Semiconducting Polymer-Based Nanocomposite for Photothermal Elimination of Staphylococcus aureus Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Disk Materials
2.2. Thermal Measurements
2.3. S. aureus Bacteria
2.4. Biofilm Development
2.5. Photothermal Therapy
2.5.1. Mild Hyperthermia with Antibiotics In Vitro
2.5.2. Mild Hyperthermia with Antibiotics In Vivo
2.5.3. Ablative Hyperthermia In Vitro
2.6. Fluorescence Imaging of Biofilms
2.7. Statistical Analysis
3. Results
3.1. Temperature Changes in BSe-Si for Mild Hyperthermia
3.2. Xen 29 Biofilm Reduction with Mild Hyperthermia and Gentamicin
3.3. Biofilm Reduction In Vivo
3.4. Temperature Changes in BSe-Si with Variable Laser Parameters for Ablative Hyperthermia
3.5. PTT for Ablative Hyperthermia
3.6. Biofilm Response to Heat Shock
3.7. Biofilm Regrowth Following Heat Shock
3.8. Microscopic Variance in Biofilms After PTT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Si | Silicone |
| NPs | Nanoparticles |
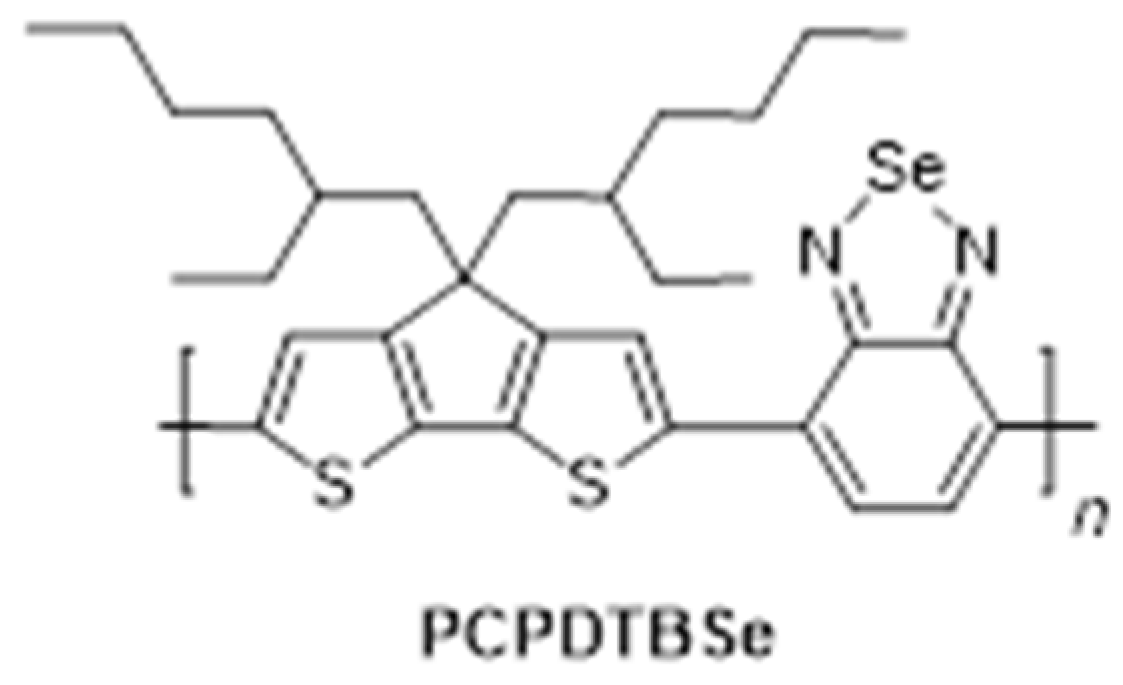
| PCPDTBSe | Poly [4,4-bis(2-ethylhexyl)-cyclopenta [2,1-b;3,4-b’]dithiophene-2,6-diyl-alt22,1,3-benzoselenadiazole-4,7-diyl] |
| BSe-Si | PCPDTBSe polymer in silicone |
| CV | Crystal violet |
| CFU | Colony-forming unit |
| PTT | Photothermal therapy |
| SSI | Surgical site infections |
| HCAI | Healthcare-associated infection |
| VAP | Ventilator-associated pneumonia |
| eDNA | Extracellular DNA |
| CLABI | Central line-associated bloodstream infection |
| CAUTI | Catheter-associated urinary tract infection |
| EPS | Extracellular polysaccharide |
| NIR | Near-infrared |
| THF | Tetrahydrofuran |
| CW | Continuous wave |
| UAMS-1 | Strain of S. aureus from the University of Arkansas Medical Sciences |
| TSB | Tryptic soy broth |
| NB1 | Nutrient broth 1 |
| ROI | Region of Interest |
| IVIS | In vivo imaging system |
| IACUC | Institutional animal care and usage committee |
| WGA | Wheat germ agglutinin |
| PI | Propidium iodide |
| SYTO9 | Green fluorescent nucleic acid stain |
| TOTO3 | Dimeric cyanine dye for DNA |
References
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health care-associated infections—An overview. IDR 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices—An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Schinabeck, M.K.; Ghannoum, M.A. Biofilm-Related Indwelling Medical Device Infections. In Biofilms, Infection, and Antimicrobial Therapy; CRC Press: Boca Raton, FL, USA, 2005; pp. 57–68. [Google Scholar]
- Shirtliff, M.; Leid, J.G.; Shirtliff, M. The Role of Biofilms in Device-Related Infections; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Slettengren, M.; Mohanty, S.; Kamolvit, W.; Van Der Linden, J.; Brauner, A. Making Medical Devices Safer: Impact of Plastic and Silicone Oil on Microbial Biofilm Formation. J. Hosp. Infect. 2020, 106, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Park, S.J.; Choi, S.; Uh, Y.; Park, J.Y.; Han, K.-H. The Influence of Urinary Catheter Materials on Forming Biofilms of Microorganisms. J. Bacteriol. Virol. 2017, 47, 32–40. [Google Scholar] [CrossRef]
- VanEpps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef]
- Gidey, K.; Gidey, M.T.; Hailu, B.Y.; Gebreamlak, Z.B.; Niriayo, Y.L. Clinical and Economic Burden of Healthcare-Associated Infections: A Prospective Cohort Study. PLoS ONE 2023, 18, e0282141. [Google Scholar] [CrossRef]
- Parra-Ruiz, J.; Vidaillac, C.; Rose, W.E.; Rybak, M.J. Activities of High-Dose Daptomycin, Vancomycin, and Moxifloxacin Alone or in Combination with Clarithromycin or Rifampin in a Novel in Vitro Model of Staphylococcus Aureus Biofilm. Antimicrob. Agents Chemother. 2010, 54, 4329–4334. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of Antibiotic Resistance in Bacterial Biofilms. Int. J. Med. Microbiol. 2002, 292, 107. [Google Scholar] [CrossRef]
- Darouiche, R.; Landon, G.; Patti, J.; Nguyen, L.; Fernau, R.; McDevitt, D.; Greene, C.; Foster, T.; Klima, M. Role of Staphylococcus Aureus Surface Adhesins in Orthopaedic Device Infections: Are Results Model-Dependent? J. Med. Microbiol. 1997, 46, 75–79. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler Jr, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Oliveira, W.; Silva, P.; Silva, R.; Silva, G.; Machado, G.; Coelho, L.; Correia, M. Staphylococcus Aureus and Staphylococcus Epidermidis Infections on Implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Chu, V.H.; Crosslin, D.R.; Friedman, J.Y.; Reed, S.D.; Cabell, C.H.; Griffiths, R.I.; Masselink, L.E.; Kaye, K.S.; Corey, G.R.; Reller, L.B. Staphylococcus Aureus Bacteremia in Patients with Prosthetic Devices: Costs and Outcomes. Am. J. Med. 2005, 118, 1416-e19. [Google Scholar] [CrossRef]
- Schilcher, K.; Andreoni, F.; Dengler Haunreiter, V.; Seidl, K.; Hasse, B.; Zinkernagel, A.S. Modulation of Staphylococcus Aureus Biofilm Matrix by Subinhibitory Concentrations of Clindamycin. Antimicrob. Agents Chemother. 2016, 60, 5957. [Google Scholar] [CrossRef]
- Gupta, T.T.; Gupta, N.K.; Pestrak, M.J.; Dusane, D.H.; Harro, J.M.; Horswill, A.R.; Stoodley, P. Staphylococcus Aureus Aggregates on Orthopedic Materials under Varying Levels of Shear Stress. Appl. Environ. Microbiol. 2020, 86, e01234-20. [Google Scholar] [CrossRef]
- Tran, N.N.; Morrisette, T.; Jorgensen, S.C.; Orench-Benvenutti, J.M.; Kebriaei, R. Current Therapies and Challenges for the Treatment of Staphylococcus Aureus Biofilm-related Infections. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2023, 43, 816–832. [Google Scholar] [CrossRef] [PubMed]
- da Silva Meira, Q.G.; de Medeiros Barbosa, I.; Athayde, A.J.A.A.; de Siqueira-Júnior, J.P.; de Souza, E.L. Influence of Temperature and Surface Kind on Biofilm Formation by Staphylococcus Aureus from Food-Contact Surfaces and Sensitivity to Sanitizers. Food Control 2012, 25, 469–475. [Google Scholar] [CrossRef]
- Crouzet, M.; Le Senechal, C.; Brözel, V.S.; Costaglioli, P.; Barthe, C.; Bonneu, M.; Garbay, B.; Vilain, S. Exploring Early Steps in Biofilm Formation: Set-up of an Experimental System for Molecular Studies. BMC Microbiol. 2014, 14, 253. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, R. Biofilms: Microbial Cities of Scientific Significance. JMEN 2014, 1, 14. [Google Scholar] [CrossRef]
- Hobley, L.; Harkins, C.; MacPhee, C.E.; Stanley-Wall, N.R. Giving Structure to the Biofilm Matrix: An Overview of Individual Strategies and Emerging Common Themes. FEMS Microbiol Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef]
- Abee, T.; Kovács, Á.T.; Kuipers, O.P.; van der Veen, S. Biofilm Formation and Dispersal in Gram-Positive Bacteria. Curr. Opin. Biotechnol. 2011, 22, 172–179. [Google Scholar] [CrossRef]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Rahal, J.J.; Simberkoff, M.S.; Kagan, K.; Moldover, N.H. Bactericidal Efficacy of Sch 20569 and Amikacin against Gentamicin-Sensitive and-Resistant Organisms. Antimicrob. Agents Chemother. 1976, 9, 595–599. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Orazi, G.; O’Toole, G.A. “It Takes a Village”: Mechanisms Underlying Antimicrobial Recalcitrance of Polymicrobial Biofilms. J. Bacteriol. 2019, 202, e00530-19. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of Medical Devices by Staphylococci. Env. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface Treatment Strategies to Combat Implant-Related Infection from the Beginning. J. Orthop. Transl. 2018, 17, 42–54. [Google Scholar] [CrossRef]
