Bio-Organic Fertilizer Modulates the Rhizosphere Microbiome to Enhance Sugarcane Growth and Suppress Smut Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Fertilizer Preparation
2.2. Experimental Design
2.3. Disease Assessment and Sample Collection
2.4. Determination of Enzyme Activities
2.5. Measurements of Soil Physicochemical Properties
2.6. DNA Extraction and High-Throughput Sequencing
2.7. Statistical Analysis
3. Results
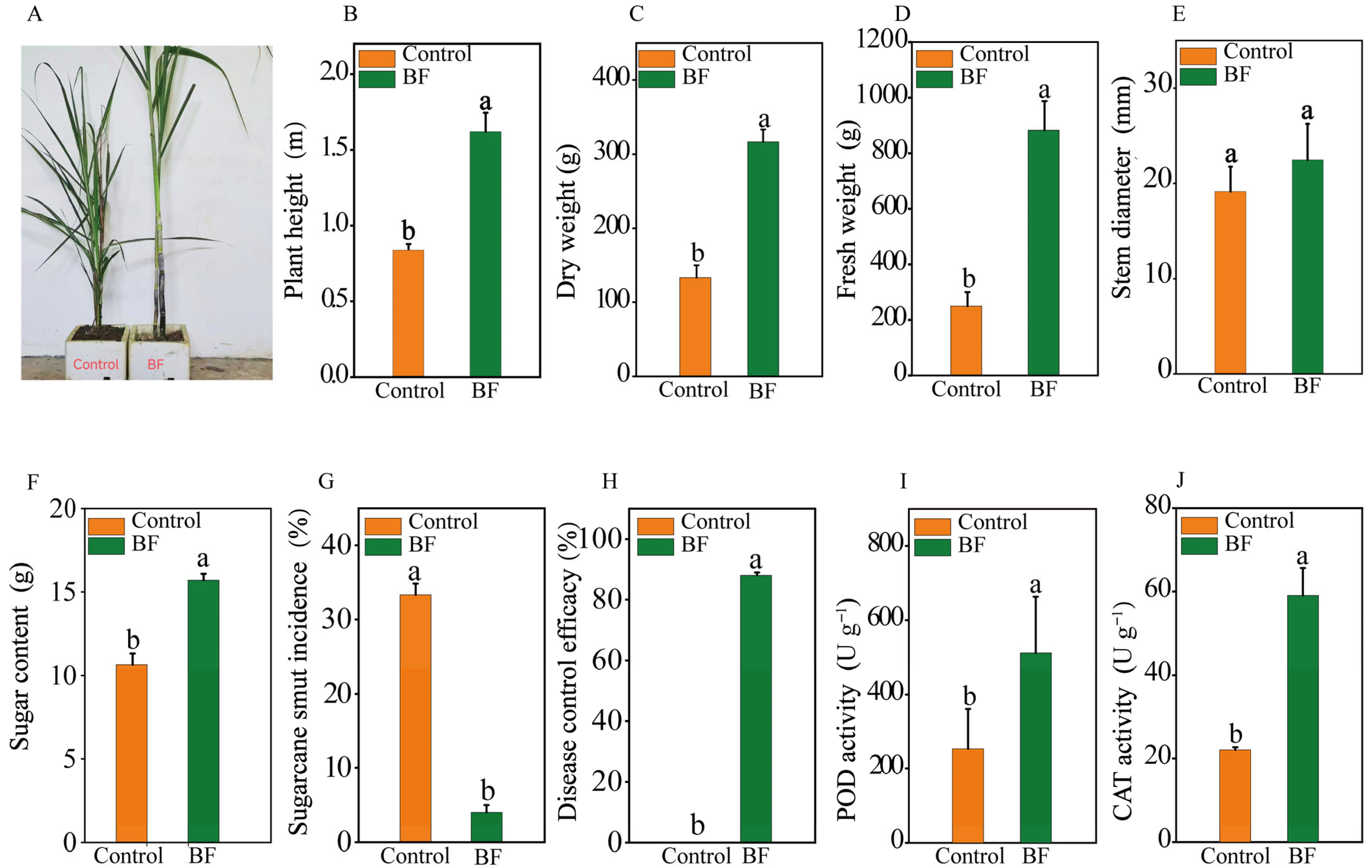
3.1. Effects of Bio-Organic Fertilizer Application on Sugarcane Growth, Disease Incidence and Enzyme Activities
3.2. Effects of Bio-Organic Fertilizer Application on Soil Physicochemical Properties
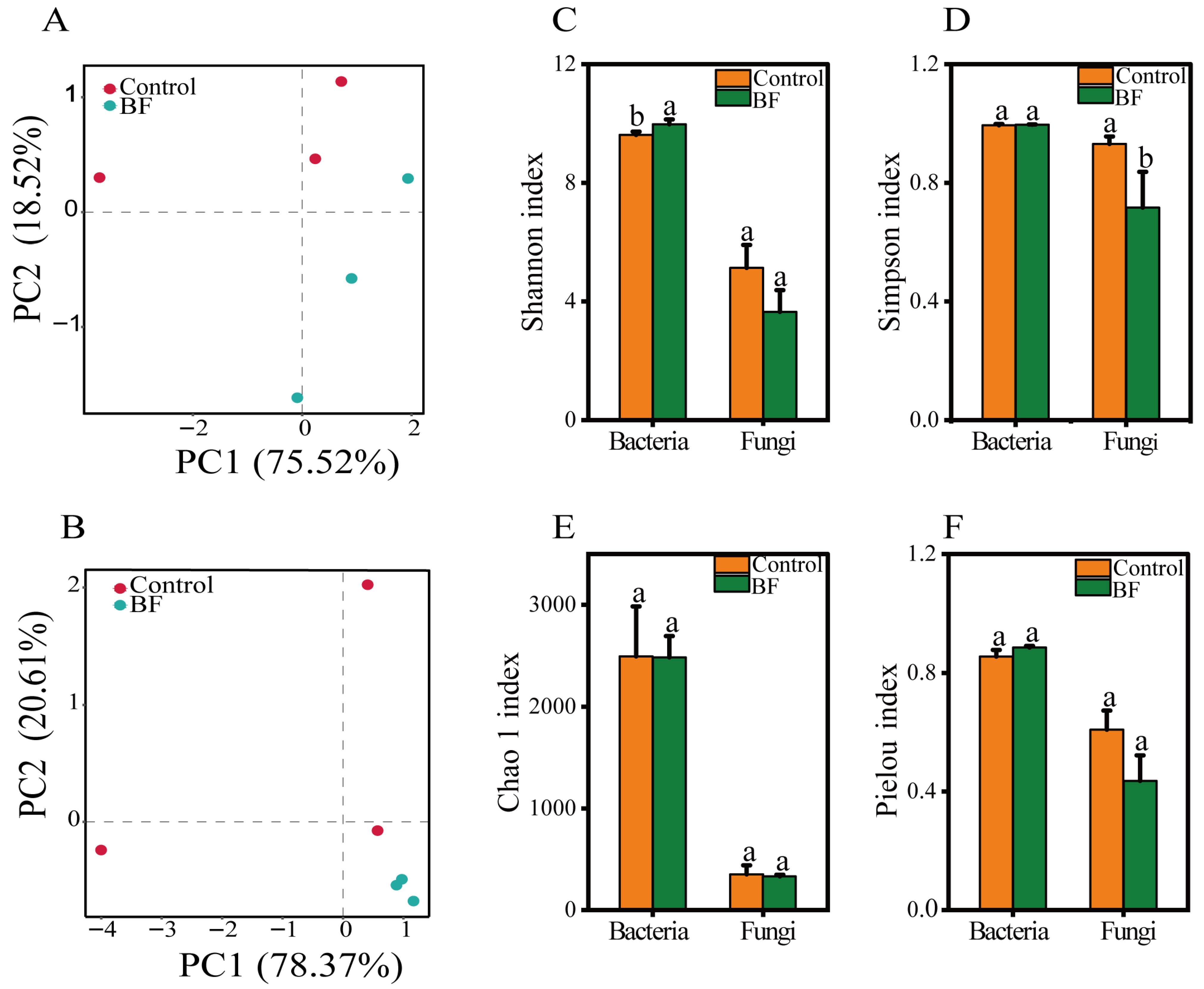
3.3. Effects of Bio-Organic Fertilizer Application on Rhizosphere Microbial Community Composition and Diversity
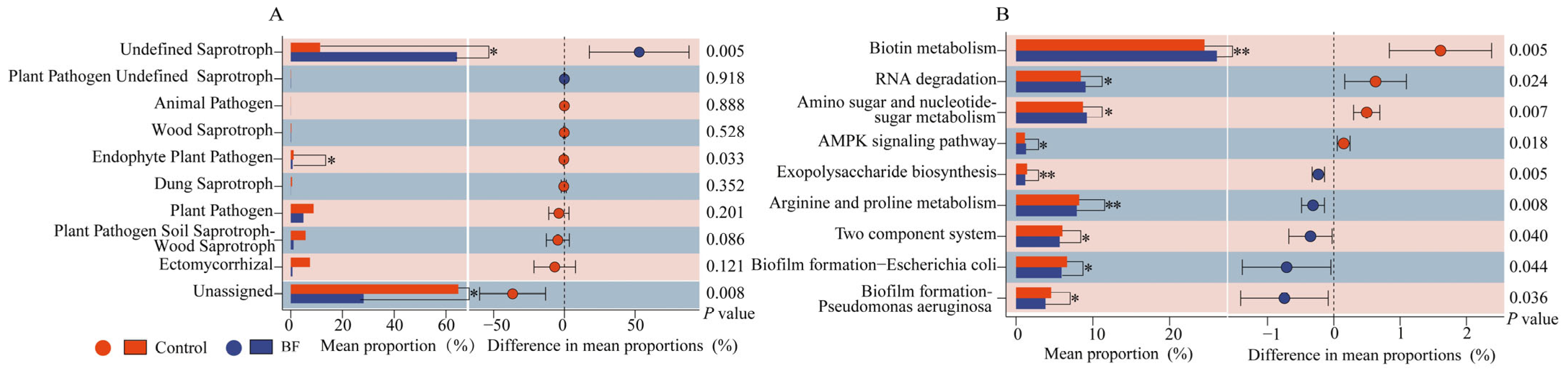
3.4. Functional Prediction of Rhizosphere Microbiota
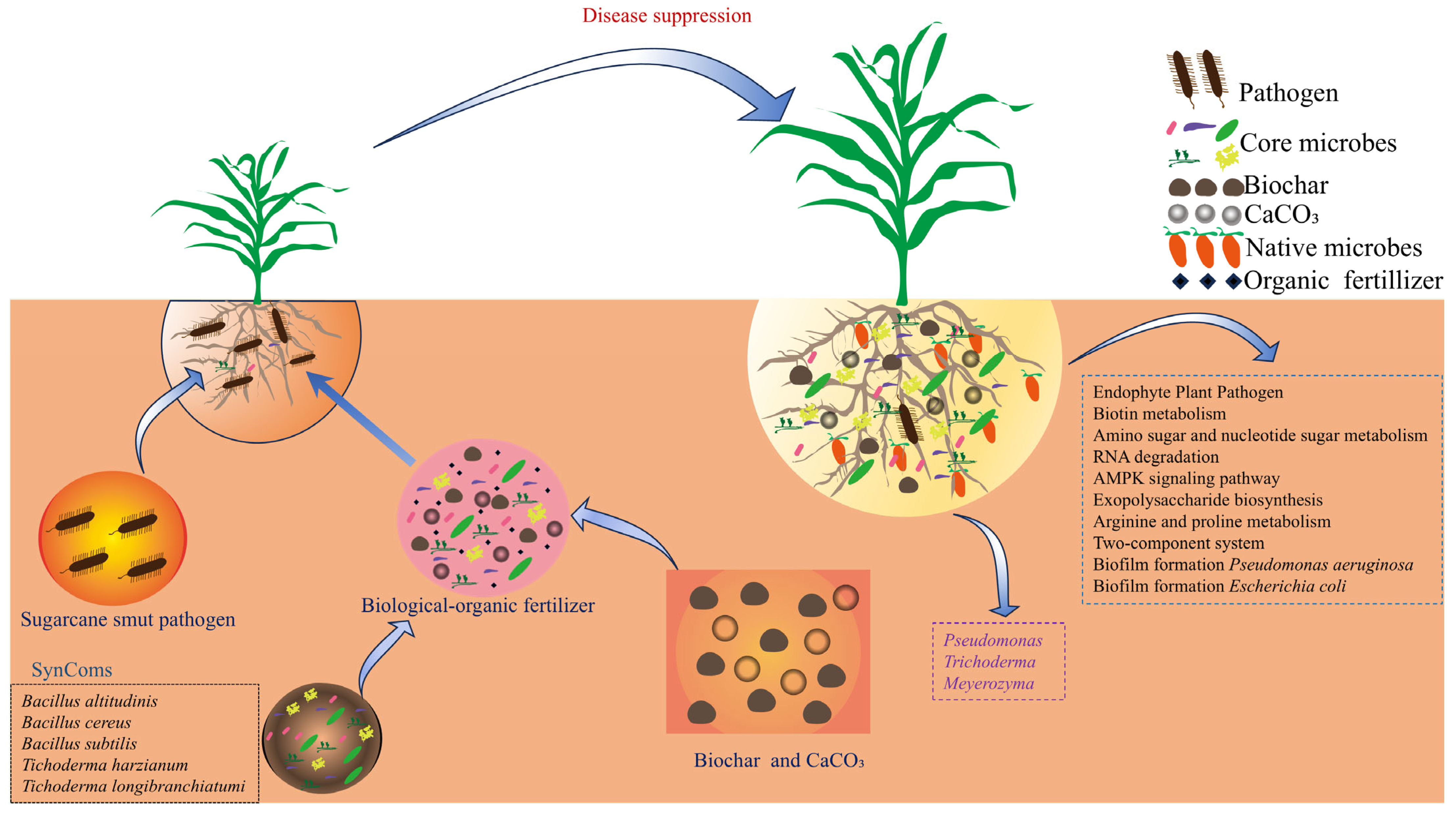
3.5. Correlations Between Rhizosphere Microbiota, Plant Growth, and Disease Suppression
4. Discussion
4.1. Bio-Organic Fertilizer Enhances Sugarcane Growth and Induces Systemic Resistance
4.2. Bio-Organic Fertilizer Modulates Rhizosphere Microbial Community Structure and Diversity
4.3. Functional Implications of Rhizosphere Microbiome Shifts
4.4. Linking Microbiome Changes to Plant Health and Disease Suppression
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, G.; Liu, P.; Wu, Q.; Zhang, S.; Zhao, P.; Zhang, Y.; Que, Y. Sugarcane breeding: A fantastic past and promising future driven by technology and methods. Front. Plant Sci. 2024, 15, 1375934. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, S.A.; Magarey, R.C.; McNeil, M.D.; Aitken, K.S. Sugarcane Smut, Caused by Sporisorium scitamineum, a Major Disease of Sugarcane: A Contemporary Review. Phytopathology 2021, 111, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Xu, L.; Wu, Q.; Liu, Y.; Ling, H.; Liu, Y.; Zhang, Y.; Guo, J.; Su, Y.; Chen, J. Genome sequencing of Sporisorium scitamineum provides insights into the pathogenic mechanisms of sugarcane smut. BMC Genom. 2014, 15, 996. [Google Scholar] [CrossRef]
