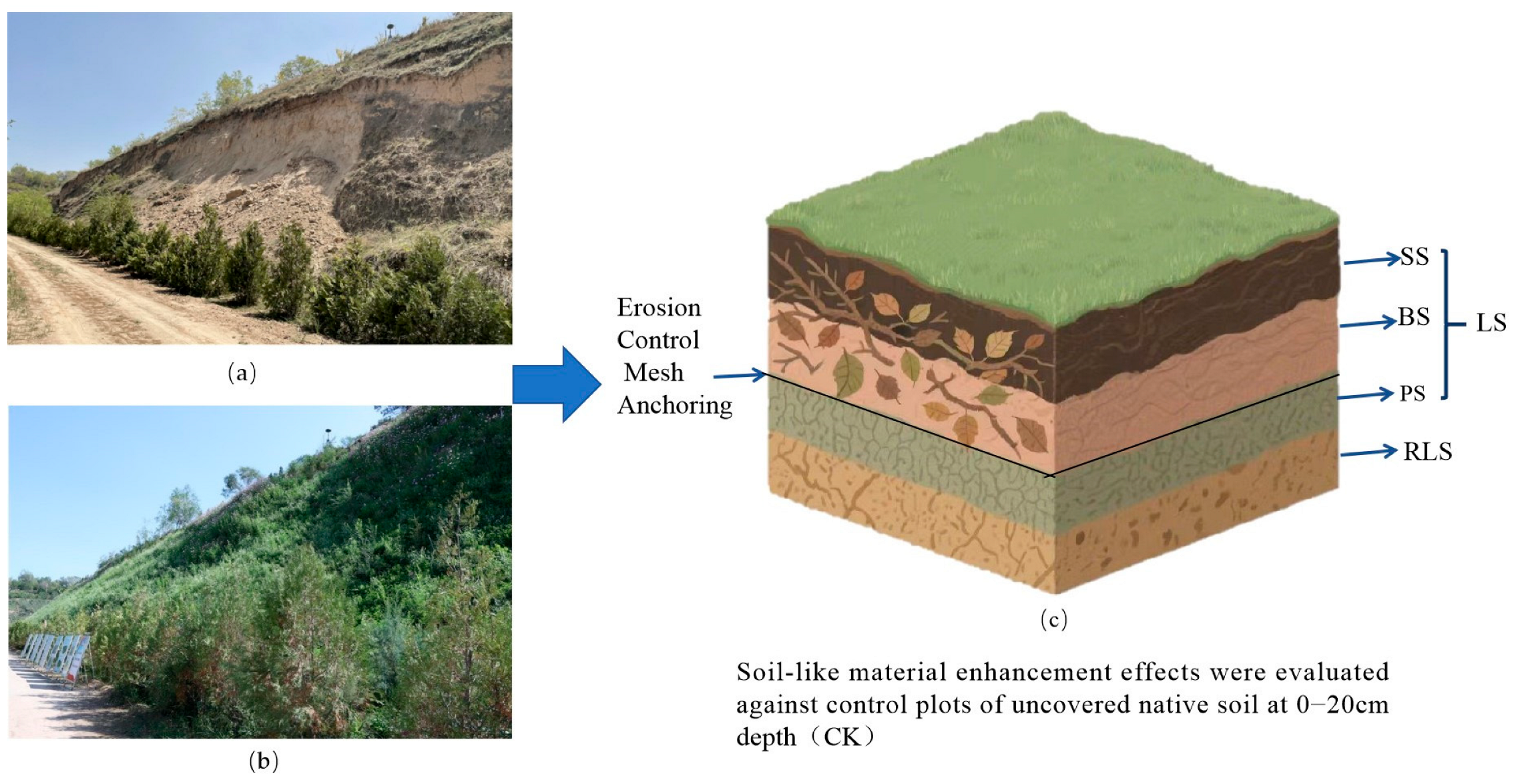
2.2. Erosion Control System
2.3. Soil Sampling
2.4. Determination of Soil Nutrient Content and Enzyme Activity
2.5. Analysis of Microbial Community Structure Based on High-Throughput Sequencing
2.6. Statistical Analysis
3. Results
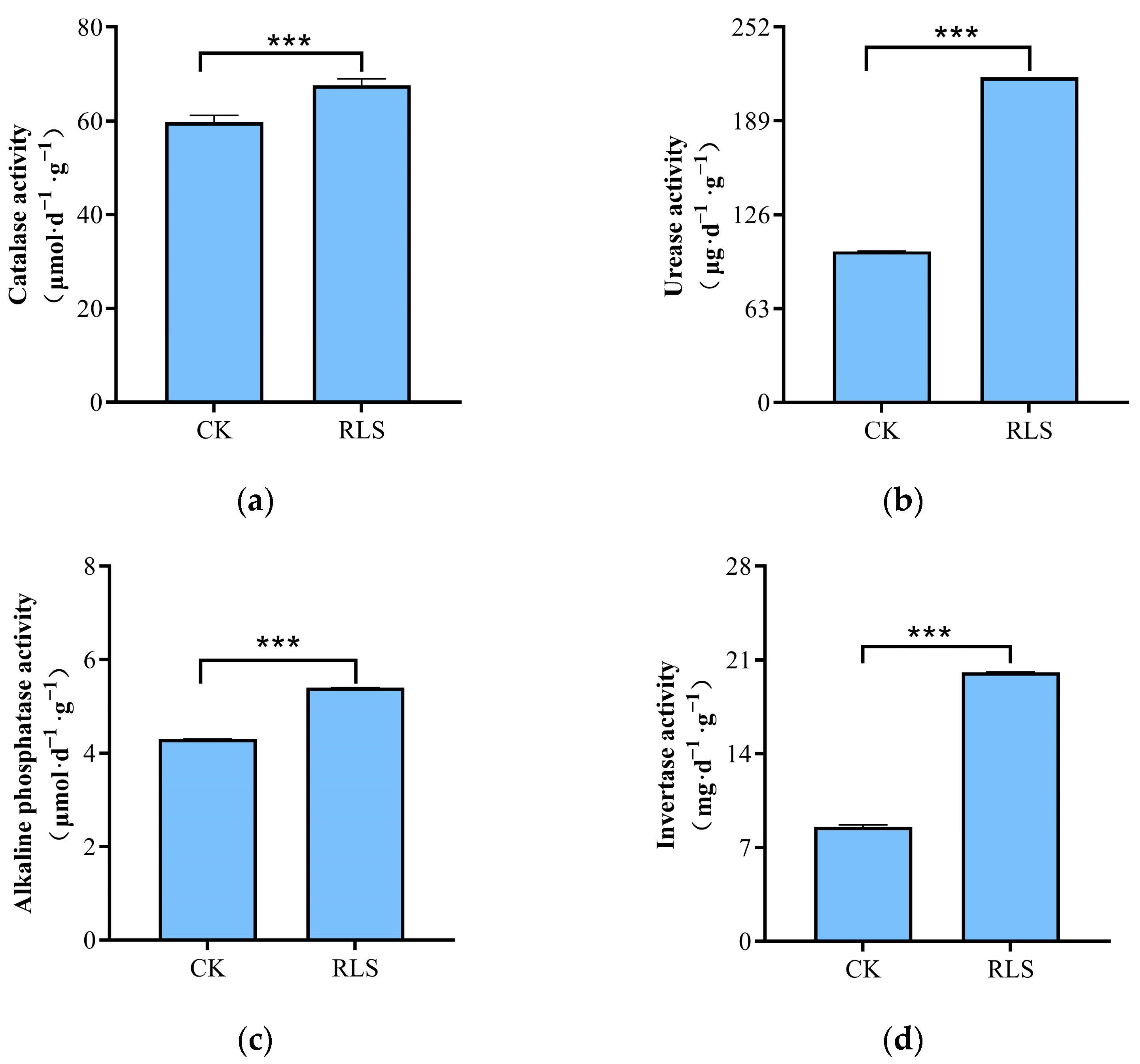
3.1. Effects of Soil-like Substrate Technology on Soil Nutrient Content and Enzyme Activity
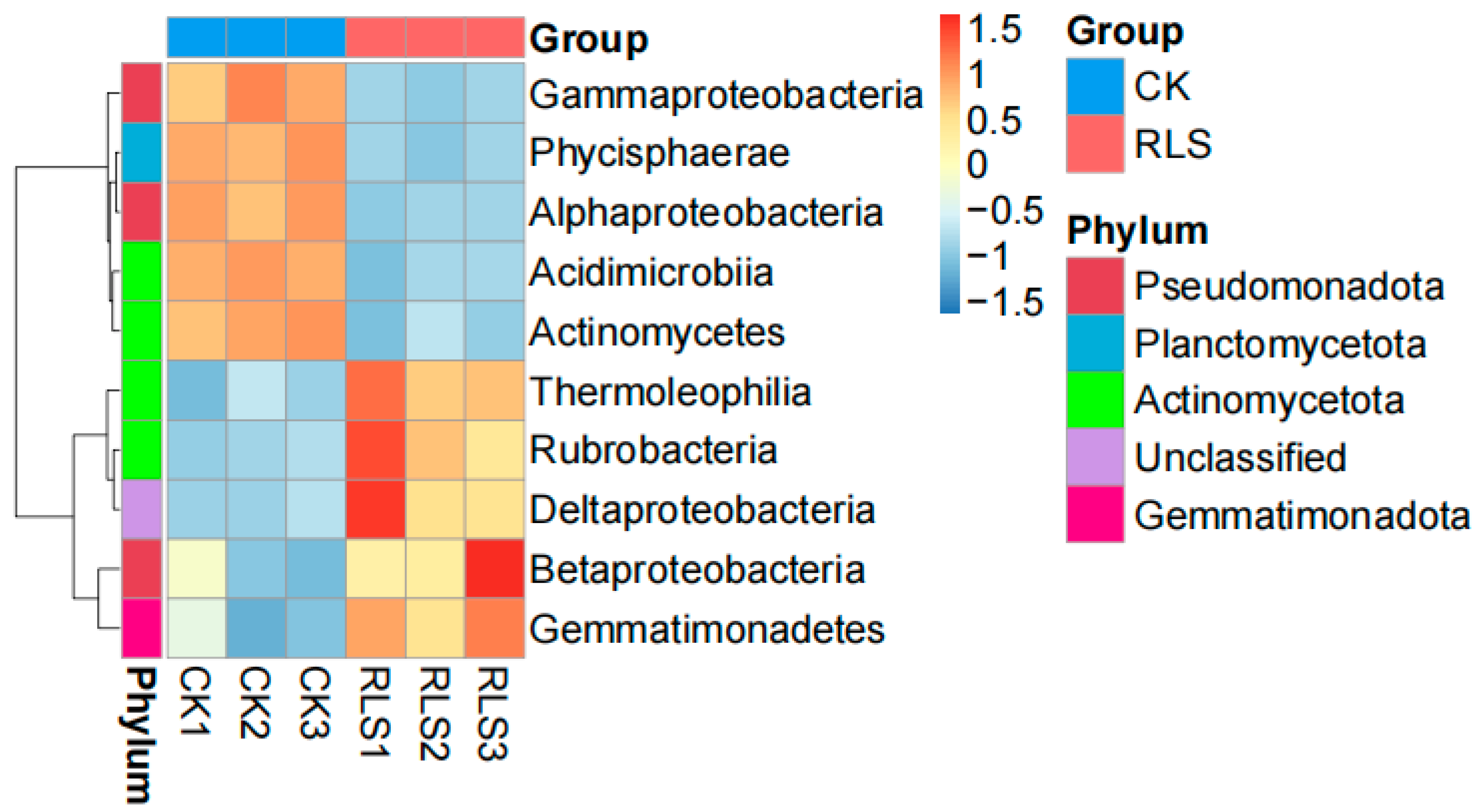
3.2. Effects of Soil-like Substrate on Soil Microorganisms
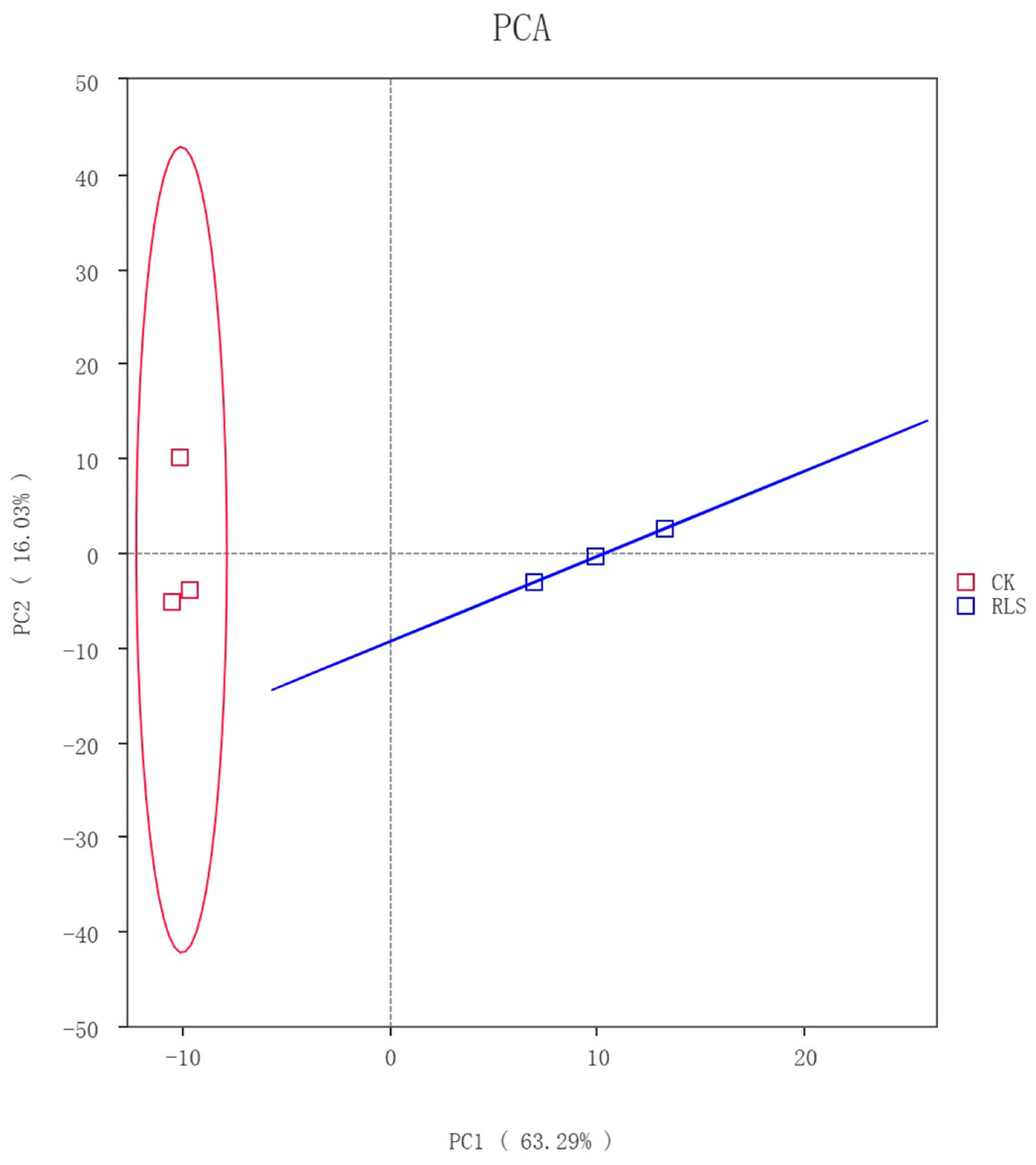
3.2.1. Principal Component Analysis of Soil-like Substrate
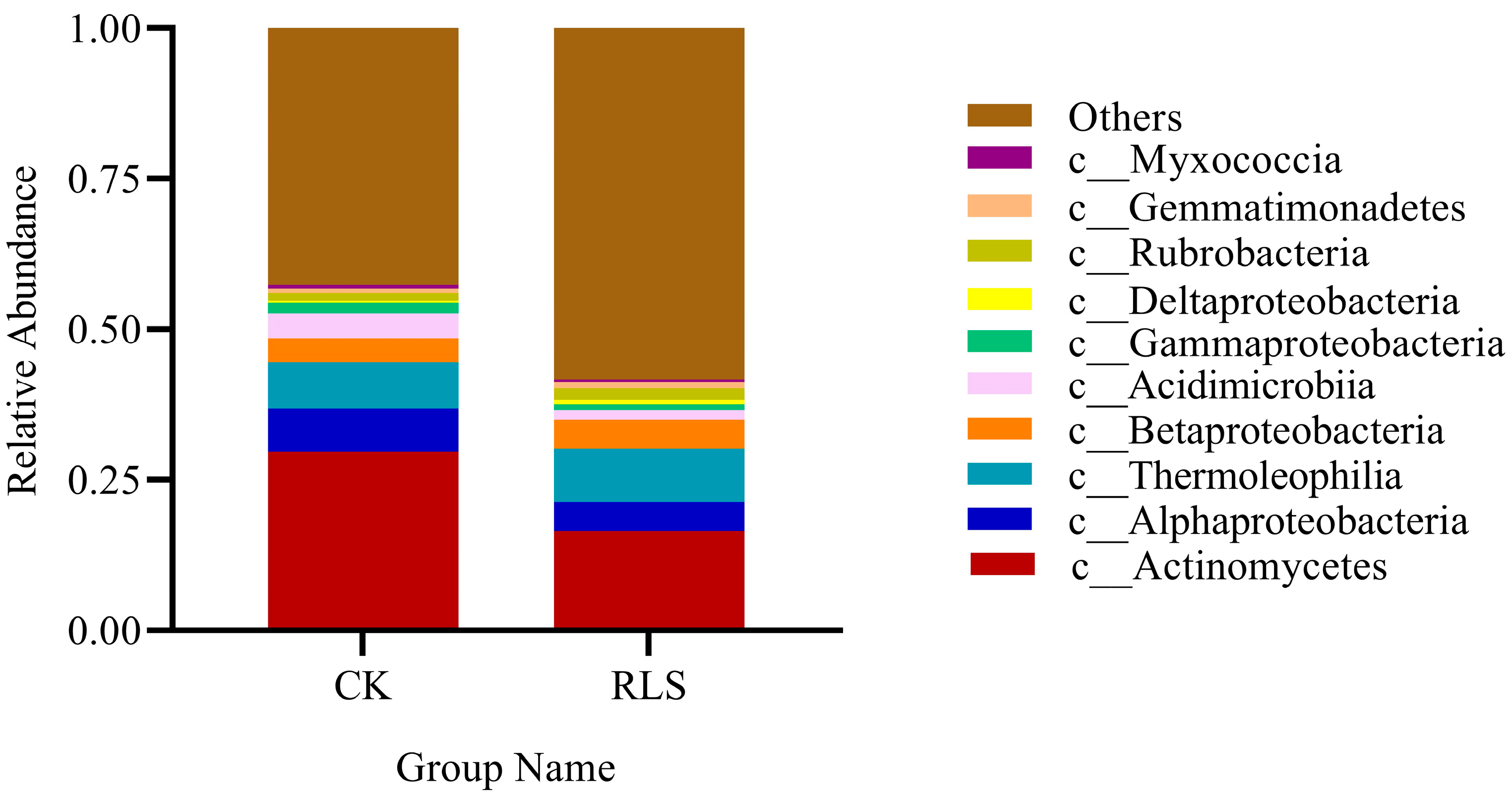
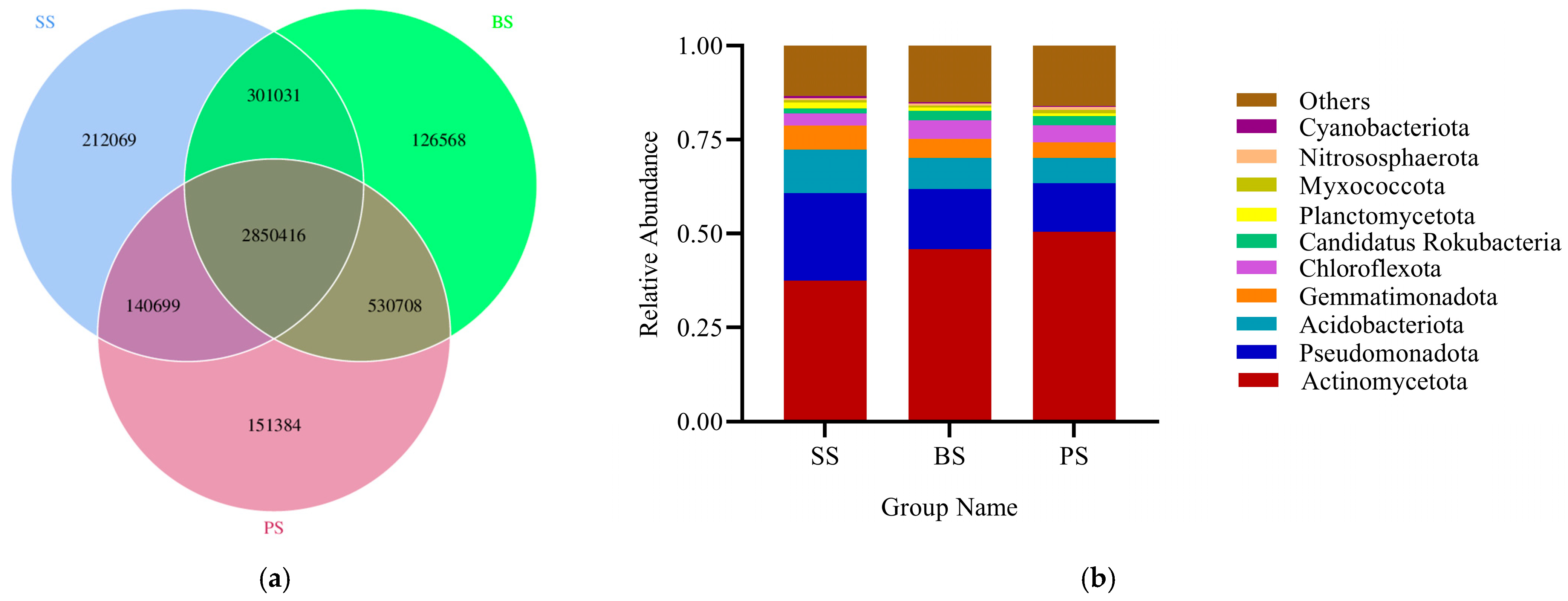
3.2.2. Characteristics of Soil Community Changes in Soil-like Substrate
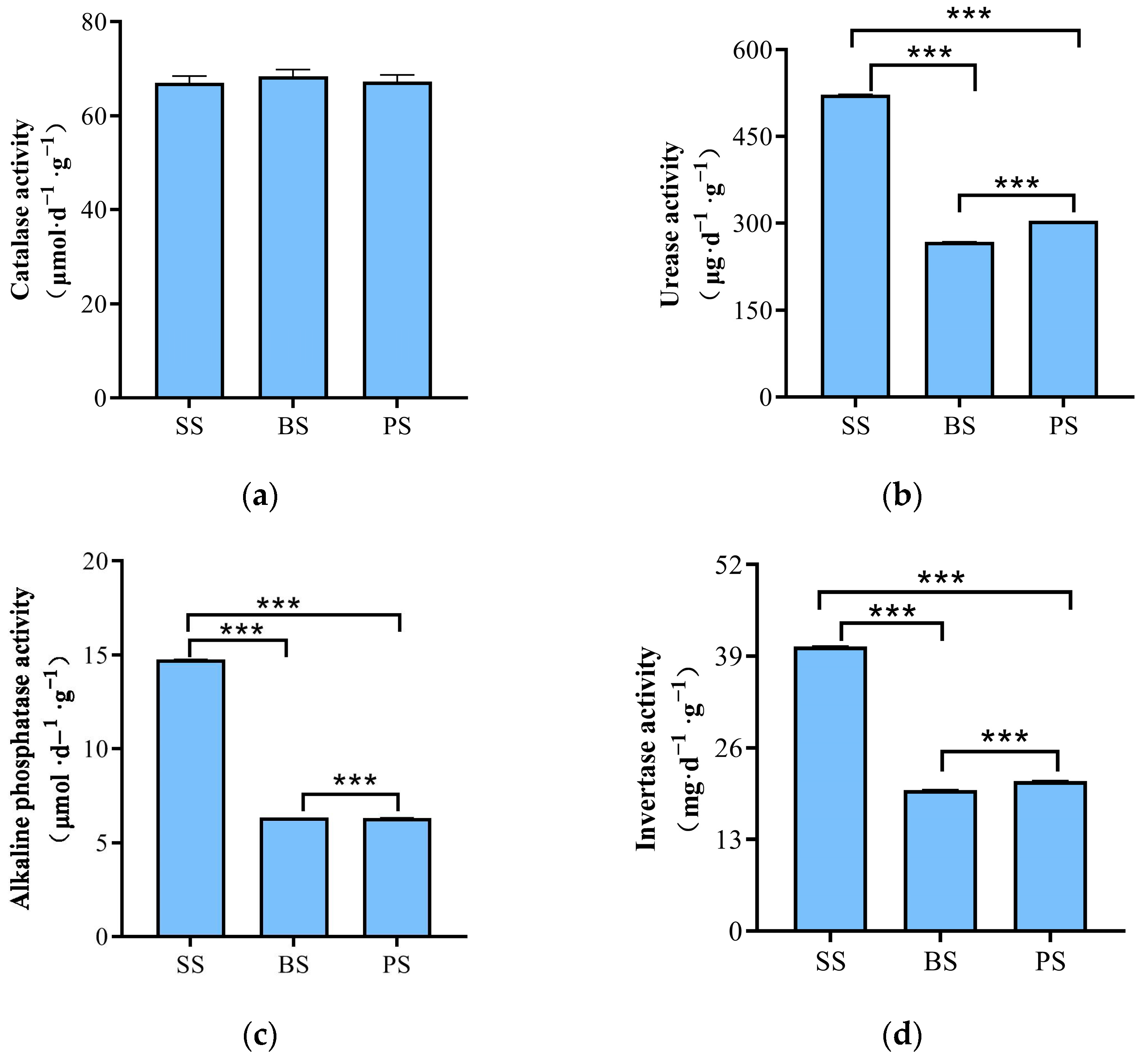
3.3. Soil Nutrient Content and Enzyme Activity in Soil-like Substrate at Different Depths
3.4. Analysis of Soil Microbial Diversity at Different Depths of Soil-like Substrate
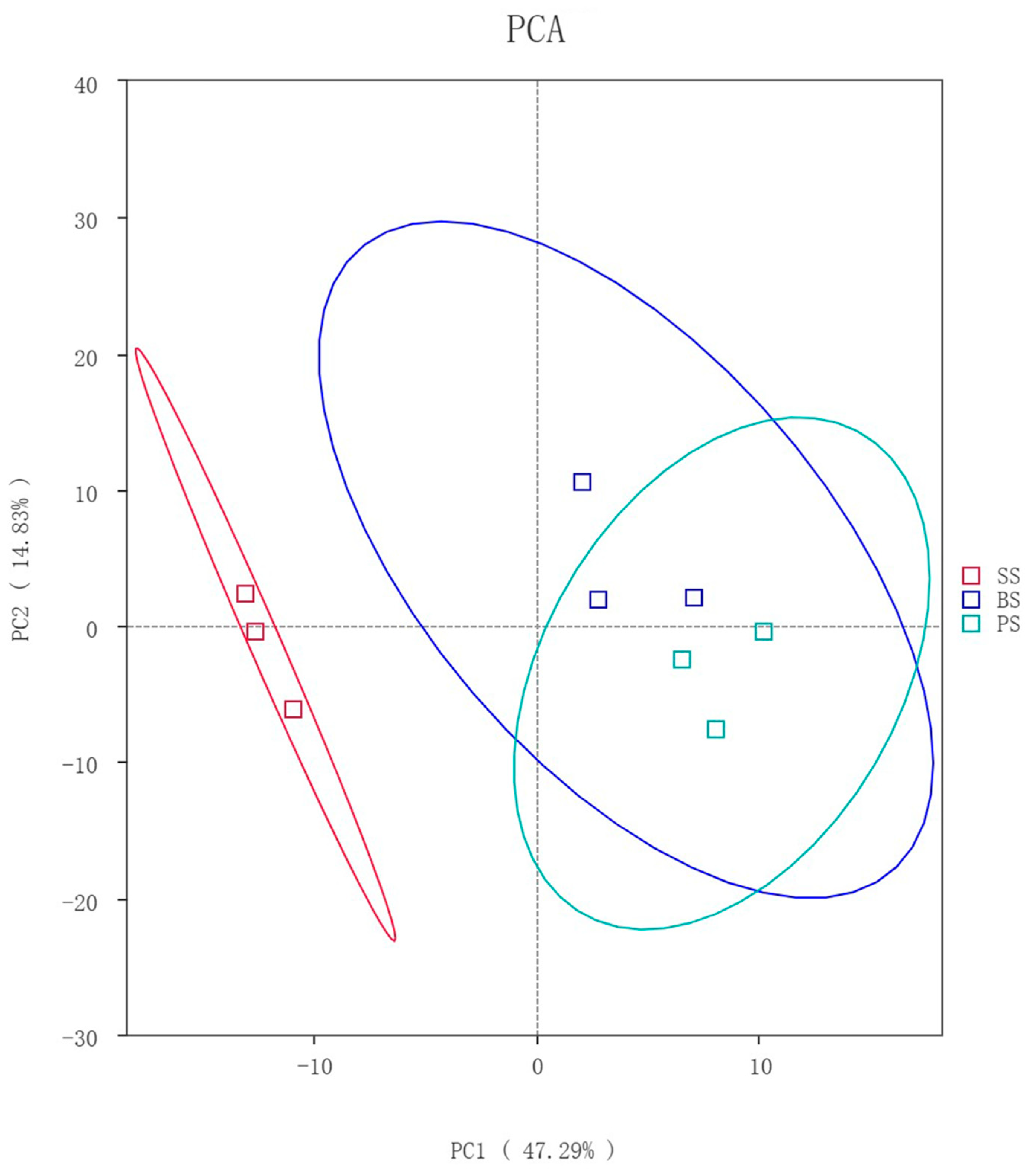
3.4.1. Principal Component Analysis of Soil Microbes at Different Depths of Soil-like Substrate
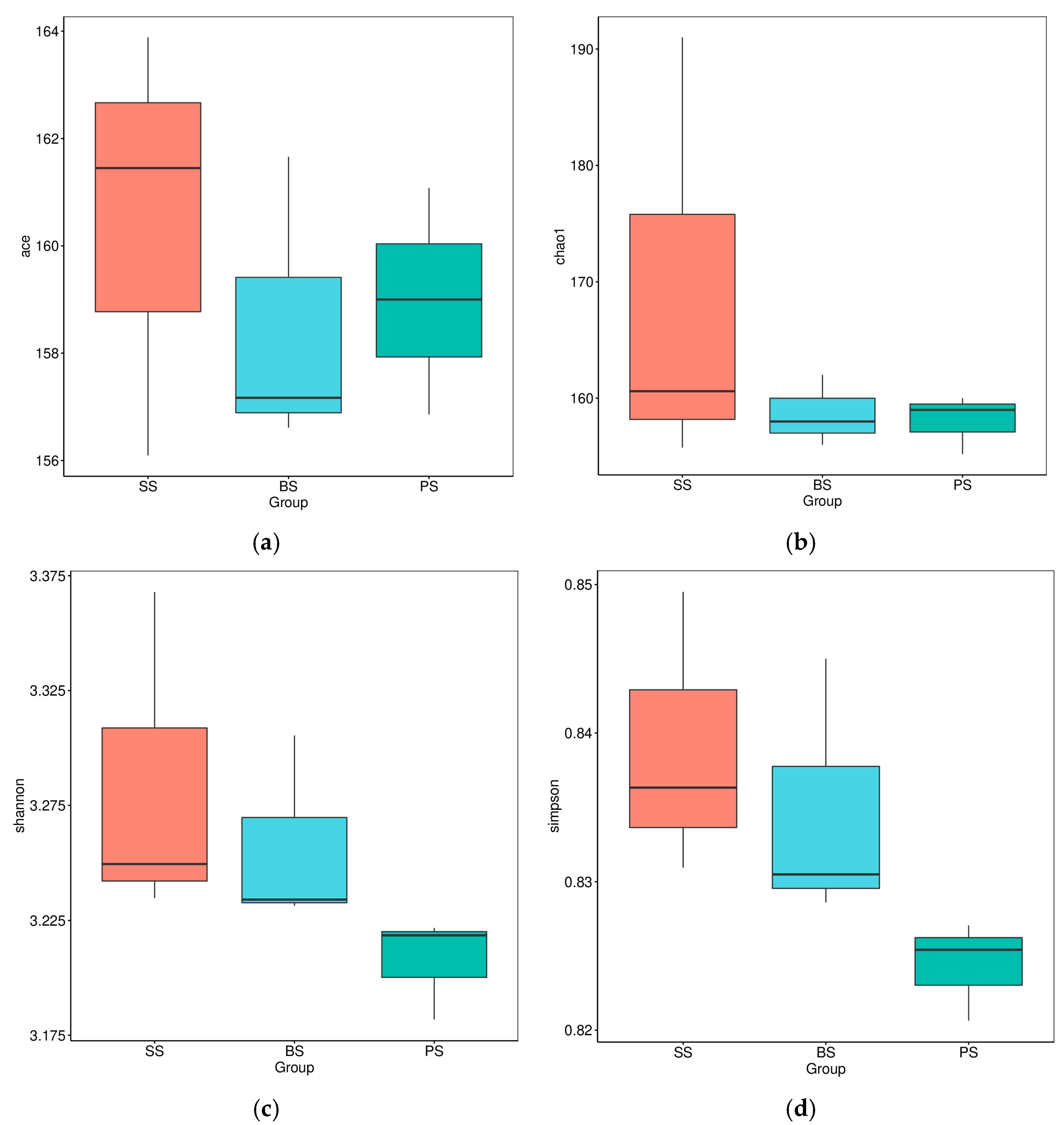
3.4.2. Analysis of Alpha Diversity Indices of Soil Microbes at Different Depths of Soil-like Substrate
3.4.3. Characteristics of Soil Community Changes at Different Depths of Soil-like Substrate
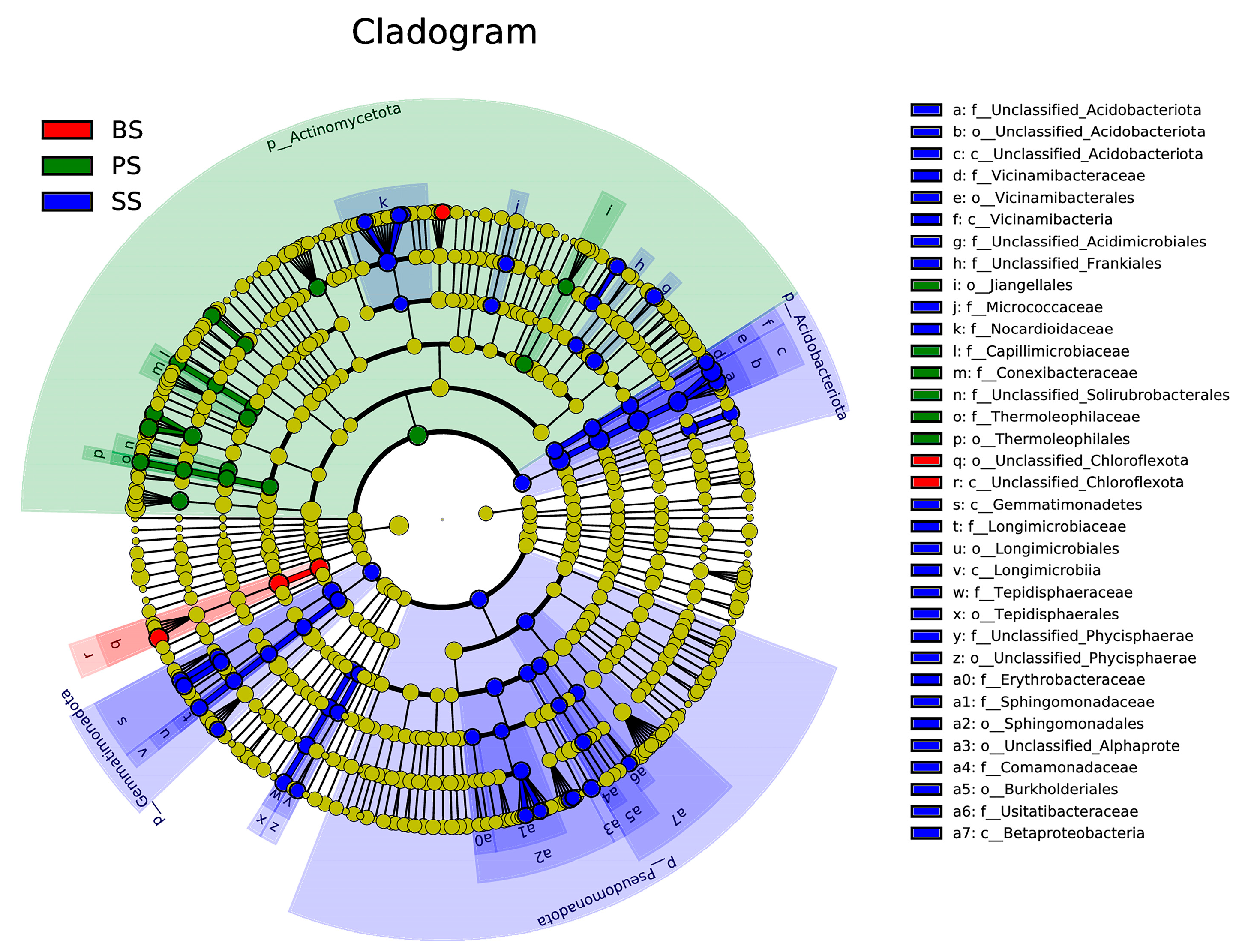
3.4.4. LEfSe Analysis of Differential Species in Soil-like Substrate at Different Depths
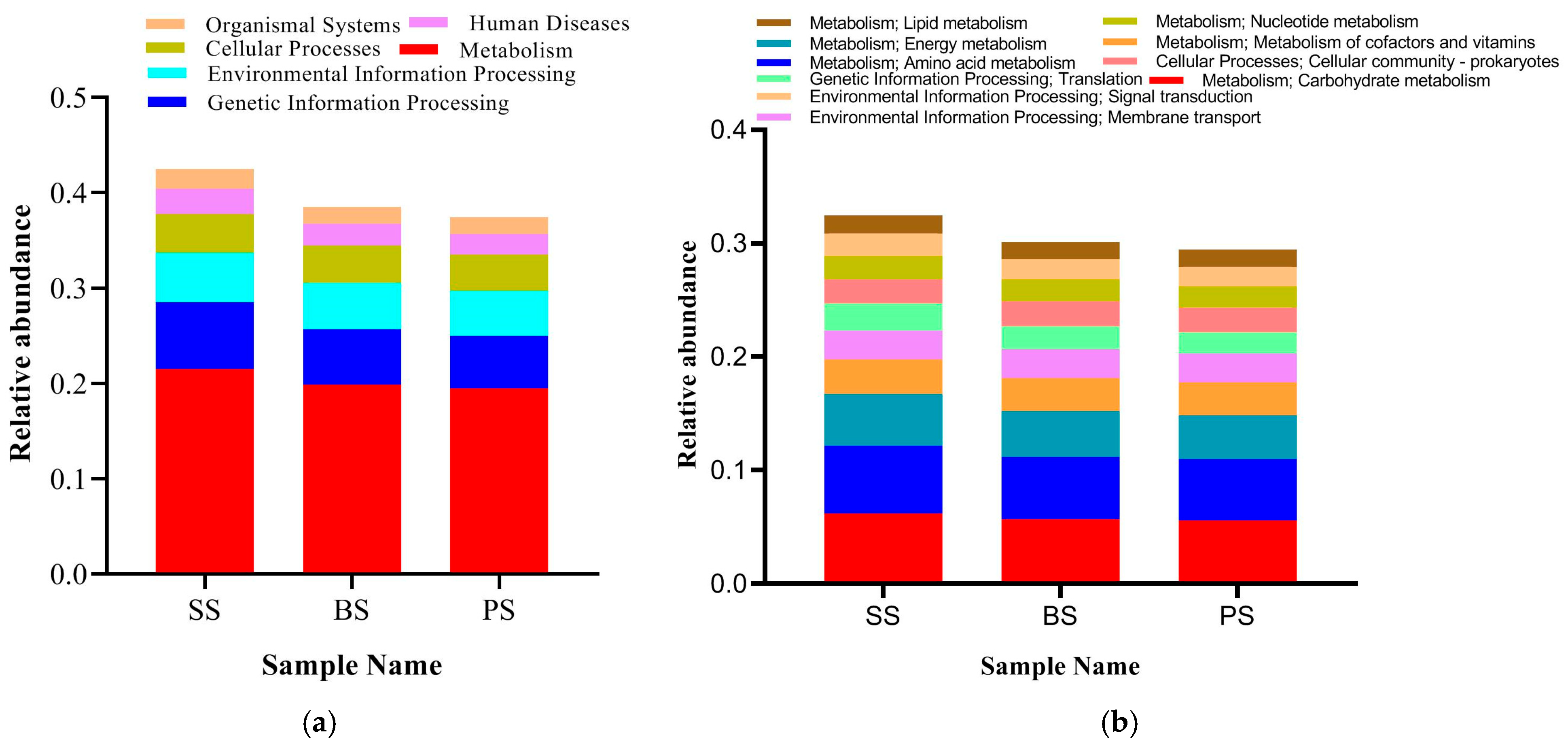
3.4.5. Analysis of Functional Characteristics of Soil Microbial Communities at Different Depths of Soil-like Substrate
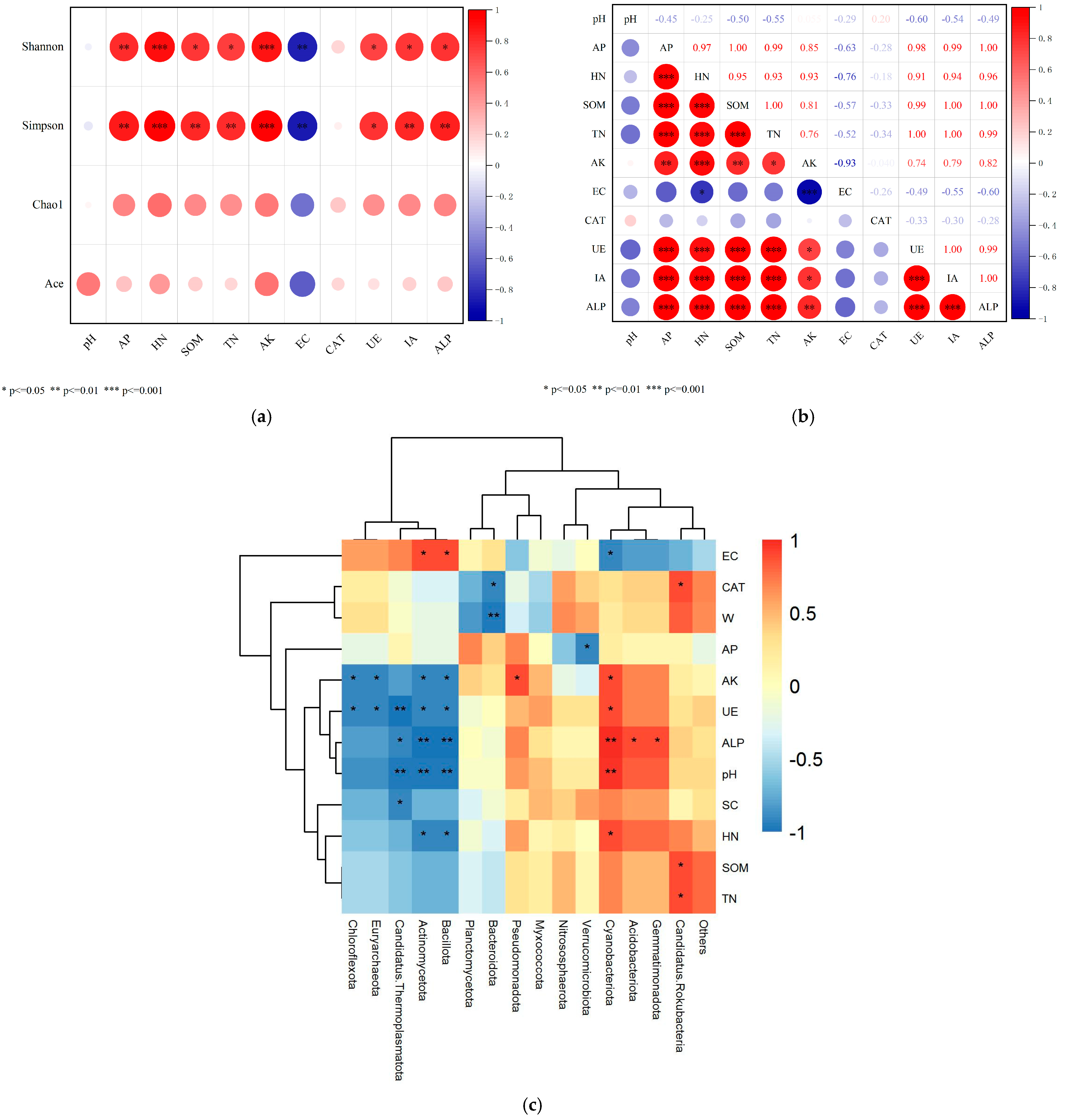
3.5. Relationships Between Soil Microbial Community Structure and Nutrient Content, Enzyme Activity
4. Discussion
4.1. Effects of Soil-like Substrate Improvement Technology on Soil Nutrient Content, Enzyme Activity, and Microbial Community Structure
