Exploring the Potential of Haematococcus pluvialis as a Source of Bioactives for Food Applications: A Review
Abstract
1. Introduction
2. Biology and Physiology of Haematococcus pluvialis
2.1. Taxonomy
2.2. Morphology and Life Cycle
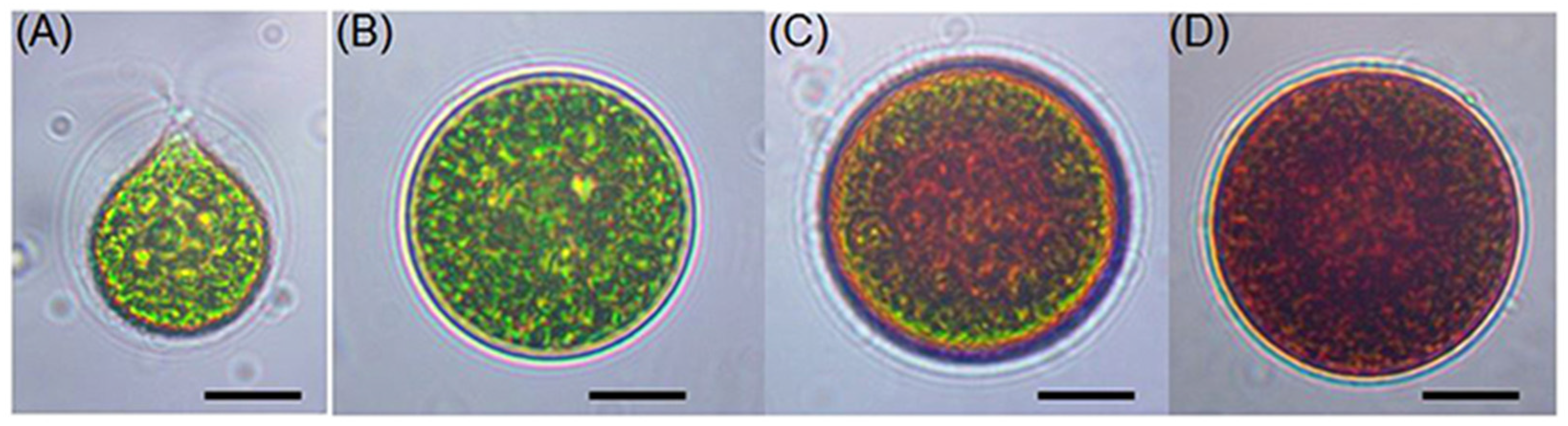
2.3. Growth Conditions and Environmental Influence
3. Bioactive Compounds from Haematococcus pluvialis
4. Cultivation Strategies for Enhanced Bioactive Production
4.1. Open Ponds vs. Photobioreactors
4.2. Environmental Stress Factors
4.3. Biotechnological Approaches (Genetic Engineering, Omics Studies)
5. Extraction and Purification of Bioactives
5.1. Conventional Extraction Methods
5.2. Emerging Green Technologies
5.3. Comparative Efficiency and Sustainability
| Extraction Method | Compound Yield (mg/g) | Toxicity | Advantages | Disadvantages | Industrial Scalability | Cost | Ref. |
|---|---|---|---|---|---|---|---|
| Organic Solvents (Acetone, Ethanol, Hexane) | Astaxanthin 15–31.4 | Use of toxic solvents, low selectivity | Simple operation | Requires cell disruption pretreatments | Widely used in labs and semi-industrial scale | Low initial cost | [21] |
| Supercritical CO2 | Astaxanthin Up to 40 | Non-toxic solvent | High selectivity, minimal residue, mild temperature | Requires high pressure | Used in industries and on a lab scale | High equipment cost | [11] |
| Ultrasound-Assisted Extraction | Astaxanthin 30–35 | Can be toxic | Fast, reduced solvent usage, efficient when combined with pretreatment | Requires specific equipment, possible localized heating | Pilot and semi-industrial scales | Low cost | [82] |
| Microwave-Assisted Extraction | Astaxanthin 28–32 | Nontoxic | Rapid process, preserves bioactives, low energy consumption | Needs specialized equipment, critical temperature control | Applied at lab or pilot scale | Low cost | [21] |
| Ionic Liquids/Natural Deep Eutectic Solvents | Astaxanthin 25–38 | Depends on the composition of the solution | High selectivity, biodegradable, and recyclable solvents | High viscosity and limited availability | Medium cost | High cost | [80] |
6. Applications in the Food Industry
6.1. Functional Ingredients (Supplements, Fortification, Beverages)
6.2. Natural Food Colorants
6.3. Antioxidant and Anti-Inflammatory Properties in Food Systems
6.4. Stability of H. pluvialis Bioproducts
6.5. Evidence from In Vitro, In Vivo, and Clinical Studies
7. Safety and Regulatory Considerations
Consumer Acceptance and Market Trends
8. Future Perspectives and Challenges
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Borowitzka Michael, A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Ali Sameh, S.; Al-Tohamy, R.; Al-Zahrani, M.; Schagerl, M.; Kornaros, M.; Sun, J. Advancements and challenges in microalgal protein production: A sustainable alternative to conventional protein sources. Microb. Cell Factories 2025, 24, 61. [Google Scholar] [CrossRef]
- Ma, S.L.; Sun, S.; Li, T.Z.; Yan, Y.J.; Wang, A.K. Application research and progress of microalgae as a novel protein resource in the future. Crit. Rev. Food Sci. Nutr. 2024, 65, 1–24. [Google Scholar] [CrossRef]
- Williamson, E.; Ross, I.L.; Wall, B.T.; Hankamer, B. Microalgae: Potential novel protein for sustainable human nutrition. Trends Plant Sci. 2024, 29, 370–382. [Google Scholar] [CrossRef]
- Prates, J.A.M. Unlocking the Functional and Nutritional Potential of Microalgae Proteins in Food Systems: A Narrative Review. Foods 2025, 14, 1524. [Google Scholar] [CrossRef] [PubMed]
