Gut Microbiome and Immune System Crosstalk in Chronic Inflammatory Diseases: A Narrative Review of Mechanisms and Therapeutic Opportunities
Abstract
1. Introduction
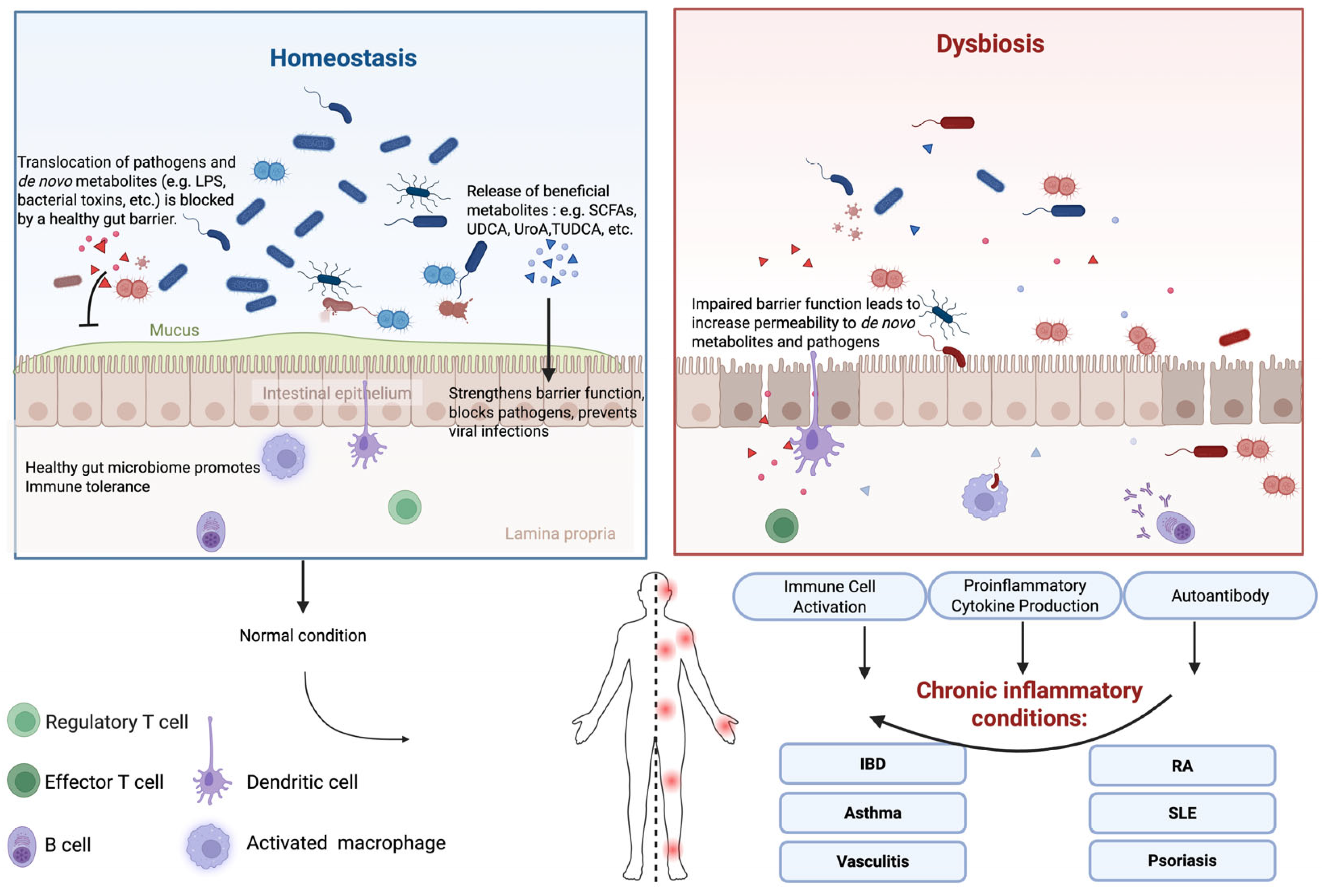
2. Overview of Gut Microbiome-Driven Immune and Inflammatory Modulation
2.1. Gut Epithelial Barrier
2.2. Interactions with Immune Cells
2.2.1. Toll-Like Receptors
2.2.2. Proinflammatory and Anti-Inflammatory Cytokines
3. Gut Microbiota and Chronic Inflammatory Diseases
3.1. Rheumatoid Arthritis (RA)
3.1.1. General Understanding of RA Pathogenesis
3.1.2. Microbiome and RA Pathogenesis
3.1.3. Therapeutics of RA Targeting Gut Microbiome: Current and Future
3.2. Inflammatory Bowel Disease (IBD)
3.2.1. Gut Microbiome and IBD Pathogenesis
3.2.2. Certain Gut Microbes and IBD
3.2.3. Current Microbiota-Targeted Interventions for IBD
3.2.4. Future Directions for Microbial Therapies of IBD
3.3. Psoriasis
3.3.1. General Understanding of Psoriasis Pathogenesis
3.3.2. Gut Microbiome and Psoriasis Pathogenesis
3.3.3. Therapeutics of Psoriasis Targeting Gut Microbiome: Current and Future
3.4. Systemic Lupus Erythematosus (SLE)
3.4.1. Disease Pathogenesis
3.4.2. Gut Microbiome and SLE
3.4.3. Microbial Therapeutic Strategies/Interventions and Future Directions
3.5. Asthma
3.5.1. Evidence of Gut Microbiome Association
3.5.2. The Gut-Lung Axis in Asthma Pathogenesis
3.5.3. Gut Microbiome-Based Therapeutics
3.5.4. Future Directions
3.6. Vasculitis
3.6.1. Gut Microbiome and Vasculitis Pathogenesis
3.6.2. Microbial Interventions and Future Direction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANAs | antinuclear antibodies |
| BEVs | microbiota-derived extracellular vesicles |
| CD | Crohn’s disease |
| COPD | chronic obstructive pulmonary disease |
| DCs | dendritic cells |
| FMT | fecal microbiota transplantation |
| FOS | fructo-oligosaccharides |
| GI | gastrointestinal |
| HDACs | histone deacetylases |
| HLA | human leukocyte antigen |
| IBD | inflammatory bowel disease |
| IgA | immunoglobulin A |
| IgAV | immunoglobulin A vasculitis |
| IL | interleukin |
| ILC2 | innate lymphoid cells type 2 |
| KD | Kawasaki disease |
| LPS | lipopolysaccharides |
| MAMPs | microbial-associated molecular patterns |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor kappa B |
| pDCs | plasmacytoid dendritic cells |
| PRRs | pattern recognition receptors |
| RA | rheumatoid arthritis |
| SCFAs | short-chain fatty acids |
| SLE | systemic lupus erythematosus |
| Th17 | T helper 17 |
| TLR | toll-like receptor |
| TNF-α | tumor necrosis factor-alpha |
| Tregs | regulatory T cells |
| TUDCA | tauroursodeoxycholic acid |
| UC | ulcerative colitis |
| UDCA | ursodeoxycholic acid |
References
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Michie, L.; O Tucker, H. Influence of Commensal Microbiota in Barrier Function of Intestinal Mucosal Epithelium. Adv. Res. Endocrinol. Metab. 2019, 1, 33–36. [Google Scholar]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Lo, B.C.; Chen, G.Y.; Núñez, G.; Caruso, R. Gut microbiota and systemic immunity in health and disease. Int. Immunol. 2021, 33, 197–209. [Google Scholar] [CrossRef]
- Dey, P. Mechanisms and implications of the gut microbial modulation of intestinal metabolic processes. npj Metab. Health Dis. 2025, 3, 24. [Google Scholar] [CrossRef]
- Ferranti, E.P.; Dunbar, S.B.P.; Dunlop, A.L.; Corwin, E.J.P. 20 things you didn’t know about the human gut microbiome. J. Cardiovasc. Nurs. 2014, 29, 479–481. [Google Scholar] [CrossRef]
- Krishnamurthy, K.H.; Pereira, M.; Bosco, J.; George, J.; Jayaraman, V.; Krishna, K.; Wang, T.; Bei, K.; Rajasekaran, J.J. Gut commensals and their metabolites in health and disease. Front. Microbiol. 2023, 8, 1244293. [Google Scholar] [CrossRef]
