Multi-Kingdom Gut Microbiome Interaction Characteristics Predict Immune Checkpoint Inhibitor Efficacy Across Pan-Cancer Cohorts
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohorts
2.2. Metagenomic Analysis
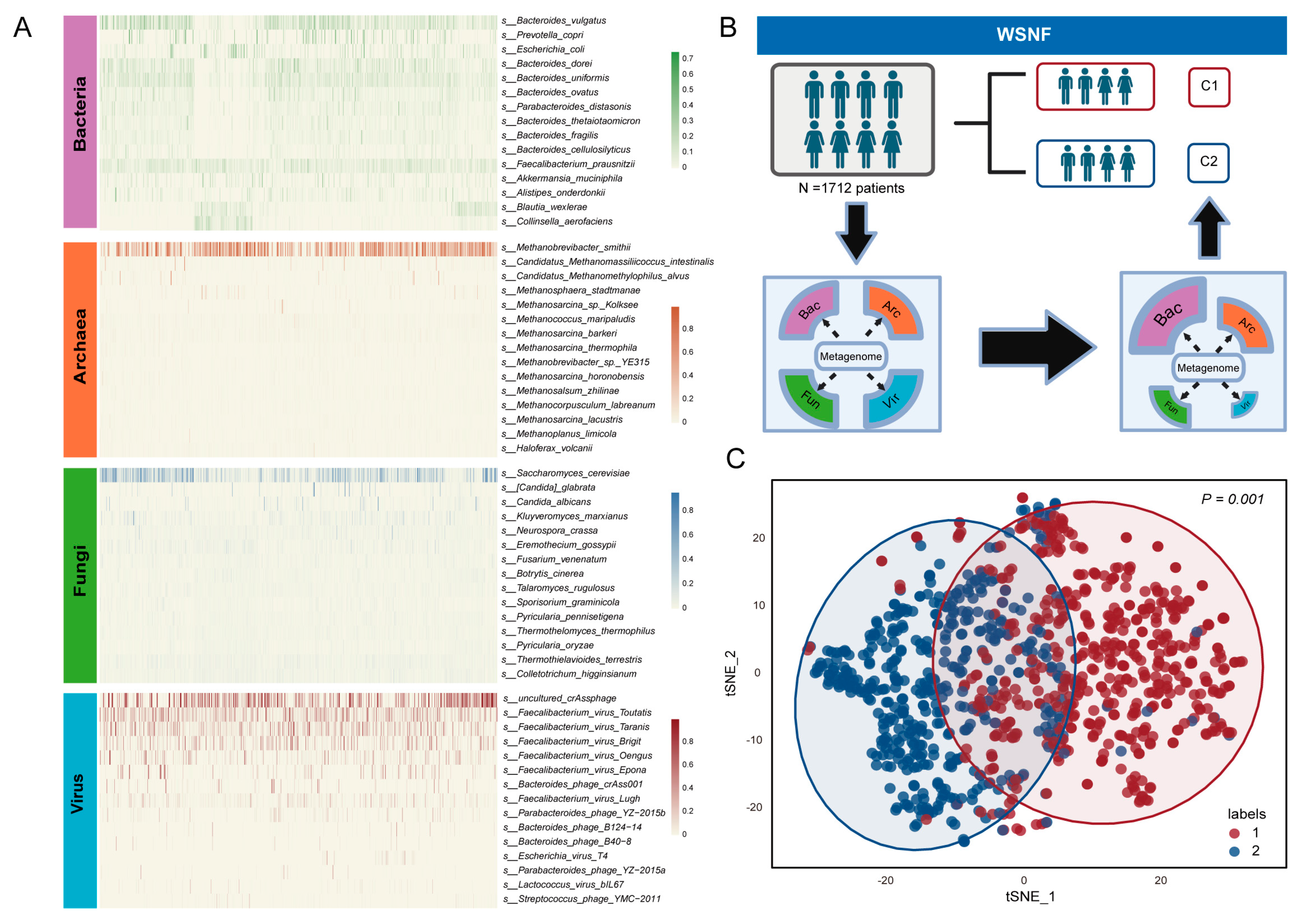
2.3. Weighted Similarity Network Fusion (WSNF) Analysis
2.4. Microbial Diversity Analysis
2.5. Survival Analysis
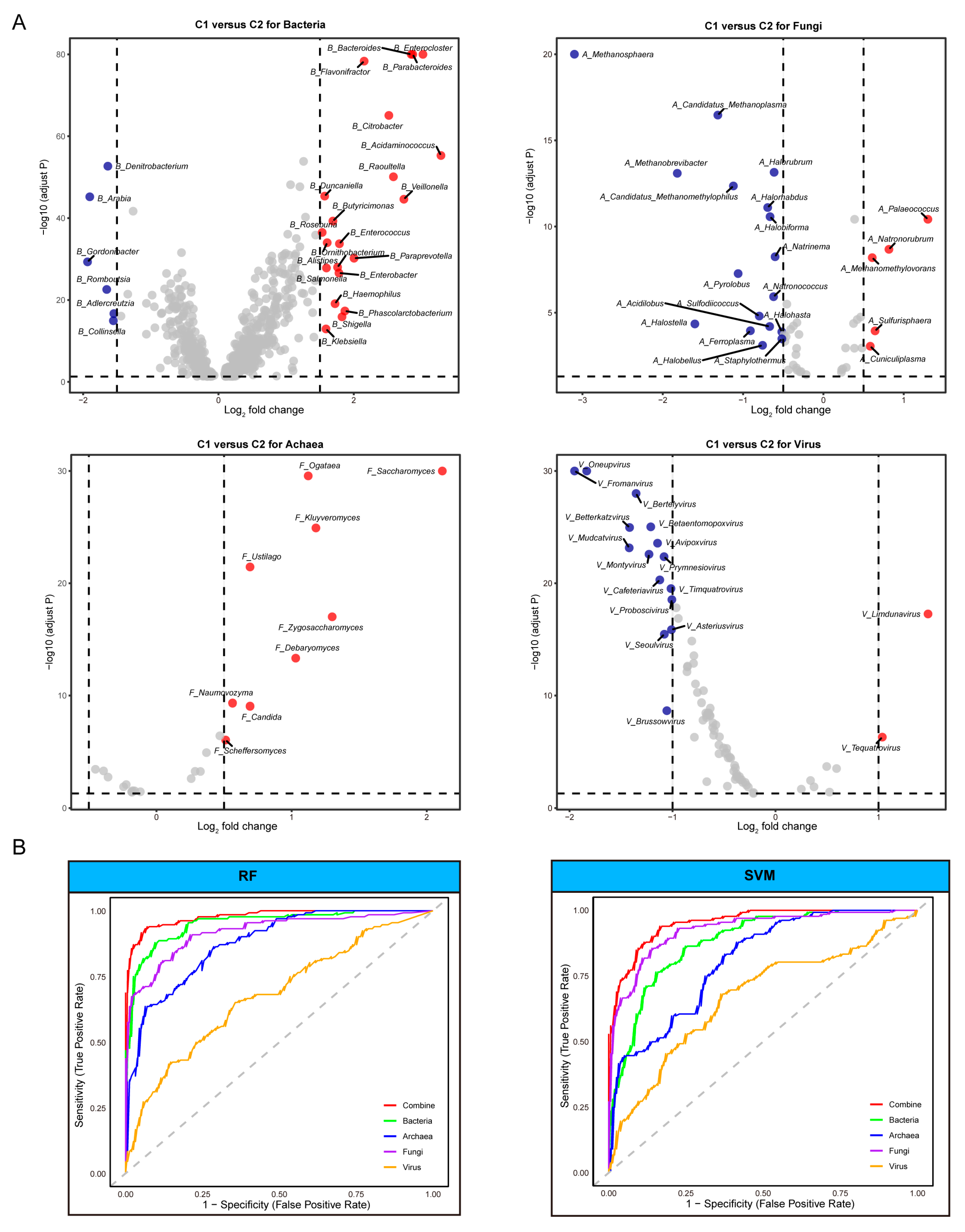
2.6. Differential Taxa Analysis and Machine Learing
2.7. Microbial Community Network Analysis
2.8. Statistical Analysis
3. Results
3.1. Multi-Kingdom Microbial Clustering Using WSNF Method
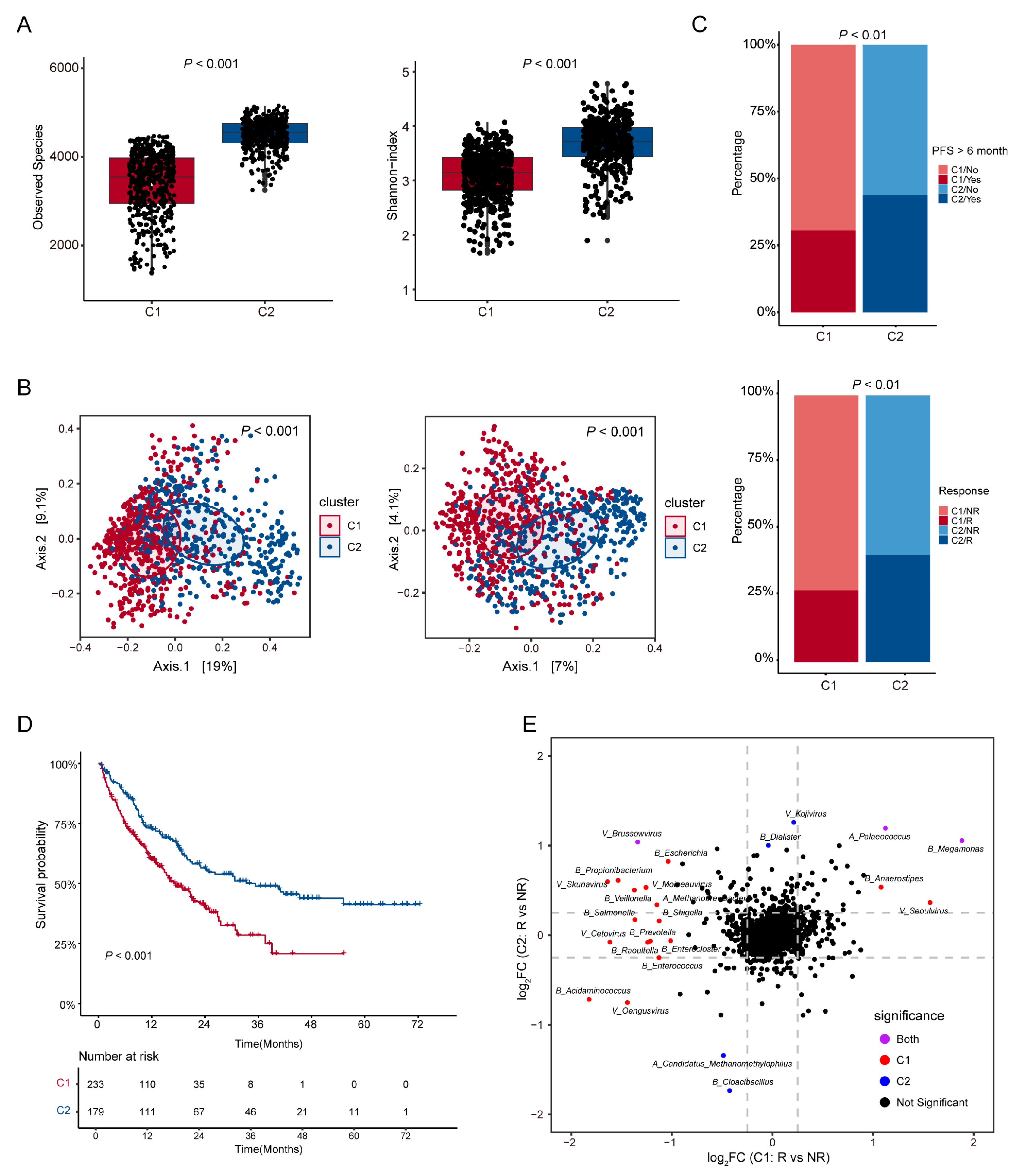
3.2. Analysis of Microbial Diversity and Its Correlation with ICI Therapy Outcomes in Patient Subtypes
3.3. Identification of Distinct Microbial Taxa and Their Effectiveness in Predicting ICI Therapy Outcomes
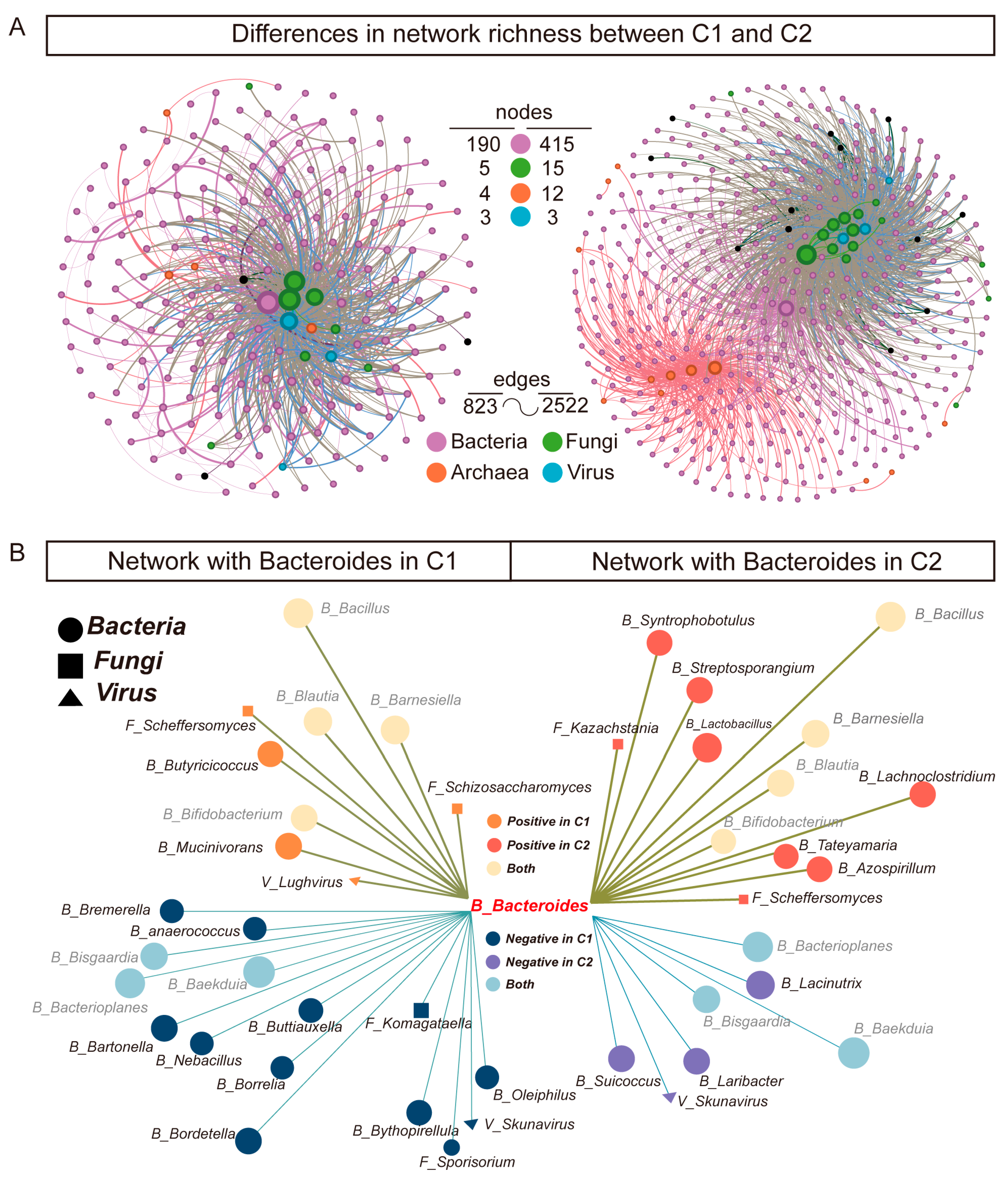
3.4. Co-Occurrence Networks of Gut Microbiota Reveal Rich Diversity in Subtype C2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34, Erratum in N. Engl. J. Med. 2015, 373, 1270–1271. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-year survival outcomes from the pacific trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 2022, 40, 1301–1311, Erratum in J. Clin. Oncol. 2022, 40, 1965. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ye, M.; Zou, Q.; Chen, P.; He, Z.; Wu, B.; He, D.; He, C.; Xue, X.; Ji, Z.; et al. Toripalimab plus axitinib versus sunitinib as first-line treatment for advanced renal cell carcinoma: Renororch, a randomized, open-label, phase III study. Ann. Oncol. 2024, 35, 190–199. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef] [PubMed]
- van Not, O.J.; Verheijden, R.J.; Eertwegh, A.J.M.v.D.; Haanen, J.B.A.G.; Aarts, M.J.B.; Berkmortel, F.W.P.J.v.D.; Blank, C.U.; Boers-Sonderen, M.J.; de Groot, J.-W.B.; Hospers, G.A.P.; et al. Association of Immune-Related Adverse Event Management with Survival in Patients With Advanced Melanoma. JAMA Oncol. 2022, 8, 1794–1801. [Google Scholar] [CrossRef]
- Simpson, R.C.; Shanahan, E.R.; Scolyer, R.A.; Long, G.V. Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2023, 20, 697–715. [Google Scholar] [CrossRef]
- Bjork, J.R.; Hospers, G.A.; Weersma, R.K. Longitudinal analysis shows how microbiome changes might influence responses to immune checkpoint blockade. Nat. Med. 2024, 30, 644–645. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H., Jr.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: A phase I trial. Nat. Med. 2023, 29, 2121–2132. [Google Scholar] [CrossRef]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A.; et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, É.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. MBio 2021, 13, e0314421. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Zeng, B.; Hu, G.; Gan, R. Epstein–Barr virus-associated gastric cancer: A distinct subtype. Cancer Lett. 2020, 495, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Wu, W.K.K.; Wong, S.H.; Sung, J.J.; Yu, J. Altered Gut Archaea Composition and Interaction With Bacteria Are Associated With Colorectal Cancer. Gastroenterology 2020, 159, 1459–1470.e5. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Peters, B.A.; Wilson, M.; Moran, U.; Pavlick, A.; Izsak, A.; Wechter, T.; Weber, J.S.; Osman, I.; Ahn, J. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 2019, 11, 61. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Wadud Khan, M.A.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Derosa, L.; Routy, B.; Maltez Thomas, A.; Iebba, V.; Zalcman, G.; Friard, S.; Mazieres, J.; Audigier-Valette, C.; Moro-Sibilot, D.; Goldwasser, F.; et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 2022, 28, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Tidjani Alou, M.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Stein-Thoeringer, C.K.; Saini, N.Y.; Zamir, E.; Blumenberg, V.; Schubert, M.-L.; Mor, U.; Fante, M.A.; Schmidt, S.; Hayase, E.; Hayase, T.; et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 2023, 29, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Maltez Thomas, A.; Bolte, L.A.; Björk, J.R.; Kist de Ruijter, L.; Armanini, F.; Asnicar, F.; Blanco-Miguez, A.; Board, R.; Calbet-Llopart, N.; et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 2022, 28, 535–544. [Google Scholar] [CrossRef]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.