Abstract
The rise of multi-drug-resistant (MDR) bacteria poses a severe global threat to public health, necessitating the development of innovative therapeutic strategies to overcome these challenges. Copper-based nanomaterials have emerged as promising agents due to their intrinsic antibacterial properties, cost-effectiveness, and adaptability for multifunctional therapeutic approaches. These materials exhibit exceptional potential in advanced antibacterial therapies, including chemodynamic therapy (CDT), photothermal therapy (PTT), and photodynamic therapy (PDT). Their unique physicochemical properties, such as controlled ion release, reactive oxygen species (ROS) generation, and tunable catalytic activity, enable them to target MDR bacteria effectively while minimizing off-target effects. This paper systematically reviews the mechanisms through which Cu-based nanomaterials enhance antibacterial efficiency and emphasizes their specific performance in the antibacterial field. Key factors influencing their antibacterial properties—such as electronic interactions, photothermal characteristics, size effects, ligand effects, single-atom doping, and geometric configurations—are analyzed in depth. By uncovering the potential of copper-based nanomaterials, this work aims to inspire innovative approaches that improve patient outcomes, reduce the burden of bacterial infections, and enhance global public health initiatives.
1. Introduction
Antibiotic resistance has emerged as a global health crisis, posing significant challenges to modern medicine and scientific research [1,2]. The increasing prevalence of bacterial infections, coupled with the declining efficacy of traditional antibiotics, has led to an alarming surge in antibiotic-resistant strains. The misuse and overuse of antibiotics, once the mainstay of treatment for bacterial infections, have accelerated the emergence of multi-drug-resistant pathogens or “superbugs”. These pathogens are resistant to multiple classes of antibiotics, rendering conventional treatments ineffective and resulting in untreatable infections, increased healthcare costs, and escalating mortality rates [3,4,5]. With once-reliable antibiotics losing their potency, the urgent need for alternative antibacterial strategies has never been more critical [6,7].
In response to this escalating threat, nanomaterials have emerged as promising candidates in the next generation of antibacterial therapies. Unlike traditional antibiotics, nanomaterials provide distinct advantages through their multifaceted mechanisms of action, particularly against drug-resistant bacterial infections [8,9,10] (Table 1). Among the various nanomaterials under investigation, copper-based nanomaterials have garnered significant attention due to their broad-spectrum antibacterial activity [11,12,13]. These materials mitigate the risk of resistance development by targeting bacteria through multiple pathways, including the disruption of cell membranes, the generation of reactive oxygen species (ROS), and interference with vital enzyme functions. Specifically, they disrupt bacterial cell membranes, generate reactive oxygen species, and interfere with critical enzyme functions. This multipronged strategy positions copper-based nanomaterials as a powerful and versatile solution to the growing challenge of antibiotic resistance [14,15,16].

Table 1.
Studies on the application of nanomaterials against drug-resistant bacteria in recent years.
In recent years, copper-based nanomaterials have been widely explored not only for antibacterial applications but also in tumor therapies (Figure 1) [33,34,35]. Despite the wide range of applications of copper nanomaterials in various fields in dental water treatment [36,37,38], there are still a limited number of relevant reviews on how to optimize the antibacterial efficiency of copper nanomaterials. Furthermore, emerging antibacterial strategies utilizing copper-based nanomaterials, such as chemodynamic therapy (CDT) [39,40], photothermal therapy (PTT) [41,42], and photodynamic therapy (PDT) [43], offer new opportunities to combat resistant pathogens. These advancements underscore the critical need to comprehensively summarize recent advances in copper-based nanomaterials for antibacterial purposes.
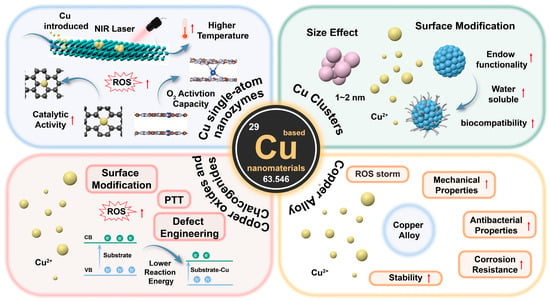
Figure 1.
Schematic illustration of antibacterial therapy applications based on construction of copper nanostructured materials. The red arrows represent an upward trend.
This review aims to address this gap by focusing on the mechanisms through which copper-based materials enhance antibacterial efficiency, their diverse applications in combating bacterial infections, and, more specifically, the role of copper oxide and copper sulfide in antibacterial therapy. Additionally, we delve into key factors influencing the antibacterial performance of these materials, including electronic interactions, photothermal properties, size effects, ligand modifications, single-atom doping, and geometric configurations. By unlocking the potential of copper-based nanomaterials, this review seeks to expand their applications in addressing drug-resistant pathogens, ultimately improving patient outcomes and advancing global public health.
2. Key Antibacterial Targets: Membrane Integrity, ROS, Metabolism/Enzymes, DNA, and Signaling Pathways
Understanding the pathways through which nanomaterials exert their antibacterial effects is essential in the rational design and development of next-generation therapeutic agents. These mechanisms can be categorized into five principal pathways: the disruption of bacterial cell membrane integrity, the generation of reactive oxygen species (ROS), interference with bacterial metabolism and enzyme function, DNA damage, and disruption to bacterial signaling pathways. Each pathway induces specific biochemical and structural disruptions, inhibiting bacterial proliferation or causing cell death (Figure 2).
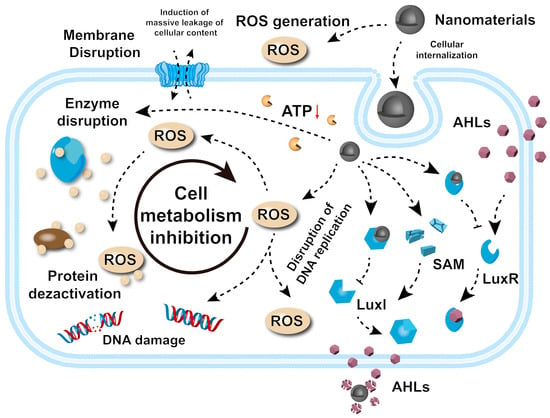
Figure 2.
Antibacterial mechanisms behind nanomaterials. The red arrow represents a downward trend.
2.1. Membrane Disruption
The destruction of bacterial membranes by nanomaterials represents a key antibacterial mechanism. The process is initiated by electrostatic interactions between positively charged nanoparticles and negatively charged components of the bacterial membrane, such as phospholipids and lipopolysaccharides in Gram-negative bacteria. These interactions bring nanoparticles into close proximity with the cell membrane, enabling further interactions. Upon contact, nanoparticles can penetrate the lipid bilayer, causing physical damage such as puncturing or thinning and compromising membrane integrity [44,45,46]. This leads to an uncontrolled flow of ions and molecules across the membrane, disrupting cellular homeostasis and function. In addition, nanoparticles may interact with and denature key membrane proteins, further exacerbating membrane instability [47]. These combined effects result in membrane collapse, the leakage of intracellular components (e.g., DNA, RNA, proteins), and eventual bacterial death. This mechanism is particularly effective against drug-resistant strains, positioning nanomaterials as potent alternatives to traditional antibiotics.
Notably, Stefan Howorka’s team has pioneered the development of cholesterol-modified DNA nanostructures, negatively charged nanostructures that preferentially bind to cholesterol-deficient bacterial membranes and attach to the membrane surface by aggregating to form barrel-like clusters, which triggers the rupture of the membrane, leading to cell death [48]. This selective disruption of bacterial membranes provides an idea for the development of novel antimicrobial agents, especially for antibiotic resistance, and small molecule or polymer drugs may be designed in the future by mimicking this principle.
2.2. Generation of Reactive Oxygen Species (ROS)
ROS have attracted attention for their broad-spectrum, efficient, and long-lasting bactericidal effects. ROS, including superoxide anions (O2−), hydroxyl radicals (·OH), and hydrogen peroxide (H2O2), are highly reactive molecules capable of inflicting extensive oxidative damage on bacterial cells, ultimately leading to cell death [49,50]. Nanomaterials facilitate ROS generation through several mechanisms. For example, titanium dioxide (TiO2) nanoparticles, when activated by UV light, form electron–hole pairs that catalyze surface redox reactions to produce ROS [51,52,53]. Similarly, silver nanoparticles (Ag) promote electron transfer from bacterial membrane components to molecular oxygen, generating O2− and disrupting bacterial respiration [54,55]. Iron oxide nanoparticles (Fe3O4) participate in Fenton and Fenton-like reactions, where Fe2+ reacts with H2O2 to generate ·OH, one of the most bactericidal potent ROS [56,57,58]. The resulting ROS damage bacterial DNA, proteins, and lipids. Lipid peroxidation impairs membrane integrity, increasing permeability and causing the leakage of bacterial contents [59]. ROS-induced DNA damage, including strand breaks and base modifications, disrupts replication and transcription, further inhibiting bacterial survival.
Yu et al. have developed an ROS nanogenerator (Fe-HMME@DHA@MPN) activated by ultrasound and gastric acid, which can effectively kill drug-resistant bacteria [60]. The nanogenerator utilizes the Fenton reaction to generate ROS and self-supplies hydrogen peroxide through encapsulated DHA to ensure the continuous generation of ROS in the absence of exogenous H2O2. The antibacterial mechanism and adhesion properties of ROS also make it highly capable of destroying the biofilm, which in turn kills drug-resistant H. pylori and destroys its biofilm. These multifaceted mechanisms make ROS-generating nanomaterials highly effective against antibiotic-resistant strains, highlighting their potential in antibacterial therapies.
2.3. Interference with Bacterial Metabolism and Enzyme Function
Nanomaterials can disrupt bacterial metabolic processes and enzymatic functions, primarily through metal-catalyzed oxidation and metal ion toxicity. In E. coli, oxidative damage often targets specific amino acids near metal-binding sites, leading to protein carbonylation and degradation [61,62]. Metalloenzymes, such as peptide deformylase (PDF) and threonine dehydrogenase (Tdh), are particularly susceptible to oxidative damage catalyzed by Fe2+, though alternative metals such as Mn2+ can mitigate this damage [63]. In addition, toxic metal ions, such as Cu+, disrupt Fe-S cluster-containing enzymes essential in bacterial metabolism, inhibiting their activity and bacterial growth [64]. Other ions, including Ag+ and Cd2+, inactivate Fe-S enzymes, though bacterial repair mechanisms can sometimes restore their function [65]. Metal substitution, such as Pb2+ replacing Zn2+ in δ-aminolaevulinic acid dehydrogenase (ALAD) or Ag+ replacing Cu2+ in superoxide dismutase, also inhibits enzyme function, preventing bacterial proliferation [66,67].
Furthermore, the researchers developed a gastric acid-responsive nanocarrier, CAMO, which inhibited Helicobacter pylori urease activity through the acid-triggered release of silver ions (Ag+), reduced bacterial resistance, and synergistically enhanced the antibacterial effect of clarithromycin [30]. At the same time, the outer probiotic layer reduces inflammation by targeting and protecting gastric epithelial cells, carrying out the dual mechanism of antibiotic potentiation and host protection, which provides a new strategy for precise delivery and synergistic therapy for the treatment of drug-resistant bacterial infections.
