Effect of Xylopia frutescens Essential Oil on the Activation of Defense Mechanisms Against Phytopathogenic Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection, Botanical Provenance and Traceability, and Location of Experiments
2.2. Obtaining the Essential Oil Xylopia frutescens Aubl.
2.3. Effects on Crops and Diseases
2.3.1. Chromatographic Analysis of Essential Oil Xylopia frutescens Aubl.
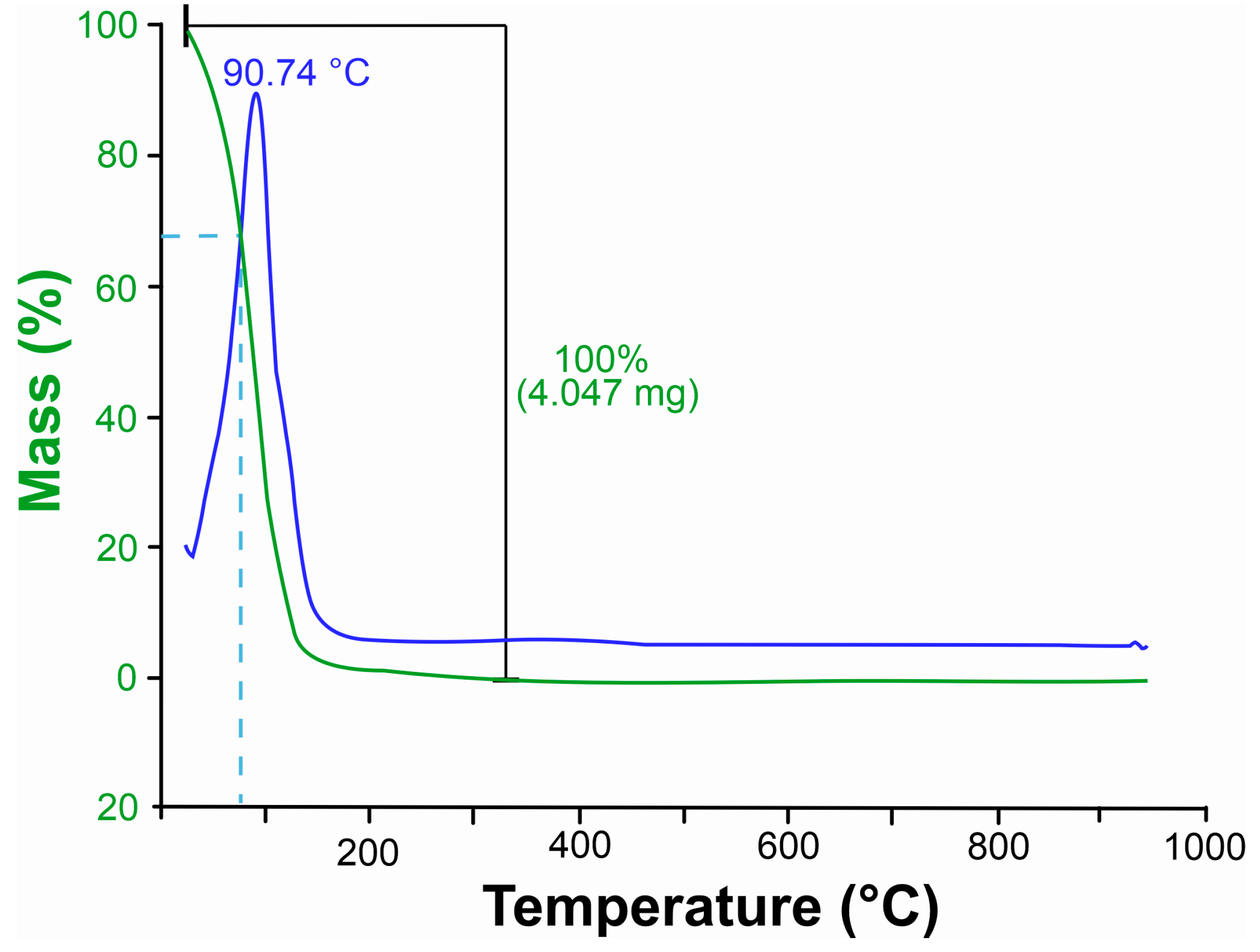
2.3.2. Thermal Analysis of Xylopia frutescens Essential Oil
2.4. Bioassays
2.4.1. Phytotoxicity
2.4.2. Inhibition of Mycelial Growth
2.4.3. Disease Control
2.5. Quantification of Organic Compounds
2.6. Enzymatic Activities in Maize and Cowpea Plants Exposed to Xylopia frutescens Essential Oil
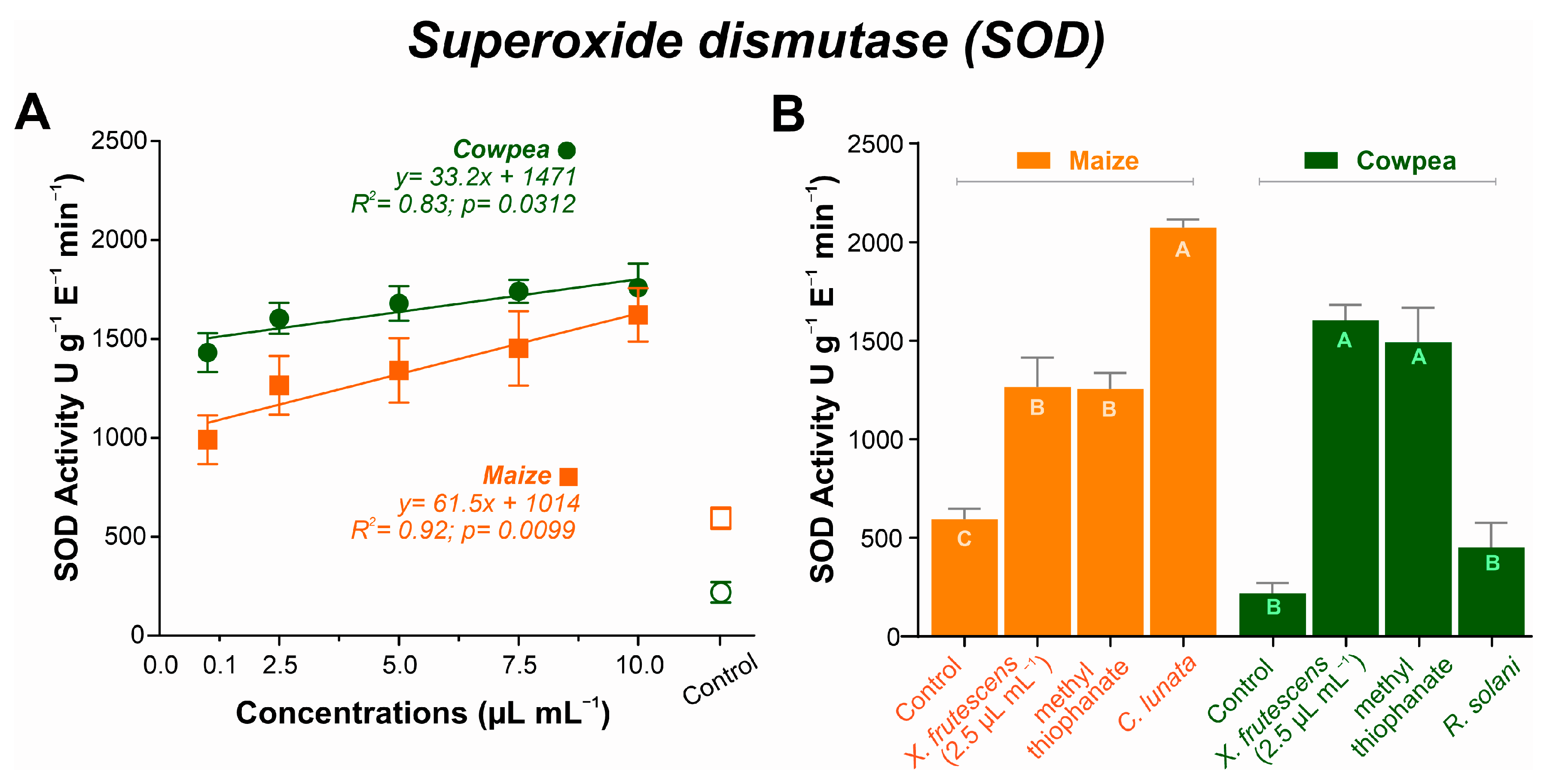
2.6.1. Superoxide Dismutase (SOD)
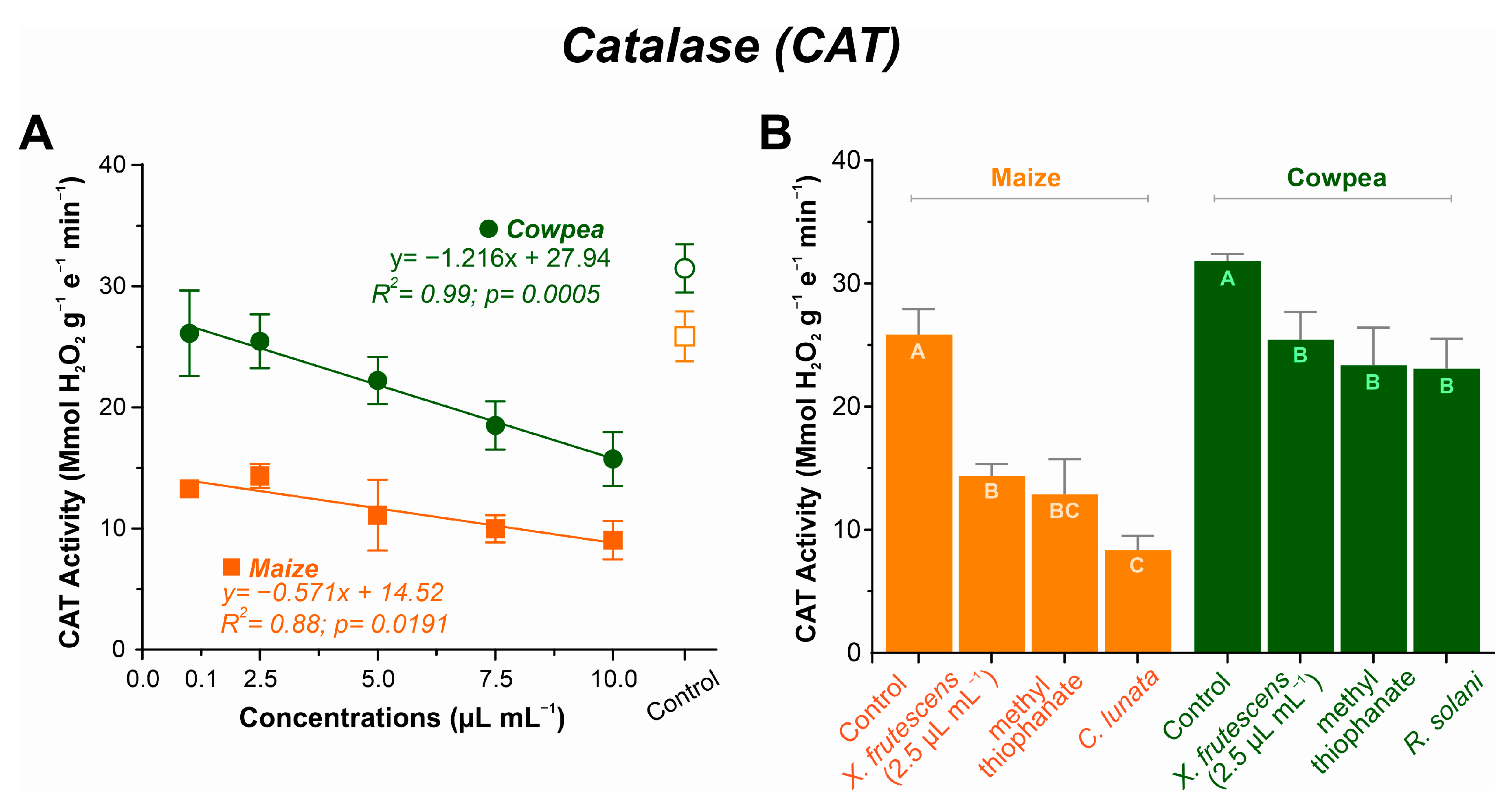
2.6.2. Catalase (CAT)
2.6.3. Ascorbate Peroxidase (APX)
2.6.4. Chitinase (CHIT)
2.7. Statistical Analysis
3. Results
3.1. Yield and Characterization of the Essential Oil
3.1.1. Chromatography of Xylopia frutescens Essential Oil
3.1.2. Thermogravimetric Analysis (TGA) of Xylopia frutescens Essential Oil
3.2. Mycelial Growth of Curvularia lunata and Rhizoctonia solani
3.3. Phytotoxic and Disease-Suppressing Effects on Maize and Cowpea Plants
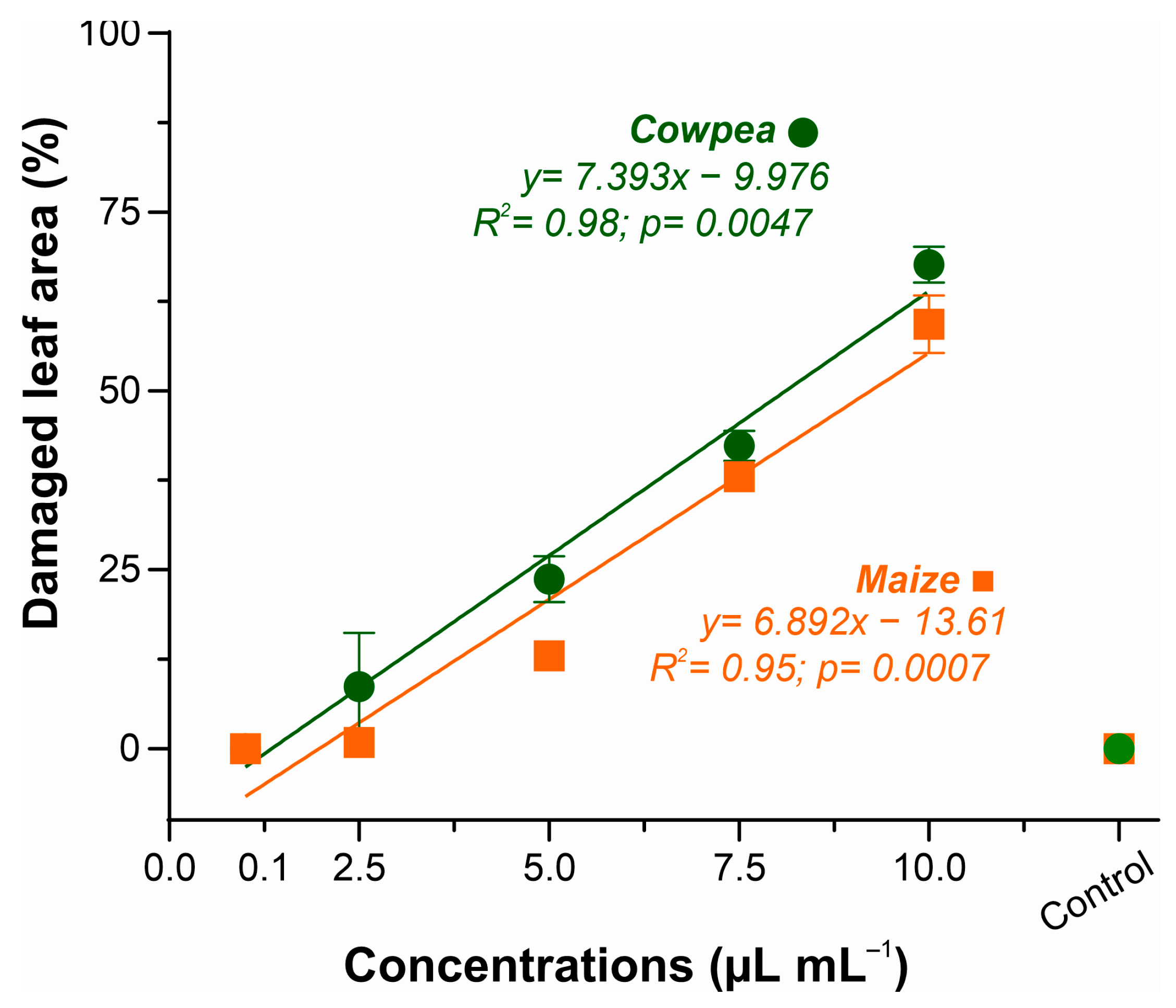
3.3.1. Phytotoxicity of Xylopia frutescens Essential Oil in Maize and Cowpea Plants
3.3.2. Disease Estimates by the Area Under the Disease Progress Curve (AUDPC)
3.4. Chlorophyll Content on Healthy and Diseased Maize and Cowpea Plants Exposed to Xylopia frutescens Essential Oil
3.5. Enzymatic Activities in Healthy and Diseased Maize and Cowpea Plants
3.5.1. Superoxide Dismutase (SOD) Expression
3.5.2. Catalase (CAT) Expression
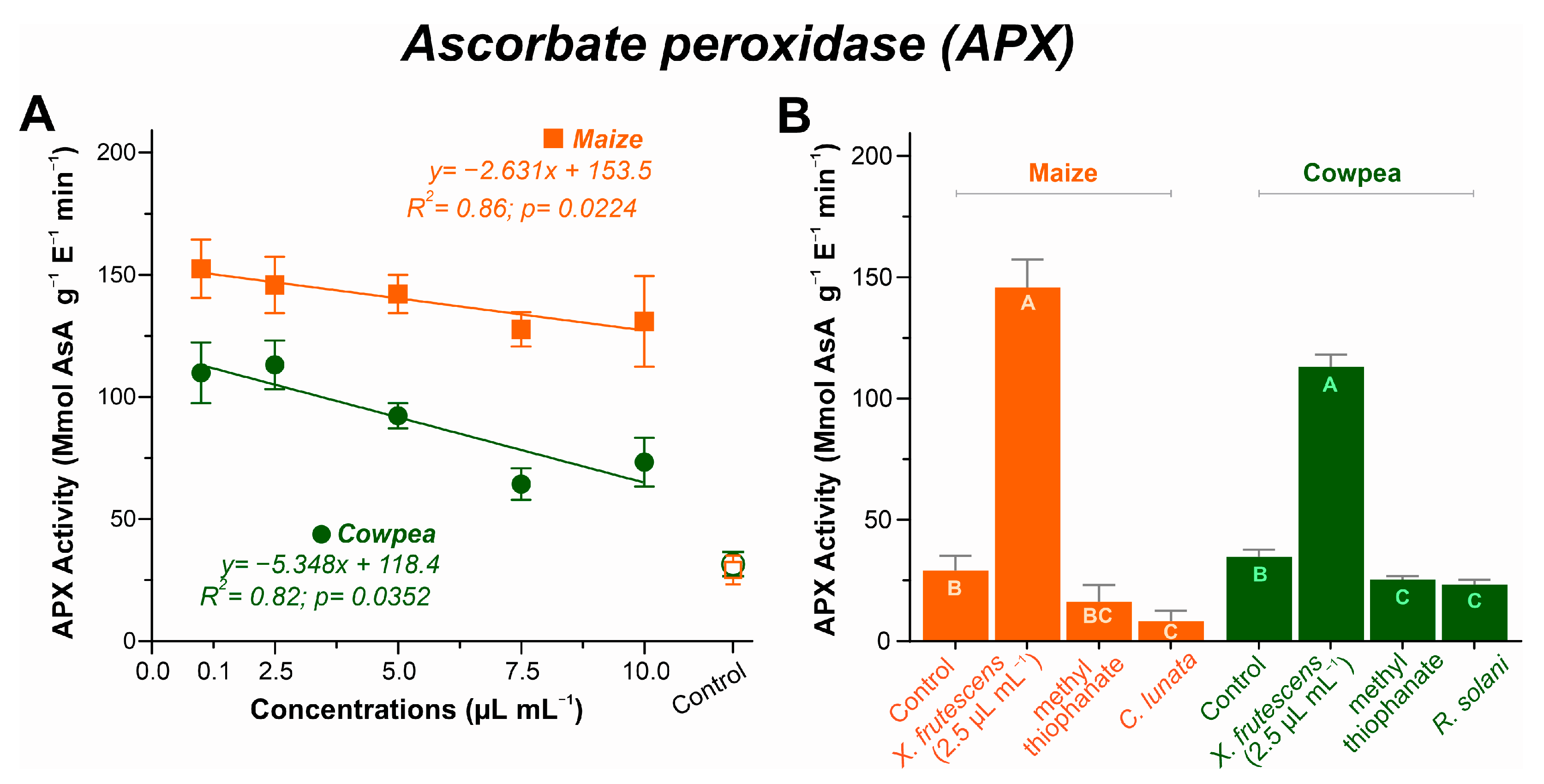
3.5.3. Ascorbate Peroxidase (APx) Activity
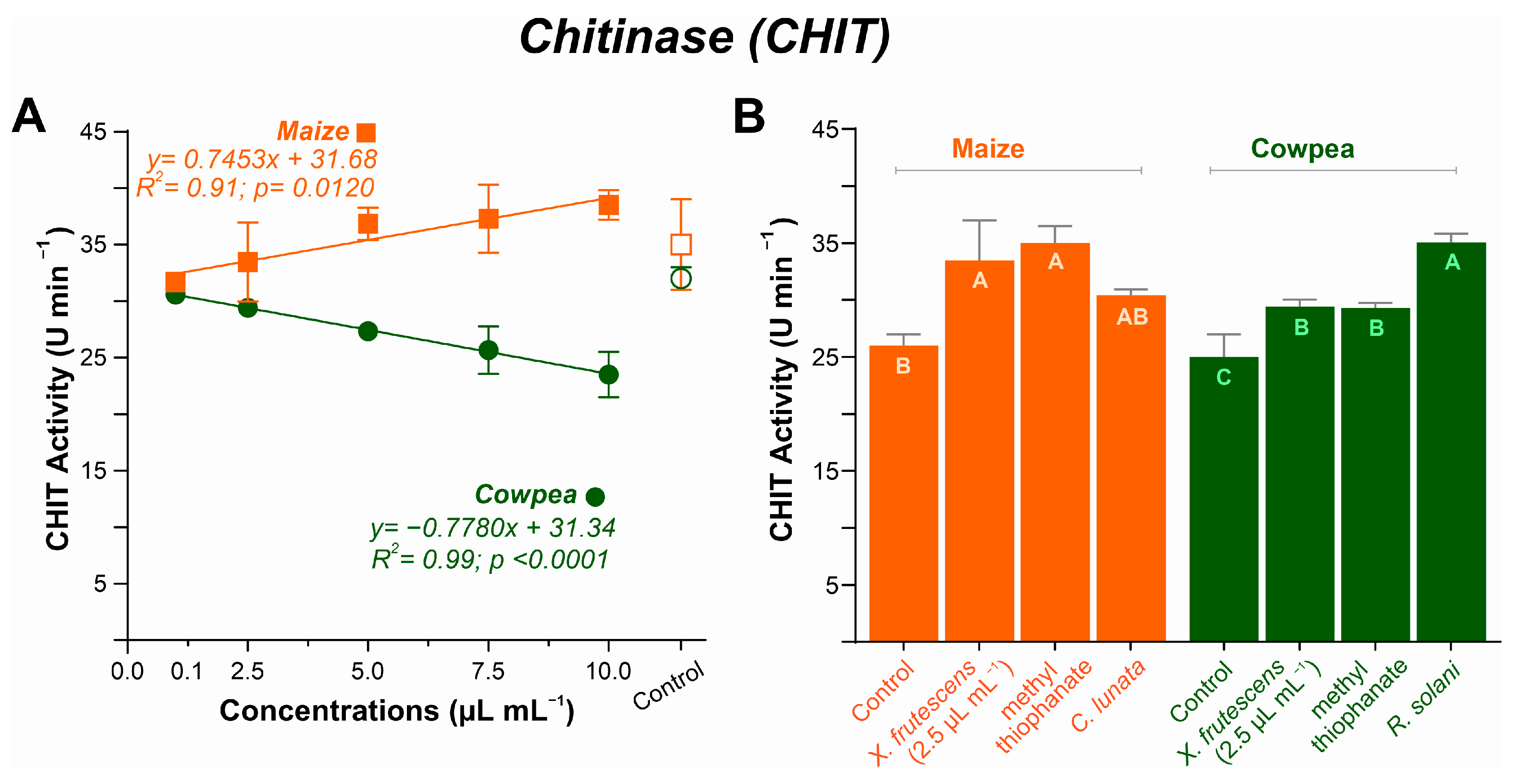
3.5.4. Chitinase (CHIT) Activity
4. Discussion
4.1. Xylopia frutescens Essential Oil
4.1.1. Essential Oil Content
4.1.2. Chromatography
4.1.3. Thermal Analysis
4.2. Mycelial Growth
4.3. Phytotoxicity and Disease-Suppressing Effects on Maize and Cowpea Plants
4.4. Chlorophyll
4.5. Enzyme Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SOD | Superoxide dismutase |
| CAT | Catalase |
| APx | Ascorbate peroxidase |
| XEO | Xylopia frutescens essential oil |
| CHIT | Chitinase |
| ROS | Reactive oxygen species |
| TGA | Thermogravimetric analysis |
| GC-MS | Gas chromatography coupled with mass spectrometry |
| EO | Essential oil |
| H2O2 | Hydrogen peroxide |
References
- Mattos, D., Jr.; Quaggio, J.; Boaretto, R. Uso de elicitores para defesa em plantas cítricas. Citrus Res. Technol. 2010, 31, 65–74. [Google Scholar] [CrossRef]
- Nóbrega, J.S.; do Nascimento, L.C. Sanidade de sementes e sua influência no controle de fitopatógenos. Res. Soc. Dev. 2020, 9, e649108101. [Google Scholar] [CrossRef]
- Gao, S.; Liu, T.; Li, Y.; Wu, Q.; Fu, K.; Chen, J. Understanding resistant germplasm-induced virulence variation through analysis of proteomics and suppression subtractive hybridization in a maize pathogen curvularia lunata. Proteomics 2012, 12, 3524–3535. [Google Scholar] [CrossRef]
- Mourão, D.S.C.; Ferreira de Souza Pereira, T.; Souza, D.J.; Chagas, A.F., Jr.; Dalcin, M.S.; Veloso, R.A.; Leão, E.U.; dos Santos, G.R. Essential oil of cymbopogon citratus on the control of the curvularia leaf spot disease on maize. Medicines 2017, 4, 62. [Google Scholar] [CrossRef]