- Ha, N.C.; Tam, L.T.; Hien, H.T.M.; Thu, N.T.H.; Hong, D.D.; Thom, L.T. Optimization of Culture Conditions for High Cell Productivity and Astaxanthin Accumulation in Vietnam’s Green Microalgae Haematococcus pluvialis HB and a Neuroprotective Activity of Its Astaxanthin. Bioengineering 2024, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.; Gedi, M.A.; Akepach, P.; Assey, H.; Zaky, A.S.; Gray, D.A. Chlamydomonas reinhardtii Is a potential food supplement with the capacity to outperform Chlorella and Spirulina. Appl. Sci. 2020, 10, 6736. [Google Scholar] [CrossRef]
- Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Inácio, L.G.; Afonso, C.; Maranhão, P. The microalga Dunaliella and its applications: A review. Appl. Phycol. 2023, 4, 99–120. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Grewe, C.; Griehl, C. Novel approaches for the production of astaxanthin from Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2012, 96, 1163–1174. [Google Scholar]
- Recht, L.; Zark, M.; BoussiBA, S. Astaxanthin as a food additive. In Functional Foods and Dietary Supplements: From Structure-Function Relationships to Health Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 37–58. [Google Scholar]
- Mularczyk, M.; Michalak, I.; Marycz, K. Astaxanthin and other Nutrients from Haematococcus pluvialis—Multifunctional Applications. Mar. Drugs 2020, 18, 459. [Google Scholar] [CrossRef] [PubMed]
- Villaró, S.; Ciard, M.; España, A.M.; Zurano, A.S.; Fernández, G.A.; Lafarga, T. Microalgae derived astaxanthin: Research and consumer trends and industrial use as food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- Nishshanka, G.K.S.H.; Liyanaarachchi, V.C.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Chang, J.S. Haematococcus pluvialis: A potential feedstock for multiple-product biorefining. J. Clean. Prod. 2022, 344, 131103. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, X.; Yu, X.; Li, S.; Wang, K.; Wei, L.; Li, R.; Qin, S. Advancements of astaxanthin production in Haematococcus pluvialis: Update insight and way forward. Biotechnol. Adv. 2025, 79, 108519. [Google Scholar] [CrossRef]
- Christabel, C.; Kim, B.; Narasimhan, A.L.; Sathiyavahisan, L.P.; Ilhamsyah, D.P.A.; Kim, E.J.; Oh, Y.K. Enhanced Cell Growth and Astaxanthin Production in Haematococcus lacustris by Mechanostimulation of Seed Cysts. Appl. Sci. 2024, 14, 10434. [Google Scholar] [CrossRef]
- Samara, C.; Biziouras, A.; Papapanagiotou, G.; Chatzidoukas, C. Extending the green stage of Haematococcus pluvialis as a crucial precursor for efficient astaxanthin production: Optimization via Taguchi design. Biochem. Eng. J. 2025, 220, 109763. [Google Scholar] [CrossRef]
- Gencer, Ö.; Turan, G. Enhancing biomass and lipid productivities of Haematococcus pluvialis for industrial raw materials products. Biotechnol. Biofuels Bioprod. 2025, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.N.H.; Shoparwe, N.F.; Yusoff, A.H.; Rahim, A.A.; Chang, C.S.; Tan, J.S.; Osan, S.N.; Arumugam, K.; Ariff, A.B.; Sulaiman, A.Z. A Review on Haematococcus pluvialis Bioprocess Optimization of Green and Red Stage Culture Conditions for the Production of Natural Astaxanthin. Biomolecules 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Kawano, S. The Cell Cycle, Morphology, and Physiology of Haematococcus pluvialis. Front. Plant Sci. 2019, 10, 1435. [Google Scholar]
- Bold, H.C.; Wynne, M.J. Introduction to the Algae: Structure and Reproduction; Prentice-Hall Inc.: Upper Saddle River, NJ, USA, 1985. [Google Scholar]
- Hazen, T.E. The Life History of Sphaerella lacustris (Haemtococcus pluvialis). Mem. Torrey Bot. Club 1899, 6, 211–246. [Google Scholar]
- Leonardi, P.I.; Popovich, C.A.; Damiani, M.C. Feedstocks for Secondgeneration Biodiesel: Microalgae’s Biology and Oil Composition. Economic Effects of Biofuel Production; Intech: London, UK, 2011; pp. 318–346. [Google Scholar] [CrossRef]