2.4. DNA Damage
Nanomaterials exhibit potent antibacterial effects by inducing bacterial DNA damage through direct and indirect mechanisms [68]. For instance, Ag nanomaterials penetrate bacterial cells and bind to DNA, causing structural damage such as strand breaks, cross-linking, and adduct formation, thereby disrupting critical processes such as replication and transcription [69]. Nanomaterials also inhibit DNA repair enzymes, including polymerases, helicases, and ligases, impairing the ability of cells to repair DNA damage and exacerbating its effects [70]. Additionally, E-CuSe inhibits the self-repair ability of bacteria by down-regulating DNA repair-related genes (such as RecA, UvrD, etc.), meaning that bacteria are unable to complete the dynamic repair of DNA damage caused by copper ion attack and eventually die due to the cumulative irreversible damage. This gene regulation mechanism cooperates with the physical effect of E-CuSe to destroy the bacterial cell membrane, breaking through the defense threshold of drug-resistant bacteria and significantly enhancing the antibacterial efficiency [45]. Collectively, these mechanisms make nanoparticles particularly effective against resistant bacteria, offering a robust alternative to conventional antibiotics.
2.5. Disruption of Bacterial Signaling (Quorum Sensing System)
Quorum sensing (QS) is a bacterial communication system that regulates physiological processes such as biofilm formation, virulence factor production, motility, and drug resistance mechanisms [71,72]. Nanomaterials have been found to disrupt QS, hindering bacterial collaboration and impairing their ability to coordinate essential functions. These disruptions can be achieved through various mechanisms: (I) the inhibition of signal molecule production (auto-inducible factors)—nanomaterials interfere with bacterial metabolic pathways involved in the synthesis of autoinducer molecules, reducing the overall signaling capacity [73]; (II) signal quenching—nanoparticles bind directly to signaling molecules, such as autoinducers, neutralizing their activity and preventing them from interacting with QS receptors [74,75]; and (III) receptor blocking—nanomaterials can obstruct QS receptors, inhibiting the detection and response to signaling molecules [76]. By disrupting QS, nanomaterials inhibit biofilm formation, reduce virulence factor production, and enhance the susceptibility of bacteria to antibacterial agents.
Notably, such mechanisms have been validated in several studies [77]. For example, researchers synthesized Zn-Nx-C nanomaterials with endostatin-like activity and demonstrated their ability to degrade AHL signaling molecules, interfere with the QS signaling pathway, and inhibit biofilm formation in a multi-drug-resistant Pseudomonas aeruginosa model. The Zn-Nx-C coating was also effective in inhibiting biofilm formation on the surface of hull materials in an aqueous environment. After 30 days of exposure in the Suhang Grand Canal, the coating achieved 80.3% biofilm inhibition efficiency. These properties make nanomaterials a promising strategy for combating drug-resistant infections and minimizing bacterial pathogenicity [78].
3. Cu-Based Nanomaterials for Biocatalytic Antibacterial Applications
In recent years, copper-based nanomaterials have demonstrated breakthrough research progress in the field of antibacterial therapeutics due to their unique compositional and structural features, providing an important scientific basis for the development of novel antibacterial strategies against drug-resistant bacterial infections [79,80,81]. In this subsection, we will systematically review recent research progress in copper-based antimicrobial nanomaterials with different chemical coordination environments and compositional features, from copper single-atom systems precisely tuned on the atomic scale (Cu-N3, Cu/PMCs) to copper nanoclusters with quantum size effects (Cu8NC, CuCs) and from redox-active copper oxides (Cu5.4O) to narrow bandgap-characterized copper sulfur compounds (CuS, Cu2−xSe), as well as other copper-doped nanomaterials. The research focuses on the analysis of the conformational relationships of the nanomaterials and the mechanism behind the antimicrobial effect, which will provide theoretical support for the rational design of new nano-antibacterial agents.
3.1. Cu Single-Atom Nanozymes
Single-atom nanomaterials (SACs) represent an innovative class of catalysts where individual metal atoms are dispersed on suitable support materials, maximizing their atomic efficiency and catalytic activity [82,83,84,85]. These cutting-edge materials have gained significant attention for diverse applications. SACs exhibit broad-spectrum antibacterial activity, including potent effects against multi-drug-resistant strains, positioning them as promising candidates to address the escalating issue of antibiotic resistance. They exhibit rapid and effective bactericidal action by disrupting bacterial cell walls, membranes, and genetic materials [41,86,87,88]. SACs are classified based on the metal type and the support structure, which can range from carbon-based materials [89,90,91] to metal oxides [92,93] and other functional nanostructures [94,95]. Among these, Cu SACs hold particular promise due to their remarkable redox activity and photothermal properties.
The rational design of nanozymes hinges on sophisticated strategies, with coordination engineering and spatial configuration optimization emerging as critical approaches to enhancing their catalytic efficiency and antibacterial performance. Coordination engineering, as a pivotal strategy, refines the coordination number and ligand environment around Cu atoms to boost POD-like activity (Figure 3a), facilitating efficient H2O2 decomposition into ·OH (Figure 3b) [96]. Cu SACs with lower coordination numbers (e.g., CuN3) exhibit superior H2O2 adsorption and dissociation compared to their CuN4 counterparts, resulting in enhanced ROS generation and antibacterial activity. Furthermore, tailored coordination structures significantly improve the structural stability and catalytic performance of SACs, ensuring adaptability to diverse reaction conditions, such as variations in energy radiation, pH, and temperature. Beyond coordination optimization, the spatial modulation of Cu atom positioning further elevates catalytic performance [88]. A recent study systematically evaluated Cu SACs with dual Cu sites on poly (heptazine imide) (PHI) nanoplatforms, comparing interlayer (CuL/PHI) and in-plane (CuP/PHI) configurations (Figure 3c). The results demonstrated that interlayer localization (CuL/PHI) notably enhances photoinduced electron transfer, O2 activation (Figure 3d), and ROS generation, achieving near-complete bactericidal activity under visible light. Notably, SACs with interlayer CuL/PHI exhibited superior antibacterial efficacy and stability over their in-plane counterparts. These findings underscore the synergistic roles of coordination engineering and atomic spatial configuration in advancing the rational design of nanozymes, offering valuable insights for biomedical applications.
Building on these advancements, recent studies have highlighted the potential of Cu doping to enhance the photothermal properties of nanomaterials, opening up new avenues for performance optimization [97,98,99]. Liu et al. demonstrated that Cu doping significantly amplifies the photothermal effect of PMCS by introducing spin–orbit coupling and d-orbital transitions (Figure 3e) [100]. These transitions facilitate energy release via inter-system crossing rather than emission. Additionally, Cu doping generates impurity energy levels that enhance dielectrophoretic absorption and induce localized surface plasmon resonance (LSPR). As a result, Cu/PMCS exhibits plasmon generation at 808 nm, a feature absent in pure PMCS (Figure 3f). Simulations further confirmed that Cu/PMCS achieves notable absorption and higher thermal output at 808 nm, attributed to the LSPR effect (Figure 3g).
Following the progress made with carbon-based copper single-atom catalysts, attention has turned to metal oxide-supported systems, such as copper anchored on ceria, which exhibit distinct advantages in catalytic and antibacterial applications [93,101,102]. Anchoring copper onto CeO2 enhances POD-like activity, as elucidated by theoretical calculations (Figure 3h,i), which reveal the mechanism behind Cu single sites promoting ROS generation [92]. Specifically, the Cu-sub configuration induces structural modifications that activate adjacent Ce sites by altering local coordination and oxygen alkalinity, thereby stabilizing reaction intermediates. Energy analyses indicate that Cu single sites markedly lower the potential determining step (PDS) energy for POD reactions, with the diCe6c@Cu-sub site exhibiting the lowest energy barrier (0.970 eV). Simultaneously, Cu single sites suppress HORAC activity by raising PDS energies at most sites. This dual effect of enhancing ROS generation while inhibiting its elimination highlights the critical role of Cu in boosting the catalytic efficiency of Cu-CeO2 systems (Figure 3j).
The exploration of nanozyme design has expanded significantly, focusing on three key strategies to optimize catalytic and antibacterial performance: coordination engineering and spatial configuration optimization, doping-induced photothermal enhancements, and the integration of metal oxide supports. These approaches not only deepen our understanding of catalytic mechanisms but also pave the way for practical applications.
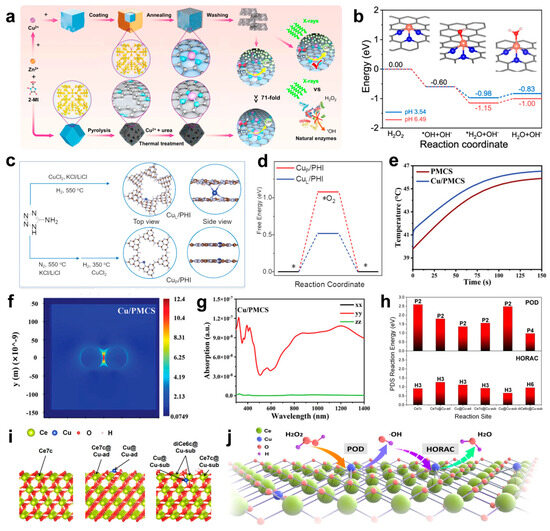
Figure 3.
(a) Schematic diagram of the synthesis strategy of CuN3-SAzyme and CuN4-SAzyme. 2-methylimidazole: 2-MI. (b) Calculated energy profiles for the dissociation of the H2O2 molecule on CuN3-SAzyme at two experimental pH values. Note that these produced ·OH radicals are very easily involved in reactions around them, which can lead to a further decrease in the energy of the whole system. Cu, orange; C, gray; N, blue; O, red; H, pink. (c) Scheme of the synthesis of CuL/PHI and CuP/PHI, N (gray), Cu (blue), H (white), and C (brown). An asterisk (*) represents the surface position of the adsorption. (d) DFT calculation of free energy of O2 adsorption (ΔG*O2) for CuP/PHI (red) and CuL/PHI (blue). (e) Heating curve under 808 nm NIR irradiation with a power density of 1 W cm−2 for 150 s plotted by simulation. (Cu/PMCS refers to Cu/PMCS-2). (f) Thermal field maps of Cu/PMCS plasmons. (g) Absorption spectra at 808 nm of Cu/PMCS. (h) The calculated PDS reaction energy of POD and HORAC processes for different reaction sites with the exact PDS labeled above the bar. (i) The calculated model of pristine CeO2 (left), CeO2 with Cu3c added on the 3O atoms on the surface (Cu-ad, middle) and CeO2 with a Ce atom substituted by Cu (Cu-sub, right). The six possible reaction sites are highlighted with a tagged arrow. (j) The proposed mechanism of regulation of catalytic activities by Cu-CeO2 single-site nanozyme. (a,b) Reproduced with permission [96]. Copyright 2024, Nature Publishing Group. (c,d) Reproduced with permission [88]. Copyright 2023, WILEY-VCH. (e–g) Reproduced with permission [100]. Copyright 2024, WILEY-VCH. (h–j) Reproduced with permission [92]. Copyright 2024, Nature Publishing Group.
Figure 3.