- Viteri, L.O.; González, M.J.; Silva, P.B.; Gomes, J.M.; Svacina, T.; Costa, L.T.M.; Valarezo, E.; Mantilla-Afanador, J.G.; Herrera, O.M.; Aguiar, R.W.S.; et al. Gaba and octopamine receptors as potential targets for fumigant actions of Bursera graveolens essential oil against Callosobruchus maculatus and Callosobruchus chinensis. J. Xenobiotics 2025, 15, 91. [Google Scholar] [CrossRef]
- Viteri Jumbo, L.O.; Haddi, K.; Faroni, L.R.D.; Heleno, F.F.; Pinto, F.G.; Oliveira, E.E. Toxicity to, oviposition and population growth impairments of Callosobruchus maculatus exposed to clove and cinnamon essential oils. PLoS ONE 2018, 13, e0207618. [Google Scholar] [CrossRef]
- de Sá, M.N.F.; de Souza Lima, J.; de Jesus, F.N.; Perez, J.O.; Gava, C.A.T. Seleção in vitro de agentes de biocontrole visando o controle de fusarium sp. Acta Bras. 2019, 3, 14–16. [Google Scholar]
- de Sousa, I.; Benchimol, R.; da Silva, C.; Santos, A.; Pinheiro, C.; Carvalho, E.d.A.; Iêda Alana Leite de Sousa, G.U.; Ana Karoliny Alves Santos, G.U.; Cássia Cristina Chaves Pinheiro, G.U.; Eudes de Arruda Carvalho, S. Potencial de biocontrole de rhizoctonia solani do feijão-caupi. Biota Amaz. 2017, 7, 86–89. [Google Scholar]
- Nechet, K.d.L.; Halfeld-Vieira, B.A. Efeito do inóculo, período de molhamento foliar e do estádio fenológico do feijão-caupi no desenvolvimento da mela. Trop. Plant Pathol. 2011, 36, 104–109. [Google Scholar] [CrossRef]
- Delaney, T.P. Genetic dissection of acquired resistance to disease. Plant Physiol. 1997, 113, 5. [Google Scholar] [CrossRef]
- Stein, R.J.; Lopes, S.I.G.; Fett, J.P. Iron toxicity in field-cultivated rice: Contrasting tolerance mechanisms in distinct cultivars. Theor. Exp. Plant Physiol. 2014, 26, 135–146. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.C.M.d.; Pedrosa, E.M.R.; Rolim, M.M.; Oliveira Filho, R.A.d.; Souza, M.A.L.M.d.; Pereira Filho, J.V. Crescimento e respostas enzimáticas do feijoeiro caupi sob estresse hídrico e nematoide de galhas. Rev. Bras. de Eng. Agrícola e Ambient. 2015, 19, 113–118. [Google Scholar] [CrossRef]
- Yadavalli, V.; Neelam, S.; Rao, A.S.; Reddy, A.R.; Subramanyam, R. Differential degradation of photosystem i subunits under iron deficiency in rice. J. Plant Physiol. 2012, 169, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.L.; Ferreira, T.P.d.S.; Dalcin, M.S.; Mourão, D.d.S.C.; Fernandes, P.R.d.S.; Neitzke, T.R.; Oliveira, J.V.d.A.; Dias, T.; Jumbo, L.O.V.; de Oliveira, E.E.; et al. Lippia sidoides cham. Compounds induce biochemical defense mechanisms against Curvularia lunata sp. in maize plants. Multidiscip. Sci. J. 2025, 8, 7. [Google Scholar] [CrossRef]
- Guilherme, E.O.; Giongo, M.V.; Araujo, S.H.C.; Ferreira, T.P.S.; Moraes, C.B.; Moura, W.S.; Viteri Jumbo, L.O.; Svacina, T.; Oliveira, A.C.S.S.; Aguiar, R.W.A.; et al. Jenipapo, Genipa americana L., essential oil and Curvularia lunata control: Potential mode-of-action; plant immune responses and selectivity against beneficial non-target organisms. Ind. Crop. Prod. 2023, 199, 116708. [Google Scholar] [CrossRef]
- Laosinwattana, C.; Wichittrakarn, P.; Teerarak, M. Chemical composition and herbicidal action of essential oil from Tagetes erecta L. Leaves. Ind. Crops Prod. 2018, 126, 129–134. [Google Scholar] [CrossRef]
- Ribeiro, J.G.; Serra, I.M.R.d.S.; Araújo, M.U.P. Use of natural products to control anthracnose caused by colletotrichum gloeosporioides in papaya. Summa Phytopathol. 2016, 42, 160–164. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Ferreira, T.d.S.; Veloso, R.; Santos, G.d.; Santos, L.d.; Ferreira, T.d.S.; Barros, A.; Possel, R.; Aguiar, R.d.S. Enzymatic activity and elicitor of phytoalexins of lippia sidoides cham. And endophytic fungi. Afr. J. Biotechnol. 2018, 17, 521–530. [Google Scholar] [CrossRef]