- Zhang, Y.; Fleur, A.S.; Feng, H. The development of live biotherapeutics against Clostridioides difficile infection towards reconstituting gut microbiota. Gut Microbes 2022, 14, 2052698. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Maciel-Fiuza, M.F.; Muller, G.C.; Campos, D.M.S.; Costa, P.D.S.S.; Peruzzo, J.; Bonamigo, R.R.; Veit, T.; Vianna, F.S.L. Role of gut microbiota in infectious and inflammatory diseases. Front. Microbiol. 2023, 14, 1098386. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Steele, J.; Chen, K.; Sun, X.; Zhang, Y.; Wang, H.; Tzipori, S.; Feng, H. Systemic dissemination of Clostridium difficile toxins A and B is associated with severe, fatal disease in animal models. J. Infect. Dis. 2011, 205, 384–391. [Google Scholar] [CrossRef]
- Spadoni, I.; Zagato, E.; Bertocchi, A.; Paolinelli, R.; Hot, E.; Di Sabatino, A.; Caprioli, F.; Bottiglieri, L.; Oldani, A.; Viale, G.; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830–834. [Google Scholar] [CrossRef]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef]
- Segui-Perez, C.; Huang, L.Z.X.; Paganelli, F.L.; Lievens, E.; Strijbis, K. Probiotic Bifidobacterium bifidum strains desialylate MUC13 and increase intestinal epithelial barrier function. Sci. Rep. 2025, 15, 8778. [Google Scholar] [CrossRef]
- Kara, S.; Volkan, B.; Erten, I. Lactobacillus rhamnosus GG can protect malnourished children. Benef. Microbes 2019, 10, 237–244. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Reytor, D.; Puebla, C.; Karahanian, E.; García, K. Use of Short-Chain Fatty Acids for the Recovery of the Intestinal Epithelial Barrier Affected by Bacterial Toxins. Front. Physiol. 2021, 12, 650313. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.W.; Sams, A. The Microbiotic Highway to Health-New Perspective on Food Structure, Gut Microbiota, and Host Inflammation. Nutrients 2018, 10, 1590. [Google Scholar] [CrossRef]
- Van den Bossche, L.; Hindryckx, P.; Devisscher, L.; Devriese, S.; Van Welden, S.; Holvoet, T.; Vilchez-Vargas, R.; Vital, M.; Pieper, D.H.; Van den Bussche, J.; et al. Ursodeoxycholic Acid and Its Taurine- or Glycine-Conjugated Species Reduce Colitogenic Dysbiosis and Equally Suppress Experimental Colitis in Mice. Appl. Environ. Microbiol. 2017, 83, e02766-16. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.; von der Weid, P.-Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef]
- Preet, R.; Islam, A.; Shim, J.; Rajendran, G.; Mitra, A.; Vishwakarma, V.; Kutz, C.; Choudhury, S.; Pathak, H.; Dai, Q.; et al. Gut commensal Bifidobacterium-derived extracellular vesicles modulate the therapeutic effects of anti-PD-1 in lung cancer. Nat. Commun. 2025, 16, 3500. [Google Scholar] [CrossRef]
- Nie, X.; Li, Q.; He, Y.; Xu, Y.; Qiao, S.; Wang, X.; Meng, F.; Xie, J.; Nie, S. Engineered bacterial extracellular vesicles for gastrointestinal diseases. J. Control. Release 2025, 385, 113972. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhang, Y.; Song, M.; Fan, J.; Feng, S.; Li, J.; Wu, Z.; Zuo, B.; Tao, S.; Liu, X. Lactobacillus alleviates intestinal epithelial barrier dysfunction through GPR43-mediated M2 macrophage polarization. Anim. Dis. 2024, 4, 20. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.; Zhu, H.; Zhao, T.; Tu, J.; Yin, X.; Yang, S.; Zhang, W.; Zhang, F.; Zhang, M.; et al. Gut microbiota promotes macrophage M1 polarization in hepatic sinusoidal obstruction syndrome via regulating intestinal barrier function mediated by butyrate. Gut Microbes 2024, 16, 2377567. [Google Scholar] [CrossRef]
- Xie, Q.; Li, Q.; Fang, H.; Zhang, R.; Tang, H.; Chen, L. Gut-Derived Short-Chain Fatty Acids and Macrophage Modulation: Exploring Therapeutic Potentials in Pulmonary Fungal Infections. Clin. Rev. Allergy Immunol. 2024, 66, 316–327. [Google Scholar] [CrossRef]
- Gaskarth, D.A.; Fan, S.; Highton, A.J.; Kemp, R.A. The microbial metabolite butyrate enhances the effector and memory functions of murine CD8+ T cells and improves anti-tumor activity. Front. Med. 2025, 12, 1577906. [Google Scholar] [CrossRef]
- Akimova, T.; Ge, G.; Golovina, T.; Mikheeva, T.; Wang, L.; Riley, J.L.; Hancock, W.W. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin. Immunol. 2010, 136, 348–363. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8(+) T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, L.; Chu, Y. Gut microbiota shape B cell in health and disease settings. J. Leukoc. Biol. 2021, 110, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, C.; Magri, G.; Cerutti, A. Intestinal IgA production and its role in host-microbe interaction. Immunol. Rev. 2014, 260, 76–85. [Google Scholar] [CrossRef]