- Czygan, F.-C. Blutregen und blutschnee: Stickstoffmangel-zellen von Haematococcus pluvialis und Chlamydomonas nivalis. Arch. Microbiol. 1970, 74, 69–76. [Google Scholar] [CrossRef]
- Burchardt, L.; Balcerkiewicz, S.; Kokociński, M.; Samardakiewicz, S.; Adamski, Z. Occurrence of Haematococcus pluvialis Flotow emend. Wille in a small artificial pool on the university campus of the Collegium Biologicum in Poznań (Poland). Biodivers. Res. Conserv. 2006, 2006, 163–166. [Google Scholar] [CrossRef]
- Bian, C.; Liu, C.; Zhang, G.; Tao, M.; Huang, D.; Wang, C.; Lou, S.; Li, H.; Shi, Q.; Hu, Z. A chromosome-level genome assembly for the astaxanthin-producing microalga Haematococcus pluvialis. Sci. Data 2023, 10, 511. [Google Scholar] [CrossRef]
- Elliott, A.M. Morphology and life history of Haematococcus pluvialis. Archiv. Protistekunde 1934, 82, 250–272. [Google Scholar]
- Fábregas, J.; Domínguez, A.; Regueiro, M.; Maseda, A. Changes in protein, carbohydrate and lipid content of Haematococcus pluvialis in response to different culture conditions and their effects on astaxanthin production. Aquaculture 2001, 198, 1–13. [Google Scholar]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Wayama, M.; Ota, S.; Matsuura, H.; Nango, N.; Hirata, A.; Kawano, S. Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS ONE 2013, 8, e53618. [Google Scholar] [CrossRef] [PubMed]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur. J. Phycol. 2002, 37, 217–226. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef]
- Brinda, B.R.; Sarada, R.; Kamath, B.S.; Ravishankar, G.A. Accumulation of astaxanthin in flagellated cells of Haematococcus pluvialis–cultural and regulatory aspects. Curr. Sci. 2004, 87, 1290–1295. [Google Scholar]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef]
- Hata, N.; Ogbonna, J.C.; Hasegawa, Y.; Taroda, H.; Tanaka, H. Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J. Appl. Phycol. 2001, 13, 395–402. [Google Scholar] [CrossRef]
- Sarada, R.; Bhattacharya, S.; Ravishankar, G. Optimization of culture conditions for growth of the green alga Haematococcus pluvialis. World J. Microbiol. Biotechnol. 2002, 18, 517–521. [Google Scholar] [CrossRef]
- Kwan, P.P.; Banerjee, S.; Shariff, M.; Yusoff, F. Influence of light on biomass and lipid production in microalgae cultivation. Aquac. Res. 2020, 52, 1337–1347. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Chaisutyakorn, P.; Gleadow, R.; Beardall, J. A comparison of photoautotrophic, heterotrophic, and mixotrophic growth for biomass production by the green alga Scenedesmus sp. (Chlorophyceae). Phycologia 2018, 57, 309–317. [Google Scholar] [CrossRef]
- de Moraes, L.B.S.; Mota, G.C.P.; dos Santos, E.P.; Campos, C.V.F.D.S.; da Silva, B.A.B.; Olivera Gálvez, A.; de Souza Bezerra, R. Haematococcus pluvialis cultivation and astaxanthin production using different nitrogen sources with pulse feeding strategy. Biomass Convers. Biorefinery 2023, 14, 16231–16243. [Google Scholar] [CrossRef]
- BByeon, H.; An, Y.; Kim, T.; Rayamajhi, V.; Lee, J.; Shin, H.; Jung, S. Effects of Four Organic Carbon Sources on the Growth and Astaxanthin Accumulation of Haematococcus lacustris. Life 2023, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Wang, Z.; Ding, Y.; Geng, Y.; Li, Y. Enhancing the production of astaxanthin by mixotrophic cultivation of Haematococcus pluvialis in open raceway ponds. Aquac. Int. 2019, 28, 625–638. [Google Scholar] [CrossRef]