- Medeiros, A.P.R.; Leite, J.J.F.; de Assis, R.M.A.; Rocha, J.P.M.; Bertolucci, S.K.V.; Pereira Pinto, J.E.B. Application of natural elicitors to promote growth, photosynthetic pigments, and the content and composition of essential oil in Melissa officinalis L. Ind. Crop. Prod. 2024, 208, 117885. [Google Scholar] [CrossRef]
- Parikh, L.; Agindotan, B.O.; Burrows, M.E. Antifungal activity of plant-derived essential oils on pathogens of pulse crops. Plant Dis. 2021, 105, 1692–1701. [Google Scholar] [CrossRef]
- Costa, L.T.M.; Smagghe, G.; Viteri Jumbo, L.O.; Santos, G.R.; Aguiar, R.W.S.; Oliveira, E.E. Selective actions of plant-based biorational insecticides: Molecular mechanisms and reduced risks to non-target organisms. Curr. Opin. Environ. Sci. Health 2025, 44, 100601. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant essential oils as biopesticides: Applications, mechanisms, innovations, and constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Lopes, J.; De Mello-Silva, R. Annonaceae do parque estadual de ibitipoca, minas gerais. Bol. de Botânica da Univ. de São Paulo 2012, 30, 157–164. [Google Scholar]
- Nascimento, A.; Maia, T.; Soares, T.; Menezes, L.; Scher, R.; Costa, E.; Cavalcanti, S.; La Corte, R. Repellency and larvicidal activity of essential oils from xylopia laevigata, Xylopia frutescens, lippia pedunculosa, and their individual compounds against aedes aegypti linnaeus. Neotrop. Entomol. 2017, 46, 223–230. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, Â.A.B.; Cascaes, M.M.; do Nascimento, L.D.; de Jesus Pereira Franco, C.; Ferreira, O.O.; Anjos, T.O.d.; Karakoti, H.; Kumar, R.; da Silva Souza-Filho, A.P.; de Oliveira, M.S.; et al. Chemical evaluation, phytotoxic potential, and in silico study of essential oils from leaves of Guatteria schomburgkiana mart. And Xylopia frutescens Aubl. (annonaceae) from the brazilian amazon. Molecules 2023, 28, 2633. [Google Scholar]
- Matejić, J.S.; Stojanović-Radić, Z.Z.; Ristić, M.S.; Veselinović, J.B.; Zlatković, B.K.; Marin, P.D.; Džamić, A.M. Chemical characterization, in vitro biological activity of essential oils and extracts of three Eryngium L. Species and molecular docking of selected major compounds. J. Food Sci. Technol. 2018, 55, 2910–2925. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Mabrouk, S.; Salah, K.B.H.; Aouni, M.; Khouja, M.L.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Correlation between chemical composition and antibacterial activity of essential oils from fifteen Eucalyptus species growing in the korbous and jbel abderrahman arboreta (north east tunisia). Molecules 2012, 17, 3044–3057. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, W.B.; Da Silva, J.C.; Araujo, M.G.S.; da Silva, A.L.L.; Vasconcelos, T.L.C.; Cabral, K.M.; Bernardo, H.L. Biological potential of plant species Xylopia frutescens: An integrative review. J. Chem. Pharm. Res. 2016, 8, 794–800. [Google Scholar]
- Ferraz, R.P.; Cardoso, G.M.; da Silva, T.B.; Fontes, J.E.d.N.; Prata, A.P.d.N.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumour properties of the leaf essential oil of Xylopia frutescens aubl.(annonaceae). Food Chem. 2013, 141, 196–200. [Google Scholar] [CrossRef]
- Garcez, W.S.; Garcez, F.R.; da Silva, L.M.; Sarmento, U.C. Substâncias de origem vegetal com atividade larvicida contra aedes aegypti. Rev. Virtual de Química 2013, 5, 363–393. [Google Scholar]
