Rhizospheric and Endophytic Plant Growth-Promoting Bacteria Associated with Coffea arabica L. and Coffea canephora Pierre ex Froehner: A Review of Their Agronomic Potential
Abstract
1. Introduction
2. Mechanisms of Action of Plant Growth-Promoting Bacteria
2.1. Direct Mechanisms of Action of Plant Growth-Promoting Bacteria
2.2. Indirect Mechanisms of Action of Plant Growth-Promoting Bacteria
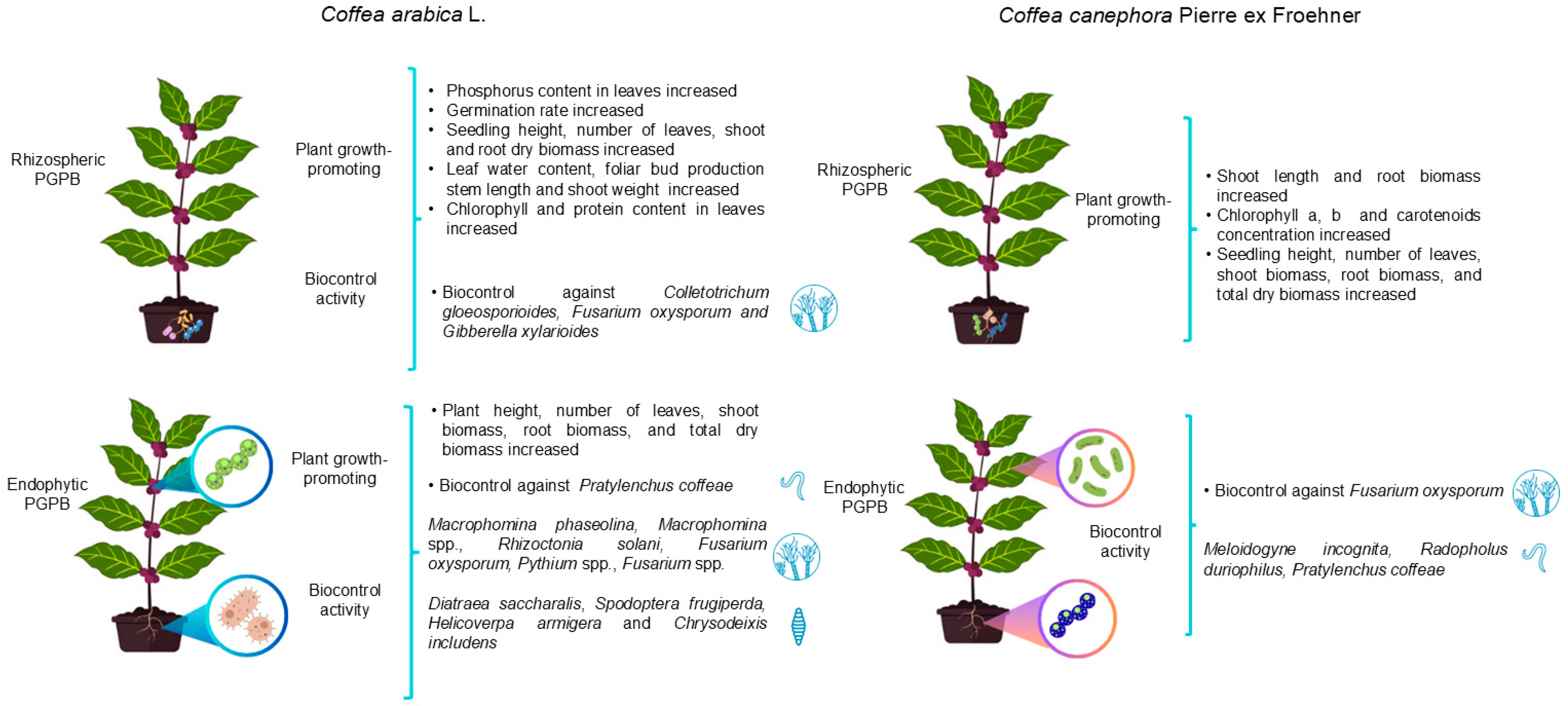
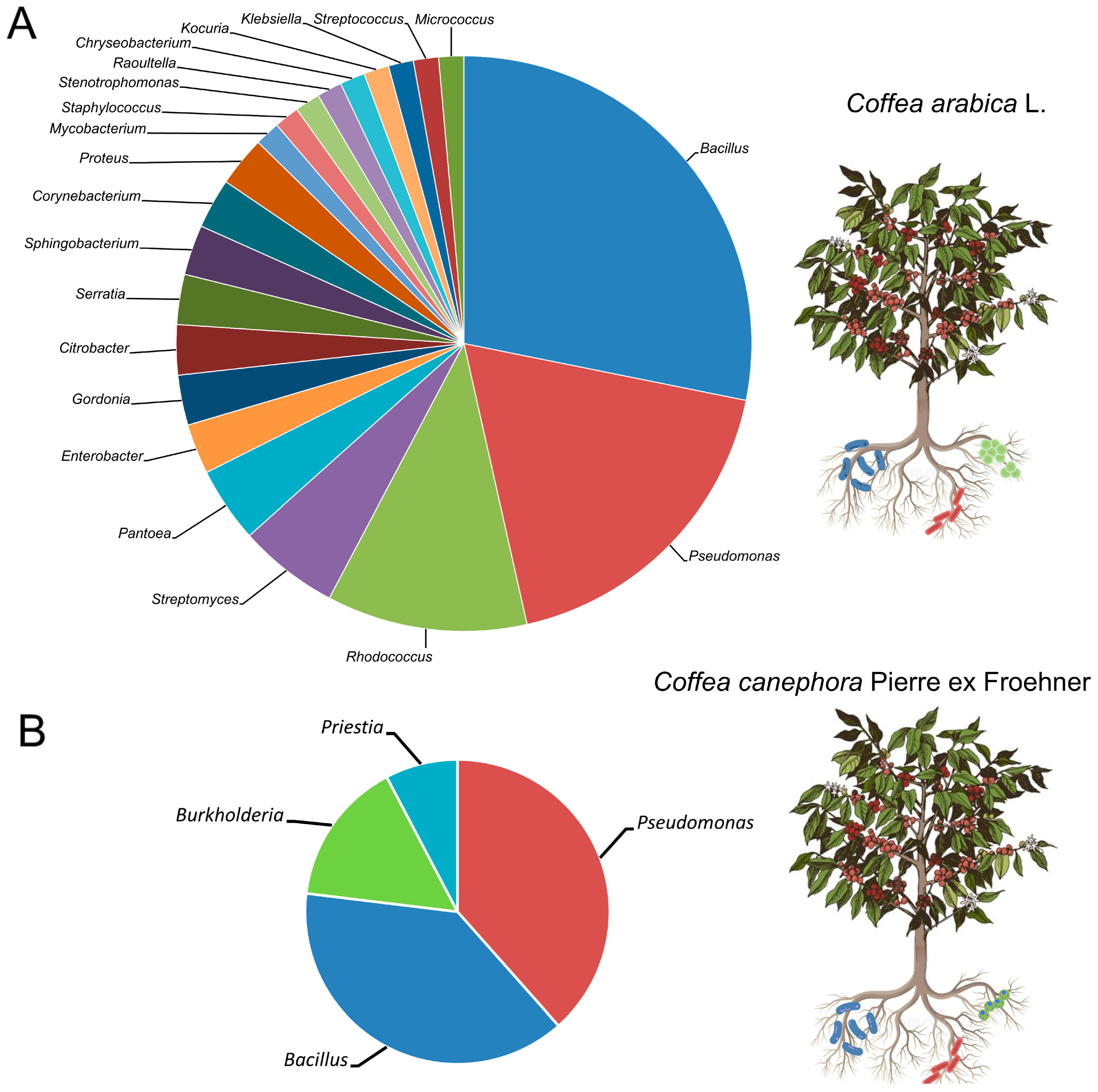
3. Plant Growth-Promoting Rhizobacteria Associated with Coffea arabica and Coffea canephora
| Genus/Species | Associated Coffee Species | Plant Growth-Promoting Traits | Effect on the Plant | Country | Reference |
|---|---|---|---|---|---|
| Bacillus subtilis Kocuria sp. | Coffea arabica var. caturra | Phosphate solubilization | Phosphorus content in leaves increased Biocontrol activity not assessed | Colombia | [56] |
| Bacillus amyloliquefaciens | Coffea arabica | Phosphate solubilization IAA, siderophore and ammonia (NH3) production ACC deaminase activity Hydrolytic enzyme secretion (chitinase, β-1,3-glucanase, protease and lipase) | Biocontrol of Colletotrichum gloeosporioides and Fusarium oxysporum enhances the germination of infected seeds | India | [41] |
| Pseudomonas spp. | Coffea arabica | Phosphate solubilization | Germination rate, shoot length, shoot and root biomass increased Chlorophyll and protein content in leaves increased Biocontrol activity not assessed | Nepal | [52] |
| Bacillus sp. | Coffea arabica | Phosphate solubilization IAA, NH3 and hydrogen cyanide (HCN) production Biological nitrogen fixation capacity Tolerance to heavy metals (Hg, Cu, Zn, Mn) and salinity. | Germination rate, root growth and seedling vigor increased Biocontrol activity not assessed | Ethiopia | [54] |
| Pseudomonas aeruginosa | Coffea canephora | IAA, gibberellic acid, kinetin and zeatin production | Shoot length and root biomass increased Chlorophyll a, chlorophyll b and carotenoid concentrations increased Inhibition of Fusarium solani F04 and suppression of egg hatching in Meloidogyne spp. | Vietnam | [50] |
| Streptomyces sp. | Coffea arabica | Phosphate and zinc solubilization Hydrolytic enzyme secretion (chitinase, protease and lipase) | Biocontrol against Gibberella xylarioides | Ethiopia | [58] |
| Enterobacter mori Bacillus spp. Pseudomonas gozinkensis | Coffea arabica | IAA-type indolic compound synthesis ACC deaminase activity Siderophore, catalase and exopolysaccharide (EPS) production | Seedling height, number of leaves, shoot biomass, root biomass and total dry biomass increased Biocontrol against Boeremia coffea | Brazil | [59] |
| Pseudomonas spp. Bacillus cereus Pseudomonas koreensis Pseudomonas sp. | Coffea canephora | IAA-type indolic compound synthesis ACC deaminase activity Siderophore, catalase and EPS production | Seedling height, number of leaves, shoot biomass, root biomass and total dry biomass increased Biocontrol against Hemileia vastatrix | Brazil | [59] |