- Pang, N.; Chen, S. Effects of C5 organic carbon and light on growth and cell activity of Haematococcus pluvialis under mixotrophic conditions. Algal Res. 2017, 21, 227–235. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- An, Y.; Kim, T.; Byeon, H.; Rayamajhi, V.; Lee, J.; Jung, S.; Shin, H. Produção aprimorada de astaxantina a partir de Haematococcus pluvialis usando um sistema híbrido de cultivo aberto-fechado. Appl. Sci. 2024, 14, 1104. [Google Scholar] [CrossRef]
- Boussiba, S.; Bing, W.; Yuan, J.-P.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga Haeamtococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Zhang, W. Chemicals to enhance microalgal growth and accumulation of high-value bioproducts. Front. Microbiol. 2015, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.A.; Hariyanto, P.; Irshad, M.; Ruqian, C.; Wulandari, S.; Hong, M.E.; Sim, S.J.; Kim, J. Strategy for high-yield astaxanthin recovery directly from wet Haematococcus pluvialis without pretreatment. Bioresour. Technol. 2022, 346, 126616. [Google Scholar] [CrossRef]
- Kim, J.H.; Affan, A.; Jang, J.; Kang, M.-H.; Ko, A.-R.; Jeon, S.-M.; Oh, C.; Heo, S.-J.; Lee, Y.-H.; Ju, S.-J.; et al. Morphological, molecular, and biochemical characterization of astaxanthin-producing green microalga Haematococcus sp. KORDI03 Haematococcaceae, Chlorophyta) isolated from Korea. J. Microbiol. Biotechnol. 2015, 25, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Krishnamurthi, M.C.; Ravi, S.; Chauhan, V.S. Valorising Haematococcus Biomass for Commercial Applications; Raja, R., Hemaiswarya, S., Narayanan, M., Kandasamy, S., Jayappriyan, K., Eds.; Haematococcus; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Hossain, A.K.M.M.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The Effect of Astaxanthin-Rich Microalgae “Haematococcus pluvialis” and Wholemeal Flours Incorporation in Improving the Physical and Functional Properties of Cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef]
- Tavares, L.H.S.; Tedesque, M.G.; Millan, R.N.; Fernades, J.B.K.; Scardoeli-Truzzi, B. Haematococcus pluvialis biomass as a replacement for fish meal in the diet of Macrobrachium amazonicum post-larvae (Heller, 1862). Acta Scientiarum. Anim. Sci. 2023, 46, e63925. [Google Scholar] [CrossRef]
- Bassani, J.C.; Martins, V.F.R.; Barbosa, J.; Coelho, M.; Sousa, C.; Steffens, J.; Backes, G.T.; Pereira, H.; Pintado, M.E.; Teixeira, P.C.; et al. Exploring the Development of a Clean-Label Vegan Burger Enriched with Fermented Microalgae. Foods 2025, 14, 2884. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Lombardi, A.T. Growth, photosynthesis and biochemical composition of Haematococcus pluvialis at various pH. J. Algal Biomass Util. 2017, 8, 1–15. [Google Scholar]
- Peled, E.; Leu, S.; Zarka, A.; Weiss, M.; Pick, U.; Khozin-Goldberg, I.; Boussiba, S. Isolation of a Novel Oil Globule Protein from the Green Alga Haematococcus pluvialis (Chlorophyceae). Lipids 2011, 46, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo, C.; Rivera, N.; Rodríguez, E.; Fernández-González, A.; Hernández Battez, A. Biodiesel derived from the microalgae Nannochloropsis gaditana and Haematococcus pluvialis: Physicochemical and tribological properties. J. Mol. Liq. 2024, 408, 125391. [Google Scholar] [CrossRef]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcuspluvialis to assess its potential use as a biodiesel feedstock. Bioresour Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef]