- Wi, Y.M.; Patel, R. Understanding Biofilms and Novel Approaches to the Diagnosis, Prevention, and Treatment of Medical Device-Associated Infections. Infect. Dis. Clin. N. Am. 2018, 32, 915–929. [Google Scholar] [CrossRef]
- Wasserman, D.D.; Creech, J.A.; Healy, M. Cooling Techniques for Hyperthermia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Partanen, A.; Yarmolenko, P.S.; Viitala, A.; Appanaboyina, S.; Haemmerich, D.; Ranjan, A.; Jacobs, G.; Woods, D.; Enholm, J.; Wood, B.J.; et al. Mild Hyperthermia with Magnetic Resonance-Guided High-Intensity Focused Ultrasound for Applications in Drug Delivery. Int. J. Hyperth. 2012, 28, 320–336, Erratum in Int. J. Hyperth. 2012, 28, 473. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Piazena, H.; Notter, M.; Thomsen, A.R.; Grosu, A.-L.; Scholkmann, F.; Pockley, A.G.; Multhoff, G. From Localized Mild Hyperthermia to Improved Tumor Oxygenation: Physiological Mechanisms Critically Involved in Oncologic Thermo-Radio-Immunotherapy. Cancers 2023, 15, 1394. [Google Scholar] [CrossRef]
- Krokidis, M.; Ahmed, I. Overview of Thermal Ablation Devices: Radiofrequency Ablation. In Interventional Radiology Techniques in Ablation; Clark, T., Sabharwal, T., Eds.; Techniques in Interventional Radiology; Springer: London, UK, 2013; pp. 5–11. ISBN 978-0-85729-094-6. [Google Scholar]
- Lepock, J.R. Protein Denaturation during Heat Shock. In Advances in Molecular and Cell Biology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 19, pp. 223–259. ISBN 1569-2558. [Google Scholar]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89–97. [Google Scholar] [CrossRef]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The Role of Hyperthermia in the Multidisciplinary Treatment of Malignant Tumors. Integr. Cancer Ther. 2019, 18, 1534735419876345. [Google Scholar] [CrossRef] [PubMed]
- Pavlovsky, L.; Sturtevant, R.A.; Younger, J.G.; Solomon, M.J. Effects of Temperature on the Morphological, Polymeric, and Mechanical Properties of Staphylococcus epidermidis Bacterial Biofilms. Langmuir 2015, 31, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Ricker, E.B.; Nuxoll, E. Synergistic effects of heat and antibiotics on Pseudomonas aeruginosa biofilms. Biofouling 2017, 33, 855–866. [Google Scholar] [CrossRef]
- Almutairi, L.A.; Yu, B.; Dyne, E.; Ojaym, A.A.; Kim, M.-H. Mild Magnetic Hyperthermia Is Synergistic with an Antibiotic Treatment against Dual Species Biofilms Consisting of S. Aureus and P. Aeruginosa by Enhancing Metabolic Activity. Int. J. Hyperth. 2023, 40, 2226845. [Google Scholar] [CrossRef]
- Alumutairi, L.; Yu, B.; Filka, M.; Nayfach, J.; Kim, M.H. Mild magnetic nanoparticle hyperthermia enhances the susceptibility of Staphylococcus aureus biofilm to antibiotics. Int. J. Hyperth. 2020, 37, 66–75. [Google Scholar] [CrossRef]
- Kim, M.-H.; Yamayoshi, I.; Mathew, S.; Liln, H.; Nayfach, J.; Simon, S.I. Magnetic Nanoparticle Targeted Hyperthermia of Cutaneous Staphylococcus aureus Infection. Ann. Biomed. Eng. 2013, 41, 598–609, Erratum in Ann. Biomed. Eng. 2013, 41, 610. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, B.; Zhang, W.; Wang, H.; Dai, Y.; Li, X.; Zhang, W.; Zhang, X.; Mao, J.; Zhao, S.; et al. A Size-Adaptive Nanomicrobicide for Synergistic Photothermal and Gaseous Dismantling of Multidrug-Resistant Biofilms. Nano Lett. 2025, 25, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Bañobre-López, M.; Espiña, B.; Rivas, J.; Azeredo, J. Effect of Magnetic Hyperthermia on the Structure of Biofilm and Cellular Viability of a Food Spoilage Bacterium. Biofouling 2013, 29, 1225–1232. [Google Scholar] [CrossRef]
- Russell, A.D. Lethal Effects of Heat on Bacterial Physiology and Structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Wu, M.C.; Deokar, A.R.; Liao, J.H.; Shih, P.Y.; Ling, Y.C. Graphene-Based Photothermal Agent for Rapid and Effective Killing of Bacteria. ACS Nano 2013, 7, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Tsuchido, T.; Katsui, N.; Takeuchi, A.; Takano, M.; Shibasaki, I. Destruction of the Outer Membrane Permeability Barrier of Escherichia Coli by Heat Treatment. Appl. Environ. Microbiol. 1985, 50, 298–303. [Google Scholar] [CrossRef]
- Lal, S.; Clare, S.E.; Halas, N.J. Nanoshell-Enabled Photothermal Cancer Therapy: Impending Clinical Impact. Acc. Chem. Res. 2008, 41, 1842–1851. [Google Scholar] [CrossRef]
- Liu, M.; He, D.; Yang, T.; Liu, W.; Mao, L.; Zhu, Y.; Wu, J.; Luo, G.; Deng, J. An Efficient Antimicrobial Depot for Infectious Site-Targeted Chemo-Photothermal Therapy. J. Nanobiotechnol. 2018, 16, 23. [Google Scholar] [CrossRef]
- Meeker, D.G.; Jenkins, S.V.; Miller, E.K.; Beenken, K.E.; Loughran, A.J.; Powless, A.; Muldoon, T.J.; Galanzha, E.I.; Zharov, V.P.; Smeltzer, M.S.; et al. Synergistic Photothermal and Antibiotic Killing of Biofilm-Associated Staphylococcus aureus Using Targeted Antibiotic-Loaded Gold Nanoconstructs. ACS Infect. Dis. 2016, 2, 241–250. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, C.M.; Coffin, R.C.; Carroll, D.L.; Levi-Polyachenko, N.H. Low Band Gap Donor-Acceptor Conjugated Polymer Nanoparticles and Their NIR-mediated Thermal Ablation of Cancer Cells. Macromol. Biosci. 2013, 13, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yates-Alston, S.; Sarkar, S.; Cochran, M.; Kuthirummal, N.; Levi, N. Hybrid Donor-Acceptor Polymer Nanoparticles and Combination Antibiotic for Mitigation of Pathogenic Bacteria and Biofilms. J. Microbiol. Methods 2021, 190, 106328. [Google Scholar] [CrossRef]
- Sarkar, S.; Graham-Gurysh, E.G.; MacNeill, C.M.; Kelkar, S.; McCarthy, B.D.; Mohs, A.; Levi-Polyachenko, N. Variable Molecular Weight Nanoparticles for Near-Infrared Fluorescence Imaging and Photothermal Ablation. ACS Appl. Polym. Mater. 2020, 2, 4162–4170. [Google Scholar] [CrossRef]
- Aksoy, I.; Kucukkececi, H.; Sevgi, F.; Metin, O.; Hatay Patir, I. Photothermal Antibacterial and Antibiofilm Activity of Black Phosphorus/Gold Nanocomposites against Pathogenic Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 26822–26831. [Google Scholar] [CrossRef]
- Behzadpour, N.; Akbari, N.; Sattarahmady, N. Photothermal Inactivation of Methicillin-resistant Staphylococcus Aureus: Anti-biofilm Mediated by a Polypyrrole–Carbon Nanocomposite. IET Nanobiotechnol. 2019, 13, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Merkl, P.; Zhou, S.; Zaganiaris, A.; Shahata, M.; Eleftheraki, A.; Thersleff, T.; Sotiriou, G.A. Plasmonic Coupling in Silver Nanoparticle Aggregates and Their Polymer Composite Films for Near-Infrared Photothermal Biofilm Eradication. ACS Appl. Nano Mater. 2021, 4, 5330–5339. [Google Scholar] [CrossRef]
- Cha, S.-H.; Bae, J.; Lee, K.J. Enhancement of Adhesion between Inorganic Nanoparticles and Polymeric Matrix in Nanocomposite by Introducing Polymeric Thin Film onto Nanoparticles. Polym. Eng. Sci. 2015, 55, 1906–1911. [Google Scholar] [CrossRef]
- Li, J.; Pan, G.; Zyryanov, G.V.; Peng, Y.; Zhang, G.; Ma, L.; Li, S.; Chen, P.; Wang, Z. Positively Charged Semiconductor Conjugated Polymer Nanomaterials with Photothermal Activity for Antibacterial and Antibiofilm Activities in Vitro and in vivo. ACS Appl. Mater. Interfaces 2023, 15, 40864–40876. [Google Scholar] [CrossRef]