(a) Schematic diagram of the synthesis strategy of CuN3-SAzyme and CuN4-SAzyme. 2-methylimidazole: 2-MI. (b) Calculated energy profiles for the dissociation of the H2O2 molecule on CuN3-SAzyme at two experimental pH values. Note that these produced ·OH radicals are very easily involved in reactions around them, which can lead to a further decrease in the energy of the whole system. Cu, orange; C, gray; N, blue; O, red; H, pink. (c) Scheme of the synthesis of CuL/PHI and CuP/PHI, N (gray), Cu (blue), H (white), and C (brown). An asterisk (*) represents the surface position of the adsorption. (d) DFT calculation of free energy of O2 adsorption (ΔG*O2) for CuP/PHI (red) and CuL/PHI (blue). (e) Heating curve under 808 nm NIR irradiation with a power density of 1 W cm−2 for 150 s plotted by simulation. (Cu/PMCS refers to Cu/PMCS-2). (f) Thermal field maps of Cu/PMCS plasmons. (g) Absorption spectra at 808 nm of Cu/PMCS. (h) The calculated PDS reaction energy of POD and HORAC processes for different reaction sites with the exact PDS labeled above the bar. (i) The calculated model of pristine CeO2 (left), CeO2 with Cu3c added on the 3O atoms on the surface (Cu-ad, middle) and CeO2 with a Ce atom substituted by Cu (Cu-sub, right). The six possible reaction sites are highlighted with a tagged arrow. (j) The proposed mechanism of regulation of catalytic activities by Cu-CeO2 single-site nanozyme. (a,b) Reproduced with permission [96]. Copyright 2024, Nature Publishing Group. (c,d) Reproduced with permission [88]. Copyright 2023, WILEY-VCH. (e–g) Reproduced with permission [100]. Copyright 2024, WILEY-VCH. (h–j) Reproduced with permission [92]. Copyright 2024, Nature Publishing Group.
3.2. Cu Clusters
Metal nanoclusters (NCs) have become an important material for the design and development of efficient dual-catalytic-site catalysts due to their rapid development in chemical catalysis. Metal clusters have excellent chemical purity, well-defined crystal structures, high specific surface areas, and uniformly distributed catalytic sites, while facilitating the achievement of targeted spot modifications, which contribute to the establishment of clear structure–property relationships [103,104,105,106]. However, although copper has attracted much attention due to its economy, the stability of Cu NCs is inferior to that of Au NCs and Ag NCs, which limits their development. How to effectively improve the stability of Cu NCs remains an urgent challenge [104,107,108,109].
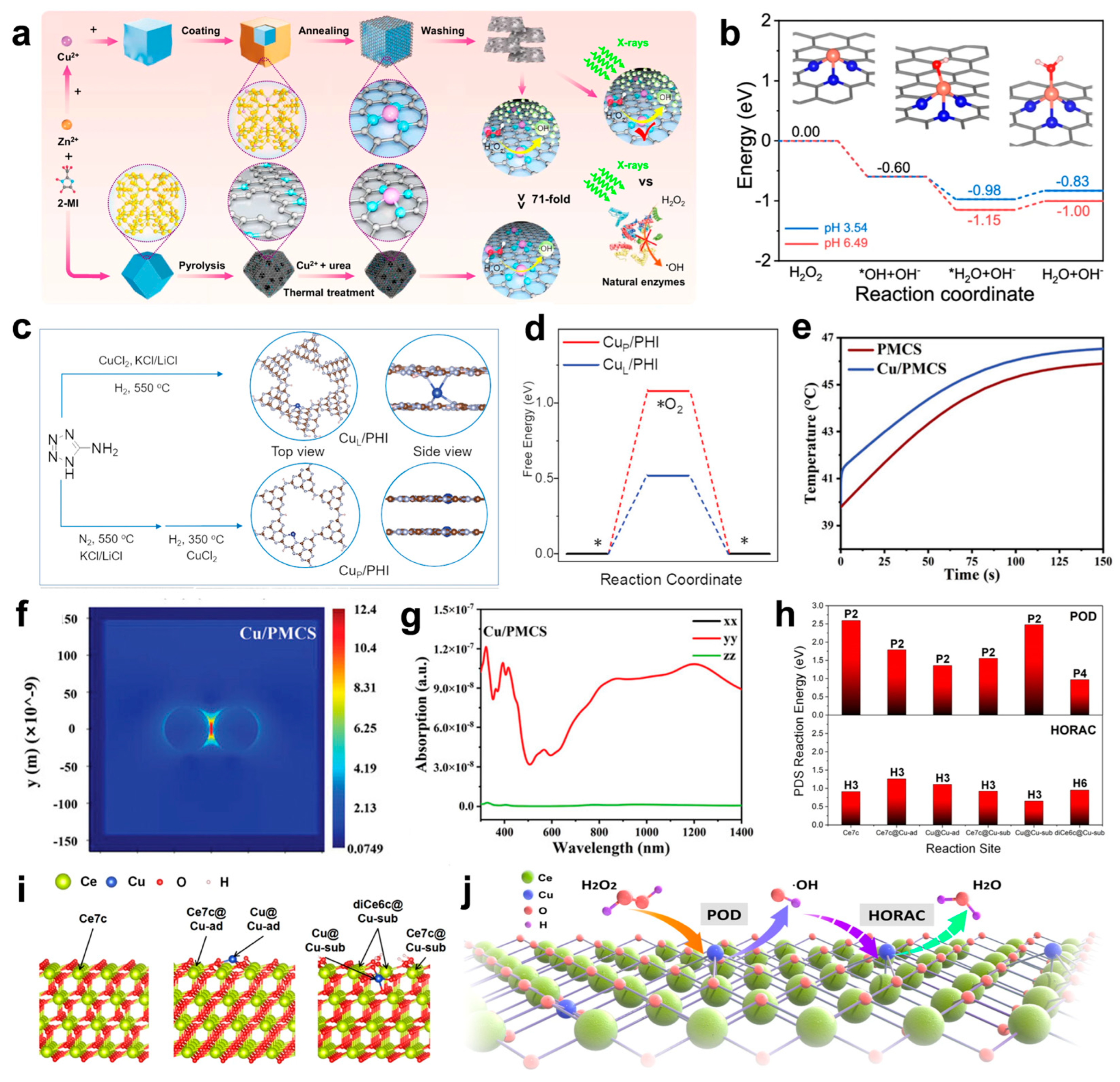
Jia et al. developed a copper nanocluster (Cu NC) catalyst with a dynamic ligand effect, characterized by high stability, atomic precision, and dynamic dual catalytic sites [110]. The designed microcrystalline Cu4NCs feature well-defined structures and demonstrate outstanding catalytic performance in the borohydride reaction of alkynes (Figure 4a), achieving superior regioselectivity, stereoselectivity, and 100% chemoselectivity. Under mild conditions (an air atmosphere, proton solvent, and room temperature), the reaction yield reached up to 99%. Notably, the catalyst achieved an exceptional turnover number (TON) of 77,786, significantly surpassing other reported catalysts (Figure 4b).
The size of metal nanoclusters (NCs) is between that of small molecules and nanoparticles, combining the unique properties of molecules and nanomaterials. As functional building blocks, metal NCs can be assembled into materials with multifunctional properties such as catalysis [111,112,113], luminescence [114], and imaging [115,116], which are widely used in the fields of catalysis, luminescence, and sensing.
Theanine peptides facilitate the efficient assembly of copper clusters (CuCs) through coordination interactions between their amino acid sequences and copper ions [117]. The peptide chain provides a stable chemical environment, promoting the aggregation and self-assembly of CuCs into nanostructures with small particle sizes and high surface activity. Serving as both a template and stabilizer, theanine peptides endow CuCs with excellent structural stability and biological activity (Figure 4c,d). The antibacterial mechanism behind CuCs involves two primary pathways. First, CuCs disrupt the bacterial cell wall and membrane structure, causing the leakage of intracellular components. Second, CuCs bind to glutathione reductase (GR) (Figure 4e), inhibiting its catalytic activity in reducing oxidized glutathione (GSSG) to reduced glutathione (GSH), thereby impairing the bacterial antioxidant defense system (Figure 4f). This leads to a significant accumulation of endogenous reactive oxygen species (ROS), which induce DNA fragmentation via genomic damage and accelerate cell wall rupture (Figure 4g), ultimately causing bacterial death. Importantly, the low toxicity of CuCs highlights their potential as a safe and effective strategy for combating multi-drug-resistant bacterial infections.
Cu nanoclusters have shown great application value in catalysis due to their well-defined structure, excellent catalytic performance, and versatility. In order to solve the problem of the insufficient stability of copper nanoclusters, strategies such as the dynamic ligand effect and peptide chain templates have been studied to significantly improve the stability and catalytic activity of copper nanoclusters and achieve high selectivity, high yield, and low-toxicity antibacterial properties, providing a new direction for catalysis and antibacterial applications.
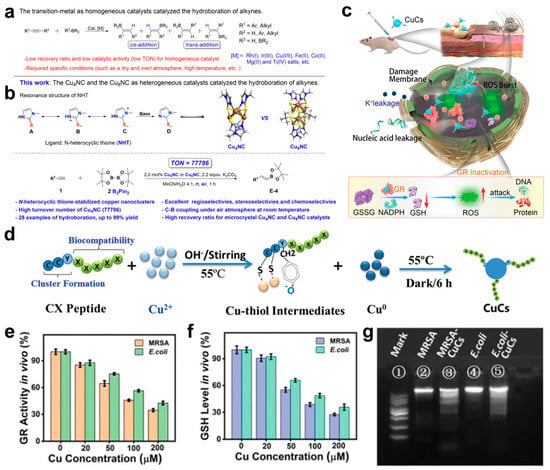
Figure 4.
(a) Transition metal-catalyzed hydroboration of alkynes. (b) This work: microcrystalline Cu4NC and Cu8NC, as heterogeneous catalysts, catalyzed the hydroboration of alkynes. R1 represents the type of functional group. (c) Schematic illustration of CuCs healing local MRSA skin wound infection. The arrows represent the changing trend. (d) Formation of CuCs. (e) Enzymatic activity of GR in MRSA and E. coli after treatment with CuCs. (f) GSH levels in MRSA and E. coli after treatment with CuCs. (g) CuCs induced DNA fragmentation: (1) DNA marker; (2) genomic DNA of untreated MRSA; (3) genomic DNA of MRSA treated with CuCs; (4) genomic DNA of untreated E. coli; and (5) genomic DNA of E. coli treated with CuCs. (a,b) Reproduced with permission [110]. Copyright 2024, Nature Publishing Group. (c–g) Reproduced with permission [117]. Copyright 2021, WILEY-VCH.
Figure 4.
(a) Transition metal-catalyzed hydroboration of alkynes. (b) This work: microcrystalline Cu4NC and Cu8NC, as heterogeneous catalysts, catalyzed the hydroboration of alkynes. R1 represents the type of functional group. (c) Schematic illustration of CuCs healing local MRSA skin wound infection. The arrows represent the changing trend. (d) Formation of CuCs. (e) Enzymatic activity of GR in MRSA and E. coli after treatment with CuCs. (f) GSH levels in MRSA and E. coli after treatment with CuCs. (g) CuCs induced DNA fragmentation: (1) DNA marker; (2) genomic DNA of untreated MRSA; (3) genomic DNA of MRSA treated with CuCs; (4) genomic DNA of untreated E. coli; and (5) genomic DNA of E. coli treated with CuCs. (a,b) Reproduced with permission [110]. Copyright 2024, Nature Publishing Group. (c–g) Reproduced with permission [117]. Copyright 2021, WILEY-VCH.
3.3. Copper Oxides and Chalcogenides
Copper oxides and chalcogenides, composed of copper and chalcogen elements such as sulfur, selenium, and tellurium, demonstrate significant antibacterial properties due to their multifunctional mechanisms and broad adaptability. Their controlled and sustained release of Cu2+ ions effectively disrupts bacterial membranes and inhibits microbial metabolism, ensuring prolonged antibacterial activity [118,119,120,121]. Moreover, these materials exhibit remarkable stability under diverse environmental conditions, including extreme pH, maintaining consistent antibacterial efficacy [120,122].