- Fukata, M.; Arditi, M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol. 2013, 6, 451–463. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Luo, R.; Yao, Y.; Chen, Z.; Sun, X. An examination of the LPS-TLR4 immune response through the analysis of molecular structures and protein-protein interactions. Cell Commun. Signal. 2025, 23, 142. [Google Scholar] [CrossRef]
- Rogier, R.; Koenders, M.I.; Abdollahi-Roodsaz, S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J. Immunol. Res. 2015, 2015, 527696. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.S. How flagellin and toll-like receptor 5 contribute to enteric infection. Infect. Immun. 2007, 75, 545–552. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.; Chen, S.; He, R.; Chao, G.; Zhang, S. TLR5 Signaling in the Regulation of Intestinal Mucosal Immunity. J. Inflamm. Res. 2023, 16, 2491–2501. [Google Scholar] [CrossRef]
- Oliviera-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- McClure, R.; Massari, P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front. Immunol. 2014, 5, 386. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.E.; Cho, K.; Stickling, C.; Reynolds, J.M. Toll-like Receptor 2 in Autoimmune Inflammation. Immune Netw. 2021, 21, e18. [Google Scholar] [CrossRef]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-Like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Smits, H.H.; Engering, A.; Van Der Kleij, D.; De Jong, E.C.; Schipper, K.; Van Capel, T.M.M.; Zaat, B.A.J.; Yazdanbakhsh, M.; Wierenga, E.A.; Van Kooyk, Y.; et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, T.; Olesińska, M.; Paradowska-Gorycka, A. Current Understanding of an Emerging Role of HLA-DRB1 Gene in Rheumatoid Arthritis-From Research to Clinical Practice. Cells 2020, 9, 1127. [Google Scholar] [CrossRef]
- Snir, O.; Widhe, M.; von Spee, C.; Lindberg, J.; Padyukov, L.; Lundberg, K.; Engström, Å.; Venables, P.J.; Lundeberg, J.; Holmdahl, R.; et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: Association with HLA-DRB1 alleles. Ann. Rheum. Dis. 2009, 68, 736–743. [Google Scholar] [CrossRef]
- Dedmon, L.E. The genetics of rheumatoid arthritis. Rheumatolog 2020, 59, 2661–2670. [Google Scholar] [CrossRef]
- Black, R.J.; Cross, M.; Haile, L.M.; Culbreth, G.T.; Steinmetz, J.D.; Hagins, H.; Kopec, J.A.; Brooks, P.M.; Woolf, A.D.; Ong, K.L.; et al. Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e594–e610. [Google Scholar] [CrossRef]
- Ferro, M.; Charneca, S.; Dourado, E.; Guerreiro, C.S.; Fonseca, J.E. Probiotic Supplementation for Rheumatoid Arthritis: A Promising Adjuvant Therapy in the Gut Microbiome Era. Front. Pharmacol. 2021, 12, 711788. [Google Scholar] [CrossRef]
- Liu, L.; Xie, S. Dietary fiber intake associated with risk of rheumatoid arthritis among U.S. adults: NHANES 2010–2020. Medicine 2023, 102, e33357. [Google Scholar] [CrossRef]
- Zeng, J.; Peng, L.; Zheng, W.; Huang, F.; Zhang, N.; Wu, D.; Yang, Y. Fecal microbiota transplantation for rheumatoid arthritis: A case report. Clin. Case Rep. 2021, 9, 906–909. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, S.; Zhang, D. Global, regional and national burden of inflammatory bowel disease in 204 countries and territories from 1990 to 2019: A systematic analysis based on the Global Burden of Disease Study 2019. BMJ Open 2023, 13, e065186. [Google Scholar] [CrossRef]
- El Hadad, J.; Schreiner, P.; Vavricka, S.R.; Greuter, T. The Genetics of Inflammatory Bowel Disease. Mol. Diagn. Ther. 2024, 28, 27–35. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109. [Google Scholar] [CrossRef]