- Zhang, X.; Geven, M.A.; Grijpma, D.W.; Gautrot, J.E.; Peijs, T. Polymer-Polymer Composites for the Design of Strong and Tough Degradable Biomaterials. Mater. Today Commun. 2016, 8, 53–63. [Google Scholar] [CrossRef]
- Coffin, R.C.; Peet, J.; Rogers, J.; Bazan, G.C. Streamlined Microwave-Assisted Preparation of Narrow-Bandgap Conjugated Polymers for High-Performance Bulk Heterojunction Solar Cells. Nat. Chem. 2009, 1, 657. [Google Scholar] [CrossRef] [PubMed]
- MacNeill, C.M.; Graham, E.G.; Levi-Polyachenko, N.H. Soft template synthesis of donor–acceptor conjugated polymer nanoparticles: Structural effects, stability, and photothermal studies. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1622–1632. [Google Scholar] [CrossRef]
- McCabe--Lankford, E.E.; Brown, T.L.; Levi-Polyachenko, N.H. Assessing fluorescence detection and effective photothermal therapy of near-infrared polymer nanoparticles using alginate tissue phantoms. Lasers Surg. Med. 2018, 50, 1040–1049. [Google Scholar] [CrossRef]
- Larkin, K.A.; Martin, J.S.; Zeanah, E.H.; True, J.M.; Braith, R.W.; Borsa, P.A. Limb Blood Flow After Class 4 Laser Therapy. J Athl. Train. 2012, 47, 178–183. [Google Scholar] [CrossRef]
- de la Barra Ortiz, H.A.; Cangas, S.A.; Herrera, A.C.; García, F.O.; Velásquez, S.V. Efficacy of Class IV Laser in the Management of Musculoskeletal Pain: A Systematic Review. Physiother. Q. 2021, 29, 1–11. [Google Scholar] [CrossRef]
- Ghimire, A.; Skelly, J.D.; Song, J. Micrococcal-Nuclease-Triggered On-Demand Release of Vancomycin from Intramedullary Implant Coating Eradicates Staphylococcus aureus Infection in Mouse Femoral Canals. ACS Cent. Sci. 2019, 5, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Baban, C.K.; Cronin, M.; Akin, A.R.; O’Brien, A.; Gao, X.; Tabirca, S.; Francis, K.P.; Tangney, M. Bioluminescent Bacterial Imaging In Vivo. J. Vis. Exp. 2012, 69, 4318. [Google Scholar] [CrossRef]
- Chang, M.H.; Cirillo, S.L.G.; Cirillo, J.D. Using Luciferase to Image Bacterial Infections in Mice. J. Vis. Exp. 2011, 48, 2547. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Willard, J.; Kadurugamuwa, J.L.; Yu, J.; Francis, K.P.; Bayer, A.S. Real-Time In Vivo Bioluminescent Imaging for Evaluating the Efficacy of Antibiotics in a Rat Staphylococcus aureus Endocarditis Model. Antimicrob. Agents Chemother. 2005, 49, 380–387. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Ji, Y. Environmental factors modulate biofilm formation by Staphylococcus aureus. Sci. Prog. 2020, 103, 0036850419898659. [Google Scholar] [CrossRef] [PubMed]
- Haasbroek, K.; Yagi, M.; Yonei, Y. Staphylococcus aureus Biofilm Inhibiting Activity of Advanced Glycation Endproduct Crosslink Breaking and Glycation Inhibiting Compounds. Antibiotics 2022, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Veeregowda, D.H.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C. Extracellular Polymeric Matrix Production and Relaxation under Fluid Shear and Mechanical Pressure in Staphylococcus aureus Biofilms. Appl. Environ. Microbiol. 2017, 84, e01516-17. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Sharma, D.; Brinsmade, S.R.; Felden, B.; Augagneur, Y. Genome Sequence of the Clinical Isolate Staphylococcus Aureus Subsp. Aureus Strain UAMS-1. Genome Announc. 2015, 3, e01584-14. [Google Scholar] [CrossRef]
- Rom, J.S.; Beenken, K.E.; Ramirez, A.M.; Walker, C.M.; Echols, E.J.; Smeltzer, M.S. Limiting protease production plays a key role in the pathogenesis of the divergent clinical isolates of Staphylococcus aureus LAC and UAMS-1. Virulence 2021, 12, 584–600. [Google Scholar] [CrossRef]
- Beenken, K.E.; Smeltzer, M.S. Staphylococcus aureus Biofilm-Associated Infections: Have We Found a Clinically Relevant Target? Microorganisms 2025, 13, 852. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Nistico, L.; Sambanthamoorthy, K.; Longwell, M.; Iannitelli, A.; Cellini, L.; Di Stefano, A.; Hall Stoodley, L.; Stoodley, P. Temporal Expression of AgrB, CidA, and AlsS in the Early Development of Staphylococcus aureus UAMS-1 Biofilm Formation and the Structural Role of Extracellular DNA and Carbohydrates. Pathog. Dis. 2014, 70, 414–422. [Google Scholar] [CrossRef]
- Harapanahalli, A.K.; Chen, Y.; Li, J.; Busscher, H.J.; van der Mei, H.C. Influence of Adhesion Force on icaA and cidA Gene Expression and Production of Matrix Components in Staphylococcus aureus Biofilms. Appl. Environ. Microbiol. 2015, 81, 3369–3378. [Google Scholar] [CrossRef]
- Alkan-Taş, B.; Berksun, E.; Taş, C.E.; Ünal, S.; Ünal, H. NIR-Responsive Waterborne Polyurethane-Polydopamine Coatings for Light-Driven Disinfection of Surfaces. Prog. Org. Coat. 2022, 164, 106669. [Google Scholar] [CrossRef]
- Jia, M.; Tan, H.; Liu, Z.; Wu, J.; Gou, X.; An, J. Polydopamine Nanoplatform for Synergistic Photothermal and Antibiotic Treatment of Catheter-Associated Urinary Tract Infection. ACS Appl. Nano Mater. 2025, 8, 13851–13860. [Google Scholar] [CrossRef]
- Yang, G.; Deng, R.; Chang, Y.; Li, H. Polydopamine-Based Surface Coating Fabrication on Titanium Implant by Combining a Photothermal Agent and TiO2 Nanosheets for Efficient Photothermal Antibacterial Therapy and Promoted Osteogenic Activity. Int. J. Biol. Macromol. 2024, 281, 136481. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Li, X.; Li, Q.; Xiu, W.; He, A.; Dai, Z.; Dong, H.; Shan, J.; Mou, Y. Simultaneous Biofilm Disruption, Bacterial Killing, and Inflammation Elimination for Wound Treatment Using Silver Embellished Polydopamine Nanoplatform. Small 2024, 20, 2400927. [Google Scholar] [CrossRef]
- Hu, X.; Ma, R.; Zhang, P.; Dong, J.; Sun, J.; Wang, W.; Liu, Q.; Kong, L.; Zhang, X.; Wang, Z.; et al. Biomimetic Metal–Organic Framework Combats Biofilm-Associated Infections via Hyperthermia-Enhanced Bacterial Metabolic Interference and Autophagy-Promoted Adaptive Immunity. Adv. Funct. Mater. 2024, 34, 2310509. [Google Scholar] [CrossRef]
- Mamone, L.; Tomás, R.; Di Venosa, G.; Gándara, L.; Durantini, E.; Buzzola, F.; Casas, A. Laser NIR Irradiation Enhances Antimicrobial Photodynamic Inactivation of Biofilms of Staphylococcus Aureus. Lasers Surg. Med. 2024, 56, 783–795. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Nie, C.; Zhang, R.; Qu, S.; Shao, Q.; Zhang, X.; Li, J.; Li, W.; Li, H.; et al. Photothermal Conjugated Polymer Microneedle with Biofilm Elimination and Angiogenesis for Diabetic Wound Healing. Nano Lett. 2025, 25, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Eltz, F.Z.; Vebber, M.C.; Aguzzoli, C.; Machado, G.; da Silva Crespo, J.; Giovanela, M. Preparation, Characterization and Application of Polymeric Thin Films Containing Silver and Copper Nanoparticles with Bactericidal Activity. J. Environ. Chem. Eng. 2020, 8, 103745. [Google Scholar] [CrossRef]
- Dowling, D.; Donnelly, K.; McConnell, M.; Eloy, R.; Arnaud, M. Deposition of Anti-Bacterial Silver Coatings on Polymeric Substrates. Thin Solid Film. 2001, 398, 602–606. [Google Scholar] [CrossRef]