Building on these properties, Peng et al. developed Cu5.4O@Hep-PEG hydrogels, a multifunctional wound dressing that incorporates amine-functionalized star-shaped polyethylene glycol (starPEG) and heparin with Cu5.4O ultrasmall nanozymes (Figure 5a) [123]. These hydrogels demonstrated a controlled release of approximately 16% Cu5.4O USNPs over 24 h in PBS, highlighting their sustained release capabilities (Figure 5b). This innovative biomaterial addresses key challenges in chronic wound healing by adsorbing pro-inflammatory chemokines such as MCP-1 and IL-8, while simultaneously scavenging reactive oxygen species (ROS) (Figure 5c). Through this dual mechanism, Cu5.4O@Hep-PEG hydrogels effectively disrupt the inflammatory feedback loop, reduce oxidative stress, and create a balanced microenvironment conducive to wound repair and regeneration (Figure 5d–g).
In addition to ion release, copper chalcogenides, such as copper sulfide (CuS), display unique photothermal and photocatalytic properties that further enhance their antibacterial efficiency. Under near-infrared (NIR) light irradiation, CuS nanoparticles absorb light and convert it into localized heat, effectively killing bacteria without causing excessive thermal damage to surrounding tissues [124,125,126]. Leveraging these distinctive properties, the CuS/NO hydrogel represents a breakthrough in wound care by integrating photothermal therapy, sustained Cu2+ release, and controlled nitric oxide (NO) delivery into a single platform (Figure 5h) [127]. This multifunctional hydrogel combines mild photothermal heating and dual-phase NO release to deliver enhanced antibacterial effects while simultaneously promoting tissue regeneration through angiogenesis and collagen deposition. As a result, the CuS/NO hydrogel offers a biocompatible, efficient, and versatile solution for treating infected wounds, addressing both infection control and wound healing in a single system. Simultaneously, the photocatalytic generation of reactive oxygen species (ROS) under light exposure provides an additional mechanism for microbial eradication. These complementary effects enable copper chalcogenides to deliver broad-spectrum antibacterial activity with high efficiency.
Beyond these functionalities, defect engineering introduces further enhancements to copper-based materials by altering their electronic structures. For instance, copper defects (VCu) in compounds like Cu2−xSe create metal-defect active centers that improve catalytic performance [128]. These centers enhance the adsorption and activation of NADH, thereby boosting dehydrogenation activity (Figure 5i). Furthermore, defect-engineered copper nanomaterials favor the efficient four-electron oxygen reduction pathway, directly converting oxygen to H2O rather than forming H2O2 via the less efficient two-electron pathway. This pathway significantly improves oxygen utilization and overall catalytic efficiency (Figure 5j,k). Theoretical studies further highlight that defect engineering accelerates electron transfer, enhancing both NADH oxidation and oxygen reduction, even under hypoxic conditions commonly present in infection sites or tumor microenvironments (Figure 5l). These advances in defect-engineered copper nanomaterials underline their superior catalytic activity, establishing them as highly effective agents in antibacterial and therapeutic applications.
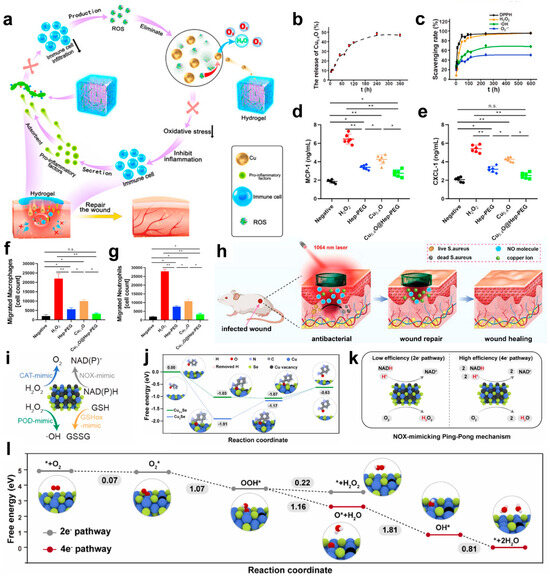
Figure 5.
(a) Schematic of Cu5.4O@Hep-PEG hydrogels in the treatment of diabetic wounds. The downward arrow indicates a decline. (b) In vitro cumulative release of Cu5.4O USNPs from Cu5.4O@Hep-PEG over time. (c) ROS levels in untreated and Hep-PEG-, Cu5.4O-, and Cu5.4O@Hep-PEG-treated 3T3 cells incubated with 250 μM H2O2. (d) Concentrations of MCP-1 and (e) CXCL-1 incubated with 100 μM H2O2 under different treatment conditions. (f) The number of macrophages and (g) neutrophils in the lower chamber of a transwell incubated with conditioned medium collected after incubation with 100 μM H2O2 under different treatment conditions. (* p < 0.05; ** p < 0.01; n.s.: no significance, One-way ANOVA). (h) Synergistic treatment with mild light-induced heat, copper ions, and NO from CuS/NO Gel for infected wound healing. (i) Schematic illustration of multienzyme mimetic activities in Cu2−xSe nanozymes. (j) Free energy diagrams of Cu2Se NPs and Cu2−xSe nanozymes during the NADH dehydrogenation process. (k) Schematic illustration of the NOX mimicking Ping-Pong mechanism on Cu2−xSe nanozymes. (l) Free energy diagrams of Cu2−xSe nanozymes for 2e− and 4e− ORR pathways. (a–g) Reproduced with permission [123]. Copyright 2021, Elsevier Ltd. (h) Reproduced with permission [127]. Copyright 2023, WILEY-VCH. (i–l) Reproduced with permission [128]. Copyright 2024, Elsevier Ltd.
Figure 5.
(a) Schematic of Cu5.4O@Hep-PEG hydrogels in the treatment of diabetic wounds. The downward arrow indicates a decline. (b) In vitro cumulative release of Cu5.4O USNPs from Cu5.4O@Hep-PEG over time. (c) ROS levels in untreated and Hep-PEG-, Cu5.4O-, and Cu5.4O@Hep-PEG-treated 3T3 cells incubated with 250 μM H2O2. (d) Concentrations of MCP-1 and (e) CXCL-1 incubated with 100 μM H2O2 under different treatment conditions. (f) The number of macrophages and (g) neutrophils in the lower chamber of a transwell incubated with conditioned medium collected after incubation with 100 μM H2O2 under different treatment conditions. (* p < 0.05; ** p < 0.01; n.s.: no significance, One-way ANOVA). (h) Synergistic treatment with mild light-induced heat, copper ions, and NO from CuS/NO Gel for infected wound healing. (i) Schematic illustration of multienzyme mimetic activities in Cu2−xSe nanozymes. (j) Free energy diagrams of Cu2Se NPs and Cu2−xSe nanozymes during the NADH dehydrogenation process. (k) Schematic illustration of the NOX mimicking Ping-Pong mechanism on Cu2−xSe nanozymes. (l) Free energy diagrams of Cu2−xSe nanozymes for 2e− and 4e− ORR pathways. (a–g) Reproduced with permission [123]. Copyright 2021, Elsevier Ltd. (h) Reproduced with permission [127]. Copyright 2023, WILEY-VCH. (i–l) Reproduced with permission [128]. Copyright 2024, Elsevier Ltd.
3.4. Copper Alloy
The antimicrobial effect of metal alloys is mainly achieved through a threefold synergistic mechanism: metal ion release [129,130,131], surface contact sterilization [132,133,134], and corrosion microenvironment regulation [135,136,137,138,139]. The metal ions represented by silver, copper, and zinc ions disrupt the bacterial membrane structure and interfere with metabolism by inducing reactive oxygen species, while the surface metal particles directly inhibit the formation of biofilms, and the ions released during the corrosion process and the change in local pH further inhibit the proliferation of microorganisms. Among them, copper alloys outperform copper oxides, sulfides, and pure copper in terms of antibacterial properties, stability, and mechanical performance [140,141]. Through alloying, the antibacterial efficiency of copper is enhanced by optimizing ion release, increasing oxidation resistance, and improving durability, while maintaining superior mechanical properties and conductivity [142,143]. This synergistic optimization of antimicrobial efficacy with mechanical properties and corrosion resistance makes copper alloys valuable for applications in the fields of infection prevention and the control of medical devices and the development of environmentally friendly materials [144,145,146,147].
The Ti-6Al-4V-xCu alloy exemplifies this advancement, achieving superior mechanical strength, corrosion resistance, antibacterial efficacy, and biocompatibility through microstructural refinement and laser powder bed fusion (L-PBF) manufacturing [148]. The addition of copper refines the microstructure by forming a Cu-rich β phase and Ti2Cu precipitates, enhancing strength through solid solution and precipitation hardening while improving wear resistance. Increased copper content boosts corrosion resistance by forming a stable oxide film and reducing galvanic corrosion, aided by L-PBF’s rapid solidification process. Antibacterial properties are achieved via controlled Cu2+ ion release and ROS generation, while the optimized copper content ensures biocompatibility, promoting cell adhesion, osteogenesis, and angiogenesis. These features make Ti-6Al-4V-xCu alloys ideal for durable, antibacterial biomedical implants.
4. Looking Ahead
Copper nanomaterials have shown remarkable potential in dealing with antibiotic-resistant bacteria but still face some challenges, and further optimization and improvement are urgently needed in the future.
However, their clinical translation still faces systemic challenges. In terms of biosafety, although surface modification techniques (e.g., polydopamine coating and polyethylene glycol functionalization) can reduce the acute toxicity of the materials to mammalian cells, the dynamics of the modified layer in complex physiological environments have not yet been clarified. This “biocompatible camouflage” may interfere with the normal clearance function of macrophages, leading to the abnormal accumulation of nanomaterials in the liver and spleen organs. More importantly, it is questionable whether this simplified paradigm can truly reflect the complex immune response network in the human body, given that existing studies have generally used in vitro cellular models to assess toxicity.
The risk of drug resistance evolution needs to be revisited. Despite the multi-targeted action profile of copper ions, the adaptive evolution of bacteria through population-sensing regulation may generate cross-resistance. In addition, the oxidative stress environment triggered by copper nanomaterials may selectively promote the formation of resistant bacterial subpopulations, and these metabolically dormant bacteria establish a temporary barrier to resistance by, for example, altering membrane permeability. What is more serious is that the current drug resistance research still sticks to the detection model of “a single strain and static concentration”, completely ignoring real evolutionary scenarios such as multi-species competition and gene-level transfer in the clinical environment. This kind of experimental design, which is detached from ecological reality, may lead to a serious underestimation of the drug resistance risk of copper nanomaterials.
At the clinical translational level, current studies have fallen into the misconception of “laboratory perfectionism”. Structural control problems in large-scale production have led to batch-to-batch fluctuations in the antimicrobial effects of nanomaterials, and such instability can lead to disastrous consequences in medical applications. A more fundamental contradiction is that the existing medical regulatory system lacks dynamic safety assessment standards for nanomaterials—the long-term biological effects of nanomaterials remain uncharted as the materials undergo evolution in the body, including protein crown formation, ion release, and morphological transformation.
5. Summary
Overall, the growing global drug resistance crisis has created an urgent need for the development of novel antimicrobial strategies that break through the existing therapeutic paradigm. Copper-based nanomaterials, with their multimodal synergistic mechanisms, such as chemical-dynamic therapy (CDT), photothermal therapy (PTT), and photodynamic therapy (PDT), can effectively address the problem of antibiotic resistance and are expected to demonstrate the advantage of spatiotemporally controllable targeted sterilization. The structure–function modulation principle, including key factors such as electronic interactions, size effects, and single-atom doping, provides theoretical support for the precise design of antimicrobial materials. The multidisciplinary integration of copper nanomaterials not only promotes the translation of basic research to the clinic but also provides an innovative interdisciplinary reference program for the global anti-drug-resistance crisis.