4.2. Analysis of Soil Nutrients, Enzyme Activity, and Microbial Community Structure and Functional Characteristics at Different Depths of Soil-like Substrate Technology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CK | The control zone |
| RLS | −20 cm depth layer of the soil-like substrate |
| LS | The soil-like substrate technology zone |
| SS | Soil-like substrate of the surface layer (0–6 cm) |
| BS | Soil-like substrate of the substratum layer (6–12 cm) |
| PS | Soil-like substrate of the vegetation mat substrate layer (12–20 cm) |
| TN | Total nitrogen |
| HN | Hydrolyzable nitrogen |
| AP | Available phosphorus |
| AK | Available potassium |
| SOM | Organic matter |
| EC | Electrical conductivity |
| UE | Urease |
| CAT | Catalase |
| ALP | Alkaline phosphatase |
| IA | Invertase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Manukovsky, N.S.; Kovalev, V.S.; Rygalov, V.Y.; Zolotukhin, I.G. Waste bioregeneration in life support CES: Development of soil organic substrate. Adv. Space Res. 1997, 20, 1827–1832. [Google Scholar] [CrossRef]
- Wen, X.; Deng, X. Current soil erosion assessment in the Loess Plateau of China: A mini-review. J. Clean. Prod. 2020, 276, 123091. [Google Scholar] [CrossRef]
- Apostolova, I.; Valcheva, M.; Sopotlieva, D.; Velev, N.; Ganeva, A.; Nekhrizov, G. Natural Vegetation Recovery on Excavated Archaeological Sites: A Case Study of Ancient Burial Mounds in Bulgaria. Sustainability 2022, 14, 7318. [Google Scholar] [CrossRef]
- Kramarczuk, P.; Musielok, Ł.; Stolarczyk, M.; Jelonkiewicz, Ł.; Nikorych, V.A.; Szymański, W. Impact of vegetation type on the content and spectroscopic properties of soil organic matter in the subalpine zone of the Bieszczady Mountains (Eastern Carpathians). Plant Soil 2024, 512, 409–428. [Google Scholar] [CrossRef]
- Geisen, S.; Heinen, R.; Andreou, E.; Lent, T.V.; Hooven, F.C.T.; Thakur, M.P. Contrasting effects of soil microbial interactions on growth–defence relationships between early-and mid-successional plant communities. New Phytol. 2022, 233, 1345–1357. [Google Scholar] [CrossRef]