- Scodelaro Bilbao, P.G.; Damiani, C.; Salvador, G.A.; Leonardi, P.I. Haematococcus pluvialis as a source of fatty acids and phytosterols: Potential nutritional and biological implications. J. Appl. Phycol. 2016, 28, 3283–3294. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Kolonas, A.; Stagos, D.; Gortzi, O. Uma revisão da sustentabilidade, composição química, compostos bioativos, atividade antioxidante e antidiabética, propriedades neuroprotetoras e benefícios para a saúde das microalgas. Biomass 2025, 5, 11. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Z.; Liu, S.; Luo, S.; He, R.; Yang, X.; Li, S.; Yang, X.; An, Y.; Lu, Y. Utilization of Microalgae and Duckweed as Sustainable Protein Sources for Food and Feed: Nutritional Potential and Functional Applications. J. Agric. Food. Chem. 2025, 73, 4466–4482. [Google Scholar] [CrossRef]
- Dragoş, N.; Bercea, V.; Bica, A.; Drug, B.; Nicoar, A.; Coman, C. Astaxanthin Production From A New Strain of Haematococcus pluvialis Grown in Batch Culture. Ann. Rom. Soc. Cell Biol. 2010, 15, 353–361. [Google Scholar]
- Yang, Y.; Kim, B.; Lee, J.Y. Astaxanthin structure, metabolism, and health benefits. J. Hum. Nutr. Food Sci. 2013, 1, 1–1003. [Google Scholar]
- Solovchenko, A.E. Recent breakthroughs in the biology of astaxanthin accumulation by microalgal cell. Photosynth. Res. 2015, 125, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.R.; Sarada, R.; Shylaja, M.D.; Ravishankar, G.A. Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J. Food Sci. Tech. 2015, 52, 6703–6710. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, A.; Wen, X.; Wang, Z.; Wang, K.; Geng, Y.; Li, Y. Application of surfactants for controlling destructive fungus contamination in mass cultivation of Haematococcus pluvialis. Bioresour. Technol. 2020, 317, 124025. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Hou, D.; Li, Y.; Fan, J.; Huang, J.; Liang, S.; Wang, W.; Pan, R.; Wang, J.; Li, S. The effective photoinduction of Haematococcus pluvialis for accumulating astaxanthin with attached cultivation. Bioresour. Technol. 2014, 163, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Xie, Q.; Lin, S.; Xu, W.; Cheung, P.C.K. Microalgae-derived astaxanthin: Bioactivities, biotechnological approaches and industrial technologies for its production. Crit. Rev. Food Sci. Nutr. 2025, 65, 7358–7392. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zheng, L.; Yang, Z.; Li, L.; Zhang, Q.; Li, L.; Chen, W.; Wang, G.; Song, L. Biomass production and astaxanthin accumulation of Haematococcus pluvialis in large-scale outdoor culture based on year-round survey: Influencing factors and physiological response. Algal Res. 2023, 71, 103070. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef]
- Wang, X.; Meng, C.; Zhang, H.; Xing, W.; Cao, K.; Zhu, B.; Zhang, C.; Sun, F.; Gao, Z. Transcriptomic and Proteomic Characterizations of the Molecular Response to Blue Light and Salicylic Acid in Haematococcus pluvialis. Mar. Drugs 2022, 20, 1. [Google Scholar] [CrossRef]
- Jin, C.; You, J.; Zhou, Z.; Liu, Q.; Zhou, X. Novel insights into saline stress on photosynthetic activity and astaxanthin production of Haematococcus pluvialis. J. Oceanol. Limnol. 2024, 43, 921–938. [Google Scholar] [CrossRef]
- Galarza, J.I.; Gimpel, J.A.; Rojas, V.; Vega, B.O.A.; Henríquez, V. Over-accumulation of astaxanthin in Haematococcus pluvialis through chloroplast genetic engineering. Algal Res. 2018, 31, 291–297. [Google Scholar] [CrossRef]
- Kayani, S.-I.; Rahman, S.; Shen, Q.; Cui, Y.; Liu, E.; Hu, X. Molecular approaches to enhance astaxanthin biosynthesis; future outlook: Engineering of transcription factors in Haematococcus pluvialis. Crit. Rev. Biotechnol. 2023, 44, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, K.; Du, F.; Xing, H.; Su, X.; Hu, T.; Chen, F.; Qin, Y.; Dong, Z.; Sun, X.; et al. Transcriptome profiling and co-expression network analysis of 96 Haematococcus pluvialis samples. Sci. Data 2025, 12, 1272. [Google Scholar] [CrossRef]