Author Contributions
Conceptualization, W.Z. and Y.Z.; investigation, Y.Z.; data curation, Z.L.; writing—original draft preparation, Y.Z.; writing—review and editing, W.Z.; funding acquisition, W.Z. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Guangxi Natural Science Fund for Distinguished Young Scholars (No. 2024GXNSFFA010008), the National Natural Science Foundation of China (No. 22469002), Advanced Innovation Teams and Xinghu Scholars Program of Guangxi Medical University, and start-up funding for high-level talents from Guangxi Medical University Cancer Hospital.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; De La Fuente-Nunez, C.; et al. Antimicrobial Resistance: A Concise Update. Lancet Microbe 2025, 6, 100947. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.; Wright, G.D. Antibacterial Drug Discovery in the Resistance Era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; Yang, F.; Low, J.L.; Wang, L.; Paulus, B.; Wang, Y.; Trampuz, A.; Cheng, C.; Haag, R. A Copper Single-Atom Cascade Bionanocatalyst for Treating Multidrug-Resistant Bacterial Diabetic Ulcer. Adv. Funct. Mater. 2023, 33, 2301986. [Google Scholar] [CrossRef]
- Raoult, D.; Leone, M.; Roussel, Y.; Rolain, J.-M. Attributable Deaths Caused by Infections with Antibiotic-Resistant Bacteria in France. Lancet Infect. Dis. 2019, 19, 128–129. [Google Scholar] [CrossRef]
- Nishshankage, K.; Fernandez, A.B.; Pallewatta, S.; Buddhinie, P.K.C.; Vithanage, M. Current Trends in Antimicrobial Activities of Carbon Nanostructures: Potentiality and Status of Nanobiochar in Comparison to Carbon Dots. Biochar 2024, 6, 2. [Google Scholar] [CrossRef]
- Dzuvor, C.K.O.; Shen, H.-H.; Haritos, V.S.; He, L. Coassembled Multicomponent Protein Nanoparticles Elicit Enhanced Antibacterial Activity. ACS Nano 2024, 18, 4478–4494. [Google Scholar] [CrossRef]
- Huang, T.; Linklater, D.; Li, X.; Gamage, S.S.B.; Alkazemi, H.; Farrugia, B.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. One-Step Synthesis of Antimicrobial Polypeptide-Selenium Nanoparticles Exhibiting Broad-Spectrum Efficacy against Bacteria and Fungi with Superior Resistance Prevention. ACS Appl. Mater. Interfaces 2024, 16, 68996–69010. [Google Scholar] [CrossRef]
- Linklater, D.P.; Ivanova, E.P. Nanostructured Antibacterial Surfaces—What Can Be Achieved? Nano Today 2022, 43, 101404. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Lan, F.; Aarons, J.; Shu, Y.; Zhou, X.; Jiao, H.; Wang, H.; Guan, Q.; Li, W. Anchoring Strategy for Highly Active Copper Nanoclusters in Hydrogenation of Renewable Biomass-Derived Compounds. Appl. Catal. B Environ. 2021, 299, 120651. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Li, X.; Gao, C.; Pu, Y.; Zhong, X.; Qian, J.; Zeng, M.; Chu, X.; Chen, Z.; et al. Embedding Reverse Electron Transfer between Stably Bare Cu Nanoparticles and Cation-vacancy CuWO4. Adv. Mater. 2024, 36, 2412570. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Cheng, J.; Ou, Y.; Deng, Y.; Zhao, Q. Copper I-II-Containing Composites and Coatings with High and Broad-Spectrum Antimicrobial Activity. Chem. Eng. J. 2024, 498, 155193. [Google Scholar] [CrossRef]
- Fathi, P.; Roslend, A.; Alafeef, M.; Moitra, P.; Dighe, K.; Esch, M.B.; Pan, D. In Situ Surface-directed Assembly of 2D Metal Nanoplatelets for Drug-free Treatment of Antibiotic-resistant Bacteria. Adv. Healthc. Mater. 2022, 11, 2102567. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shan, J.; Jin, X.; Sun, W.; Cheng, L.; Chen, X.-L.; Wang, X. Nanoarchitectonics of in Situ Antibiotic-Releasing Acicular Nanozymes for Targeting and Inducing Cuproptosis-like Death to Eliminate Drug-Resistant Bacteria. ACS Nano 2024, 18, 24327–24349. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Yuan, Z.; Guo, J. Copper Nanoparticles and Copper Ions Promote Horizontal Transfer of Plasmid-Mediated Multi-Antibiotic Resistance Genes across Bacterial Genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; He, S.; Han, X.; Wang, A.; Zhang, F.; Deng, J.; Long, X.; Lin, J.; Feng, Y.; et al. Enhancing Antibiotic-Resistant Bacterial Infection Therapy: Self-Assembling Gemini Quaternary Ammonium-Functionalized Peptide Nanoassemblies with Multiple Antibacterial Mechanisms. ACS Nano 2025, 19, 6977–6992. [Google Scholar] [CrossRef]
- Wu, X.; Xing, Z.; Huang, H.; Ding, Z.; Gao, Y.; Adeli, M.; Ma, L.; Ma, T.; Cheng, C.; Zhao, C. Bacteriophage-like Nanobiocatalysts with Spiky Topography and Dual-Atom Sites for Treating Drug-Resistant Bacteria. ACS Nano 2024. [Google Scholar] [CrossRef]
- Zhang, H.; Li, D.; Ren, H.; Ma, Z.; Meng, S.; Qiao, Y.; Yang, J.; Wang, Y.; Zhou, Q.; Xie, L. A Bioinspired Virus-like Mechano–Bactericidal Nanomotor for Ocular Multidrug-resistant Bacterial Infection Treatment. Adv. Mater. 2025, 37, 2408221. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, W.; Liu, L.; Gao, Y.; Zhang, C.; Chen, L.; Lv, F.; Xi, J.; Du, T.; Luo, L.; et al. Spin Polarization Induced by Atomic Strain of MBene Promotes the ·O2– Production for Groundwater Disinfection. Nat. Commun. 2025, 16, 197. [Google Scholar] [CrossRef]
- Fu, S.; Yi, X.; Li, Y.; Li, Y.; Qu, X.; Miao, P.; Xu, Y. Berberine and Chlorogenic Acid-Assembled Nanoparticles for Highly Efficient Inhibition of Multidrug-Resistant Staphylococcus Aureus. J. Hazard. Mater. 2024, 473, 134680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ji, H.; Liu, C.; Bing, W.; Wang, Z.; Qu, X. A Multinuclear Metal Complex Based DNase-mimetic Artificial Enzyme: Matrix Cleavage for Combating Bacterial Biofilms. Angew. Chem. 2016, 128, 10890–10894. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, X.; Hou, S.; Zhang, L.; Liu, X.; Yang, G.; Wang, Y.; Hao, M.; Zhang, W.; Wang, J.; et al. A Light-Responsive Multilayered 3D Porous Ga2O3 Hydrogel for Photocatalytic Antibacterial Therapy Promoting Healing of MDR S. Aureus-Infected Wounds. J. Mater. Sci. Technol. 2025, 225, 188–202. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, R.; Gao, R.; Zhao, Y.; Yodsanit, N.; Zhu, M.; Burger, J.C.; Ye, M.; Tong, Y.; Gong, S. Multimodal Nanoimmunotherapy Engages Neutrophils to Eliminate Staphylococcus Aureus Infections. Nat. Nanotechnol. 2024, 19, 1032–1043. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, W.; Zhong, L.; Yang, Y.; Chen, Y.; Hou, Q.; Yu, P.; Jiang, X. Phenylboronic Acid-Modified Gold Nanoclusters as a Nanoantibiotic to Treat Vancomycin-Resistant Enterococcus faecalis-Caused Infections. ACS Nano 2023, 17, 19685–19695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xu, W.; Chen, W.; Li, B.; Li, G.; Deng, H.; Zhang, L.; Shao, C.; Shan, A. Self-Assembling Peptide with Dual Function of Cell Penetration and Antibacterial as a Nano Weapon to Combat Intracellular Bacteria. Sci. Adv. 2025, 11, eads3844. [Google Scholar] [CrossRef]
- Zou, P.; Huang, L.; Li, Y.; Liu, D.; Che, J.; Zhao, T.; Li, H.; Li, J.; Cui, Y.; Yang, G.; et al. Phase-separated Nano-antibiotics Enhanced Survival in Multidrug-resistant Escherichia coli Sepsis by Precise Periplasmic Ec DsbA Targeting. Adv. Mater. 2024, 36, 2407152. [Google Scholar] [CrossRef]
- Ye, Y.; Zheng, Q.; Wang, Z.; Wang, S.; Lu, Z.; Chu, Q.; Liu, Y.; Yao, K.; Wei, B.; Han, H.; et al. Metal-Phenolic Nanoparticles Enhance Low Temperature Photothermal Therapy for Bacterial Biofilm in Superficial Infections. J. Nanobiotechnol. 2024, 22, 713. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xue, B.; Ruan, S.; Guo, J.; Huang, Y.; Geng, X.; Wang, D.; Zhou, C.; Zheng, J.; Yuan, Z. Supercharged Precision Killers: Genetically Engineered Biomimetic Drugs of Screened Metalloantibiotics against Acinetobacter baumanni. Sci. Adv. 2024, 10, eadk6331. [Google Scholar] [CrossRef]
- Fan, H.; Wong, K.I.; Ma, Y.; Li, M.; Li, H.; Wei, L.; Wang, S.; Yao, M.; Lu, M. Biodegradable Acid-responsive Nanocarrier for Enhanced Antibiotic Therapy against Drug-resistant Helicobacter pylori via Urease Inhibition. Adv. Funct. Mater. 2025, 35, 2412893. [Google Scholar] [CrossRef]
- Wang, T.; Teng, R.; Wu, M.; Ge, Z.; Liu, Y.; Yang, B.; Li, C.; Fan, Z.; Du, J. A Polypeptosome Spray to Heal Antibiotic-Resistant Bacteria-Infected Wound by Photocatalysis-Induced Metabolism-Interference. ACS Nano 2024, 18, 35620–35631. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, M.; Yang, Y.; Tang, Z.; Guo, Y.; Zhou, J.; Gui, Y.; Huang, R.; Cai, J.; Yu, B.; et al. Metal Ion and Antibiotic Co-Loaded Nanoparticles for Combating Methicillin-Resistant Staphylococcus aureus-Induced Osteomyelitis. ACS Nano 2025, 19, 5253–5268. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Perero, S.; Lembo, D.; Ferraris, M.; Balagna, C. Antibacterial and Antiviral Activities of Silver Nanocluster/Silica Composite Coatings Deposited onto Air Filters. ACS Appl. Mater. Interfaces 2024, 16, 3955–3965. [Google Scholar] [CrossRef] [PubMed]
- Duarte Bernardino, C.; Lee, M.; Ren, Q.; Ruehle, B. Facile Spray-Coating of Antimicrobial Silica Nanoparticles for High-Touch Surface Protection. ACS Appl. Mater. Interfaces 2025, 17, 12507–12519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Mohammadniaei, M.; Zheng, T.; Zhang, Q.; Ashley, J.; Liu, S.; Sun, Y.; Tang, B.Z. Upregulating Aggregation-Induced-Emission Nanoparticles with Blood-Tumor-Barrier Permeability for Precise Photothermal Eradication of Brain Tumors and Induction of Local Immune Responses. Adv. Mater. 2021, 33, 2008802. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2021, 12, 141. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, S.; Xu, X.; Du, Q. Copper-Containing Nanoparticles: Mechanism of Antimicrobial Effect and Application in Dentistry-a Narrative Review. Front. Surg. 2022, 9, 905892. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial Applications of Copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef]