- Pijls, B.G.; Sanders, I.M.J.G.; Kujiper, E.J.; Nelissen, R.G.H.H. Induction heating for eradicating Staphylococcus epidermidis from biofilm. Bone Jt. Res. 2020, 9, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Sturtevant, R.A.; Sharma, P.; Pavlovsky, L.; Stewart, E.J.; Solomon, M.J.; Younger, J.G. Thermal Augmentation of Vancomycin against Staphylococcal Biofilms. Shock 2015, 44, 121–127. [Google Scholar] [CrossRef]
- van der Vinne, V.; Pothecary, C.A.; Wilcox, S.L.; McKillop, L.E.; Benson, L.A.; Kolpakova, J.; Tam, S.K.E.; Krone, L.B.; Fisk, A.S.; Wilson, T.S.; et al. Continuous and Non-Invasive Thermography of Mouse Skin Accurately Describes Core Body Temperature Patterns, but Not Absolute Core Temperature. Sci. Rep. 2020, 10, 20680. [Google Scholar] [CrossRef]
- Song, Y.; Zhu, J.; Lv, Y.; Liu, H.; Kang, L.; Shen, F.; Zhang, C.; Jiang, W.; Yu, J.; Wu, D. Temperature-Triggered Reversible Adhesion Hydrogel with Responsive Drug Release, Mild Photothermal Therapy, and Biofilm Clearance for Skin Infection Healing. ACS Appl. Mater. Interfaces 2025, 17, 19417–19435. [Google Scholar] [CrossRef]
- Stewart, G.R.; Young, D.B. Heat-shock proteins and the host–pathogen interaction during bacterial infection. Curr. Opin. Immunol. 2004, 16, 506–510. [Google Scholar] [CrossRef]
- Chmielewski, R.A.N.; Frank, J.F. A Predictive Model for Heat Inactivation of Listeria monocytogenes Biofilm on Buna-N Rubber. LWT–Food Sci. Technol. 2006, 39, 11–19. [Google Scholar] [CrossRef]
- Xue, Y.; Niu, W.; Wang, M.; Chen, M.; Guo, Y.; Lei, B. Engineering a Biodegradable Multifunctional Antibacterial Bioactive Nanosystem for Enhancing Tumor Photothermo-Chemotherapy and Bone Regeneration. ACS Nano 2020, 14, 442–453, Erratum in ACS Nano 2021, 15, 15395–15396. [Google Scholar] [CrossRef]
- Rediske, A.M.; Hymas, W.C.; Wilkinson, R.; Pitt, W.G. Ultrasonic Enhancement of Antibiotic Action on Several Species of Bacteria. J. Gen. Appl. Microbiol. 1998, 44, 283–288. [Google Scholar] [CrossRef]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A Review of the Influence of Treatment Strategies on Antibiotic Resistant Bacteria and Antibiotic Resistance Genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Alves-Barroco, C.; Rivas-García, L.; Fernandes, A.R.; Baptista, P.V. Light Triggered Enhancement of Antibiotic Efficacy in Biofilm Elimination Mediated by Gold-Silver Alloy Nanoparticles. Front. Microbiol. 2022, 13, 841124. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.E.d.A.; Araújo, A.R.d.; Piancastelli, A.C.C.; Pinotti, M. Effects of Low-Power Light Therapy on Wound Healing: LASER x LED. Bras. Dermatol. 2014, 89, 616–623. [Google Scholar] [CrossRef]
- Afroz, Z.; Jobayer, M.; Ahmed, S.; Anwar, S.; Miah, M.R.A. Central Venous Catheter-Related Bloodstream Infections (CVC-BSI) in Patients of Clinically Suspected Septicemia. Bangladesh Med. Res. Counc. Bull. 2015, 41, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Allon, M. Dialysis Catheter-Related Bacteremia: Treatment and Prophylaxis. Am. J. Kidney Dis. 2004, 44, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Richardson, I.P.; Sturtevant, R.; Heung, M.; Solomon, M.J.; Younger, J.G.; VanEpps, J.S. Hemodialysis Catheter Heat Transfer for Biofilm Prevention and Treatment. ASAIO J. 2016, 62, 92–99. [Google Scholar] [CrossRef]
- Izzo, I.; Lania, D.; Bella, D.; Formaini Marioni, C.; Coccaglio, R.; Colombini, P. Catheter Associated Urinary Tract Infection (CA-UTI) Incidence in an Internal Medicine Ward of a Northern Italian Hospital. Infez Med. 2015, 23, 243–246. [Google Scholar]
- Fireman, P. Otitis Media and Eustachian Tube Dysfunction: Connection to Allergic Rhinitis. J. Allergy Clin. Immunol. 1997, 99, s787–s797. [Google Scholar] [CrossRef]
- Winter, L.; Oberacker, E.; Paul, K.; Ji, Y.; Oezerdem, C.; Ghadjar, P.; Thieme, A.; Budach, V.; Wust, P.; Niendorf, T. Magnetic Resonance Thermometry: Methodology, Pitfalls and Practical Solutions. Int. J. Hyperth. 2016, 32, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.D.B.; Castro, S.d.M.; Pavam, M.V.; Lima, E.M.; Mendanha, S.A.; Bakuzis, A.F. Near Infrared Thermoluminescent Liposome Sensor for Real--Time Monitoring of Photothermal Therapy and Magnetic Hyperthermia. Adv. Mater. Technol. 2025, 10, 2401598. [Google Scholar] [CrossRef]
- Desclides, M.; Ozenne, V.; Bour, P.; Faller, T.; Machinet, G.; Pierre, C.; Chemouny, S.; Quesson, B. Real-Time Automatic Temperature Regulation during in vivo MRI-Guided Laser-Induced Thermotherapy (MR-LITT). Sci. Rep. 2023, 13, 3279. [Google Scholar] [CrossRef]
- De Tommasi, F.; Massaroni, C.; Grasso, R.F.; Carassiti, M.; Schena, E. Temperature Monitoring in Hyperthermia Treatments of Bone Tumors: State-of-the-Art and Future Challenges. Sensors 2021, 21, 5470. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, H.F.; Capistrano, G.; Mello, F.M.; Zufelato, N.; Silveira-Lacerda, E.; Bakuzis, A.F. Precise determination of the heat delivery during in vivo magnetic nanoparticle hyperthermia with infrared thermography. Phys. Med. Biol. 2017, 62, 4062. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Kong, L.; Dai, Y.; Tang, J.; Mei, J.; Qian, Z.; Ma, Y.; Li, Q.; Ju, S.; Wang, J.; et al. Bioresponsive nano-antibacterials for H2S-sensitized hyperthermia and immunomodulation against refractory implant–related infections. Sci. Adv. 2022, 8, eabn1701. [Google Scholar] [CrossRef]
- Chopra, R.; Shaikh, S.; Chatzinoff, Y.; Munaweera, I.; Cheng, B.; Daly, S.M.; Xi, Y.; Bing, C.; Burns, D.; Greenberg, D.E. Employing High-Frequency Alternating Magnetic Fields for the Non-Invasive Treatment of Prosthetic Joint Infections. Sci. Rep. 2017, 7, 7520. [Google Scholar] [CrossRef]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Tawre, M.S.; Kamble, E.E.; Kumkar, S.N.; Mulani, M.S.; Pardesi, K.R. Antibiofilm and Antipersister Activity of Acetic Acid against Extensively Drug Resistant Pseudomonas Aeruginosa PAW1. PLoS ONE 2021, 16, e0246020. [Google Scholar] [CrossRef]
- Silva, A.R.; Melo, L.F.; Pereira, A. How Biofilm History Affects the Impact of Thermal Disinfection on Biofilm Control and Regrowth. Heat Mass Transf. 2025, 61, 71. [Google Scholar] [CrossRef]
- Dhar, Y.; Han, Y. Current Developments in Biofilm Treatments: Wound and Implant Infections. Eng. Regen. 2020, 1, 64–75. [Google Scholar] [CrossRef]
- Järbrink, K.; Ni, G.; Sönnergren, H.; Schmidtchen, A.; Pang, C.; Bajpai, R.; Car, J. Prevalence and Incidence of Chronic Wounds and Related Complications: A Protocol for a Systematic Review. Syst. Rev. 2016, 5, 152. [Google Scholar] [CrossRef] [PubMed]
- James, G.A.; Swogger, E.; Wolcott, R.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in Chronic Wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Schirra, M.; D’hallewin, G.; Ben-Yehoshua, S.; Fallik, E. Host–Pathogen Interactions Modulated by Heat Treatment. Postharvest Biol. Technol. 2000, 21, 71–85. [Google Scholar] [CrossRef]
- Miao, H.; Shen, R.; Zhang, W.; Lin, Z.; Wang, H.; Yang, L.; Liu, X.; Lin, N. Near-infrared Light Triggered Silk Fibroin Scaffold for Photothermal Therapy and Tissue Repair of Bone Tumors. Adv. Funct. Mater. 2021, 31, 2007188. [Google Scholar] [CrossRef]
- Henderson, T.A. Can Infrared Light Really Be Doing What We Claim It Is Doing? Infrared Light Penetration Principles, Practices, and Limitations. Front. Neurol. 2024, 15, 1398894. [Google Scholar] [CrossRef]
 Si-G is biofilm grown on silicone and treated with gentamicin alone.