- Jacob, V.; Crawford, C.; Cohen-Mekelburg, S.; Viladomiu, M.; Putzel, G.G.; Schneider, Y.; Chabouni, F.; O’neil, S.; Bosworth, B.; Woo, V.; et al. Single Delivery of High-Diversity Fecal Microbiota Preparation by Colonoscopy Is Safe and Effective in Increasing Microbial Diversity in Active Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhang, S.; Wang, R.; Wu, X.; Chen, Y.; Wen, Q.; Cui, B.; Zhang, F.; Li, P. Fecal microbiota transplantation improves bile acid malabsorption in patients with inflammatory bowel disease: Results of microbiota and metabolites from two cohort studies. BMC Med. 2025, 23, 511. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Report on Psoriasis. World Health Organization. 2016. Available online: https://iris.who.int/handle/10665/204417 (accessed on 28 October 2025).
- Yan, F.; Tao, J.; Liu, J.; Chen, Y.; Huang, Z. Cross-tissue transcriptome-wide association study reveals novel psoriasis susceptibility genes. J. Transl. Autoimmun. 2025, 10, 100286. [Google Scholar] [CrossRef]
- Patel, H.A.; Revankar, R.R.; Pedroza, S.T.; Graham, S.; Feldman, S.R. The Genetic Susceptibility to Psoriasis and the Relationship of Linked Genes to Our Treatment Options. Int. J. Mol. Sci. 2023, 24, 12310. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yao, X.; Huang, Y.; Lu, Q. Global epidemiology of systemic lupus erythematosus: A comprehensive systematic analysis and modelling study. Ann. Rheum. Dis. 2023, 82, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Yi, P.; Zhu, M.; Zhou, W.; Zhang, B.; Yi, X.; Long, H.; Zhang, G.; Wu, H.; Tsokos, G.C.; et al. Safety and efficacy of fecal microbiota transplantation for treatment of systemic lupus erythematosus: An EXPLORER trial. J. Autoimmun. 2022, 130, 102844, Erratum in J. Autoimmun. 2022, 131, 102862. [Google Scholar] [CrossRef]
- Widhani, A.; Djauzi, S.; Suyatna, F.D.; Dewi, B.E. Changes in Gut Microbiota and Systemic Inflammation after Synbiotic Supplementation in Patients with Systemic Lupus Erythematosus: A Randomized, Double-Blind, Placebo-Controlled Trial. Cells 2022, 11, 3419. [Google Scholar] [CrossRef]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Liang, Y.; Fan, Y.; Wang, W. Genetic polymorphisms in genes involved in the type I interferon system (STAT4 and IRF5): Association with Asian SLE patients. Clin. Rheumatol. 2024, 43, 2403–2416. [Google Scholar] [CrossRef]
- Oh, J.; Kim, S.; Kim, M.S.; Abate, Y.H.; ElHafeez, S.A.; Abdelkader, A.; Abdi, P.; Abdulah, D.M.; Aboagye, R.G.; Abolhassani, H.; et al. Global, regional, and national burden of asthma and atopic dermatitis, 1990–2021, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 425–446. [Google Scholar] [CrossRef]
- Ntontsi, P.; Photiades, A.; Zervas, E.; Xanthou, G.; Samitas, K. Genetics and Epigenetics in Asthma. Int. J. Mol. Sci. 2021, 22, 2412. [Google Scholar] [CrossRef]
- Watts, R.A.; Hatemi, G.; Burns, J.C.; Mohammad, A.J. Global epidemiology of vasculitis. Nat. Rev. Rheumatol. 2022, 18, 22–34. [Google Scholar] [CrossRef]
- Onouchi, Y. The genetics of Kawasaki disease. Int. J. Rheum. Dis. 2018, 21, 26–30. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Zamani, M.R.; Shabgah, A.G.; Hosseini, S.; Mollaei, F.; Hosseini, N.; Sahebkar, A. Behçet’s disease: An immunogenetic perspective. J. Cell. Physiol. 2019, 234, 8055–8074. [Google Scholar] [CrossRef]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef]
- Romero-Figueroa, M.D.S.; Ramírez-Durán, N.; Montiel-Jarquín, A.J.; Horta-Baas, G. Gut-joint axis: Gut dysbiosis can contribute to the onset of rheumatoid arthritis via multiple pathways. Front. Cell. Infect. Microbiol. 2023, 13, 1092118. [Google Scholar] [CrossRef]
- Maresz, K.J.; Hellvard, A.; Sroka, A.; Adamowicz, K.; Bielecka, E.; Koziel, J.; Gawron, K.; Mizgalska, D.; A Marcinska, K.; Benedyk, M.; et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 2013, 9, e1003627. [Google Scholar] [CrossRef]
- Scher, J.U.; Sczesnak, A.; Longman, R.S.; Segata, N.; Ubeda, C.; Bielski, C.; Rostron, T.; Cerundolo, V.; Pamer, E.G.; Abramson, S.B.; et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2013, 2, e01202. [Google Scholar] [CrossRef]
- Chriswell, M.E.; Lefferts, A.R.; Clay, M.R.; Hsu, A.R.; Seifert, J.; Feser, M.L.; Rims, C.; Bloom, M.S.; Bemis, E.A.; Liu, S.; et al. Clonal IgA and IgG autoantibodies from individuals at risk for rheumatoid arthritis identify an arthritogenic strain of Subdoligranulum. Sci. Transl. Med. 2022, 14, eabn5166. [Google Scholar] [CrossRef]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Xu, H.; Liu, M.; Cao, J.; Li, X.; Fan, D.; Xia, Y.; Lu, X.; Li, J.; Ju, D.; Zhao, H. The Dynamic Interplay between the Gut Microbiota and Autoimmune Diseases. J. Immunol. Res. 2019, 2019, 7546047. [Google Scholar] [CrossRef]