- Manivasagan, P.; Thambi, T.; Joe, A.; Han, H.-W.; Seo, S.-H.; Jun Jeon, Y.; Conde, J.; Jang, E.-S. Progress in Nanomaterial-Based Synergistic Photothermal-Enhanced Chemodynamic Therapy in Combating Bacterial Infections. Prog. Mater. Sci. 2024, 144, 101292. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, X.; Su, Q.; Zhao, C.; Cao, J.; Yue, Y.; Li, S.; Chen, X.; Yin, J.; Deng, Y.; et al. Ultrasound-Activated Piezo-Hot Carriers Trigger Tandem Catalysis Coordinating Cuproptosis-like Bacterial Death against Implant Infections. Nat. Commun. 2024, 15, 1643. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Q.; Zha, Z.; Zhu, D.; Zheng, L.; Shi, L.; Wei, X.; Lian, L.; Wu, K.; Cheng, L. Copper Single-Atom Catalysts with Photothermal Performance and Enhanced Nanozyme Activity for Bacteria-infected Wound Therapy. Bioact. Mater. 2021, 6, 4389–4401. [Google Scholar] [CrossRef]
- Mei, J.; Xu, D.; Wang, L.; Kong, L.; Liu, Q.; Li, Q.; Zhang, X.; Su, Z.; Hu, X.; Zhu, W.; et al. Biofilm Microenvironment-responsive Self-assembly Nanoreactors for All-stage Biofilm Associated Infection through Bacterial Cuproptosis-like Death and Macrophage Re-rousing. Adv. Mater. 2023, 35, 2303432. [Google Scholar] [CrossRef] [PubMed]
- Caselli, L.; Traini, T.; Micciulla, S.; Sebastiani, F.; Köhler, S.; Nielsen, E.M.; Diedrichsen, R.G.; Skoda, M.W.A.; Malmsten, M. Antimicrobial Peptide Coating of TiO2 Nanoparticles for Boosted Antimicrobial Effects. Adv. Funct. Mater. 2024, 34, 2405047. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Lee, D.H.; Nguyen, P.T.; Le, P.G.; Kim, M.I. Foldable Paper Microfluidic Device Based on Single Iron Site-Containing Hydrogel Nanozyme for Efficient Glucose Biosensing. Chem. Eng. J. 2023, 454, 140541. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Y.; Huang, C.; Xu, Y.; Xu, Y. Electrogenerated Copper Selenide with Positive Charge to Efficiently Capture and Combat Drug-Resistant Bacteria for Wound Healing. J. Colloid. Interface Sci. 2023, 634, 852–863. [Google Scholar] [CrossRef]
- Rotello, V.M. Nanomaterials for Fighting Multidrug-Resistant Biofilm Infections. BME Front. 2023, 4, 0017. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Yurkiv, V.; Ghildiyal, P.; Wang, Y.; Amiri, A.; Sorokina, L.V.; Zachariah, M.R.; Shokuhfar, T.; Shahbazian-Yassar, R. In Situ Microscopic Studies on the Interaction of Multi-Principal Element Nanoparticles and Bacteria. ACS Nano 2023, 17, 5880–5893. [Google Scholar] [CrossRef]
- Bennett, I.D.; Burns, J.R.; Ryadnov, M.G.; Howorka, S.; Pyne, A.L.B. Lipidated DNA Nanostructures Target and Rupture Bacterial Membranes. Small 2024, 20, 2207585. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, S.; Zhai, Y.; Huang, R.; Pei, S.; Lin, F.; Zhang, W. Ultrafine Ru Clusters with GOx Enable Glucose-Activated Cascaded Reaction for Bacterial-Infected Diabetic Wound Healing. Chem. Eng. J. 2024, 488, 150991. [Google Scholar] [CrossRef]
- Chen, S.; Huang, F.; Mao, L.; Zhang, Z.; Lin, H.; Yan, Q.; Lu, X.; Shi, J. High Fe-Loading Single-Atom Catalyst Boosts ROS Production by Density Effect for Efficient Antibacterial Therapy. Nano-Micro Lett. 2025, 17, 32. [Google Scholar] [CrossRef]
- Lv, K.; Hou, M.; Kou, Y.; Yu, H.; Liu, M.; Zhao, T.; Shen, J.; Huang, X.; Zhang, J.; Mady, M.F.; et al. Black Titania Janus Mesoporous Nanomotor for Enhanced Tumor Penetration and Near-Infrared Light-Triggered Photodynamic Therapy. ACS Nano 2024, 18, 13910–13923. [Google Scholar] [CrossRef]
- Wang, R.; Shi, M.; Xu, F.; Qiu, Y.; Zhang, P.; Shen, K.; Zhao, Q.; Yu, J.; Zhang, Y. Graphdiyne-Modified TiO2 Nanofibers with Osteoinductive and Enhanced Photocatalytic Antibacterial Activities to Prevent Implant Infection. Nat. Commun. 2020, 11, 4465. [Google Scholar] [CrossRef]
- Li, Z.; Wang, E.; Zhang, Y.; Luo, R.; Gai, Y.; Ouyang, H.; Deng, Y.; Zhou, X.; Li, Z.; Feng, H. Antibacterial Ability of Black Titania in Dark: Via Oxygen Vacancies Mediated Electron Transfer. Nano Today 2023, 50, 101826. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Zhang, P.; Bi, J.; Nguyen, N.H.; Dang, Y.; Xu, Z.; Wang, H.; Ninan, N.; Bright, R.; Pham, T.; et al. Silver—gallium Nano-amalgamated Particles as a Novel, Biocompatible Solution for Antibacterial Coatings. Adv. Funct. Mater. 2024, 34, 2310539. [Google Scholar] [CrossRef]
- Pugazhendhi, A.S.; Neal, C.J.; Ta, K.M.; Molinari, M.; Kumar, U.; Wei, F.; Kolanthai, E.; Ady, A.; Drake, C.; Hughes, M.; et al. A Neoteric Antibacterial Ceria-Silver Nanozyme for Abiotic Surfaces. Biomaterials 2024, 307, 122527. [Google Scholar] [CrossRef]
- Guo, L.; Tang, Y.; Wang, L.; Zhou, R.; Wang, S.; Xu, H.; Yang, X.; Zhang, J.; Chen, J.; Xu, C.; et al. Synergetic Antibacterial Nanoparticles with Broad-Spectrum for Wound Healing and Lung Infection Therapy. Adv. Funct. Mater. 2024, 34, 2403188. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhong, Y.; Zhang, Q.; Wu, Y.; Huang, J.; Pang, K.; Zhou, Y.; Xiao, T.; Wu, Z.; et al. pH-Responsive and Nanoenzyme-Loaded Artificial Nanocells Relieved Osteomyelitis Efficiently by Synergistic Chemodynamic and Cuproptosis Therapy. Biomaterials 2025, 313, 122762. [Google Scholar] [CrossRef]
- Li, F.; Huang, K.; Chang, H.; Liang, Y.; Zhao, J.; Yang, S.; Liu, F. A Polydopamine Coated Nanoscale FeS Theranostic Platform for the Elimination of Drug-Resistant Bacteria via Photothermal-Enhanced Fenton Reaction. Acta Biomater. 2022, 150, 380–390. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Liu, J.; Lin, Y.; Jiao, J.; Chen, B.; Wang, W.; Wu, S.; Li, C. Photothermal Therapy with Regulated Nrf2/NF-κB Signaling Pathway for Treating Bacteria-Induced Periodontitis. Bioact. Mater. 2022, 9, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, Z.; Yan, J.; Bu, C.; Peng, C.; Li, C.; Mao, R.; Zhang, J.; Wang, Z.; Chen, S.; et al. Gastric Acid-responsive ROS Nanogenerators for Effective Treatment of Helicobacter pylori Infection without Disrupting Homeostasis of Intestinal Flora. Adv. Sci. 2023, 10, 2206957. [Google Scholar] [CrossRef]
- Sumner, E.R.; Shanmuganathan, A.; Sideri, T.C.; Willetts, S.A.; Houghton, J.E.; Avery, S.V. Oxidative Protein Damage Causes Chromium Toxicity in Yeast. Microbiology 2005, 151, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free Radical-Mediated Oxidation of Free Amino Acids and Amino Acid Residues in Proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Anjem, A.; Imlay, J.A. Mononuclear Iron Enzymes Are Primary Targets of Hydrogen Peroxide Stress. J. Biol. Chem. 2012, 287, 15544–15556. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The Iron-Sulfur Clusters of Dehydratases Are Primary Intracellular Targets of Copper Toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.F.; Imlay, J.A. Silver(I), Mercury(II), Cadmium(II), and Zinc(II) Target Exposed Enzymic Iron-Sulfur Clusters When They Toxify Escherichia Coli. Appl. Environ. Microbiol. 2012, 78, 3614–3621. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Elsey, S.P.; Hausinger, R.P. Fructose-1,6-bisphosphate Aldolase (Class II) Is the Primary Site of Nickel Toxicity in Escherichia coli. Mol. Microbiol. 2011, 82, 1291–1300. [Google Scholar] [CrossRef]
- Ogunseitan, O.A.; Yang, S.; Ericson, J. Microbial D-Aminolevulinate Dehydratase as a Biosensor of Lead Bioavailability in Contaminated Environments. Soil Biol. Biochem. 2000, 32, 1899–1906. [Google Scholar] [CrossRef]
- Wang, Y.; Malkmes, M.J.; Jiang, C.; Wang, P.; Zhu, L.; Zhang, H.; Zhang, Y.; Huang, H.; Jiang, L. Antibacterial Mechanism and Transcriptome Analysis of Ultra-Small Gold Nanoclusters as an Alternative of Harmful Antibiotics against Gram-Negative Bacteria. J. Hazard. Mater. 2021, 416, 126236. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, T.; Qu, S.; Li, Q.; Liu, B.; Zhou, W. G-quadruplex/Hemin DNAzyme-functionalized Silver Nanoclusters with Synergistic Antibacterial and Wound Healing Capabilities for Infected Wound Management. Small 2024, 20, 2307220. [Google Scholar] [CrossRef]
- Li, X.; Deng, F.; Zheng, R.; Liu, L.; Liu, Y.; Kong, R.; Chen, A.; Yu, X.; Li, S.; Cheng, H. Carrier Free Photodynamic Synergists for Oxidative Damage Amplified Tumor Therapy. Small 2021, 17, 2102470. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Sajid Jamal, Q.M.; Khan, H.M.; Jalal, M.; Ahmad, H.; Mahdi, A.A. Antiquorum Sensing Activity of Silver Nanoparticles in P. Aeruginosa: An in Silico Study. In Silico Pharmacol. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Azimi, S.; Klementiev, A.D.; Whiteley, M.; Diggle, S.P. Bacterial Quorum Sensing during Infection. Annu. Rev. Microbiol. 2020, 74, 201–219. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum Sensing Signal–Response Systems in Gram-Negative Bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Ibrahim, K.S.; Duraisamy, S.; Sivaji, I.; Kandasamy, S.; Kumarasamy, A.; Kumar, N.S. Antiquorum Sensing and Antibiofilm Potential of Biosynthesized Silver Nanoparticles of Myristica Fragrans Seed Extract against MDR Salmonella Enterica Serovar Typhi Isolates from Asymptomatic Typhoid Carriers and Typhoid Patients. Environ. Sci. Pollut. Res. 2020, 27, 2844–2856. [Google Scholar] [CrossRef] [PubMed]