| Serratia spp. Stenotrophomonas sp. Sphingobacterium spp. Raoultella sp. Chryseobacterium sp. Pantoea spp. Pseudomonas spp. | Coffea arabica var. Costa Rica 95 | ACC deaminase activity Phosphate solubilization IAA and siderophore production Biological nitrogen fixation capacity | Promotion of root development, relative foliar water content, aerial biomass and foliar bud production Biocontrol activity not assessed | Mexico | [25] |
4. Endophytic Plant Growth-Promoting Bacteria in Coffea arabica and Coffea canephora
| Genus/Species | Associated Coffee Species | Plant Organ of Isolation | Plant Growth-Promoting Traits | Effect on the Plant | Country | Reference |
|---|---|---|---|---|---|---|
| Bacillus subtilis Bacillus anthracis | Coffea arabica | Root | Proteolytic activity | Biocontrol against Pratylenchus coffeae | Indonesia | [63] |
| Bacillus spp. Burkholderia spp. Luteibacter sp. Kitasatospora sp. Lechevalieria sp. Streptomyces sp. | Coffea canephora | Root | Phosphate solubilization Indolic compounds (IAA-type) synthesis Siderophore, HCN, esterase, lipase, gelatinase and chitinase production | Biocontrol against Fusarium oxysporum, Radopholus duriophilus and Pratylenchus coffeae | Vietnam | [42] |
| Bacillus spp. Curtobacterium spp. Brachybacterium sp. Methylobacterium sp., Paracoccus sp. | Coffea canephora | Seed | Phosphate solubilization Indolic compounds (IAA-type) synthesis Siderophore, HCN, esterase, lipase, gelatinase and chitinase production | Biocontrol against Fusarium oxysporum, Radopholus duriophilus and Pratylenchus coffeae | Vietnam | [42] |
| Enterobacter hormaechei Pseudomonas putida | Coffea arabica | Root | Phosphate solubilization Indolic compounds (IAA-type) synthesis Siderophore, HCN, esterase, catalase and exopolysaccharides production ACC deaminase activity | Plant height, number of leaves, shoot dry biomass, root dry biomass and total dry biomass increased Biocontrol against Hemileia vastatrix and Boeremia coffeae | Brazil | [59] |
| Staphylococcus succinus Lecrercia adecarboxylata | Coffea arabica | Root | Biofilm formation and proteolytic activity | Number of leaves, root dry mass, shoot biomass, stem length, shoot weight and phosphorus content in leaves increased | Colombia | [68] |
5. Possible Roles of Host Filtering, Management and Edaphic Constraints in Shaping Coffea Bacterial Consortia
6. Practical Implementation Challenges of Plant Growth-Promoting Bacteria in Coffee Cultivation
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozzola, M.; Charles, S.; Ferretti, T.; Gerakari, E.; Manson, H.; Rosser, N.; von der Goltz, P. The Coffee Guide; International Trade Centre: Geneva, Switzerland, 2021; pp. 2–10. [Google Scholar]