- Kriegel, M.A. Subdoligranulum chews up joints: How a gut pathobiont can instigate arthritis. Trends Immunol. 2023, 44, 4–6. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef]
- Alipour, B.; Homayouni-Rad, A.; Vaghef-Mehrabany, E.; Sharif, S.K.; Vaghef-Mehrabany, L.; Asghari-Jafarabadi, M.; Nakhjavani, M.R.; Mohtadi-Nia, J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: A randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014, 17, 519–527. [Google Scholar] [CrossRef]
- Gupta, V.K.; Cunningham, K.Y.; Hur, B.; Bakshi, U.; Huang, H.; Warrington, K.J.; Taneja, V.; Myasoedova, E.; Davis, J.M.; Sung, J. Gut microbial determinants of clinically important improvement in patients with rheumatoid arthritis. Genome Med. 2021, 13, 149. [Google Scholar] [CrossRef]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef]
- Salim, S.Y.; Soderholm, J.D. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 362–381. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kanai, T. The gut microbiota and inflammatory bowel disease. Semin. Immunopathol. 2015, 37, 47–55. [Google Scholar] [CrossRef]
- Kittana, H.; Gomes-Neto, J.C.; Heck, K.; Geis, A.L.; Muñoz, R.R.S.; Cody, L.A.; Schmaltz, R.J.; Bindels, L.B.; Sinha, R.; Hostetter, J.M.; et al. Commensal Escherichia coli strains can promote intestinal inflammation via differential interleukin-6 production. Front. Immunol. 2018, 9, 2318. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Lacy, D.B. The role of toxins in Clostridium difficile infection. FEMS Microbiol. Rev. 2017, 41, 723–750. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef]
- Bretin, A.; Lucas, C.; Larabi, A.; Dalmasso, G.; Billard, E.; Barnich, N.; Bonnet, R.; Nguyen, H.T.T. AIEC infection triggers modification of gut microbiota composition in genetically predisposed mice, contributing to intestinal inflammation. Sci. Rep. 2018, 8, 12301. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Garcia-Gil, L.J. Escherichia coli in chronic inflammatory bowel diseases: An update on adherent invasive Escherichia coli pathogenicity. World J. Gastrointest. Pathophysiol. 2014, 5, 213–227. [Google Scholar] [CrossRef]
- Fava, F.; Danese, S. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J. Gastroenterol. 2011, 17, 557–566. [Google Scholar] [CrossRef]
- Dolan, K.T.; Chang, E.B. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nutr. Food Res. 2017, 61, 1600129. [Google Scholar] [CrossRef]
- Kaur, L.; Gordon, M.; Baines, P.A.; Iheozor-Ejiofor, Z.; Sinopoulou, V.; Akobeng, A.K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2020, 3, CD005573. [Google Scholar] [CrossRef]
- Cao, X.; Tao, S.; Wang, W.; Wu, S.; Hong, Y.; Wang, X.; Ma, Y.; Qian, H.; Zha, Z. Ternary inulin hydrogel with long-term intestinal retention for simultaneously reversing IBD and its fibrotic complication. Nat. Commun. 2024, 15, 8428. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Yang, Z.; Zhao, K.; Li, S.; He, N. The role of functional oligosaccharides as prebiotics in ulcerative colitis. Food Funct. 2022, 13, 6875–6893. [Google Scholar] [CrossRef]
- Bueno-Hernandez, N.; Yamamoto-Furusho, J.K.; Mendoza-Martínez, V.M. Nutrition in Inflammatory Bowel Disease: Strategies to Improve Prognosis and New Therapeutic Approaches. Diseases 2025, 13, 139. [Google Scholar] [CrossRef]
- Wang, C.; Gu, Y.; Chu, Q.; Wang, X.; Ding, Y.; Qin, X.; Liu, T.; Wang, S.; Liu, X.; Wang, B.; et al. Gut microbiota and metabolites as predictors of biologics response in inflammatory bowel disease: A comprehensive systematic review. Microbiol. Res. 2024, 282, 127660. [Google Scholar] [CrossRef]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef]
- Li, Y.; Chen, G.; Hu, X.; Bao, Y.; Wu, C.; Zeng, N.; Jiang, F. Assessing causal relationships between gut microbiota and psoriasis: Evidence from two sample Mendelian randomization analysis. Sci. Rep. 2024, 14, 8831. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the Gut-Skin Axis in Atopic Dermatitis. Allergy Asthma Immunol Res. 2018, 10, 354–362. [Google Scholar] [CrossRef]