-F.; Li, J.; Lu, M.; et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating species abundance in metagenomics data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef]
- Aogáin, M.M.; Narayana, J.K.; Tiew, P.Y.; Ali, N.A.B.M.; Yong, V.F.L.; Jaggi, T.K.; Lim, A.Y.H.; Keir, H.R.; Dicker, A.J.; Thng, K.X.; et al. Integrative microbiomics in bronchiectasis exacerbations. Nat. Med. 2021, 27, 688–699. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Wang, X.; Fang, Y.; Liang, W.; Wong, C.C.; Qin, H.; Gao, Y.; Liang, M.; Song, L.; Zhang, Y.; Fan, M.; et al. Fusobacterium nucleatum facilitates anti-PD-1 therapy in microsatellite stable colorectal cancer. Cancer Cell 2024, 42, 1729–1746.e8. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Deng, H.; Li, Z.; Tan, Y.; Guo, Z.; Liu, Y.; Wang, Y.; Yuan, Y.; Yang, R.; Bi, Y.; Bai, Y.; et al. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci. Rep. 2016, 6, 29401. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.; Xie, M.; Huang, P.; Yu, Y.; Sun, Q.; Shangguan, W.; Li, W.; Zhu, Z.; Xue, J.; et al. Gut microbiota parabacteroides distasonis enchances the efficacy of immunotherapy for bladder cancer by activating anti-tumor immune responses. BMC Microbiol. 2024, 24, 237. [Google Scholar] [CrossRef]