- Jiang, S.; Song, M.; Du, H.; Wang, F.; Song, T.; Chen, H.; Zeng, F.; Peng, W. Soil Properties Regulate Soil Microbial Communities During Forest Succession in a Karst Region of Southwest China. Microorganisms 2024, 12, 2136. [Google Scholar] [CrossRef] [PubMed]
- Marenga, W.; Mapoka, M.; Ultra, V.U.; Rantong, G.; Nduna, B.; Tsidu, G.M. Short-term impacts of wildfire on vegetation recovery, soil chemical properties and community-level physiological profiling in a savanna ecosystem of Botswana. Front. For. Glob. Change 2025, 8, 1605456. [Google Scholar] [CrossRef]
- Karthik, A.; Hussainy, S.A.H.; Rajasekar, M. Comprehensive Study on Biochar and its Effect on Soil Properties: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 459–477. [Google Scholar] [CrossRef]
- Cascone, S. Green Roof Design: State of the Art on Technology and Materials. Sustainability 2019, 11, 3020. [Google Scholar] [CrossRef]
- Bajiu, A.Z.; Gao, K.; Zeng, G.Y.; He, Y.H. Impact of Intercropping Five Medicinal Plants on Soil Nutrients, Enzyme Activity, and Microbial Community Structure in Camellia oleifera Plantations. Microorganisms 2024, 12, 1616. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.L.; Chen, J.; Han, C.; Zhao, Y.; Yang, X.Q.; Li, J.P. Soil extracellular enzyme stoichiometry reveals the nutrient limitations of soil microbial metabolism under precipitation changes in Ningxia desert steppe of China. Eur. J. Soil Biol. 2025, 127, 103774. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhu, J.H.; Ma, J.J.; Wang, S.L.; Zuo, L.Z.; Yang, Z.L. Close correlation between sugarcane ratoon decline and rhizosphere ecological factors. Sci. Rep. 2024, 14, 20738. [Google Scholar] [CrossRef]
- Huang, T.; An, S.S.; Huang, W.Y.; Liu, B.Y. Loess Plateau Cropland: Evolution and Ecological Impacts over Four Millennia. Land 2025, 14, 1015. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.X.; Shao, M.G.; et al. Erosion reduces soil microbial diversity, network complexityand multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Huang, J.H.; Huang, T.Y.; Chen, J.; Li, G.Q.; Wang, Z.J.; Huo, N. Nematode Community Characteristics Indicate Soil Restoration under Different Revegetation Approaches in the Semiarid Area of the Chinese Loess Plateau. Forests 2023, 14, 1886. [Google Scholar] [CrossRef]