- Hu, C.; Cui, D.; Sun, X.; Shi, J.; Xu, N. Primary metabolism is associated with the astaxanthin biosynthesis in the green algae Haematococcus pluvialis under light stress. Algal Res. 2020, 46, 101768. [Google Scholar] [CrossRef]
- Acheampong, A.; Li, L.; Elsherbiny, S.M.; Wu, Y.; Swallah, M.S.; Quaye, P.B.; Huang, Q. A crosswalk on the genetic and conventional strategies for enhancing astaxanthin production in Haematococcus pluvialis. Crit. Rev. Biotechnol. 2023, 44, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Pitacco, W.; Samorì, C.; Pezzolesi, L.; Gori, V.; Grillo, A.; Tiecco, M.; Vagnoni, M.; Galletti, P. Extraction of astaxanthin from Haematococcus pluvialis with hydrophobic deep eutectic solvents based on oleic acid. Food Chem. 2022, 379, 132156. [Google Scholar] [CrossRef]
- Bauer, A.; Minceva, M. Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid–liquid chromatography. RSC Adv. 2019, 9, 22779–22789. [Google Scholar] [CrossRef]
- Irshad, M.; Hong, M.E.; Myint, A.A.; Kim, J.; Sim, S.J. Safe and complete extraction of astaxanthin from Haematococcus pluvialis by efficient mechanical disruption of cyst cell wall. Int. J. Food Eng. 2019, 15, 20190128. [Google Scholar] [CrossRef]
- Tan, Y.; Ye, Z.; Wang, M.; Manzoor, M.F.; Aadil, R.M.; Tan, X.; Liu, Z. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. Sci. World J. 2014, 2014, 694305. [Google Scholar] [CrossRef]
- Huang, H.; Lin, X.; Li, W.; Liu, F.; Gao, J. Enhanced selective extraction of bioactive components using novel microemulsions. Sep. Purif. Technol. 2025, 219, 135427. [Google Scholar] [CrossRef]
- Yousefi, M.; Khorshidian, N.; Khanniri, E.; Mortazavian, A.M. Microalgae added to beverages, dairy, prebiotic, and probiotic products. In Handbook of Food and Feed from Microalgae: Production, Application, Regulation, and Sustainability; Elsevier Inc.: New York, NY, USA, 2023; pp. 335–347. [Google Scholar] [CrossRef]
- Batista, A.P.; Nune, M.C.; Fradinho, P.; Gouveia, L.; Sousa, I.; Raymundo, A.; Franco, J.M. Novel foods with microalgal ingredients-Effect of gel setting conditions on the linear viscoelasticity of Spirulina and Haematococcus gels. J. Food Eng. 2012, 110, 182–189. [Google Scholar] [CrossRef]
- Yıldız, S.; Reisoglu, Ş.; Aydin, S. Evaluation of functional quality of water kefir with Haematococcus pluvialis biomass and colour pigment astaxanthin. Int. J. Food Sci. Technol. 2024, 59, 4326–4335. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Zhu, L.; Zhao, X. Effects of Haematococcus pluvialis Addition on the Sensory Properties of Plant-Based Meat Analogues. Foods 2023, 12, 3435. [Google Scholar] [CrossRef]
- Wang, M.; Yin, Z.; Zeng, M. Construction of 3D printable Pickering emulsion gels using complexes of fiber polysaccharide-protein extracted from Haematococcus pluvialis residues and gelatin for fat replacer. Food Hydrocoll. 2022, 137, 108350. [Google Scholar] [CrossRef]
- Singh, T.; Pandey, V.K.; Dash, K.K.; Zanwar, S.; Singh, R. Natural bio-colorant and pigments: Sources and applications in food processing. J. Agric. Food Res. 2023, 12, 100628. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Caicedo-Paz, A.V.; Farias, F.O.; Tropea, A.; La Tella, R.; Guzmán-Flores, J.M.; Mondello, L.; Herculano, R.D.; ELL Filho, P.; Piazza, R.D.; et al. Comparative analysis of bacterial and microalgal natural astaxanthin: Part I—Focus on composition, molecular interactions, antioxidant activities, physicochemical and biological functions. Algal Res. 2024, 85, 103862. [Google Scholar] [CrossRef]