Si-G is biofilm grown on silicone and treated with gentamicin alone.  BSe-G is biofilm grown on BSe-Si and treated with gentamicin alone.
BSe-G is biofilm grown on BSe-Si and treated with gentamicin alone.  Si-3 is biofilm on silicone and treated with 3 W for 25 s.
Si-3 is biofilm on silicone and treated with 3 W for 25 s.  BSe-3 is biofilm on BSe-Si and treated with 3 W for 25 s.
BSe-3 is biofilm on BSe-Si and treated with 3 W for 25 s.  Si-5 is biofilm on silicone and treated with 5 W for 12 s.
Si-5 is biofilm on silicone and treated with 5 W for 12 s.  BSe-5 is biofilm on BSe-Si and treated with 5 W for 12 s.
BSe-5 is biofilm on BSe-Si and treated with 5 W for 12 s.  Si-G-3 is a biofilm on silicone and treated with both gentamicin and 3 W for 25 s.
Si-G-3 is a biofilm on silicone and treated with both gentamicin and 3 W for 25 s.  BSe-G-3 is biofilm on BSe-Si and treated with both gentamicin and 3 W for 25 s.
BSe-G-3 is biofilm on BSe-Si and treated with both gentamicin and 3 W for 25 s.  Si-G-5 is biofilm on silicone and treated with both gentamicin and 5 W for 12 s.
Si-G-5 is biofilm on silicone and treated with both gentamicin and 5 W for 12 s.  BSe-G-5 is biofilm on BSe-Si and treated with both gentamicin and 5 W for 12 s. (B) The number of viable colony-forming units following treatment for the same treatment groups used in A. * denotes statistical significance compared to the respective Si-G or BSe-G controls, with p < 0.05.
BSe-G-5 is biofilm on BSe-Si and treated with both gentamicin and 5 W for 12 s. (B) The number of viable colony-forming units following treatment for the same treatment groups used in A. * denotes statistical significance compared to the respective Si-G or BSe-G controls, with p < 0.05.
 Si-G is biofilm grown on silicone and treated with gentamicin alone.
Si-G is biofilm grown on silicone and treated with gentamicin alone.  BSe-G is biofilm grown on BSe-Si and treated with gentamicin alone.
BSe-G is biofilm grown on BSe-Si and treated with gentamicin alone.  Si-3 is biofilm on silicone and treated with 3 W for 25 s.
Si-3 is biofilm on silicone and treated with 3 W for 25 s.  BSe-3 is biofilm on BSe-Si and treated with 3 W for 25 s.
BSe-3 is biofilm on BSe-Si and treated with 3 W for 25 s.  Si-5 is biofilm on silicone and treated with 5 W for 12 s.
Si-5 is biofilm on silicone and treated with 5 W for 12 s.  BSe-5 is biofilm on BSe-Si and treated with 5 W for 12 s.
BSe-5 is biofilm on BSe-Si and treated with 5 W for 12 s.  Si-G-3 is a biofilm on silicone and treated with both gentamicin and 3 W for 25 s.
Si-G-3 is a biofilm on silicone and treated with both gentamicin and 3 W for 25 s.  BSe-G-3 is biofilm on BSe-Si and treated with both gentamicin and 3 W for 25 s.
BSe-G-3 is biofilm on BSe-Si and treated with both gentamicin and 3 W for 25 s.  Si-G-5 is biofilm on silicone and treated with both gentamicin and 5 W for 12 s.
Si-G-5 is biofilm on silicone and treated with both gentamicin and 5 W for 12 s.  BSe-G-5 is biofilm on BSe-Si and treated with both gentamicin and 5 W for 12 s. (B) The number of viable colony-forming units following treatment for the same treatment groups used in A. * denotes statistical significance compared to the respective Si-G or BSe-G controls, with p < 0.05.
BSe-G-5 is biofilm on BSe-Si and treated with both gentamicin and 5 W for 12 s. (B) The number of viable colony-forming units following treatment for the same treatment groups used in A. * denotes statistical significance compared to the respective Si-G or BSe-G controls, with p < 0.05.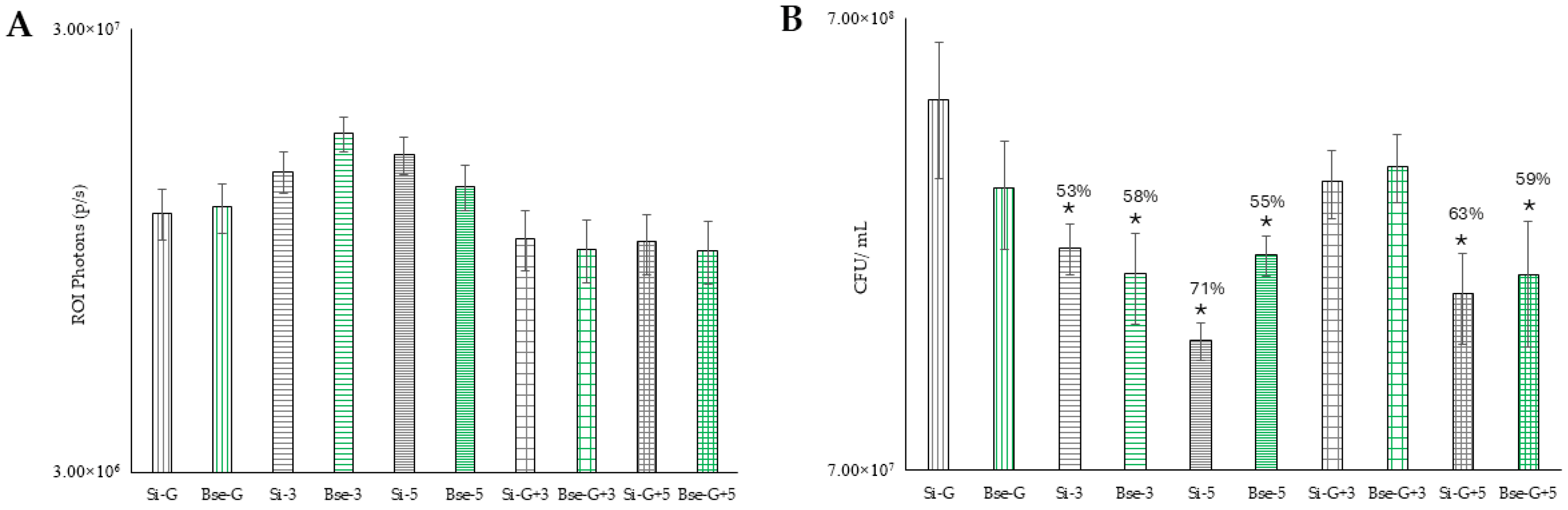
 Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. NaCl + L represents treatment with systemic saline plus exposure to 800 nm laser light at 3 W for 25 s.
Biofilm grown on BSe-Si. NaCl + L represents treatment with systemic saline plus exposure to 800 nm laser light at 3 W for 25 s.  Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. NL/NG represents treatment with no laser, but with systemic delivery of gentamicin.
Biofilm grown on BSe-Si. NL/NG represents treatment with no laser, but with systemic delivery of gentamicin.  Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. G + L represents treatment with both gentamicin and exposure to 800 nm laser light at 3 W for 25 s.
Biofilm grown on BSe-Si. G + L represents treatment with both gentamicin and exposure to 800 nm laser light at 3 W for 25 s.  Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. (B) Percent change in photon flux of the regions of interest (areas of animal flank where the disks were implanted) after PTT, as compared to before treatment, with the same groups from Part A evaluated. * denotes statistical significance compared to the BSe-Si NL/NG control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
Biofilm grown on BSe-Si. (B) Percent change in photon flux of the regions of interest (areas of animal flank where the disks were implanted) after PTT, as compared to before treatment, with the same groups from Part A evaluated. * denotes statistical significance compared to the BSe-Si NL/NG control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
 Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. NaCl + L represents treatment with systemic saline plus exposure to 800 nm laser light at 3 W for 25 s.
Biofilm grown on BSe-Si. NaCl + L represents treatment with systemic saline plus exposure to 800 nm laser light at 3 W for 25 s.  Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. NL/NG represents treatment with no laser, but with systemic delivery of gentamicin.
Biofilm grown on BSe-Si. NL/NG represents treatment with no laser, but with systemic delivery of gentamicin.  Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. G + L represents treatment with both gentamicin and exposure to 800 nm laser light at 3 W for 25 s.
Biofilm grown on BSe-Si. G + L represents treatment with both gentamicin and exposure to 800 nm laser light at 3 W for 25 s.  Biofilm grown on Si.