- Hao, H.J.; Liang, Y.J.; Pian, D.; Zhang, Y.; Chen, Y.X.; Lai, H.T.; Yu, Z.C.; Sailike, A.J.; Wang, R.; Cao, L.; et al. Macroaggregate is crucial in soil carbon and nitrogen accumulation under different vegetation types in the Loess Plateau, China. For. Ecol. Manag. 2024, 569, 122161. [Google Scholar] [CrossRef]
- Collings, J.A.; Shoemaker, L.G.; Diez, J.M. Environmental context alters plant–soil feedback effects on plant coexistence. Ecology 2025, 106, e70170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guo, X.Q.; Shan, Y.J.; Lu, X.; Cao, J.J. Effects of land-use patterns on soil microbial diversity and composition in the Loess Plateau, China. J. Arid. Land 2024, 16, 415–430. [Google Scholar] [CrossRef]
- Liu, X.R.; Feng, T.J.; Zhang, Y.F.; Liu, Y.B.; Wang, P. Vegetation restoration affects soil hydrological processes in typical natural and planted forests on the Loess Plateau. J. Hydrol. 2025, 650, 132465. [Google Scholar] [CrossRef]
- Tan, X.Y.; Jia, Y.W.; Niu, C.W.; Yang, D.W.; Lu, W.; Hao, C.F. Response of water-use efficiency to phenology in the natural forest and grassland of the Loess Plateau in China. Sci. China Earth Sci. 2023, 66, 2081–2096. [Google Scholar] [CrossRef]
- Fu, F.Y.; Wang, S.A.; Wu, X.T.; Chen, S.Y.; Tan, Z.M.; Ye, C.C.; Grünzweig, J.M. Integrating hydrological impacts for cost-effective dryland ecological restoration. Commun. Earth Environ. 2025, 6, 667. [Google Scholar] [CrossRef]
- Wang, Y.N.; Xie, J.X.; Fu, L.W.; Singh, B. Determinants of carbon emission: A multiple scale decomposition of Gansu Province. PLoS ONE 2024, 19, e0309467. [Google Scholar] [CrossRef]