- Muñoz, C.C.; Cobos, C.A.; Muñoz, J.F. Predicción del rendimiento de cultivos de café: Un mapeo sistemático. Ing. Compet. 2023, 25, e-30513171. [Google Scholar] [CrossRef]
- Worku, M. Production, productivity, quality and chemical composition of Ethiopian coffee. Cogent Food Agric. 2023, 9, 2196868. [Google Scholar] [CrossRef]
- Pohlan, H.; Janssens, J. Growth and production of coffee. In Soils, Plant Growth and Crop Production, 1st ed.; Verheye, W.H., Ed.; Eolss Publishers: Oxford, UK, 2010; pp. 102–134. [Google Scholar]
- de Sousa, L.P.; Guerreiro-Filho, O.; Mondego, J.M.C. The rhizosphere microbiomes of five species of coffee trees. Microbiol. Spectr. 2022, 10, e0044422. [Google Scholar] [CrossRef]
- Jha, S.; Bacon, C.M.; Philpott, S.M.; Méndez, V.E.; Läderach, P.; Rice, R.A. Shade coffee: Update on a disappearing refuge for biodiversity. BioScience 2014, 64, 416–428. [Google Scholar] [CrossRef]
- Lory, J.A. Agricultural Phosphorus and Water Quality. 2018. Available online: https://extension.missouri.edu/publications/g9181 (accessed on 15 October 2024).
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture—Systems at Breaking Point; Synthesis Report; FAO: Rome, Italy, 2021; pp. 2–60. ISBN 978-92-5-135327-1. [Google Scholar]
- Singh, D.; Thapa, S.; Geat, N.; Mehriya, M.L.; Rajawat, M. Biofertilizers: Mechanisms and application. In Biofertilizers; Rakshit, A., Meena, V., Parihar, M., Singh, H., Singh, A., Eds.; Woodhead Publishing: Cambridge, UK, 2021; Volume 1, pp. 151–166. [Google Scholar] [CrossRef]
- Abdelaal, K.; AlKahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Poria, V.; Debiec-Andrzejewska, K.; Fiodor, A.; Lyzohub, M.; Ajijah, N.; Singh, S.; Pranaw, K. Plant growth-promoting bacteria (PGPB) integrated phytotechnology: A sustainable approach for remediation of marginal lands. Front. Plant Sci. 2022, 13, 999866. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.H.; Harris, R.F. The ecology and biogeography of microorganism on plant surfaces. Annu. Rev. Phytopathol. 2000, 38, 145–180. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zhimo, Y.; Biasi, A.; Salim, S.; Feygenberg, O.; Wisniewski, M.; Droby, S. Endophytic microbiome in the carposphere and its importance in fruit physiology and pathology. In Postharvest Pathology; Spadaro, D., Droby, S., Gullino, M., Eds.; Springer: Cham, Switzerland, 2021; Volume 11, pp. 73–88. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent advances in bacterial amelioration of plant drought and salt stress. Biology 2023, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef]
- Kumar, R.; Swapnil, P.; Meena, M.; Selpair, S.; Yadav, B.G. Plant growth-promoting rhizobacteria (PGPR): Approaches to alleviate abiotic stresses for enhancement of growth and development of medicinal plants. Sustainability 2022, 14, 15514. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S. Plant Growth-Promoting Rhizobacteria (PGPR) and their action mechanisms in availability of nutrients to plants. In Phyto-Microbiome in Stress Regulation; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 147–203. [Google Scholar] [CrossRef]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef]