- Che, R.; Tang, D.; Fu, B.; Yan, F.; Yan, M.; Wu, Y.; Yan, J.; Huang, K.-J.; Ya, Y.; Tan, X. Dual-modal improved biosensing platform for sugarcane smut pathogen based on biological enzyme-Mg2+ DNAzyme coupled with DNA transporter cascading hybridization chain reaction. Int. J. Biol. Macromol. 2024, 286, 138403. [Google Scholar] [CrossRef]
- Collins, O.C.; Mugabi, F.; Duffy, K.J. Dynamics and Control of Sugarcane Smut Using Deterministic and Stochastic Epidemic Models. Nat. Resour. Model. 2024, 38, e12417. [Google Scholar] [CrossRef]
- Rajput, M.A.; Rajput, N.A.; Syed, R.N.; Lodhi, A.M.; Que, Y. Sugarcane Smut: Current Knowledge and the Way Forward for Management. J. Fungi 2021, 7, 1095. [Google Scholar] [CrossRef]
- Bhattacharyya, C.; Roy, R.; Tribedi, P.; Ghosh, A.; Ghosh, A. Chapter 11—Biofertilizers as substitute to commercial agrochemicals. In Agrochemicals Detection, Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann: Amsterdam, The Netherlands, 2020; pp. 263–290. [Google Scholar]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Nabi, M. Chapter eleven—Role of microorganisms in plant nutrition and soil health. In Sustainable Plant Nutrition; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Fribourg, Switzerland, 2023; pp. 263–282. [Google Scholar]
- Sagar, L.; Singh, S.; Sharma, A.; Maitra, S.; Attri, M.; Sahoo, R.K.; Ghasil, B.P.; Shankar, T.; Gaikwad, D.J.; Sairam, M. Role of soil microbes against abiotic stresses induced oxidative stresses in plants. In Microbial Symbionts and Plant Health: Trends and Applications for Changing Climate; Mathur, P., Kapoor, R., Roy, S., Eds.; Springer Nature: Singapore, 2023; pp. 149–177. [Google Scholar]