- Dambuza, I.M.; Brown, G.D. Fungi accelerate pancreatic cancer. Nature 2019, 574, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Magiatis, P.; Stathopoulou, K.; Bassukas, I.D.; Alexopoulos, E.C.; Velegraki, A.; Skaltsounis, A.-L. AhR Ligands, Malassezin, and indolo[3,2-b]carbazole are selectively produced by Malassezia furfur strains isolated from seborrheic dermatitis. J. Investig. Dermatol. 2008, 128, 1620–1625. [Google Scholar] [CrossRef]
- Luo, J.-L.; Maeda, S.; Hsu, L.-C.; Yagita, H.; Karin, M. Inhibition of NF-κB in cancer cells converts inflammation-induced tumor growth mediated by TNFα to TRAIL-mediated tumor regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef]
- Thomas, A.M.; Manghi, P.; Asnicar, F.; Pasolli, E.; Armanini, F.; Zolfo, M.; Beghini, F.; Manara, S.; Karcher, N.; Pozzi, C.; et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 2019, 25, 667–678, Erratum in Nat. Med. 2019, 25, 1948. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; An, H.; He, Y.; Fu, G.; Jiang, Z. Comprehensive analysis of microbiota signature across 32 cancer types. Front. Oncol. 2023, 13, 1127225. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, M.; Lau, H.C.-H.; Zeng, R.; Zhang, R.; Wang, L.; Li, Q.; Wang, Y.; Chen, D.; Jiang, L.; et al. Effects of gut microbiota on immune checkpoint inhibitors in multi-cancer and as microbial biomarkers for predicting therapeutic response. Med 2025, 6, 100530. [Google Scholar] [CrossRef]