- Queiroga Neto, V.; Bora, P.S.; Diniz, Z.N.; Cavalheiro, J.M.O.; Queiroga, K.F. Dipteryx lacunifera seed oil: Characterization and thermal stability. Ciência E Agrotecnologia 2009, 33, 1601–1607. [Google Scholar] [CrossRef]
- Santos, J.; Santos, I.; Conceição, M.; Porto, S.; Trindade, M.; Souza, A.G.; Prasad, S.; Fernandes, V.J., Jr.; Araujo, A. Thermoanalytical, kinetic and rheological parameters of commercial edible vegetable oils. J. Therm. Anal. Calorim. 2004, 75, 419–428. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Tatini, D.; Bisozzi, F.; Costantini, S.; Fattori, G.; Boldrini, A.; Baglioni, M.; Bonechi, C.; Donati, A.; Tozzi, C.; Riccaboni, A.; et al. Geographical origin authentication of leaves and drupes from olea europaea via 1h nmr and excitation–emission fluorescence spectroscopy: A data fusion approach. Molecules 2025, 30, 3208. [Google Scholar] [CrossRef] [PubMed]
- Seixas, P.T.L.; Castro, H.C.; Santos, G.R.; Cardoso, D.P. Controle fitopatológico do fusarium subglutinans pelo óleo essencial do capim-citronela (Cymbopogon nardus L.) e do composto citronelal. Rev. Bras. Plantas Med. 2011, 13, 523–526. [Google Scholar] [CrossRef]
- Moura, W.S.; Oliveira, E.E.; Haddi, K.; Corrêa, R.F.T.; Piau, T.B.; Moura, D.S.; Santos, S.F.; Grisolia, C.K.; Ribeiro, B.M.; Aguiar, R.W.S. Cassava starch-based essential oil microparticles preparations: Functionalities in mosquito control and selectivity against non-target organisms. Ind. Crops Prod. 2021, 162, 113289. [Google Scholar] [CrossRef]
- Freitas, S.P.; Moreira, J.G.; Freitas, I.L.J.; Freitas Júnior, S.P.; Amaral Júnior, A.T.; Silva, V.Q.R. Fitotoxicidade de herbicidas a diferentes cultivares de milho-pipoca. Planta Daninha 2009, 27, 1095–1103. [Google Scholar] [CrossRef]
- Cogliatti, M.; Juan, V.F.; Bongiorno, F.; Dalla Valle, H.; Rogers, W.J. Control of grassy weeds in annual canarygrass. Crop Prot. 2011, 30, 125–129. [Google Scholar] [CrossRef]
- Santos, G.R.d.; Café-Filho, A.C.; Leão, F.F.; César, M.; Fernandes, L.E. Progresso do crestamento gomoso e perdas na cultura da melancia. Hortic. Bras. 2005, 23, 228–232. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents; Portland Press Ltd.: London, UK, 1983. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Havir, E.A.; McHale, N.A. Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of active oxygen in chloroplasts. In Molecular Biology of Free Radical Scavenging Systems; Kyle, D.O.C., Arntzen, C., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1992; pp. 127–287. [Google Scholar]
- Wirth, S.J.; Wolf, G.A. Dye-labelled substrates for the assay and detection of chitinase and lysozyme activity. J. Microbiol. Methods 1990, 12, 197–205. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2001; pp. iii + 456. [Google Scholar]
- NIST. Chemistry Webbook; Linstrom, P.J., Mallard, W.G., Eds.; NIST: Gaithersburg, MD, USA, 2018; Volume 69. [Google Scholar]
- Shaner, G.; Finney, R.E. The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathology 1977, 67, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.S.; Andrade, E.H.A.; Zoghbi, M.d.G.B.; Luz, A.I.R.; Maia, J.G.S. Sesquiterpenes of amazonian piper species. Acta Amaz. 1998, 28, 127–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H.; Huang, Y.; Xia, P. Optimization of plant density and harvest time to maximize volatile oil accumulation in two aromatic plants. Agronomy 2024, 14, 1676. [Google Scholar] [CrossRef]