- Kebede, M.T.; Mengstie, G.Y. Optimization the efficacy of plant growth-promoting rhizobacteria via genetic engineering. Discov. Plants 2025, 2, 154. [Google Scholar] [CrossRef]
- Oger, P.M.; Mansouri, H.; Nesme, X.; Dessaux, Y.M.E. Engineering root exudation of Lotus toward the production of two novel carbon compounds leads to the selection of distinct microbial populations in the rhizosphere. Microb. Ecol. 2004, 47, 96–103. [Google Scholar] [CrossRef]
- Khan, A.; Panthari, D.; Sharma, R.S.; Punetha, A.; Singh, A.V.; Upadhayay, V.K. Chapter 6—Biofertilizers: A microbial-assisted strategy to improve plant growth and soil health. In Advanced Microbial Techniques in Agriculture, Environment, and Health Management; Chandra Pandey, S., Pande, V., Sati, D., Samant, M., Eds.; Academic Press: New York, NY, USA, 2023; pp. 97–118. [Google Scholar]
- Bhattacharjee, R.; Dey, U. Biofertilizer, a way towards organic agriculture: A review. Afr. J. Microbiol. Res. 2014, 8, 2332–2343. [Google Scholar] [CrossRef]
- Wu, K.; Yuan, S.; Wang, L.; Shi, J.; Zhao, J.; Shen, B.; Shen, Q. Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol. Fertil. Soils 2014, 50, 961–971. [Google Scholar] [CrossRef]
- Chen, C.; Lv, Q.; Tang, Q. Impact of bio-organic fertilizer and reduced chemical fertilizer application on physical and hydraulic properties of cucumber continuous cropping soil. Biomass Convers. Biorefin. 2022, 14, 921–930. [Google Scholar] [CrossRef]
- Hou, J.; Yi, G.; Hao, Y.; Li, L.; Shen, L.; Zhang, Q. The effect of combined application of biochar and phosphate fertilizers on phosphorus transformation in saline-alkali soil and its microbiological mechanism. Sci. Total Environ. 2024, 951, 175610. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Glob. Change Biol. Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, R.; Shen, Y.; Yuan, J.; Wang, L.; Luo, X.; Raza, W.; Ling, N.; Huang, Q.; Shen, Q. Development of a novel bio-organic fertilizer for plant growth promotion and suppression of rhizome rot in ginger. Biol. Control 2017, 114, 97–105. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, C.; Yang, X.; Mei, X.; Ran, W.; Shen, Q.; Xu, Y. Biocontrol of Fusarium wilt disease for Cucumis melo melon using bio-organic fertilizer. Appl. Soil Ecol. 2011, 47, 67–75. [Google Scholar] [CrossRef]
- Herrera-Vasquez, A.; Schlechter, R.; Armijo-Godoy, G.; Monteoliva, M.I. Editorial: Pathogen suppression by plant-associated microbiota. Front. Plant Sci. 2025, 16, 1604449. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Li, D. Unearthing the mechanisms underlying calcium carbonate therapies for eliminating pathogens during composting. Chem. Eng. J. 2023, 451, 139087. [Google Scholar] [CrossRef]
- Liu, Q.; He, X.; Wang, K.; Li, D. Biochar drives humus formation during composting by regulating the specialized metabolic features of microbiome. Chem. Eng. J. 2023, 458, 141380. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, Z.; Tang, S.; Xie, Q.; He, X.; Li, D. Synthetic microbial community enhances lignocellulose degradation during composting by assembling fungal communities. Bioresour. Technol. 2025, 419, 132068. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, Z.; Lv, F.; Bie, X.; Liu, S.; Ding, Z.; Xu, W. A newly isolated organic solvent tolerant Staphylococcus saprophyticus M36 produced organic solvent-stable lipase. Curr. Microbiol. 2006, 53, 510–515. [Google Scholar] [CrossRef]
- Sa, R.; He, S.; Han, D.; Liu, M.; Yu, Y.; Shang, R.; Song, M. Isolation and identification of a new biocontrol bacteria against Salvia miltiorrhiza root rot and optimization of culture conditions for antifungal substance production using response surface methodology. BMC Microbiol 2022, 22, 231. [Google Scholar] [CrossRef] [PubMed]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- de Souza, R.S.C.; Armanhi, J.S.L.; Arruda, P. From Microbiome to Traits: Designing Synthetic Microbial Communities for Improved Crop Resiliency. Front. Plant Sci. 2020, 11, 1179. [Google Scholar] [CrossRef]