- da Silva, L.C.; Barbosa, C.K.; Franco, K.S., Jr. Evaluation of the effect of Azospirillum brasilense inoculation on arabic coffee seedlings. Coffee Sci. 2020, 15, 1–3. [Google Scholar] [CrossRef]
- Svolacchia, N.; Sabatini, S. Cytokinins. Curr. Biol. 2023, 33, R1–R15. [Google Scholar] [CrossRef]
- Palberg, D.; Kisiała, A.; Jorge, G.L.; Emery, R.J.N. A survey of Methylobacterium species and strains reveals widespread production and varying profiles of cytokinin phytohormones. BMC Microbiol. 2022, 22, 49. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Gamalero, E.; Lingua, G.; Glick, B.M. Ethylene, ACC, and the plant growth-promoting enzyme ACC deaminase. Biology 2023, 12, 1043. [Google Scholar] [CrossRef]
- Moon, Y.S.; Ali, S. A fruitful decade of bacterial ACC deaminase biotechnology: Apragmatic approach towards abiotic stress relief in plants. Theor. Exp. Plant Physiol. 2022, 34, 109–129. [Google Scholar] [CrossRef]
- Jasso-Arreola, Y.; Ibarra, J.A.; Rosas-Cárdenas, F.d.F.; Estrada-de los Santos, P. Beneficial effects of ACC deaminase-producing rhizobacteria on the drought stress resistance of Coffea arabica L. Plants 2025, 14, 1084. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil. Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Schwember, A.R.; Schulze, J.; del Pozo, A.; Cabeza, R.A. Regulation of symbiotic nitrogen fixation in legume root nodules. Plants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.J.; Conde, D.; Perron, N.; Schmidt, H.; Dervinis, C.; Venado, R.; Ané, J.M.; Kirst, M. Investigating biological nitrogen fixation via single-cell transcriptomics. J. Exp. Bot. 2025, 76, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.I.d.; Pereira, M.C.; Carvalho, A.M.X.d.; Buttrós, V.H.; Pasqual, M.; Dória, J. Phosphorus-solubilizing microorganisms: A key to sustainable agriculture. Agriculture 2023, 13, 462. [Google Scholar] [CrossRef]
- Hii, Y.S.; San, C.Y.; Lau, S.W.; Danquah, M.K. Isolation and characterisation of phosphate solubilizing microorganisms from peat. Biocatal. Agric. Biotechnol. 2020, 26, 101643. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Flores, A.; Rojas-Sánchez, B.; Urtis-Flores, C.A.; Morales-Cedeño, L.R.; Valencia-Marin, M.F.; Chávez-Avila, S.; Rojas-Solis, D.; Santoyo, G. Plant growth-promoting bacteria as bioinoculants: Attributes and challenges for sustainable crop improvement. Agronomy 2021, 11, 1167. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S. Phosphate-solubilizing microorganisms: Mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 2020, 21, 49–68. [Google Scholar] [CrossRef]