- Malaka, M.J.; Araya, N.A.; Soundy, P.; du Plooy, C.P.; Araya, H.T.; Jansen Van Rensburg, W.S.; Watkinson, E.; Levember, E.; Wadiwala, E.; Amoo, S.O. Biomass, essential oil yield, and composition of marjoram as influenced by interactions of different agronomic practices under controlled conditions. Plants 2023, 12, 173. [Google Scholar] [CrossRef]
- Mendes, R.F.V. Composição química e atividades biológicas dos óleos essenciais de Xylopia frutescens Aubl.(annonaceae). Master’s Thesis, Universidade Federal de Pernambuco, Pernambuco, Brasil, 2018. Available online: https://attena.ufpe.br/bitstream/123456789/33584/1/DISSERTA%C3%87%C3%83O%20Raudiney%20Frankilin%20Vasconcelos%20Mendes.pdf (accessed on 6 November 2025).
- Uc-Cachón, A.H.; Calvo-Irabien, L.M.; Dzul-Beh, A.d.J.; Dzib-Baak, H.E.; Grijalva-Arango, R.; Molina-Salinas, G.M. Potential anti-infectious activity of essential oil chemotypes of lippia origanoides kunth on antibiotic-resistant Staphylococcus aureus strains. Plants 2024, 13, 1172. [Google Scholar] [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Alencar Santos, J.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Góis Ruiz, A.L.T.; Ann Foglio, M.; de Carvalho, J.E.; et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of psidium guineense sw. and spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Martins, G.A.; Bicas, J.L. Antifungal activity of essential oils of tea tree, oregano, thyme, and cinnamon, and their components. Braz. J. Food Technol. 2024, 27, e2023071. [Google Scholar] [CrossRef]
- Khalil, M.; Khizar, M.; Alshaya, D.S.; Hameed, A.; Muhammad, N.; Binyameen, M.; Azeem, M.; Hussain, M.; Abbas, Q.; Attia, K.A.; et al. Insecticidal and repellent activity of essential oils from seven different plant species against Tribolium castaneum (coleoptera: Tenebrionidae). Insects 2024, 15, 755. [Google Scholar] [CrossRef]
- Mukhtar, M.; Khan, H.A.; Naz, S. Antibacterial profiling of zanthoxylum armatum extracts: A comprehensive computational and experimental study. Nat. Prod. Commun. 2024, 19, 1934578X241237911. [Google Scholar] [CrossRef]
- Doorandishan, M.; Gholami, M.; Ebrahimi, P.; Jassbi, A.R. Spathulenol as the most abundant component of essential oil of Moluccella aucheri (boiss.) scheen. Nat. Volatiles Amp Essent. Oils 2021, 8, 37–41. [Google Scholar] [CrossRef]
- Hassid, A.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.F.; El Khatib, S. A review on the versatile applications of plant-based essential oils in food flavoring, culinary uses and health benefits. Discov. Food 2025, 5, 130. [Google Scholar] [CrossRef]
- Rodrigues, M.; Mazzafera, P. Essential oils from Eucalyptus species: A review of their activities, applications, and the brazilian market. Acta Bot. Bras. 2025, 39, e20240111. [Google Scholar] [CrossRef]
- Melo, E.; Michels, F.; Arakaki, D.; Lima, N.; Gonçalves, D.; Cavalheiro, L.; Oliveira, L.; Caires, A.; Hiane, P.; Nascimento, V. First study on the oxidative stability and elemental analysis of babassu (Attalea speciosa) edible oil produced in brazil using a domestic extraction machine. Molecules 2019, 24, 4235. [Google Scholar] [CrossRef]
- Portella, A.C.F.; Munaro, M.; Ascêncio, S.D.; Siqueira, C.d.A.; Ferreira, T.P.d.S.; Aguiar, R.W.d.S. Caracterização físico-química do óleo essencial da siparuna guianensis aublet. Química Nova 2014, 37, 844–849. [Google Scholar]