- Swem, L.R.; Swem, D.L.; O’Loughlin, C.T.; Gatmaitan, R.; Zhao, B.; Ulrich, S.M.; Bassler, B.L. A Quorum-Sensing Antagonist Targets Both Membrane-Bound and Cytoplasmic Receptors and Controls Bacterial Pathogenicity. Mol. Cell 2009, 35, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, G.; Yang, Y.; Wang, N.; Zhang, Y.; Su, Y.; Zhao, F.; Wu, J.; Wang, L.; Lin, Y.; et al. Nanomaterials Regulate Bacterial Quorum Sensing: Applications, Mechanisms, and Optimization Strategies. Adv. Sci. 2024, 11, 2306070. [Google Scholar] [CrossRef]
- Gao, M.; Xu, B.; Huang, Y.; Cao, J.; Yang, L.; Liu, X.; Djumaev, A.; Wu, D.; Shoxiddinova, M.; Cai, X.; et al. Nano-enabled Quenching of Bacterial Communications for the Prevention of Biofilm Formation. Angew. Chem. 2023, 135, e202305485. [Google Scholar] [CrossRef]
- Singh, H.; Dan, A.; Prasanna Kumari, B.; Dave, H.; Parsaila, N.; Navale, A.; Darban, Z.; Yadav, I.; Goyal, P.; Misra, S.K.; et al. Copper-MOF and Tannic Acid-Empowered Composite Cryogel as a Skin Substitute for Accelerated Deep Wound Healing. Biomater. Adv. 2024, 164, 213983. [Google Scholar] [CrossRef]
- Tripathi, A.; Park, J.; Pho, T.; Champion, J.A. Dual Antibacterial Properties of Copper-coated Nanotextured Stainless Steel. Small 2024, 20, 2311546. [Google Scholar] [CrossRef]
- Duvanova, E.; Krasnou, I.; Krumme, A.; Mikli, V.; Radio, S.; Rozantsev, G.M.; Karpichev, Y. Development of Functional Composite Cu(II)-Polyoxometalate/PLA with Antimicrobial Properties. Molecules 2022, 27, 2510. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Liao, S.; Jiang, C.; Wang, L.; Tang, Y.; Wu, G.; Dai, G.; Chen, L. Quantitative Proteomics Reveals the Mechanism of Silver Nanoparticles against Multidrug-Resistant Pseudomonas aeruginosa Biofilms. J. Proteome Res. 2020, 19, 3109–3122. [Google Scholar] [CrossRef]
- Huang, L.; Pu, H.; Sun, D. Spatiotemporally Guided Single-atom Bionanozyme for Targeted Antibiofilm Treatment. Small 2024, 20, 2407747. [Google Scholar] [CrossRef] [PubMed]
- Panáček, D.; Belza, J.; Hochvaldová, L.; Baďura, Z.; Zoppellaro, G.; Šrejber, M.; Malina, T.; Šedajová, V.; Paloncýová, M.; Langer, R.; et al. Single Atom Engineered Antibiotics Overcome Bacterial Resistance. Adv. Mater. 2024, 2410652. [Google Scholar] [CrossRef]
- Ji, Y.; Yu, Z.; Yan, L.; Song, W. Research progress in preparation, modification and application of biomass-based single-atom catalysts. China Powder Sci. Technol. 2023, 29, 100–107. [Google Scholar]
- Liu, X.; Chen, M.; Ma, J.; Liang, J.; Li, C.; Chen, C.; He, H. Advances in the synthesis strategies of carbon-based single-atom catalysts and their electrochemical applications. China Powder Sci. Technol. 2024, 30, 35–45. [Google Scholar]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-atom Nanozyme for Wound Disinfection Applications. Angew. Chem. 2019, 131, 4965–4970. [Google Scholar] [CrossRef]
- Zhai, Y.; Pei, S.; Qin, X.; Zhang, L.; Liu, X.; Cai, S.; Zhang, W. Ultralow Dose Iron-copper Bimetallic Single-atom Nanozymes for Efficient Photothermal-chemodynamic Antibacterial and Wound Healing. Adv. Healthc. Mater. 2025, 14, 2403920. [Google Scholar] [CrossRef]
- Ou, H.; Qian, Y.; Yuan, L.; Li, H.; Zhang, L.; Chen, S.; Zhou, M.; Yang, G.; Wang, D.; Wang, Y. Spatial Position Regulation of Cu Single Atom Site Realizes Efficient Nanozyme Photocatalytic Bactericidal Activity. Adv. Mater. 2023, 35, 2305077. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, R.; Zhao, H.; Qi, H.; Li, J.; Li, J.; Zhou, X.; Wang, A.; Fan, K.; Yan, X.; et al. Bioinspired Copper Single-atom Nanozyme as a Superoxide Dismutase-like Antioxidant for Sepsis Treatment. Exploration 2022, 2, 20210267. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Li, X.; Wu, X.; Yuan, T.; Yang, Y. Simulated Enzyme Activity and Efficient Antibacterial Activity of Copper-Doped Single-Atom Nanozymes. Langmuir 2022, 38, 6860–6870. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, J.; Han, N.; Ma, W.; Zhang, D.; Yao, M.; Wang, X.; Chen, Y. Peroxidase-like Active Cu-ZIF-8 with Rich Copper(I)-Nitrogen Sites for Excellent Antibacterial Performances toward Drug-Resistant Bacteria. Nano Res. 2024, 17, 7427–7435. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, L.; Liu, X.; Ye, C.; Zhu, P.; Tan, T.; Wang, D.; Wang, Y. Tuning Oxidant and Antioxidant Activities of Ceria by Anchoring Copper Single-Site for Antibacterial Application. Nat. Commun. 2024, 15, 1010. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Zhao, Y.; Liu, J.-J. Precisely Engineered Bionic Cu-Doped Titanium Dioxide Nanoarrays/Fluorine-Doped Tin Oxide with Excellent Antibacterial and Antifouling Properties. Tungsten 2024, 6, 522–528. [Google Scholar] [CrossRef]
- Dai, X.; Liu, H.; Cai, B.; Liu, Y.; Song, K.; Chen, J.; Ni, S.; Kong, L.; Zhan, J. A Bioinspired Atomically Thin Nanodot Supported Single-Atom Nanozyme for Antibacterial Textile Coating. Small 2023, 19, 2303901. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Ma, J.; Wang, Y.; Xie, L.; Yan, X.; Shi, L.; Li, Y.; Liu, Y. Single Copper Atom Photocatalyst Powers an Integrated Catalytic Cascade for Drug-Resistant Bacteria Elimination. ACS Nano 2023, 17, 2980–2991. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, X.; Li, Q.; Fu, Q.; Wang, B.; Li, B.; Wang, S.; Chang, Q.; Xiang, H.; Ye, C.; et al. Enhancing Radiation-Resistance and Peroxidase-like Activity of Single-Atom Copper Nanozyme via Local Coordination Manipulation. Nat. Commun. 2024, 15, 6174. [Google Scholar] [CrossRef]
- Wu, L.; Lin, H.; Cao, X.; Tong, Q.; Yang, F.; Miao, Y.; Ye, D.; Fan, Q. Bioorthogonal Cu Single-atom Nanozyme for Synergistic Nanocatalytic Therapy, Photothermal Therapy, Cuproptosis and Immunotherapy. Angew. Chem. 2024, 136, e202405937. [Google Scholar] [CrossRef]
- Xing, Y.; Xiu, J.; Zhou, M.; Xu, T.; Zhang, M.; Li, H.; Li, X.; Du, X.; Ma, T.; Zhang, X. Copper Single-Atom Jellyfish-like Nanomotors for Enhanced Tumor Penetration and Nanocatalytic Therapy. ACS Nano 2023, 17, 6789–6799. [Google Scholar] [CrossRef]
- Yu, Q.; Zhou, J.; Song, J.; Zhou, H.; Kang, B.; Chen, H.; Xu, J. A Cascade Nanoreactor of Metal-protein-polyphenol Capsule for Oxygen-mediated Synergistic Tumor Starvation and Chemodynamic Therapy. Small 2023, 19, 2206592. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Xing, S.; Zhang, Y.; Shangguan, L.; Wei, C.; Peng, F.; Liu, X. Copper-Zinc Bimetallic Single-Atom Catalysts with Localized Surface Plasmon Resonance-Enhanced Photothermal Effect and Catalytic Activity for Melanoma Treatment and Wound-Healing. Adv. Sci. 2023, 10, 2207342. [Google Scholar] [CrossRef]
- Fu, Y.; Lu, K.; Hu, A.; Huang, J.; Guo, L.; Zhou, J.; Zhao, J.; Prezhdo, O.V.; Liu, M. dz2 Band Links Frontier Orbitals and Charge Carrier Dynamics of Single-Atom Cocatalyst-Aided Photocatalytic H2 Production. J. Am. Chem. Soc. 2023, 145, 28166–28175. [Google Scholar] [CrossRef]
- Liu, H.-X.; Gao, Z.; Yan, H.; Li, S.-Q.; Wang, W.-W.; Qin, X.; Sun, H.; Cui, J.; Jia, C.-J. Ensemble of Single-Atom Catalysis and Defect Engineering in Cu1/CeO2 Nanozymes for Tumor Therapy. Sci. China Chem. 2023, 66, 2590–2599. [Google Scholar] [CrossRef]
- Wen, S.; Zhang, C.; Liu, L.-J.; Wang, Z.; Sun, D.; He, J. Stable and Recyclable Copper Nanoclusters with Exposed Active Sites for Broad-scope Protosilylation in Open Air. Angew. Chem. Int. Ed. 2024, e202416851. [Google Scholar]
- Mu, W.-L.; Li, L.; Cong, X.-Z.; Chen, X.; Xia, P.; Liu, Q.; Wang, L.; Yan, J.; Liu, C. Hierarchical Assembly of High-Nuclearity Copper(I) Alkynide Nanoclusters: Highly Effective CO2 Electroreduction Catalyst toward Hydrocarbons. J. Am. Chem. Soc. 2024, 146, 28131–28140. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sagadevan, A.; Murugesan, K.; Nastase, S.A.F.; Maity, B.; Bodiuzzaman, M.; Shkurenko, A.; Hedhili, M.N.; Yin, J.; Mohammed, O.F.; et al. Multiple Neighboring Active Sites of an Atomically Precise Copper Nanocluster Catalyst for Efficient Bond-Forming Reactions. Mater. Horiz. 2024, 11, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, C.; Qin, L.; Wang, L.; Qiao, L.; Chi, K.; Tang, Z. Atomically Precise Cu Nanoclusters: Recent Advances, Challenges, and Perspectives in Synthesis and Catalytic Applications. Nano-Micro Lett. 2025, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Han, B.-L.; Liu, Z.; Feng, L.; Wang, Z.; Gupta, R.K.; Aikens, C.M.; Tung, C.-H.; Sun, D. Polymorphism in Atomically Precise Cu23 Nanocluster Incorporating Tetrahedral [Cu4 ]0 Kernel. J. Am. Chem. Soc. 2020, 142, 5834–5841. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, C.; Han, B.; Wang, Z.; Liu, Y.; Xue, Q.; Tung, C.-H.; Sun, D. Assembly of Air-Stable Copper(I) Alkynide Nanoclusters Assisted by Tripodal Polydentate Phosphoramide Ligands. Nat. Synth. 2024, 3, 517–526. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, Q.-G.; Dong, J.-P.; Zhang, H.; Li, B.; Wang, R.; Zang, S.-Q. Assembly of Copper-Clusters into a Framework: Enhancing the Structural Stability and Photocatalytic HER Performance. Chem. Commun. 2023, 59, 3067–3070. [Google Scholar] [CrossRef]
- Jia, T.; Ai, J.; Li, X.; Zhang, M.-M.; Hua, Y.; Li, Y.-X.; Sun, C.-F.; Liu, F.; Huang, R.-W.; Wang, Z.; et al. Atomically Precise Copper Clusters with Dual Sites for Highly Chemoselective and Efficient Hydroboration. Nat. Commun. 2024, 15, 9551. [Google Scholar] [CrossRef]