- Xia, S.; Xue, Y.; Xue, C.; Jiang, X.; Li, J. Structural and rheological properties of meat analogues from Haematococcus pluvialis residue-pea protein by high moisture extrusion. LWT 2022, 154, 112756. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; An, H.; Kovacs, Z.; Abddollahi, M.; Sun, Z.; Zhang, G.; Li, C. Utilizing Haematococcus pluvialis to simulate animal meat color in high-moisture meat analogues: Texture quality and color stability. Food Res. Int. 2023, 175, 113685. [Google Scholar] [CrossRef]
- Bassani, J.C.; Santos, V.A.Q.; Barbosa-Dekker, A.M.; Dekker, R.F.; da Cunha, M.A.A.; Pereira, E.A. Microbial cell encapsulation as a strategy for the maintenance of stock cultures. LWT 2019, 102, 411–417. [Google Scholar] [CrossRef]
- Oslan, S.N.H.; Tan, J.S.; Oslan, S.N.; Matanjun, P.; Mokhtar, R.A.M.; Shapawi, R.; Huda, N. Haematococcus pluvialis as a potential source of astaxanthin with diverse applications in industrial sectors: Current research and future directions. Molecules 2021, 26, 6470. [Google Scholar] [CrossRef]
- Seo, J.-K.; Parvin, R.; Park, J.; Yang, H.-S. Utilization of astaxanthin as a synthetic antioxidant replacement for emulsified sausages. Antioxidants 2021, 10, 407. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Morais, A.M.M.B.; Morais, R.M.S.C. Effects of spray-drying and storage on astaxanthin content of Haematococcus pluvialis biomass. World J. Microbiol. Biotechnol. 2012, 28, 1253–1257. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent advances in astaxanthin micro/nanoencapsulation to improve its stability and functionality as a food ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef]
- Pudžiuvelytė, L.; Petrauskaitė, E.; Stabrauskienė, J.; Bernatonienė, J. Spray-Drying Microencapsulation of Natural Bioactives: Advances in Sustainable Wall Materials. Pharmaceuticals 2025, 18, 963. [Google Scholar] [CrossRef]
- 102 Chik, M.W.; Affandi, M.M.R.M.M.; Chellammal, H.S.J.; Hazalin, N.A.M.N.; Singh, G.K.S. Astaxanthin Nanoemulsion and Nanoparticle Formulations and Their Therapeutic Potential: A Review. Adv. Pharmacol. Pharm. 2025, 13, 342–354. [Google Scholar] [CrossRef]
- Lee, M.-C.; Huang, C.-Y.; Huang, J.; Chang, C.-Y.; Lee, P.-T.; Nan, F.-H. The Effect of Dietary Supplementation with Haematococcus pluvialis for Enhanced Pigmentation in Amphiprion ocellaris. Aquac. Res. 2023, 2023, 5542730. [Google Scholar] [CrossRef]
- Spiller, G.A.; Dewell, A. Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: A randomized clinical trial. J. Med. Food 2003, 6, 51–56. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.K.; Ahmed, R.; Chikkaputtaiah, C.; Velmurugan, N. Commercialization of Haematococcus-Based Products: Current Status and Future Forecast; Raja, R., Hemaiswarya, S., Narayanan, M., Kandasamy, S., Jayappriyan, K., Eds.; Haematococcus; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Fennema, O.R. Food Chemistry, 3rd ed.; Marcel Dekker Inc.: New York, NY, USA, 1996; p. 1069. [Google Scholar]
- Torregrosa-Crespo, J.; Montero, Z.; Fuentes, J.L.; García-Galbis, M.R.; Garbayo, I.; Vílchez, C.; Martínez-Espinosa, R.M. Exploring the valuable carotenoids for the large-scale production by marine microorganisms. Mar. Drugs 2018, 16, 203. [Google Scholar] [CrossRef] [PubMed]
- EC No 178/2002 Food Safety. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02002R0178-20180701 (accessed on 29 August 2025).
- EC No 852/2004 Food Hygiene. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32004R0852 (accessed on 29 August 2025).
- EU 2015/2283 Novel Foods. Available online: https://eur-lex.europa.eu/eli/reg/2015/2283/oj (accessed on 29 August 2025).
- FD&C Act. Available online: https://www.fda.gov/regulatory-information/laws-enforced-fda/federal-food-drug-and-cosmetic-act-fdc-act (accessed on 1 September 2025).