- Polak, K.; Bergler-Czop, B.; Szczepanek, M.; Wojciechowska, K.; Frątczak, A.; Kiss, N. Psoriasis and Gut Microbiome-Current State of Art. Int. J. Mol. Sci. 2021, 22, 4529. [Google Scholar] [CrossRef]
- Benhadou, F.; Mintoff, D.; Schnebert, B.; Thio, H.B. Psoriasis and Microbiota: A Systematic Review. Diseases 2018, 6, 47. [Google Scholar] [CrossRef]
- Buhaș, M.C.; Gavrilaș, L.I.; Candrea, R.; Cătinean, A.; Mocan, A.; Miere, D.; Tătaru, A. Gut Microbiota in Psoriasis. Nutrients 2022, 14, 2970. [Google Scholar] [CrossRef]
- Polak, K.; Muszyński, T.; Frątczak, A.; Meznerics, F.; Bánvölgyi, A.; Kiss, N.; Miziołek, B.; Bergler-Czop, B. Study of gut microbiome alterations in plaque psoriasis patients compared to healthy individuals. Postep. Dermatol Alergol 2024, 41, 378–387. [Google Scholar] [CrossRef]
- Yunusbayev, B.; Bogdanova, A.; Nadyrchenko, N.; Danilov, L.; Bogdanov, V.; Sergeev, G.; Altinbaev, R.; Bilalov, F.; Yunusbaeva, M. Gut dysbiosis narrative in psoriasis: Matched-pair approach identifies only subtle shifts correlated with elevated fecal calprotectin. Microbiol. Spectr. 2025, 13, e0138224. [Google Scholar] [CrossRef]
- Yegorov, S.; Babenko, D.; Kozhakhmetov, S.; Akhmaltdinova, L.; Kadyrova, I.; Nurgozhina, A.; Nurgaziyev, M.; Good, S.V.; Hortelano, G.H.; Yermekbayeva, B.; et al. Psoriasis Is Associated with Elevated Gut IL-1α and Intestinal Microbiome Alterations. Front. Immunol. 2020, 11, 571319. [Google Scholar] [CrossRef]
- Mao, M.; Yuan, Y.; Li, R.; Kuang, Y.; Lu, Y.; Zhu, W.; Chen, W. Modulation of gut propionate and intestinal mucosal protection by Bifidobacterium longum: Mitigating methotrexate side effects without compromising the efficacy of psoriasis therapy. Int. Immunopharmacol. 2025, 149, 114196. [Google Scholar] [CrossRef]
- Tan, J.; Taitz, J.; Nanan, R.; Grau, G.; Macia, L. Dysbiotic Gut Microbiota-Derived Metabolites and Their Role in Non-Communicable Diseases. Int. J. Mol. Sci. 2023, 24, 15256. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ho, H.J.; Tseng, C.H.; Chen, Y.F.; Wang, S.T.; Shieh, J.J.; Wu, C.Y. Short-chain fatty acids ameliorate imiquimod-induced skin thickening and IL-17 levels and alter gut microbiota in mice: A metagenomic association analysis. Sci. Rep. 2024, 14, 17495. [Google Scholar] [CrossRef]
- Groeger, D.; O’mAhony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef]
- Navarro-López, V.; Martínez-Andrés, A.; Ramírez-Boscá, A.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Carrión-Gutiérrez, M.A.; Prieto-Merino, D.; Codoñer-Cortés, F.; Ramón-Vidal, D.; Genovés-Martínez, S.; et al. Efficacy and Safety of Oral Administration of a Mixture of Probiotic Strains in Patients with Psoriasis: A Randomized Controlled Clinical Trial. Acta Derm. Venereol. 2019, 99, 1078–1084. [Google Scholar] [CrossRef]
- Buhaș, M.C.; Candrea, R.; Gavrilaș, L.I.; Miere, D.; Tătaru, A.; Boca, A.; Cătinean, A. Transforming Psoriasis Care: Probiotics and Prebiotics as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 11225. [Google Scholar] [CrossRef]
- Roach, L.A.; Meyer, B.J.; Fitton, J.H.; Winberg, P. Oral Supplementation with Algal Sulphated Polysaccharide in Subjects with Inflammatory Skin Conditions: A Randomised Double-Blind Placebo-Controlled Trial and Baseline Dietary Differences. Mar. Drugs 2023, 21, 379. [Google Scholar] [CrossRef]
- Suriano, E.S.; Souza, M.D.M.; Kobata, C.M.; Santos, F.H.Y.; Mimica, M.J. Efficacy of an adjuvant Lactobacillus rhamnosus formula in improving skin lesions as assessed by PASI in patients with plaque psoriasis from a university-affiliated, tertiary-referral hospital in São Paulo (Brazil): A parallel, double-blind, randomized clinical trial. Arch. Dermatol. Res. 2023, 315, 1621–1629. [Google Scholar] [CrossRef]
- Yin, G.; Li, J.F.; Sun, Y.F.; Ding, X.; Zeng, J.Q.; Zhang, T.; Peng, L.H.; Yang, Y.S.; Zhao, H. Fecal microbiota transplantation as a novel therapy for severe psoriasis. Zhonghua Nei Ke Za Zhi 2019, 58, 782–785. [Google Scholar]