Biofilm grown on Si.  Biofilm grown on BSe-Si. (B) Percent change in photon flux of the regions of interest (areas of animal flank where the disks were implanted) after PTT, as compared to before treatment, with the same groups from Part A evaluated. * denotes statistical significance compared to the BSe-Si NL/NG control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
Biofilm grown on BSe-Si. (B) Percent change in photon flux of the regions of interest (areas of animal flank where the disks were implanted) after PTT, as compared to before treatment, with the same groups from Part A evaluated. * denotes statistical significance compared to the BSe-Si NL/NG control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.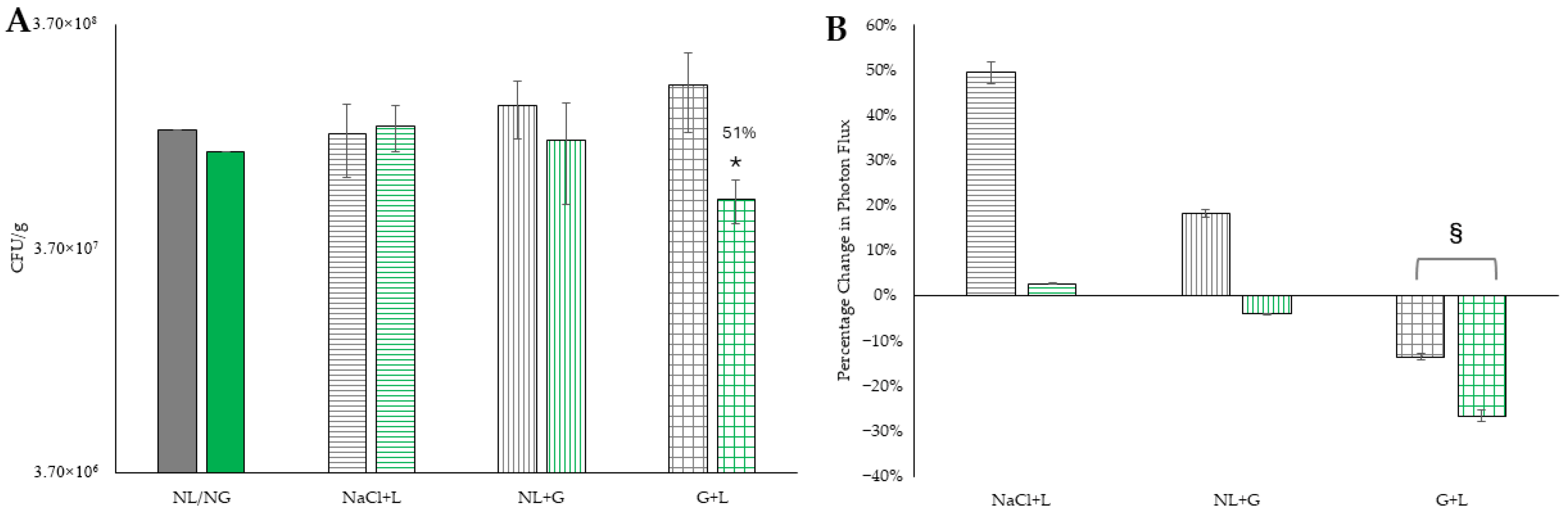
 Si, 1 W (dotted gray line);
Si, 1 W (dotted gray line);  BSe-Si, 1 W (dotted green line);
BSe-Si, 1 W (dotted green line);  Si, 3 W (dashed gray line);
Si, 3 W (dashed gray line);  BSe-Si, 3 W (dashed green line);
BSe-Si, 3 W (dashed green line);  Si, 5 W (solid gray line);
Si, 5 W (solid gray line);  BSe-Si-5 W (solid green line).
BSe-Si-5 W (solid green line).
 Si, 1 W (dotted gray line);
Si, 1 W (dotted gray line);  BSe-Si, 1 W (dotted green line);
BSe-Si, 1 W (dotted green line);  Si, 3 W (dashed gray line);
Si, 3 W (dashed gray line);  BSe-Si, 3 W (dashed green line);
BSe-Si, 3 W (dashed green line);  Si, 5 W (solid gray line);
Si, 5 W (solid gray line);  BSe-Si-5 W (solid green line).
BSe-Si-5 W (solid green line).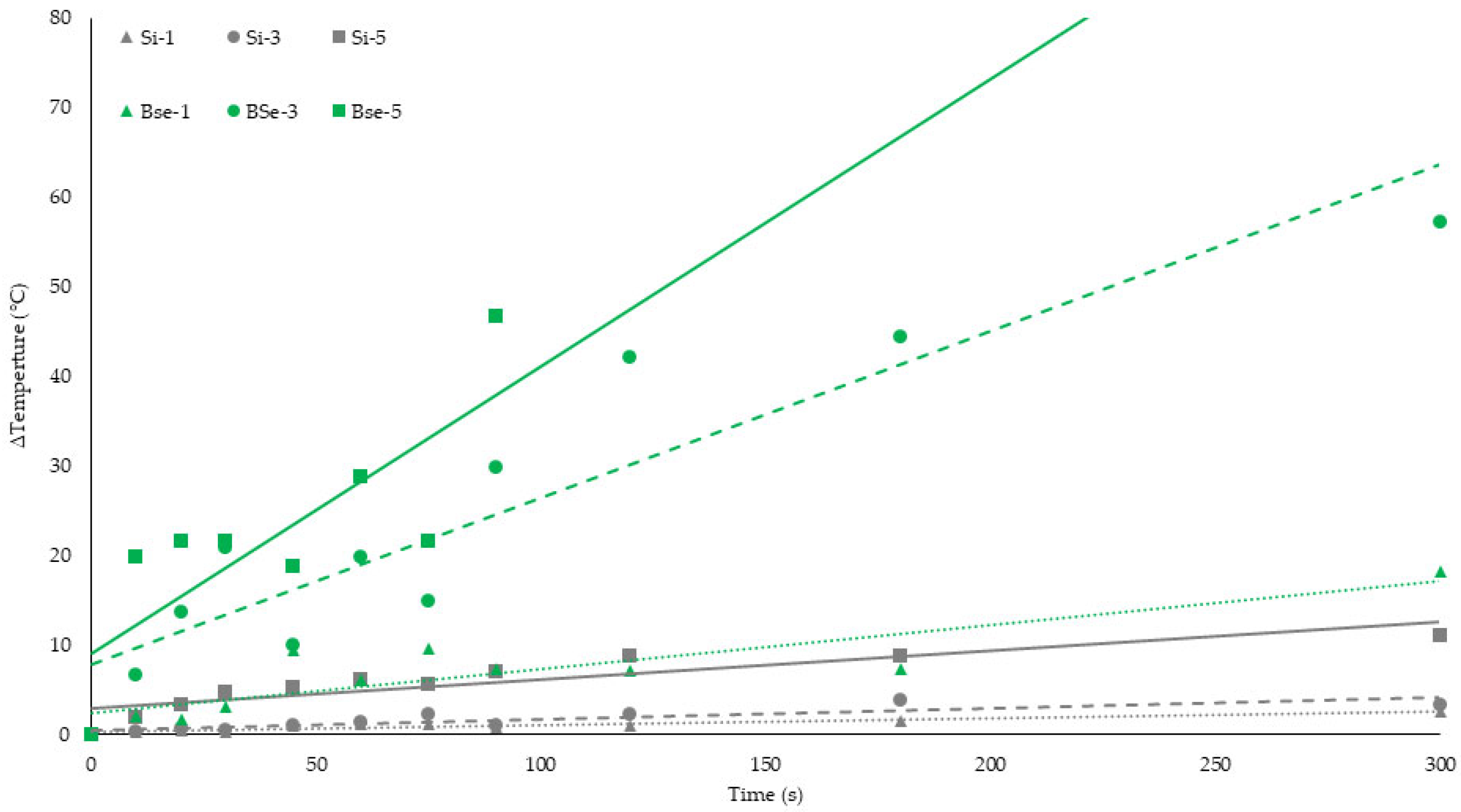
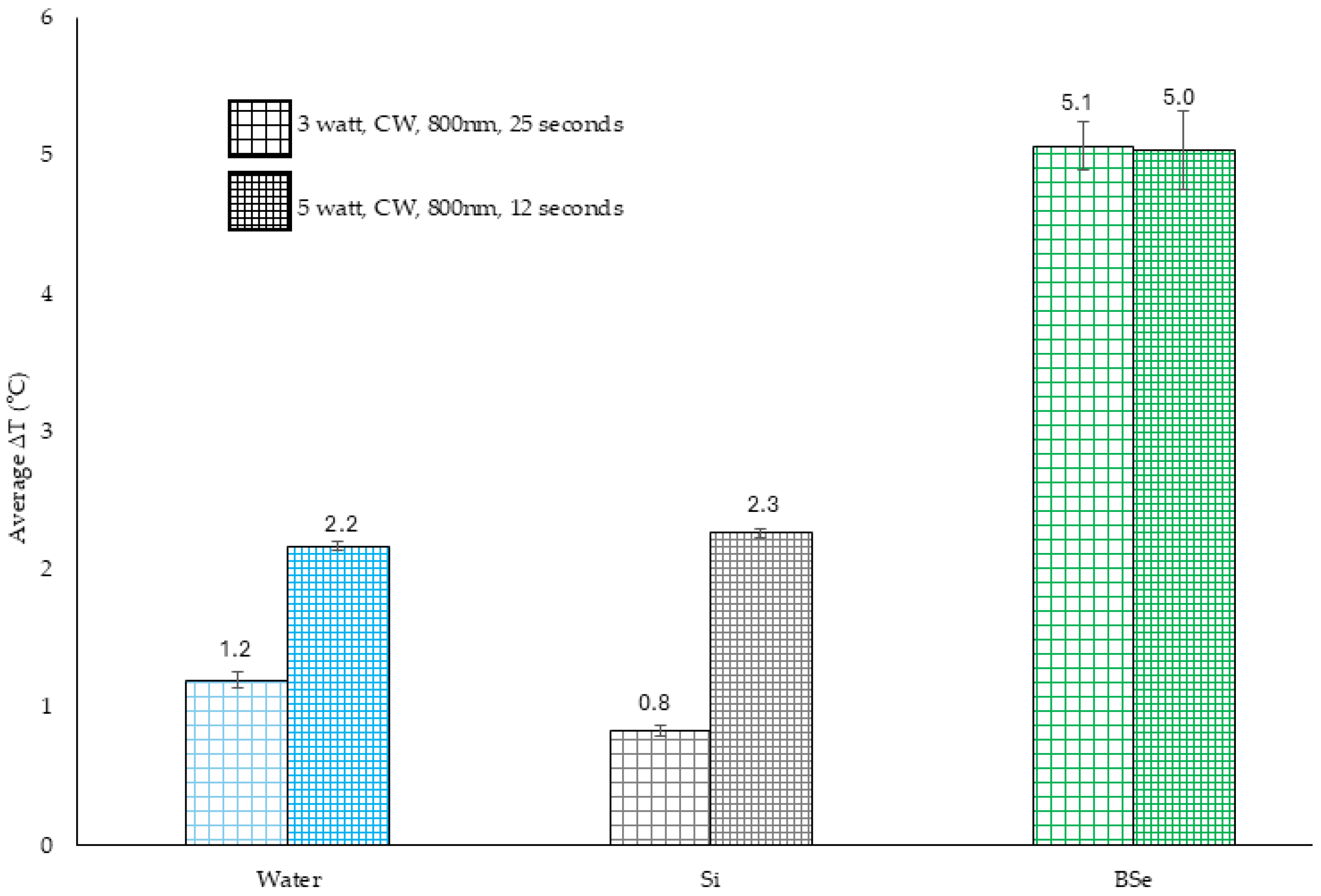
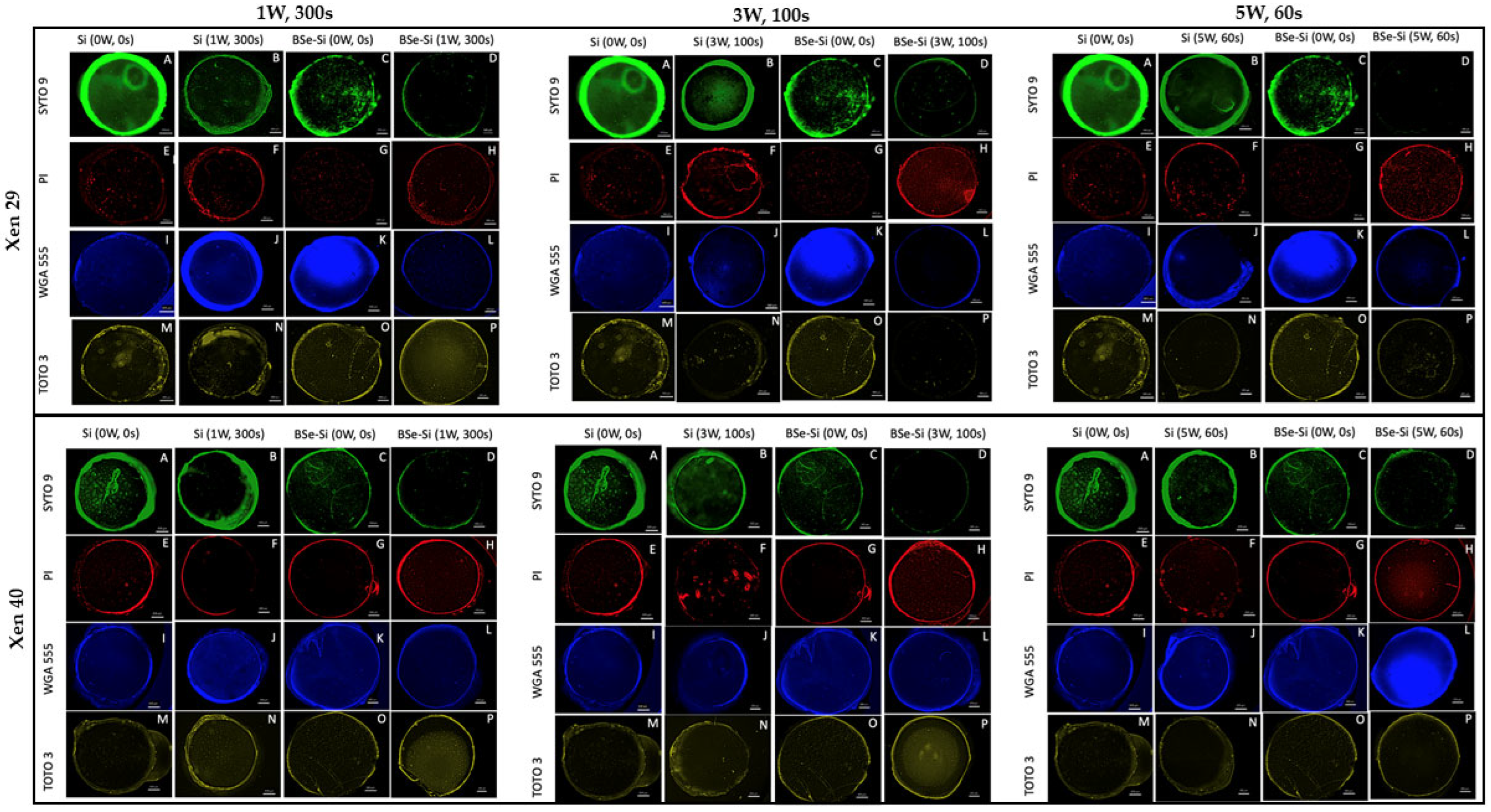
 Si, no light;
Si, no light;  BSe-Si, no light;
BSe-Si, no light;  Si exposed to 1 W for 300 s;
Si exposed to 1 W for 300 s;  BSe-Si exposed to 1 W for 300 s;
BSe-Si exposed to 1 W for 300 s;  Si exposed to 3 W for 100 s;
Si exposed to 3 W for 100 s;  BSe-Si exposed to 3 W for 100 s;
BSe-Si exposed to 3 W for 100 s;  Si exposed to 5 W for 60 s;
Si exposed to 5 W for 60 s;  BSe-Si exposed to 5 W for 60 s. ΔT indicates the measured temperature change for the respective material under the specific laser parameters. * denotes statistical significance compared to the 0 W, 0 s control group, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