- Pan, L.; Cai, B. Phosphate-solubilizing bacteria: Advances in their physiology, molecular mechanisms and microbial community effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Sahgal, M.; Sharma, K.; Enshasy, H.A.E.; Gafur, A.; Alfarraj, S.; Ansari, M.J.; Sayyed, R.Z. Optimization and identification of siderophores produced by Pseudomonas monteilii strain MN759447 and its antagonism toward fungi associated with mortality in Dalbergia sissoo plantation forest. Front. Plant Sci. 2022, 13, 984522. [Google Scholar] [CrossRef]
- Pandey, S.S. The role of iron in phytopathogenic microbe–plant interactions: Insights into virulence and host immune response. Plants 2023, 12, 3173. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore—A boon to agricultural sciences. Biol. Control 2020, 144, 104214. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Jung, H.; Bon, K.; Jeong, A.; Lee, H. Exploiting bacterial genera as biocontrol agents: Mechanisms, interactions and applications in sustainable agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Sritongon, N.; Boonlue, S.; Mongkolthanaruk, W.; Jogloy, S.; Riddech, N. The combination of multiple plant growth promotion and hydrolytic enzyme-producing rhizobacteria and their effect on Jerusalem artichoke growth improvement. Sci. Rep. 2023, 13, 5917. [Google Scholar] [CrossRef]
- Reddy, E.; Reddy, G.; Goudar, V.; Sriramula, A.; Swarnalatha, G.; Tawaha, A.; Sayyed, R. Hydrolytic enzyme-producing plant growth-promoting rhizobacteria (PGPR) in plant growth promotion and biocontrol. In Secondary Metabolites and Volatiles of PGPR in Plant-Growth Promotion; Sayyed, R.Z., Uarrota, V.G., Eds.; Springer: Cham, Switzerland, 2022; pp. 303–310. [Google Scholar] [CrossRef]
- Kejela, T.; Thakkar, V.R.; Thakor, P. Bacillus species (BT42) isolated from Coffea arabica L. rhizosphere antagonizes Colletotrichum gloeosporioides and Fusarium oxysporum and also exhibits multiple plant growth promoting activity. BMC Microbiol. 2016, 16, 277. [Google Scholar] [CrossRef]
- Duong, B.; Nguyen, H.X.; Phan, H.V.; Colella, S.; Trinh, P.Q.; Hoang, G.T.; Nguyen, T.T.; Marraccini, P.; Lebrun, M.; Duponnois, R. Identification and characterization of Vietnamese coffee bacterial endophytes displaying in vitro antifungal and nematicidal activities. Microbiol. Res. 2021, 242, 126613. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S.; Glick, B.R. Hydrogen cyanide production by soil bacteria: Biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 2022, 32, 15–38. [Google Scholar] [CrossRef]
- Muleta, D.; Assefa, F.; Granhall, U. In vitro antagonism of rhizobacteria isolated from Coffea arabica L. against emerging fungal coffee pathogens. Eng. Life Sci. 2007, 7, 577–586. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic Microbiol. 2020, 60, 828–861. [Google Scholar] [CrossRef]