- Xin, L.L.; Jia, J.S.; Hu, W.H.; Zeng, H.Q.; Chen, C.D.; Wu, B. Decomposition and Decoupling Analysis of CO2 EmissionsBased on LMDI and Two-Dimensional Decoupling Model in Gansu Province, China. Int. J. Environ. Res. Public Health 2021, 18, 6013. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.N.; Zhang, Y.F.; Wang, G.L.; Zhang, Z.Q. Soil physicochemical properties and microorganisms jointly regulate the variations of soil carbon and nitrogen cycles along vegetation restoration on the Loess Plateau, China. Plant Soil 2024, 494, 413–436. [Google Scholar] [CrossRef]
- Robertson, A.D.; Paustian, K.; Ogle, S.; Wallenstein, M.D.; Lugato, E.; Cotrufo, M.F. Unifying soil organic matter formation and persistence frameworks: The MEMS model. Biogeosciences 2019, 16, 1225–1248. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Xia, D.M. Soil and Fertilizer Science; Shanghai Jiao Tong University Press: Shanghai, China, 2007. [Google Scholar]
- Kiprotich, K.; Muema, E.; Wekesa, C.; Ndombi, T.; Muoma, J.; Omayio, D.; Ochieno, D.; Motsi, H.; Mncedi, S.; Tarus, J. Unveiling the roles, mechanisms and prospects of soil microbial communities in sustainable agriculture. Discov. Soil 2025, 2, 10. [Google Scholar] [CrossRef]
- Singh, N.; Asha; Maurya, V.K.; Singh, D. Soil Enzyme Activities as Bioindicators of Soil Health: Implications for Sustainable Agriculture. J. Nat. Res. Cons. Manag. 2024, 5, 124–136. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.Z.; Miao, F.; Li, Z.Y.; Tang, W.; Sun, J. Assessing the effect of soil salinization on soil microbial respiration and diversities under incubation conditions. Appl. Soil Ecol. 2020, 155, 103671. [Google Scholar] [CrossRef]
- Ragab, H. Relationship between Soil pH and Microbial Community Composition in Agricultural Soils. Am. J. Nat. Sci. 2024, 5, 44–54. [Google Scholar]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Yuan, F.; Yin, S.; Xu, Y.; Xiang, L.; Wang, H.; Li, Z.; Fan, K.; Pan, G. The Richness and Diversity of Catalases in Bacteria. Front. Microbiol. 2021, 12, 645477. [Google Scholar] [CrossRef]