- Abd-Elnabi, A.D.; El-Sawy, E.A.F.; Badawy, M.E.I. Plant oil nano-emulsions as a potential solution for pest control in sustainable agriculture. Neotrop. Entomol. 2025, 54, 35. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.; Jorf, S.A.M.; Nowroozi, G.; Sedghi, M.; Naseriyeh, T.; Rahmani, S.; Fathi, J.; Kahrizi, D. Nano-encapsulated ajwain essential oil elicits resistance against early blight in tomatoes (Solanum lycopersicum L.). Cell. Mol. Biol. 2025, 71, 1–5. [Google Scholar] [CrossRef]
- Magor, E.; Wilson, M.D.; Wong, H.; Cresswell, T.; Sánchez-Palacios, J.T.; Bell, R.W.; Penrose, B. Selected adjuvants increase the efficacy of foliar biofortification of iodine in bread wheat (Triticum aestivum L.) grain. Front. Plant Sci. 2023, 14, 1246945. [Google Scholar] [CrossRef]
- Chen, W.; Dong, L.; Tan, Q.; Yi, G.; Zhang, F.; Luo, J.; Mei, Y.; Jiang, W.; Li, X. An adjuvant that increases the adhesion of pesticides on plant surfaces and improves the efficiency of pest control: Polyethylene glycol sol-gel polymer. React. Funct. Polym. 2023, 192, 105722. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Scaiano, J.C.; Gallardo-Cabrera, C.; Puertas-Mejía, M.A. Photoprotection and photostability of a new lignin-gelatin-baccharis antioquensis-based hybrid biomaterial. Antioxidants 2021, 10, 1904. [Google Scholar] [CrossRef]
- Anyamele, T.; Ugbogu, E.A.; Nwankwo, V.C.; Ibe, C. A review of the traditional uses, phytochemistry and toxicological profile of Xylopia aethiopica a. Rich. Pharmacol. Res. Nat. Prod. 2023, 1, 100001. [Google Scholar]
- Okigbo, R.; Nmeka, I. Control of yam tuber rot with leaf extracts of Xylopia aethiopica and Zingiber officinale. Afr. J. Biotechnol. 2005, 4, 804–807. [Google Scholar]
- Cascaes, M.M.; Carneiro, O.d.S.; Nascimento, L.D.d.; de Moraes, Â.A.B.; de Oliveira, M.S.; Cruz, J.N.; Guilhon, G.M.S.P.; Andrade, E.H.d.A. Essential oils from annonaceae species from brazil: A systematic review of their phytochemistry, and biological activities. Int. J. Mol. Sci. 2021, 22, 12140. [Google Scholar] [CrossRef] [PubMed]
- Iwu, M.W.; Duncan, A.R.; Okunji, C.O. New antimicrobials of plant origin. In Perspectives on New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 1999; Volume 457, p. 462. [Google Scholar]
- Ootani, M.A.; dos Reis, M.R.; Cangussu, A.S.R.; Capone, A.; Fidelis, R.R.; Oliveira, W.; Barros, H.B.; Portella, A.C.F.; de Souza Aguiar, R.; dos Santos, W.F. Phytotoxic effects of essential oils in controlling weed species Digitaria horizontalis and Cenchrus echinatus. Biocatal. Agric. Biotechnol. 2017, 12, 59–65. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef]
- Hendges, C.; Stangarlin, J.R.; Zamban, V.C.; Kuhn, O.J.; Tartaro, E.L.; Carmelo, D.B. Antifungal activity against Alternaria solani and control of early blight in tomato by essential oil of citronella. Rev. Ceres 2023, 70, 101–111. [Google Scholar] [CrossRef]
- Grosso, C.; Coelho, J.A.; Urieta, J.S.; Palavra, A.M.; Barroso, J.G. Herbicidal activity of volatiles from coriander, winter savory, cotton lavender, and thyme isolated by hydrodistillation and supercritical fluid extraction. J. Agric. Food Chem. 2010, 58, 11007–11013. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Setia, N.; Kohli, R.K.; Kaur, S.; Yadav, S.S. Alternative control of littleseed canary grass using eucalypt oil. Agron. Sustain. Dev. 2007, 27, 171–177. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Zhang, Z.; He, K. The physiological and biochemical photosynthetic properties of lycium ruthenicum murr in response to salinity and drought. Sci. Hortic. 2019, 256, 108530. [Google Scholar] [CrossRef]