- Lim, M.Y.; Hong, S.; Nam, Y.-D. Understanding the role of the gut microbiome in solid tumor responses to immune checkpoint inhibitors for personalized therapeutic strategies: A review. Front. Immunol. 2024, 15, 1512683. [Google Scholar] [CrossRef]
- Gunjur, A.; Shao, Y.; Rozday, T.; Klein, O.; Mu, A.; Haak, B.W.; Markman, B.; Kee, D.; Carlino, M.S.; Underhill, C.; et al. A gut microbial signature for combination immune checkpoint blockade across cancer types. Nat. Med. 2024, 30, 797–809. [Google Scholar] [CrossRef]
- Huang, X.-Z.; Gao, P.; Song, Y.-X.; Xu, Y.; Sun, J.-X.; Chen, X.-W.; Zhao, J.-H.; Wang, Z.-N. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: A pooled analysis of 2740 cancer patients. Oncoimmunology 2019, 8, e1665973. [Google Scholar] [CrossRef] [PubMed]
- Fidelle, M.; Rauber, C.; Silva, C.A.C.; Tian, A.-L.; Lahmar, I.; de La Varende, A.-L.M.; Zhao, L.; Thelemaque, C.; Lebhar, I.; Messaoudene, M.; et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 2023, 380, eabo2296. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.-M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.; Kim, S.; Cho, B.; Kim, S.-Y.; Do, E.-J.; Bae, D.-J.; Kim, S.; Kweon, M.-N.; Song, J.S.; et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 2024, 32, 1380–1393.e9. [Google Scholar] [CrossRef]