- Sartorio, M.G.; Repizo, G.D.; Cortez, N. Catalases of the polyextremophylic Andean isolate Acinetobacter sp. Ver 3 confer adaptive response to H2O2 and UV radiation. FEBS J. 2020, 287, 4525–4539. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.A.; Shu, A.P.; Song, W.F.; Shi, W.C.; Li, M.C.; Zhang, W.X.; Li, Z.Z.; Liu, G.R.; Yuan, F.S.; Zhang, S.X.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, F.F.; Cao, J.B.; Liu, T.; Zhang, Y. Responses of Soil Quality and Microbial Community Composition to Vegetation Restoration in Tropical Coastal Forests. Biology 2025, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; Deforest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Ma, K.W.; Niu, Y.; Jia, Y.; Ordon, J.; Copeland, C.; Emonet, A.; Geldner, N.; Guan, R.; Stolze, S.C.; Nakagami, H.; et al. Coordination of microbe–host homeostasis by crosstalk with plant innate immunity. Nat. Plants 2021, 7, 814–825. [Google Scholar] [CrossRef]
- Aqeel, M.; Ran, J.; Hu, W.; Irshad, M.K.; Dong, L.; Akram, M.A.; Eldesoky, G.E.; Aljuwayid, A.M.; Chuah, L.F.; Deng, J. Plant-soil-microbe interactions in maintaining ecosystem stability and coordinated turnover under changing environmental conditions. Chemosphere 2023, 318, 137924. [Google Scholar] [CrossRef]
- Xi, Q.J.; Gao, H.K.; Wang-Erlandsson, L.; Dong, J.Z.; Fenicia, F.; Savenije, H.H.G.; Hrachowitz, M. Terrestrial ecosystems enhanced root zone water storage capacity in response to climate change over the past four decades. Sci. Bull. 2025, 70, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Sokol, N.W.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef]
- Aguilar, C.; Alwali, A.; Mair, M.; Rodriguez-Orduña, L.; Contreras-Peruyero, H.; Modi, R.; Roberts, C.; Sélem-Mojica, N.; Licona-Cassani, C.; Parkinson, E.I. Actinomycetota bioprospecting from ore-forming environments. Microb. Genom. 2024, 10, 001253. [Google Scholar] [CrossRef]
- Ibrahimi, M.; Loqman, S.; Jemo, M.; Hafidi, M.; Lemee, L.; Ouhdouch, Y. The potential of facultative predatory Actinomycetota spp. and prospects in agricultural sustainability. Front. Microbiol. 2023, 13, 1081815. [Google Scholar] [CrossRef]