- Alves, M.C.S.; Medeiros Filho, S.; Manoel Neto, A.; Brito, R.C.; Araujo, R.C. Allelopathic effect of essential oils of medicinal plants in Bidens pilosa L. Rev. Bras. Plantas Med. 2014, 16, 731–736. [Google Scholar] [CrossRef]
- Alaraidh, I.A.; Alsahli, A.A.; Abdel Razik, E.S. Alteration of antioxidant gene expression in response to heavy metal stress in Trigonella foenum-graecum L. S. Afr. J. Bot. 2018, 115, 90–93. [Google Scholar] [CrossRef]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.d.C.G.; De Paula, S.O.; Minim, V.P.R.; Bressan, J. Estresse oxidativo: Conceito, implicações e fatores modulatórios. Rev. de Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Dias, B.L.; Dalcin, M.S.; Costa, P.F.d.; Santos, P.R.R.d.; Sousa, R.R.d.; Dias, F.R.; Mourão, D.d.S.C.; Santos, G.R.d. Morinda citrifolia compounds such elicitor substances of biochemical responses in melon plants: Morinda citrifolia compounds such elicitor substances of biochemical responses in melon plants. Comun. Sci. 2023, 14, e3919. [Google Scholar] [CrossRef]
- Feierabend, J. Catalases in plants: Molecular and functional properties and role in stress defence. Antioxid. React. Oxyg. Species Plants 2005, 101–140. [Google Scholar] [CrossRef]
- Vasconcelos, A.C.F.d.; Zhang, X.; Ervin, E.H.; Kiehl, J.d.C. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Sci. Agric. 2009, 66, 395–402. [Google Scholar] [CrossRef]
- Souza, E.M.D.E.; Souza, R.C.D.; Melo, J.F.B.; Costa, M.M.D.; Souza, S.A.D.; Souza, A.M.D.; Copatti, C.E. Cymbopogon flexuosus essential oil as an additive improves growth, biochemical and physiological responses and survival against Aeromonas hydrophila infection in nile tilapia. An. Acad. Bras. Ciências 2020, 92, e20190140. [Google Scholar] [CrossRef]
- Wang, L.; Ma, R.; Yin, Y.; Jiao, Z. Antioxidant response of Arabidopsis thaliana seedlings to oxidative stress induced by carbon ion beams irradiation. J. Environ. Radioact. 2018, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Yano, S.; Rattanakit, N.; Wakayama, M.; Tachiki, T. A chitinase indispensable for formation of protoplast of schizophyllum commune in basidiomycete-lytic enzyme preparation produced by bacillus circulans ka-304. Biosci. Biotechnol. Biochem. 2004, 68, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Demartelaere, A.C.F.; Preston, H.A.F.; da Mata, T.C.; da Costa, W.P.L.B.; Nicolini, C.; de Assis Gomes, W.; de Medeiros, D.C.; de Paiva Silva, T.P.; de Souza, J.B.; de Paiva, L.L.; et al. Utilização de extratos no controle da antracnose em pós-colheita de mangifera indica. Braz. J. Dev. 2021, 7, 4872–4892. [Google Scholar] [CrossRef]


| Compound | Formula | Classification | * Ri Experimental | * Ri Literature [48,49] | % |
|---|---|---|---|---|---|
| α-Pinene | C10H16 | Hydrocarbon c | 948 | 939 | 9.6 |
| Bicyclo[3.1.0]hex-2-ene, 4-methylene-1-(1-methylethyl)- (dehydrosabinene-type) | C10H14 | Hydrocarbon c | 879 | 879 | 1.3 |
| β-Pinene | C10H16 | Hydrocarbon c | 943 | 980 | 11.0 |
| p-Cymene | C10H14 | Hydrocarbon e | 1042 | 1026 | 1.3 |
| Eucalyptol (1,8-cineole) | C10H18O | Ether a | 1059 | 1032 | 1.8 |
| α-Campholenal | C10H16O | Aldehyde a | 1155 | 1155 | 1.5 |
| Bicyclo[3.1.1]heptan-2-one, 6,6-dimethyl-, (1R)- (nopinone-type) | C9H14O | ketone a | 1047 | 1047 | 2.4 |
| Bicyclo[3.1.1] heptan-3-ol, 6,6-dimethyl-2-methylene- (Pinocarveol/isopinocarveol) | C10H16O | Alcohol a | 1131 | 1184 | 13.8 |
| Verbenol | C10H16O | Alcohol a | 1136 | 1136 | 4.3 |
| Pinocarvone | C10H14O | Ketone a | 1114 | 1162 | 5.8 |