- Castillo, N.E.T.; Acosta, Y.A.; Parra-Arroyo, L.; Martínez-Prado, M.A.; Rivas-Galindo, V.M.; Iqbal, H.M.N.; Bonaccorso, A.D.; Melchor-Martínez, E.M.; Parra-Saldívar, R. Towards an eco-friendly coffee rust control: Compilation of natural alternatives from a nutritional and antifungal perspective. Plants 2022, 11, 2745. [Google Scholar] [CrossRef]
- Mihai, R.A.; Ortiz-Pillajo, D.C.; Iturralde-Proano, K.M.; Vinueza-Pullotasig, M.Y.; Sisa-Tolagasí, L.A.; Villares-Ledesma, M.L.; Melo-Heras, E.J.; Cubi-Insuaste, N.S.; Catana, R.D. Comprehensive assessment of coffee varieties (Coffea arabica L.; Coffea canephora L.) from Coastal, Andean, and Amazonian Regions of Ecuador; a holistic evaluation of metabolism, antioxidant capacity and sensory attributes. Horticulturae 2024, 10, 200. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E. The role of plant growth promoting rhizobacteria in plant drought stress responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef]
- Mmotla, K.; Sibanyoni, N.; Allie, F. Exploring the intricacies of plant growth promoting rhizobacteria interactions: An omics review. Ann. Microbiol. 2025, 75, 5. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Wang, S.-L.; Nguyen, A.D.; Doan, M.D.; Tran, D.M.; Nguyen, T.H.; Ngo, V.A.; Doan, C.T.; Tran, T.N.; Do, V.C. Potential application of rhizobacteria isolated from the Central Highland of Vietnam as an effective biocontrol agent of robusta coffee nematodes and as a bio-fertilizer. Agronomy 2021, 11, 1887. [Google Scholar] [CrossRef]
- Suharjono, S.; Yuliatin, E. Bacteria communities of coffee plant rhizosphere and their potency as plant growth promoting. Biodiversitas 2022, 23, 5822–5834. [Google Scholar] [CrossRef]
- Kunwar, V.; Chimouriya, S.; Lamichhane, J. Isolation and characterization of phosphate solubilizing bacteria from rhizosphere of coffee plant and evaluating their effects on growth and development of coffee seedlings. Biotechnol. Ind. J. 2018, 14, 173. [Google Scholar]
- Navarro, G.V.D.; Quirong, D.D.; Maghanoy, G.A.; Cortes, A.D. Characterization and identification of rhizobacteria associated with Liberica and Robusta coffee rhizosphere. Technol. Hortic. 2023, 3, 24. [Google Scholar] [CrossRef]
- Abawari, R.A.; Tuji, F.A.; Yadete, D.M. Multi traits of phosphate solublizing bacterial and fungal isolates and evaluation of their potential as biofertilizer agent for coffee production. Int. J. Appl. Agric. Sci. 2021, 7, 1–15. [Google Scholar] [CrossRef]
- Teshome, B.; Wassie, M.; Abatneh, E. Isolation, screening and biochemical characterization of phosphate-solubilizing rhizobacteria associated with Coffea arabica L. J. Fertil. Pestic. 2017, 8, 188. [Google Scholar] [CrossRef]
- Cisneros, C.A.; Menjivar, J.C. Influencia de microorganismos solubilizadores de fósforo del suelo y su absorción por plántulas de café. Bioagro 2016, 28, 95–106. [Google Scholar]