- Lin, A.; Huang, L.; Jiang, A.; Zhu, L.; Mou, W.; Li, Y.; Zhang, C.; Liu, Z.; Zhang, J.; Cheng, Q.; et al. Microbiota boost immunotherapy? A meta-analysis dives into fecal microbiota transplantation and immune checkpoint inhibitors. BMC Med. 2025, 23, 341. [Google Scholar] [CrossRef]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef]
- Han, K.; Nam, J.; Xu, J.; Sun, X.; Huang, X.; Animasahun, O.; Achreja, A.; Jeon, J.H.; Pursley, B.; Kamada, N.; et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat. Biomed. Eng. 2021, 5, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
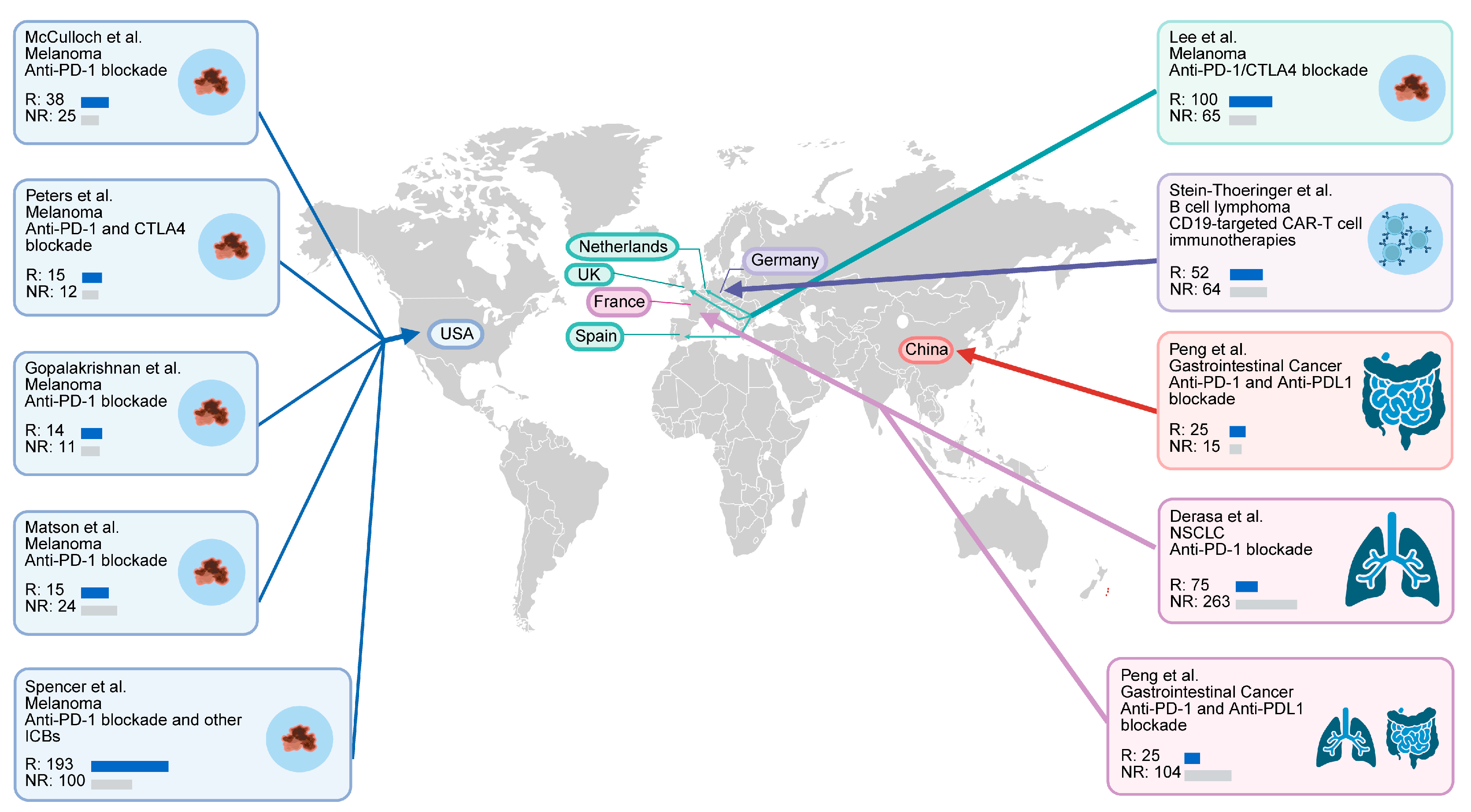
| Cohort | Sequencing Type | Country | Cancer Type | Treatment | Patients Included (Responder/Non_Responder) |
|---|---|---|---|---|---|
| PRJNA762360 | Metagenomic sequencing | USA | Melanoma | Anti-PD-1 blockade | 63 (38/25) |
| PRJEB43119 | Metagenomic sequencing | Netherlands, Spain, UK | Melanoma | Combination of anti-PD-1 and anti-CTLA-4 blockade | 165 (100/65) |
| PRJNA615114 | Metagenomic sequencing | China | Gastrointestinal cancer | Anti PD-1/PD-L1 blockade | 114 (70/44) |
| PRJNA751792 | Metagenomic sequencing | France | NSCLC | Anti-PD-1 blockade | 337 (75/262) |
| PRJNA541981 | Metagenomic sequencing | USA | Melanoma | Anti-PD-1 blockade/anti-CTLA4 blockade/combination of anti-PD-1 and antiCTLA-4 blockade | 113 (58/55) |
| PRJEB54704 | Metagenomic sequencing | Germany, USA | B cell lymphoma | CD19-targeted CAR-T cell immunotherapies | 351 (149/202) |
| PRJEB22893 | Metagenomic sequencing | USA | Melanoma | Anti-PD-1 blockade | 25 (14/11) |