- Chen, H.-L.; Zeng, Y.-B.; Zhang, Z.-Y.; Kong, C.-Y.; Zhang, S.-L.; Li, Z.-M.; Huang, J.-T.; Xu, Y.-Y.; Mao, Y.-Q.; Cai, P.-R.; et al. Gut and Cutaneous Microbiome Featuring Abundance of Lactobacillus reuteri Protected Against Psoriasis-Like Inflammation in Mice. J. Inflamm. Res. 2021, 14, 6175–6190. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zheng, X.; Luo, Q.; Li, X.; Yang, Y.; Bi, Y.; Chen, Y.; Han, L.; Chen, H.; Lu, C. Network pharmacology and gut microbiota insights: Unraveling Shenling Baizhu powder’s role in psoriasis treatment. Front. Pharmacol. 2024, 15, 1362161. [Google Scholar] [CrossRef]
- Crow, M.K. Pathogenesis of systemic lupus erythematosus: Risks, mechanisms and therapeutic targets. Ann. Rheum. Dis. 2023, 82, 999–1014. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Costenbader, K.H. Ultraviolet radiation and systemic lupus erythematosus. Lupus 2014, 23, 588–595. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Costenbader, K.H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 2016, 28, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 16, 565–579. [Google Scholar] [CrossRef]
- Dema, B.; Charles, N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies 2016, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Childs, S.G. The pathogenesis of systemic lupus erythematosus. Orthop. Nurs. 2006, 25, 140–145. [Google Scholar]
- Silverman, G.J.; Azzouz, D.F.; Alekseyenko, A.V. Systemic Lupus Erythematosus and dysbiosis in the microbiome: Cause or effect or both? Curr. Opin. Immunol. 2019, 61, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. A clinical update on the significance of the gut microbiota in systemic autoimmunity. J. Autoimmun. 2016, 74, 85–93. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Guo, F.; Huang, Y.; Li, A.; Chen, S.; Chen, J.; Chen, J.; Liu, H.-F.; Pan, Q. Gut Microbiota Dysbiosis in Systemic Lupus Erythematosus: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Front. Immunol. 2021, 12, 799788. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Yan, C.; Huang, R.; Li, K.; Du, Y.; Jin, X.; Zhu, G.; Zeng, H.; Li, B. Alterations of the microbiome across body sites in systemic lupus erythematosus: A systematic review and meta-analysis. Lupus 2024, 33, 1345–1357. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, J.; Zhou, M.; Jing, S.; Zhao, X.; Mao, P.; Qian, J.; Huang, C.; Tian, Z.; Wang, Q.; et al. The LPS induced pyroptosis exacerbates BMPR2 signaling deficiency to potentiate SLE-PAH. FASEB J. 2021, 35, e22044. [Google Scholar] [CrossRef]
- Xin, Y.; Gao, C.; Wang, L.; Liu, Q.; Lu, Q. Lipopolysaccharide released from gut activates pyroptosis of macrophages via Caspase 11-Gasdermin D pathway in systemic lupus erythematosus. MedComm 2024, 5, e610. [Google Scholar] [CrossRef]
- Vieira, S.M.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 2018, 359, 1156–1161, Erratum in Science 2018, 360, eaat9922. [Google Scholar] [CrossRef] [PubMed]
- Zegarra-Ruiz, D.F.; El Beidaq, A.; Iñiguez, A.J.; Di Ricco, M.; Manfredo Vieira, S.; Ruff, W.E.; Mubiru, D.; Fine, R.L.; Sterpka, J.; Greiling, T.M.; et al. A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity. Cell Host Microbe 2019, 25, 113–127.e6. [Google Scholar] [CrossRef]
- Ali, A.Y.; Zahran, S.A.; Eissa, M.; Kashef, M.T.; Ali, A.E. Gut microbiota dysbiosis and associated immune response in systemic lupus erythematosus: Impact of disease and treatment. Gut Pathog. 2025, 17, 10. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, Y.J.; Park, J.S.; Lee, E.B.; Song, Y.W. CD47 Potentiates Inflammatory Response in Systemic Lupus Erythematosus. Cells 2021, 10, 1151. [Google Scholar] [CrossRef]
- Parodi, E.; Novi, M.; Bottino, P.; La Porta, E.; Merlotti, G.; Castello, L.M.; Gotta, F.; Rocchetti, A.; Quaglia, M. The Complex Role of Gut Microbiota in Systemic Lupus Erythematosus and Lupus Nephritis: From Pathogenetic Factor to Therapeutic Target. Microorganisms 2025, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Banaki, R.; Faezi, S.T.; Esmaeilzadeh, A.; Mahmoudi, M.; Farhadi, E.; Alikhani, M. The Effect of Probiotic Yogurt Containing Lactobacillus rhamnosus and Bifidobacterium bifidum on Disease Activity and Disability in Patients with Systemic Lupus Erythematosus: A Randomized Controlled Trial. Iran. J. Allergy Asthma Immunol. 2025, 24, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef] [PubMed]