- Satyaprakash, M.; Nikitha, T.; Chaitanya, I.; Reddi, E.U.B. Phosphate solubilizing rhizobacteria with Coffea arabica L. in coffee plantations of north eastern Ghats, Visakhapatnam District, Andhara Pradesh, India. Int. J. Curr. Res. 2017, 9, 49779–49783. [Google Scholar]
- Nuguse, M.; Kejela, T. Actinomycetes isolated from rhizosphere of wild Coffea arabica L. showed strong biocontrol activities against coffee wilt disease. PLoS ONE 2024, 19, e0306837. [Google Scholar] [CrossRef]
- de Souza, M.P.P.; Pereira, M.A.; Braghini, M.T.; de Sousa, L.; Costa, J.M.; Alves, F.R.; da Silveira, A.P. Beneficial bacteria improve seedling growth and nutrition and promote biological control of coffee diseases. J. Appl. Microbiol. 2025, 136, lxaf050. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Nguyen, T.T.H.; Nguyen, T.D.; Le, T.A.; Pham, T.T.V.; Dam, T.T.H.; Nguyen, T.H. Isolation and identification of IAA-producing rhizobacteria from Robusta coffee plantations in Dak Nong province. Vietnam J. Sci. Technol. Eng. 2025. [Google Scholar] [CrossRef]
- Ali, M.A.; Ahmed, T.; Ibrahim, E.; Rizwan, M.; Chong, K.P.; Yong, J.W.H. A review on mechanisms and prospects of endophytic bacteria in biocontrol of plant pathogenic fungi and their plant growth-promoting activities. Heliyon 2024, 10, e31573. [Google Scholar] [CrossRef]
- da Silva Bandeira, O.N.; da Silva Bandeira, R.; de Souza, C.R.B. Systematic review and meta-analysis of the potential effects of endophytic bacteria Klebsiella on plant growth promotion and biocontrol of pathogens. World J. Microbiol. Biotechnol. 2025, 41, 89. [Google Scholar] [CrossRef]
- Asyiah, I.; Soekarto, S.; Husain, M.; Hindersah, R.; Mudakir, I.; Narulita, E.; Nurasyiah, I. The endophytic bacteria isolation as biological control agent of Pratylenchus coffeae. Asian J. Microbiol. Biotechnol. Environ. Sci. 2018, 20, 165–171. [Google Scholar]
- Hoang, H.; Tran, L.H.; Nguyen, T.H.; Nguyen, D.A.T.; Nguyen, H.H.T.; Pham, N.B.; Trinh, P.Q.; de Boer, T.; Brouwer, A.; Chu, H.H. Ocurrence of endophytic bacteria in Vietnamese Robusta coffee roots and their effects on plant parasitic nematodes. Symbiosis 2020, 80, 75–84. [Google Scholar] [CrossRef]
- Pratiwi, E.R.; Ardyati, T.; Suharjono, S. Plant growth-promoting endophytic bacteria of Coffea canephora and Coffea arabica L. in UB Forest. J. Exp. Life Sci. 2020, 10, 119–126. [Google Scholar] [CrossRef]
- Sharan, K.; Aswin, A.N.; Rajan, S.S. The distinctive endophytic bacterial isolates obtained from offee (Coffea arabica L.) RCRS, Thandigudi. Int. J. Agric. Environ. Biotechnol. 2022, 15, 753–763. [Google Scholar] [CrossRef]
- de Castro, M.T.; de Lima Ferreira, A.D.D.; do Nascimento, I.N.; Rocha, G.T.; Celestino, M.F.; Freire, Í.A.; Moreira, I.C.; Gomes, G.C.; dos Reis Cunha, B.; Linhares, S.C.; et al. Endophytic Bacillus spp. of coffee plants (Coffea arabica L.) and its potential in the biocontrol of phytopathogenic fungi and Lepidoptera larvae. Egypt. J. Biol. Pest Control 2025, 35, 8. [Google Scholar] [CrossRef]