- Wang, C.Q.; Kuzyakov, Y. Mechanisms and implications of bacterial-fungal competition for soil resources. ISME J. 2024, 18, wrae073. [Google Scholar] [CrossRef]
- Kong, L.J.; Zhang, L.; Wang, Y.N.; Huang, Z.B. Impact of Ecological Restoration on the Physicochemical Properties and Bacterial Communities in Alpine Mining Area Soils. Microorganisms 2024, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, L.; Jing, M.; Wang, K.; Song, C.; Song, Y. Nitrogen addition restricts key soil ecological enzymes and nutrients by reducing microbial abundance and diversity. Sci. Rep. 2025, 15, 5560. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Song, Y.J.; An, Y.X.; Lu, Y.L.; Zhong, G.H. Soil Microorganisms: Their Role in Enhancing Crop Nutrition and Health. Diversity 2024, 16, 734. [Google Scholar] [CrossRef]
- Brzeszcz, J.; Kaszycki, P. Aerobic bacteria degrading both n-alkanes and aromatic hydrocarbons: An undervalued strategy for metabolic diversity and flexibility. Biodegradation 2018, 29, 359–407. [Google Scholar] [CrossRef]
| Sample Name | pH | TN (g·kg−1) | SOM (g·kg−1) | AP (mg·kg−1) | HN (mg·kg−1) | AK (mg·L−1) | EC (mS·cm−1) |
|---|---|---|---|---|---|---|---|
| CK | 7.94 b ± 0.02 | 3.7 b ± 0.01 | 6.66 b ± 0.22 | 7.8 b ± 0.12 | 24.80 b ± 0.80 | 11.087 b ± 0.19 | 1.88 a ± 0.05 |
| RLS | 8.41 a ± 0.05 | 8.8 a ± 0.01 | 13.5 a ± 0.10 | 12.1 a ± 0.12 | 57.21 a ± 4.02 | 18.559 a ± 0.23 | 0.329 b ± 0.003 |
| Phylum | Class | Microbial Abundance of the Sample | Increase or Decrease | |
|---|---|---|---|---|
| CK | RLS | In Percentage (%) | ||
| Actinobacteria | Rubrobacteria | 0.012 | 0.019 | 37.19 |
| Proteobacteria | Deltaproteobacteria | 0.004 | 0.008 | 54.48 |
| Actinobacteria | Actinomycetes | 0.297 | 0.165 | −44.45 |
| Proteobacteria | Betaproteobacteria | 0.04 | 0.048 | 15.97 |
| Gemmatimonadetes | Gemmatimonadetes | 0.008 | 0.011 | 27.66 |
| Actinobacteria | Thermoleophilia | 0.076 | 0.088 | 13.29 |
| Proteobacteria | Alphaproteobacteria | 0.072 | 0.049 | −31.33 |