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Capelli, G.C.; Cysewski, G. The Worlds’ Best Kept Health Secret Natural Astaxanthin; Cyanotech Corporation: Kailua-Kona, HI, USA, 2013. [Google Scholar]
- EFSA Panel on Nutrition; Novel Foods and Food Allergens (NDA). Safety of astaxanthin for its use as a novel food in food supplements. EFSA J. 2020, 18, e05993. [Google Scholar] [CrossRef]
- de Oliveira, A.P.F.; Bragotto, A.P.A. Microalgae-based products: Food and public health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- European Commission; European Climate; Infrastructure and Environment Executive Agency; Bruce, F. #EU4Algae–Overview of Education, Youth & Business Support for the European Algae Sector–Summary Report, October 2023; Publications Office of the European Union: Luxembourg, 2024. [CrossRef]
- Olsen, M.L.; Olsen, K.; Jensen, P.E. Consumer acceptance of microalgae as a novel food-Where are we now? And how to get further. Physiol. Plant. 2024, 176, e14337. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.F.; Bianchini, C.B.; Wanderley, B.R.d.S.M.; Fritzen-Freire, C.B.; Bezerra, P.Q.M.; Nunes, I.L. Analysis of the knowledge and attitudes of Brazilian consumers regarding microalgae: A strategy to assess the development potential of new foods. Food Sci. Technol. 2024, 44, e000365. [Google Scholar] [CrossRef]

| Composition Content (% DW) | Green Stage | Red Stage |
|---|---|---|
| Proteins | 29–45 | 17–25 |
| Lipids (% of total) | 20–25 | 32–37 |
| Neutral lipids | 59 | 51.9–53.5 |
| Phospholipids | 23.7 | 20.6–21.1 |
| Glycolipids | 11.5 | 25.7–26.5 |
| Carbohydrates | 15–17 | 36–40 |
| Carotenoids (% of total) | 0.5 | 2–5 |
| Neoxanthin | 8.3 | n.d. |
| Violaxanthin | 12.5 | n.d. |
| β-Carotene | 16.7 | 1.0 |
| Lutein | 56.3 | 0.5 |
| Zeaxanthin | 6.3 | n.d. |
| Astaxanthin (incl. esters) | n.d. | 81.2 |
| Adonixanthin | n.d. | 0.4 |
| Adonirubin | n.d. | 0.6 |
| Canthaxanthin | n.d. | 5.1 |
| Echinenone | n.d. | 0.2 |
| Chlorophylls | 1.5–2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassani, J.C.; da Cunha, S.; de Assis Leite, D.C.; Endres, C.M.; Pelisser, C.; Meneghetti, K.L.; Bombo, G.; Morais, A.M.M.B.; Morais, R.M.S.C.; Backes, G.T.; et al. Exploring the Potential of Haematococcus pluvialis as a Source of Bioactives for Food Applications: A Review. Microorganisms 2025, 13, 2606. https://doi.org/10.3390/microorganisms13112606
Bassani JC, da Cunha S, de Assis Leite DC, Endres CM, Pelisser C, Meneghetti KL, Bombo G, Morais AMMB, Morais RMSC, Backes GT, et al. Exploring the Potential of Haematococcus pluvialis as a Source of Bioactives for Food Applications: A Review. Microorganisms. 2025; 13(11):2606. https://doi.org/10.3390/microorganisms13112606
Chicago/Turabian StyleBassani, Joseane C., Sthéfani da Cunha, Deborah Catharine de Assis Leite, Creciana M. Endres, Crivian Pelisser, Karine L. Meneghetti, Gabriel Bombo, Alcina M. M. B. Morais, Rui M. S. C. Morais, Geciane T. Backes, and et al. 2025. "Exploring the Potential of Haematococcus pluvialis as a Source of Bioactives for Food Applications: A Review" Microorganisms 13, no. 11: 2606. https://doi.org/10.3390/microorganisms13112606
APA StyleBassani, J. C., da Cunha, S., de Assis Leite, D. C., Endres, C. M., Pelisser, C., Meneghetti, K. L., Bombo, G., Morais, A. M. M. B., Morais, R. M. S. C., Backes, G. T., & Steffens, J. (2025). Exploring the Potential of Haematococcus pluvialis as a Source of Bioactives for Food Applications: A Review. Microorganisms, 13(11), 2606. https://doi.org/10.3390/microorganisms13112606