- Joseph, M.; Rahman Pathiripparambath, M.S.; Thomas, V.; Tharayil, H.; Jayasree, R.S.; Nair, L.V. Porphyrin and Doxorubicin Mediated Nanoarchitectonics of Copper Clusters: A Bimodal Theranostics for Cancer Diagnosis and Treatment in Vitro. J. Mater. Chem. B 2024, 12, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, P.; Wang, T.; Li, J.; Yun, K.; Zhang, X.; Yang, X. A Copper Nanocluster-Based Multifunctional Nanoplatform for Augmented Chemo/Chemodynamic/Photodynamic Combination Therapy of Breast Cancer. Pharmacol. Res. 2023, 187, 106632. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, F.; Shi, L.; Tang, Q.; Li, B.; Wang, X.; Jin, Y. Nanotrains of DNA Copper Nanoclusters That Triggered a Cascade Fenton-like Reaction and Glutathione Depletion to Doubly Enhance Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 37280–37290. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, M.; Yao, L.-Y. Copper(I) Cluster of Aggregation-Induced Emission and X-Ray Scintillator Characteristic. Chem. Res. Chin. Univ. 2024, 40, 887–893. [Google Scholar] [CrossRef]
- Sharma, N.; Mohammad, W.; Le Guével, X.; Shanavas, A. Gold Nanoclusters as High Resolution NIR-II Theranostic Agents. Chem. Biomed. Imaging 2024, 2, 462–480. [Google Scholar] [CrossRef]
- Jia, T.; Guan, Z.-J.; Zhang, C.; Zhu, X.-Z.; Chen, Y.-X.; Zhang, Q.; Yang, Y.; Sun, D. Eight-Electron Superatomic Cu31 Nanocluster with Chiral Kernel and NIR-II Emission. J. Am. Chem. Soc. 2023, 145, 10355–10363. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Shu, Q.; Xu, C.; Zheng, Q.; Guo, Z.; Wang, C.; Hao, Z.; Liu, X.; Wang, G.; et al. Copper Clusters: An Effective Antibacterial for Eradicating Multidrug-Resistant Bacterial Infection In Vitro and In Vivo. Adv. Funct. Mater. 2021, 31, 2008720. [Google Scholar] [CrossRef]
- Akhtar, N.; Choi, C.; Ateeq, M.; Fazil, P.; Shah, N.S.; Khan, J.A.; Al-Sehemi, A.G.; Zada, A.; Ali Shah, M.I.; Ikram, R.; et al. Visible Light Active CdS/CuO Nanocomposites for Photocatalytic Degradation of Ciprofloxacin, H2 Production and Antimicrobial Activity. Chem. Eng. J. 2025, 507, 160336. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, X.; Zhao, Z.; Sun, S.; Cheng, W.; Yu, H.; Chang, X.; Wang, B. CuO Nanozymes Catalyze Cysteine and Glutathione Depletion Induced Ferroptosis and Cuproptosis for Synergistic Tumor Therapy. Small 2024, 20, 2400326. [Google Scholar] [CrossRef]
- Gao, X.; Wei, M.; Ma, D.; Yang, X.; Zhang, Y.; Zhou, X.; Li, L.; Deng, Y.; Yang, W. Engineering of a Hollow-structured Cu2−XS Nano-homojunction Platform for near Infrared-triggered Infected Wound Healing and Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2106700. [Google Scholar] [CrossRef]
- Chen, X.; Huang, N.; Wang, D.; Zhang, M.; Deng, X.; Guo, F.; Yi, B.; Yuan, C.; Zhou, Q. Sulfated Chitosan-Modified CuS Nanocluster: A Versatile Nanoformulation for Simultaneous Antibacterial and Bone Regenerative Therapy in Periodontitis. ACS Nano 2024, 18, 14312–14326. [Google Scholar] [CrossRef]
- Li, L.; Xie, Y.; Wang, J.; Sun, Q.; Gao, M.; Li, C. Biofilm Microenvironment-Activated Multimodal Therapy Nanoplatform for Effective Anti-Bacterial Treatment and Wound Healing. Acta Biomater. 2024, 183, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; He, D.; Ge, X.; Lu, Y.; Chai, Y.; Zhang, Y.; Mao, Z.; Luo, G.; Deng, J.; Zhang, Y. Construction of Heparin-Based Hydrogel Incorporated with Cu5.4O Ultrasmall Nanozymes for Wound Healing and Inflammation Inhibition. Bioact. Mater. 2021, 6, 3109–3124. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Qiu, Y.; Lin, M.; Cui, K.; Chen, G.; Chen, Y.; Fan, C.; Zhang, Y.; Xu, L.; Chen, H.; et al. CuS Nanoparticles as a Photodynamic Nanoswitch for Abrogating Bypass Signaling to Overcome Gefitinib Resistance. Nano Lett. 2019, 19, 3344–3352. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, L.; Qiu, Y.; Liu, X.; Zhang, J.; Gao, Y.; Ge, L. Hollow Cubic CuSe@CdS with Tunable Size for Plasmon-Induced Vis-NIR Driven Photocatalytic Properties. Nanoscale 2024, 16, 8151–8161. [Google Scholar] [CrossRef]
- Wu, W.; Pu, Y.; Gao, S.; Shen, Y.; Zhou, M.; Yao, H.; Shi, J. Bacterial Metabolism-Initiated Nanocatalytic Tumor Immunotherapy. Nano-Micro Lett. 2022, 14, 220. [Google Scholar] [CrossRef]
- Jia, P.; Zou, Y.; Jiang, J. S-nitrosylated CuS Hybrid Hydrogel Patches with Robust Antibacterial and Repair-promoting Activity for Infected Wound Healing. Small 2024, 20, 2307629. [Google Scholar] [CrossRef]
- Liu, J.; Yu, B.; Rong, M.; Sun, W.; Lu, L. A New Strategy to Fight Tumor Heterogeneity: Integrating Metal-Defect Active Centers within NADH Oxidase Nanozymes. Nano Today 2024, 54, 102113. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Ren, L.; Shen, M.; Yang, K. Study on Antibacterial Performance of Cu-Bearing Cobalt-Based Alloy. Mater. Lett. 2014, 129, 88–90. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; et al. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem. Res. Toxicol. 2015, 28, 1815–1822. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Li, M.; Yu, P.; Chu, X.; Li, L.; Tan, G.; Wang, Y.; Chen, X.; Zhang, Y.; et al. The Synergistic Antibacterial Activity and Mechanism of Multicomponent Metal Ions-Containing Aqueous Solutions against Staphylococcus Aureus. J. Inorg. Biochem. 2016, 163, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, G.; Li, H.; Yang, L.; Wang, X.; Qin, G.; Zhang, E. Anti-Bacterium Influenced Corrosion Effect of Antibacterial Ti-3Cu Alloy in Staphylococcus aureus Suspension for Biomedical Application. Mater. Sci. Eng. C 2019, 94, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of Nano/Micro-Ag Compound Particles on the Bio-Corrosion, Antibacterial Properties and Cell Biocompatibility of Ti-Ag Alloys. Mater. Sci. Eng. C 2017, 75, 906–917. [Google Scholar] [CrossRef]
- Liu, R.; Tang, Y.; Zeng, L.; Zhao, Y.; Ma, Z.; Sun, Z.; Xiang, L.; Ren, L.; Yang, K. In Vitro and in Vivo Studies of Anti-Bacterial Copper-Bearing Titanium Alloy for Dental Application. Dent. Mater. 2018, 34, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.Y.; Wyatt, E.; Upadhyayula, S.; Whall, A.; Nuñez, V.; Vullev, V.I.; Liu, H. Degradation and Antibacterial Properties of Magnesium Alloys in Artificial Urine for Potential Resorbable Ureteral Stent Applications. J. Biomed. Mater. Res. 2014, 102, 781–792. [Google Scholar] [CrossRef]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T.A. Alkaline pH Homeostasis in Bacteria: New Insights. Biochim. Biophys. Acta (BBA)—Biomembr. 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Burne, R.A.; Marquis, R.E. Alkali Production by Oral Bacteria and Protection against Dental Caries. FEMS Microbiol. Lett. 2000, 193, 1–6. [Google Scholar] [CrossRef]
- Reece, S.Y.; Nocera, D.G. Proton-Coupled Electron Transfer in Biology: Results from Synergistic Studies in Natural and Model Systems. Annu. Rev. Biochem. 2009, 78, 673–699. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, X.; Liu, J.; Dai, W.; Luo, L.; Jiang, Z.; Gong, S.; Zhao, Y.; Li, Z. Designing Ni2MnSn Heusler Magnetic Nanoprecipitate in Copper Alloy for Increased Strength and Electromagnetic Shielding. Nat. Commun. 2024, 15, 10494. [Google Scholar] [CrossRef]
- Qin, S.; Sun, G.; Mao, X.; Zhang, X.; Zhou, B.; Li, M.; Chang, Y.; Zhang, Y.; Wang, L.; Li, M. Ultrafine-Grained Copper Alloy with Remarkable Improvement in Strength and Thermal Stability at High Temperatures. Mater. Sci. Eng. A 2024, 908, 146763. [Google Scholar] [CrossRef]
- Ciliveri, S.; Bandyopadhyay, A. Additively Manufactured SiO2 and Cu-Added Ti Implants for Synergistic Enhancement of Bone Formation and Antibacterial Efficacy. ACS Appl. Mater. Interfaces 2024, 16, 3106–3115. [Google Scholar] [CrossRef]
- Liu, F.; Liu, X.; Xie, G.; Wu, Y.; Chen, C. Studies on Thermal Stability, Softening Behavior and Mechanism of an ADS Copper Alloy at Elevated Temperatures. J. Mater. Sci. Technol. 2024, 186, 79–90. [Google Scholar] [CrossRef]
- Ke, J.G.; Liu, R.; Xie, Z.M.; Zhang, L.C.; Wang, X.P.; Fang, Q.F.; Liu, C.S.; Wu, X.B. Ultrahigh Strength, Thermal Stability and High Thermal Conductivity in Hierarchical Nanostructured Cu-W Alloy. Acta Mater. 2024, 264, 119547. [Google Scholar] [CrossRef]
- Mahmoudi, P.; Akbarpour, M.R.; Lakeh, H.B.; Jing, F.; Hadidi, M.R.; Akhavan, B. Antibacterial Ti–Cu Implants: A Critical Review on Mechanisms of Action. Mater. Today Bio 2022, 17, 100447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Chen, L.; Gong, F.; Yang, X.; Han, Z.; Wang, Y.; Ge, J.; Gao, X.; Li, Y.; Zhong, X.; et al. PtCu Nanosonosensitizers with Inflammatory Microenvironment Regulation for Enhanced Sonodynamic Bacterial Elimination and Tissue Repair. Adv. Funct. Mater. 2023, 33, 2212489. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Su, L.; Wang, M.; Jiang, Y.; Gong, S.; Xiao, Z.; Li, Z. The Electrochemical Corrosion Behavior and Antibacterial Properties of Cu-xFe Alloy. J. Mater. Res. Technol. 2024, 33, 698–713. [Google Scholar] [CrossRef]
- Ju, J.; Zan, R.; Shen, Z.; Wang, C.; Peng, P.; Wang, J.; Sun, B.; Xiao, B.; Li, Q.; Liu, S.; et al. Remarkable Bioactivity, Bio-Tribological, Antibacterial, and Anti-Corrosion Properties in a Ti-6Al-4V-xCu Alloy by Laser Powder Bed Fusion for Superior Biomedical Implant Applications. Chem. Eng. J. 2023, 471, 144656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).