| p-Mentha-1,5-dien-8-ol | C10H16O | Alcohol a | 1125 | 1125 | 1.9 |
| Bicyclo[3.1.1] hept-2-ene-2-carboxaldehyde, 6,6-dimethyl- (myrtenal) | C10H14O | Aldehyde a | 1136 | 1170 | 12.5 |
| Bicyclo[3.1.1] hept-2-ene-2-methanol, 6,6-dimethyl- (myrtenol) | C10H16O | Alcohol a | 1191 | 1198 | 6.0 |
| Bicyclo[3.1.1] hept-3-en-2-one, 4,6,6-trimethyl- (Verbenone/2-pinen-4-one) | C10H14O | ketone a | 1119 | 1204 | 3.0 |
| Cyclohexene, 4-ethenyl-4-methyl-3-(1-methylethenyl)-1-(1-methylethyl)- (sesquiterpene hydrocarbon) | C15H24 | Hydrocarbon d | 1377 | 1377 | 0.9 |
| Germacrene D | C15H24 | Hydrocarbon d | 1515 | 1515 | 4.0 |
| (-)-Spathulenol | C15H24O | Alcohol b | 1536 | 1536 | 12.9 |
| Caryophyllene oxide | C15H24O | Epoxide b | 1507 | 1507 | 1.1 |
| Isospathulenol | C15H24O | Alcohol b | 1623 | 1623 | 4.1 |
| Total identified (%) | 99.2 | ||||
| a Oxygenated monoterpenes (%) | 50.0 | ||||
| b Oxigenated sesquiterpenes (%) | 20.0 | ||||
| c Monoterpene (%) | 15.0 | ||||
| d Sesquiterpene (%) | 10.0 | ||||
| e Aromatic monoterpene (%) | 5.0 |
| Fungi | Treatment | Mycelial Growth Rate (mm/Day ± SE) | MMGR (mm/Day) | ||||
|---|---|---|---|---|---|---|---|
| Day 2 | Day 4 | Day 6 | Day 8 | Day 10 | |||
| Curvularia lunata | Control (water and Tween 80) | 12.2 ± 0.3 | 13.2 ± 0.1 | 9.2 ± 0.4 | 8.4 ± 0.3 | 2.2 ± 0 | 9.0 |
| Xylopia frutescens (2.5 µL mL−1) | 6.2 ± 0.3 | 6.4 ± 0.3 | 9.4 ± 0.5 | 10.2 ± 1.0 | 10.9 ± 0.1 | 8.6 | |
| Methyl thiophanate (20 µg mL−1) | 10.8 ± 0.9 | 11.2 ± 0.7 | 11.4 ± 0.4 | 6.5 ± 1.3 | 4.6 ± 0.6 | 9.0 | |
| Rhizoctonia solani | Control (water and Tween 80) | 35.8 ± 0.3 | 9.7 ± 0.5 | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 9.0 |
| Xylopia frutescens (2.5 µL mL−1) | 0 ± 0 | 5.8 ± 0.3 | 9.4 ± 0.5 | 13.2 ± 1.0 | 13.2 ± 0.8 | 8.5 | |
| Methyl thiophanate (20 µg mL−1) | 35.8 ± 0.9 | 9.2 ± 0.7 | 0.0 ± 0 | 0.0 ± 0 | 0.0 ± 0 | 9.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourão, D.d.S.C.; Dias, B.L.; Dalcin, M.S.; Viteri, L.O.; Gonzales, M.A.; Fernandes, P.R.S.; Silva, V.B.; Costa, M.A.; González, M.J.; Amaral, A.G.; et al. Effect of Xylopia frutescens Essential Oil on the Activation of Defense Mechanisms Against Phytopathogenic Fungi. Microorganisms 2025, 13, 2571. https://doi.org/10.3390/microorganisms13112571
Mourão DdSC, Dias BL, Dalcin MS, Viteri LO, Gonzales MA, Fernandes PRS, Silva VB, Costa MA, González MJ, Amaral AG, et al. Effect of Xylopia frutescens Essential Oil on the Activation of Defense Mechanisms Against Phytopathogenic Fungi. Microorganisms. 2025; 13(11):2571. https://doi.org/10.3390/microorganisms13112571
Chicago/Turabian StyleMourão, Dalmarcia de Souza C., Bruna L. Dias, Mateus S. Dalcin, Luis O. Viteri, Manuel A. Gonzales, Paulo R. S. Fernandes, Vitória B. Silva, Mariana A. Costa, Maria J. González, Ana G. Amaral, and et al. 2025. "Effect of Xylopia frutescens Essential Oil on the Activation of Defense Mechanisms Against Phytopathogenic Fungi" Microorganisms 13, no. 11: 2571. https://doi.org/10.3390/microorganisms13112571
APA StyleMourão, D. d. S. C., Dias, B. L., Dalcin, M. S., Viteri, L. O., Gonzales, M. A., Fernandes, P. R. S., Silva, V. B., Costa, M. A., González, M. J., Amaral, A. G., Nascimento, I. R. d., Moraes, C. B. d., Gomes, V. T. S., Câmara, M. P., Silva, M. G. d., Café-Filho, A. C., Moura, W. S., & Santos, G. R. d. (2025). Effect of Xylopia frutescens Essential Oil on the Activation of Defense Mechanisms Against Phytopathogenic Fungi. Microorganisms, 13(11), 2571. https://doi.org/10.3390/microorganisms13112571