BSe-Si exposed to 5 W for 60 s. ΔT indicates the measured temperature change for the respective material under the specific laser parameters. * denotes statistical significance compared to the 0 W, 0 s control group, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
 Si, no light;
Si, no light;  BSe-Si, no light;
BSe-Si, no light;  Si exposed to 1 W for 300 s;
Si exposed to 1 W for 300 s;  BSe-Si exposed to 1 W for 300 s;
BSe-Si exposed to 1 W for 300 s;  Si exposed to 3 W for 100 s;
Si exposed to 3 W for 100 s;  BSe-Si exposed to 3 W for 100 s;
BSe-Si exposed to 3 W for 100 s;  Si exposed to 5 W for 60 s;
Si exposed to 5 W for 60 s;  BSe-Si exposed to 5 W for 60 s. ΔT indicates the measured temperature change for the respective material under the specific laser parameters. * denotes statistical significance compared to the 0 W, 0 s control group, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
BSe-Si exposed to 5 W for 60 s. ΔT indicates the measured temperature change for the respective material under the specific laser parameters. * denotes statistical significance compared to the 0 W, 0 s control group, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
 Control biofilms maintained at 37 °C.
Control biofilms maintained at 37 °C.  Biofilms treated with 50 °C for 60 s.
Biofilms treated with 50 °C for 60 s.  Biofilms treated with 50 °C for 100 s.
Biofilms treated with 50 °C for 100 s.  Biofilms treated with 64 °C for 60 s.
Biofilms treated with 64 °C for 60 s.  Biofilms treated with 64 °C for 100 s. (C) Xen 29 and (D) Xen 40 biofilm mass following hyperthermia in a hot water bath. * denotes statistical significance compared to 37 °C control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
Biofilms treated with 64 °C for 100 s. (C) Xen 29 and (D) Xen 40 biofilm mass following hyperthermia in a hot water bath. * denotes statistical significance compared to 37 °C control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
 Control biofilms maintained at 37 °C.
Control biofilms maintained at 37 °C.  Biofilms treated with 50 °C for 60 s.
Biofilms treated with 50 °C for 60 s.  Biofilms treated with 50 °C for 100 s.
Biofilms treated with 50 °C for 100 s.  Biofilms treated with 64 °C for 60 s.