| Sample Name | pH | TN (g·kg−1) | SOM (g·kg−1) | AP (mg·kg−1) | HN (mg·kg−1) | AK (mg·L−1) | EC (mS·cm−1) |
|---|---|---|---|---|---|---|---|
| SS | 8.6 b ± 0.01 | 1.29 a ± 0.001 | 22.40 a ± 0.35 | 79.9 a ± 2.02 | 99.38 a ± 0.93 | 27.64 a ± 0.63 | 0.188 b ± 0.01 |
| BS | 8.76 a ± 0.03 | 0.9 c ± 0.0004 | 14.2 b ± 0.13 | 7.5 b ± 0.16 | 73.56 b ± 3.24 | 21.72 b ± 0.39 | 0.195 b ± 0.01 |
| PS | 8.6 b ± 0.04 | 0.93 b ± 0.001 | 14.4 b ± 0.16 | 3.5 c ± 0.24 | 63.79 c ± 2.32 | 13.84 c ± 0.11 | 0.243 a ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.; Tan, Q.; Han, B.; Li, R.; Gu, L.; Wang, X.; Li, Y.; Zhang, Q. Soil-like Substrate Technology Improves Soil Nutrient Content and Enzyme Activity, Enhancing Soil Microbial Community Structure and Restoring Soils in Ecologically Sensitive Areas of the Loess Plateau. Microorganisms 2025, 13, 2621. https://doi.org/10.3390/microorganisms13112621
Bai G, Tan Q, Han B, Li R, Gu L, Wang X, Li Y, Zhang Q. Soil-like Substrate Technology Improves Soil Nutrient Content and Enzyme Activity, Enhancing Soil Microbial Community Structure and Restoring Soils in Ecologically Sensitive Areas of the Loess Plateau. Microorganisms. 2025; 13(11):2621. https://doi.org/10.3390/microorganisms13112621
Chicago/Turabian StyleBai, Gexue, Qingqing Tan, Bingbing Han, Ruidong Li, Lijun Gu, Xiaojing Wang, Yan Li, and Quanfang Zhang. 2025. "Soil-like Substrate Technology Improves Soil Nutrient Content and Enzyme Activity, Enhancing Soil Microbial Community Structure and Restoring Soils in Ecologically Sensitive Areas of the Loess Plateau" Microorganisms 13, no. 11: 2621. https://doi.org/10.3390/microorganisms13112621
APA StyleBai, G., Tan, Q., Han, B., Li, R., Gu, L., Wang, X., Li, Y., & Zhang, Q. (2025). Soil-like Substrate Technology Improves Soil Nutrient Content and Enzyme Activity, Enhancing Soil Microbial Community Structure and Restoring Soils in Ecologically Sensitive Areas of the Loess Plateau. Microorganisms, 13(11), 2621. https://doi.org/10.3390/microorganisms13112621