- Sun, X.; Xu, S.; Lu, H. Non-Destructive Identification and Estimation of Granulation in Honey Pomelo Using Visible and Near-Infrared Transmittance Spectroscopy Combined with Machine Vision Technology. Appl. Sci. 2020, 10, 5399. [Google Scholar] [CrossRef]
- Spitzer, C.M.; Wardle, D.A.; Lindahl, B.D.; Sundqvist, M.K.; Gundale, M.J.; Fanin, N.; Kardol, P. Root traits and soil micro-organisms as drivers of plant–soil feedbacks within the sub-arctic tundra meadow. J. Ecol. 2021, 110, 466–478. [Google Scholar] [CrossRef]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [CrossRef] [PubMed]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Aloo, B.N.; Tripathi, V.; Makumba, B.A.; Mbega, E.R. Plant growth-promoting rhizobacterial biofertilizers for crop production: The past, present, and future. Front. Plant Sci. 2022, 13, 1002448. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, L.; Lv, W.; Zhang, H.; Zhang, Y.; Zhang, H.; Zhang, H.; Zhu, Z.; Ge, T.; Zhang, W. Long-term bioorganic and organic fertilization improved soil quality and multifunctionality under continuous cropping in watermelon. Agric. Ecosyst. Environ. 2024, 359, 108721. [Google Scholar] [CrossRef]
- Montalba, R.; Arriagada, C.; Alvear, M.; Zúñiga, G.E. Effects of conventional and organic nitrogen fertilizers on soil microbial activity, mycorrhizal colonization, leaf antioxidant content, and Fusarium wilt in highbush blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2010, 125, 775–778. [Google Scholar] [CrossRef]