Biofilms treated with 64 °C for 60 s.  Biofilms treated with 64 °C for 100 s. (C) Xen 29 and (D) Xen 40 biofilm mass following hyperthermia in a hot water bath. * denotes statistical significance compared to 37 °C control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
Biofilms treated with 64 °C for 100 s. (C) Xen 29 and (D) Xen 40 biofilm mass following hyperthermia in a hot water bath. * denotes statistical significance compared to 37 °C control, with p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.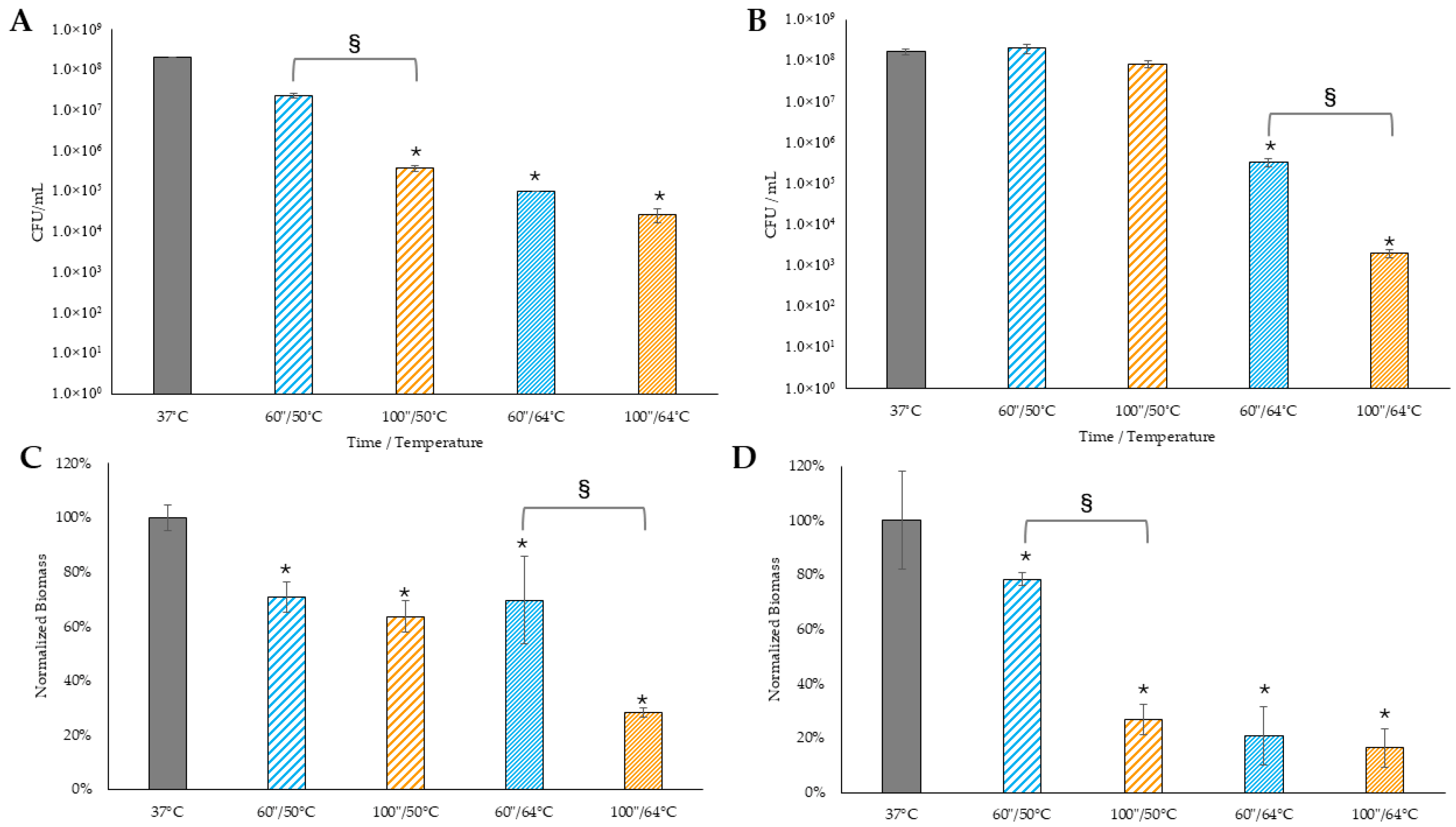
 Control biofilms maintained at 37 °C.
Control biofilms maintained at 37 °C.  Biofilms treated with 50 °C for 60 s.
Biofilms treated with 50 °C for 60 s.  Biofilms treated with 50 °C for 100 s.
Biofilms treated with 50 °C for 100 s.  Biofilms treated with 64 °C for 60 s.
Biofilms treated with 64 °C for 60 s.  Biofilms treated with 64 °C for 100 s. * denotes statistical significance compared to 37 °C control, and p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
Biofilms treated with 64 °C for 100 s. * denotes statistical significance compared to 37 °C control, and p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
 Control biofilms maintained at 37 °C.
Control biofilms maintained at 37 °C.  Biofilms treated with 50 °C for 60 s.
Biofilms treated with 50 °C for 60 s.  Biofilms treated with 50 °C for 100 s.
Biofilms treated with 50 °C for 100 s.  Biofilms treated with 64 °C for 60 s.
Biofilms treated with 64 °C for 60 s.  Biofilms treated with 64 °C for 100 s. * denotes statistical significance compared to 37 °C control, and p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.
Biofilms treated with 64 °C for 100 s. * denotes statistical significance compared to 37 °C control, and p < 0.05. § indicates statistical significance between the two groups, with p < 0.05.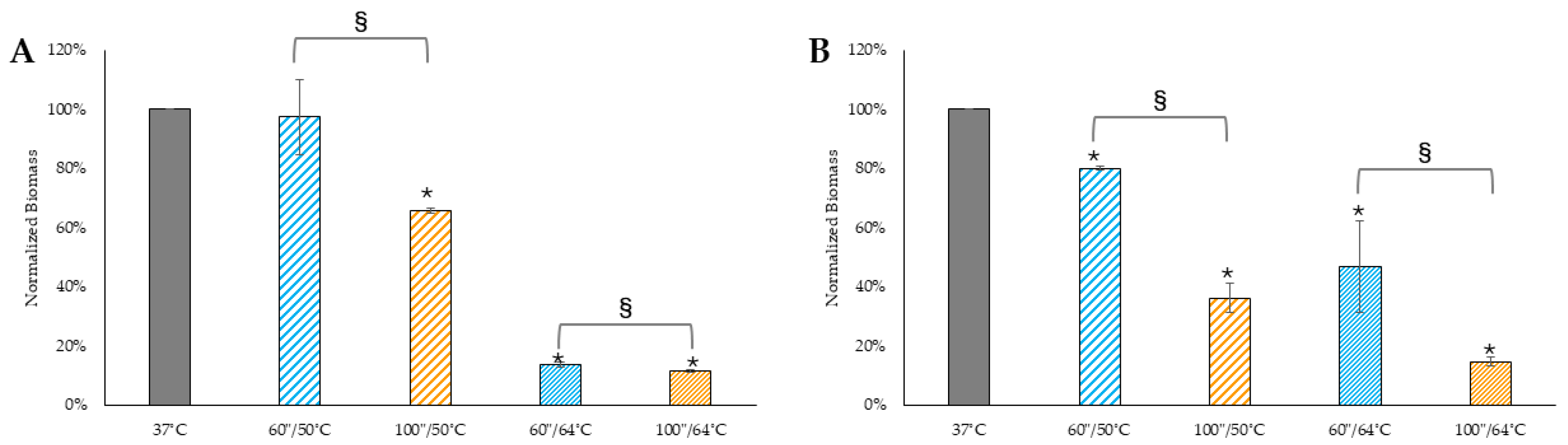
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, P.; Vargas, E.; Green, S.; Greer, M.; Yates-Alston, S.; Esposito, M.; Tan, L.; Levi, N. Semiconducting Polymer-Based Nanocomposite for Photothermal Elimination of Staphylococcus aureus Biofilm. Microorganisms 2025, 13, 2568. https://doi.org/10.3390/microorganisms13112568
Sanchez P, Vargas E, Green S, Greer M, Yates-Alston S, Esposito M, Tan L, Levi N. Semiconducting Polymer-Based Nanocomposite for Photothermal Elimination of Staphylococcus aureus Biofilm. Microorganisms. 2025; 13(11):2568. https://doi.org/10.3390/microorganisms13112568
Chicago/Turabian StyleSanchez, Pedro, Erica Vargas, Stan Green, Madison Greer, Shaina Yates-Alston, Mariana Esposito, Li Tan, and Nicole Levi. 2025. "Semiconducting Polymer-Based Nanocomposite for Photothermal Elimination of Staphylococcus aureus Biofilm" Microorganisms 13, no. 11: 2568. https://doi.org/10.3390/microorganisms13112568
APA StyleSanchez, P., Vargas, E., Green, S., Greer, M., Yates-Alston, S., Esposito, M., Tan, L., & Levi, N. (2025). Semiconducting Polymer-Based Nanocomposite for Photothermal Elimination of Staphylococcus aureus Biofilm. Microorganisms, 13(11), 2568. https://doi.org/10.3390/microorganisms13112568