| PRJEB22863 | Metagenomic sequencing | France | NSCLC, RCC | Anti-PD-1/PD-L1 blockade | 217 (36/181) |
| PRJNA399742 | Metagenomic sequencing | USA | Melanoma | Majority with anti-PD-1 blockade, a small portion with anti CTLA-4 blockade | 172 (11/18) |
| PRJNA397906 | Metagenomic sequencing | USA | Melanoma | Anti-PD-1 blockade and other immune checkpoint blockades | 44 (19/20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, T.; Zhu, Z. Multi-Kingdom Gut Microbiome Interaction Characteristics Predict Immune Checkpoint Inhibitor Efficacy Across Pan-Cancer Cohorts. Microorganisms 2025, 13, 2595. https://doi.org/10.3390/microorganisms13112595
Qiao T, Zhu Z. Multi-Kingdom Gut Microbiome Interaction Characteristics Predict Immune Checkpoint Inhibitor Efficacy Across Pan-Cancer Cohorts. Microorganisms. 2025; 13(11):2595. https://doi.org/10.3390/microorganisms13112595
Chicago/Turabian StyleQiao, Tong, and Zhenjun Zhu. 2025. "Multi-Kingdom Gut Microbiome Interaction Characteristics Predict Immune Checkpoint Inhibitor Efficacy Across Pan-Cancer Cohorts" Microorganisms 13, no. 11: 2595. https://doi.org/10.3390/microorganisms13112595
APA StyleQiao, T., & Zhu, Z. (2025). Multi-Kingdom Gut Microbiome Interaction Characteristics Predict Immune Checkpoint Inhibitor Efficacy Across Pan-Cancer Cohorts. Microorganisms, 13(11), 2595. https://doi.org/10.3390/microorganisms13112595