- Wu, H.-S.; Yang, X.-N.; Fan, J.-Q.; Miao, W.-G.; Ling, N.; Xu, Y.-c.; Huang, Q.-W.; Shen, Q. Suppression of Fusarium wilt of watermelon by a bio-organic fertilizer containing combinations of antagonistic microorganisms. BioControl 2008, 54, 287–300. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Nahar, K.; Hossain, M.; Mahmud, J.; Hossen, M.; Masud, A.; Moumita; Fujita, M. Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Leontidou, K.; Genitsaris, S.; Papadopoulou, A.; Kamou, N.; Bosmali, I.; Matsi, T.; Madesis, P.; Vokou, D.; Karamanoli, K.; Mellidou, I. Plant growth promoting rhizobacteria isolated from halophytes and drought-tolerant plants: Genomic characterisation and exploration of phyto-beneficial traits. Sci. Rep. 2020, 10, 14857. [Google Scholar] [CrossRef]
- Preston, G.M. Plant perceptions of plant growth-promoting Pseudomonas. Philos. Trans. R. Soc. B-Biol. Sci. 2004, 359, 907–918. [Google Scholar] [CrossRef]
- Alattas, H.; Glick, B.R.; Murphy, D.V.; Scott, C. Harnessing Pseudomonas spp. for sustainable plant crop protection. Front. Microbiol. 2024, 15, 1485197. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- De Marco, L.; Epis, S.; Capone, A.; Martin, E.; Bozic, J.; Crotti, E.; Ricci, I.; Sassera, D. The Genomes of Four Meyerozyma caribbica Isolates and Novel Insights into the Meyerozyma guilliermondii Species Complex. G3 Genes Genomes Genet. 2018, 8, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Wang, S.-Y.; Wang, C.-X.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Antagonistic mechanisms of yeasts Meyerozyma guilliermondii and M. caribbica for the control of plant pathogens: A review. Biol. Control 2023, 186, 105333. [Google Scholar] [CrossRef]
- Bonanomi, G.; Lorito, M.; Vinale, F.; Woo, S.L. Organic Amendments, Beneficial Microbes, and Soil Microbiota: Toward a Unified Framework for Disease Suppression. Annu. Rev. Phytopathol. 2018, 56, 1–20. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The plant endosphere world—Bacterial life within plants. Environ. Microbiol 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Roux, C.; Etienne, T.A.; Hajnsdorf, E.; Ropers, D.; Carpousis, A.J.; Cocaign-Bousquet, M.; Girbal, L. The essential role of mRNA degradation in understanding and engineering E. coli metabolism. Biotechnol. Adv. 2022, 54, 107805. [Google Scholar] [CrossRef]