- Ramos-Cabrera, E.V.; Delgado-Espinosa, Z.Y.; Murillo-Muñoz, R.A.; Muñoz-Diaz, V.E.; Hoyos-García, J. Evaluación de bacterias endofíticas solubilizadoras de fósforo en café, una alternativa sostenible. Biotecnol. Sect. Agropecu. Agroind. 2021, 19, 94–107. [Google Scholar] [CrossRef]
- Ogata-Gutierrez, K.; Chumpitaz-Segovia, C.; Lirio-Paredes, J.; Zuniga-Davila, D. Antifungal activity of phyllospheric bacteria isolated from Coffea arabica against Hemileia vastatrix. Microorganisms 2024, 12, 582. [Google Scholar] [CrossRef]
- Asad, S.; Priyashantha, A.K.H.; Tibpromma, S.; Luo, Y.; Zhang, J.; Fan, Z.; Zhao, L.; Shen, K.; Niu, C.; Lu, L.; et al. Coffee-associated endophytes: Plant growth promotion and crop protection. Biology 2023, 12, 911. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of microbial inoculants by encapsulation in natural polysaccharides: Focus on beneficial properties of carrier additives and derivatives. Front. Plant Sci. 2020, 11, 270. [Google Scholar] [CrossRef]
- Liu, X.; Mei, S.; Salles, J.F. Inoculated microbial consortia perform better than single strains in living soil: A meta-analysis. App. Soil Ecol. 2023, 190, 105011. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 an (EC) No 1107/2009. EUR-Lex-Access to European Union Law. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj/eng?utm_source (accessed on 20 October 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-López, M.; Bautista-Cruz, A.; Toledo-López, A.; Aquino-Bolaños, T. Rhizospheric and Endophytic Plant Growth-Promoting Bacteria Associated with Coffea arabica L. and Coffea canephora Pierre ex Froehner: A Review of Their Agronomic Potential. Microorganisms 2025, 13, 2567. https://doi.org/10.3390/microorganisms13112567
Ramírez-López M, Bautista-Cruz A, Toledo-López A, Aquino-Bolaños T. Rhizospheric and Endophytic Plant Growth-Promoting Bacteria Associated with Coffea arabica L. and Coffea canephora Pierre ex Froehner: A Review of Their Agronomic Potential. Microorganisms. 2025; 13(11):2567. https://doi.org/10.3390/microorganisms13112567
Chicago/Turabian StyleRamírez-López, Marisol, Angélica Bautista-Cruz, Arcelia Toledo-López, and Teodulfo Aquino-Bolaños. 2025. "Rhizospheric and Endophytic Plant Growth-Promoting Bacteria Associated with Coffea arabica L. and Coffea canephora Pierre ex Froehner: A Review of Their Agronomic Potential" Microorganisms 13, no. 11: 2567. https://doi.org/10.3390/microorganisms13112567
APA StyleRamírez-López, M., Bautista-Cruz, A., Toledo-López, A., & Aquino-Bolaños, T. (2025). Rhizospheric and Endophytic Plant Growth-Promoting Bacteria Associated with Coffea arabica L. and Coffea canephora Pierre ex Froehner: A Review of Their Agronomic Potential. Microorganisms, 13(11), 2567. https://doi.org/10.3390/microorganisms13112567