- Silwal, P.; Kim, J.K.; Yuk, J.M.; Jo, E.K. AMP-Activated Protein Kinase and Host Defense against Infection. Int. J. Mol. Sci. 2018, 19, 3495. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]



| Variables | Control | Bio-Organic Fertilizer |
|---|---|---|
| pH | 6.82 ± 0.04 b | 6.99 ± 0.04 a |
| Soil organic carbon (g/kg) | 21.73 ± 2.88 a | 21.46 ± 0.60 a |
| Total N (g/kg) | 1.91 ± 0.02 a | 2.08 ± 0.24 a |
| Total P (g/kg) | 2.62 ± 0.33 a | 2.38 ± 0.10 a |
| Total K (g/kg) | 25.17 ± 3.02 a | 20.33 ± 1.30 a |
| Available N (mg/kg) | 200.1 ± 20.5 a | 190.3 ± 18.7 a |
| Available P (mg/kg) | 51.2 ± 0.9 a | 63.4 ± 1.2 a |
| Available K (mg/kg) | 880.5 ± 10.1 b | 2130.7 ± 60.3 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; He, X.; Liu, Q.; Gao, F.; Zeng, C.; Li, D. Bio-Organic Fertilizer Modulates the Rhizosphere Microbiome to Enhance Sugarcane Growth and Suppress Smut Disease. Microorganisms 2025, 13, 2563. https://doi.org/10.3390/microorganisms13112563
Chen F, He X, Liu Q, Gao F, Zeng C, Li D. Bio-Organic Fertilizer Modulates the Rhizosphere Microbiome to Enhance Sugarcane Growth and Suppress Smut Disease. Microorganisms. 2025; 13(11):2563. https://doi.org/10.3390/microorganisms13112563
Chicago/Turabian StyleChen, Fei, Xunyang He, Qiumei Liu, Fulai Gao, Chaozhen Zeng, and Dejun Li. 2025. "Bio-Organic Fertilizer Modulates the Rhizosphere Microbiome to Enhance Sugarcane Growth and Suppress Smut Disease" Microorganisms 13, no. 11: 2563. https://doi.org/10.3390/microorganisms13112563
APA StyleChen, F., He, X., Liu, Q., Gao, F., Zeng, C., & Li, D. (2025). Bio-Organic Fertilizer Modulates the Rhizosphere Microbiome to Enhance Sugarcane Growth and Suppress Smut Disease. Microorganisms, 13(11), 2563. https://doi.org/10.3390/microorganisms13112563