- Frati, F.; Salvatori, C.; Incorvaia, C.; Bellucci, A.; Di Cara, G.; Marcucci, F.; Esposito, S. The Role of the Microbiome in Asthma: The Gut(-)Lung Axis. Int. J. Mol. Sci. 2018, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Lee-Sarwar, K.A.; Kelly, R.S.; Lasky-Su, J.; Zeiger, R.S.; O’COnnor, G.T.; Sandel, M.T.; Bacharier, L.B.; Beigelman, A.; Laranjo, N.; Gold, D.R.; et al. Integrative analysis of the intestinal metabolome of childhood asthma. J. Allergy Clin. Immunol. 2019, 144, 442–454. [Google Scholar] [CrossRef]
- Durack, J.; Kimes, N.E.; Lin, D.L.; Rauch, M.; McKean, M.; McCauley, K.; Panzer, A.R.; Mar, J.S.; Cabana, M.D.; Lynch, S.V. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat. Commun. 2018, 9, 707. [Google Scholar] [CrossRef]
- Zhernakova, A.; Yassour, M.; Hall, L.J.; Collado, M.C. Unlocking the power of human milk and infant feeding: Understanding how nutrition and early microbiota interaction shapes health programming. Cell Host Microbe 2025, 33, 820–835. [Google Scholar] [CrossRef]
- Ozcan, E.; Sela, D.A. Inefficient Metabolism of the Human Milk Oligosaccharides Lacto-N-tetraose and Lacto-N-neotetraose Shifts Bifidobacterium longum subsp. infantis Physiology. Front. Nutr. 2018, 5, 46. [Google Scholar] [CrossRef]
- Lee, S.H.; Gong, Y.N.; Ryoo, E. Clostridium difficile colonization and/or infection during infancy and the risk of childhood allergic diseases. Korean J. Pediatr. 2017, 60, 145–150. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Chiu, C.J.; Tsai, C.Y.; Lee, Y.R.; Liu, W.L.; Chuang, H.L.; Huang, M.T. Short-chain fatty acids ameliorate allergic airway inflammation via sequential induction of PMN-MDSCs and Treg cells. J. Allergy Clin. Immunol. Glob. 2023, 2, 100163. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mikami, D.; Kimura, H.; Kamiyama, K.; Morikawa, Y.; Yokoi, S.; Kasuno, K.; Takahashi, N.; Taniguchi, T.; Iwano, M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem. Biophys. Res. Commun. 2017, 486, 499–505. [Google Scholar] [CrossRef]
- Imoto, Y.; Kato, A.; Takabayashi, T.; Sakashita, M.; Norton, J.E.; Suh, L.A.; Carter, R.G.; Weibman, A.R.; Hulse, K.E.; Stevens, W.; et al. Short-chain fatty acids induce tissue plasminogen activator in airway epithelial cells via GPR41&43. Clin. Exp. Allergy 2018, 48, 544–554. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, X.; Dong, B.; Tan, H.; Li, Q.; Zhang, J.; Su, H.; Sun, X. Obesity-related asthma and its relationship with microbiota. Front. Cell. Infect. Microbiol. 2023, 13, 1303899. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Wu, Y.-X.; Jie, Z.-J.; Li, X.-J.; Zhang, J. Genetic evidence on the causality between gut microbiota and various asthma phenotypes: A two-sample Mendelian randomization study. Front. Cell. Infect. Microbiol. 2023, 13, 1270067. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Bao, Y.; Cao, S.; Jiang, P.; Zhang, Z.; Li, L.; Kang, Y.; Wu, Q. The investigation of the role of oral-originated Prevotella-induced inflammation in childhood asthma. Front. Microbiol. 2024, 15, 1400079. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.L.; Wang, C.; Li, G.-B.; Zhao, T.; Zhou, R.-L.; Chen, J. Antibiotic administration aggravates asthma by disrupting gut microbiota and the intestinal mucosal barrier in an asthma mouse model. Exp. Ther. Med. 2024, 27, 157. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.; Lin, P.; Gao, P.; Wang, Z.; Guo, K.; Qin, S.; Zhou, C.; Wang, B.; Wu, E.; Khan, N.; et al. Gut Microbiota Regulate Gut-Lung Axis Inflammatory Responses by Mediating ILC2 Compartmental Migration. J. Immunol. 2021, 207, 257–267. [Google Scholar] [CrossRef]
- Kabil, A.; Nayyar, N.; Brassard, J.; Li, Y.; Chopra, S.; Hughes, M.R.; McNagny, K.M. Microbial intestinal dysbiosis drives long-term allergic susceptibility by sculpting an ILC2-B1 cell-innate IgE axis. J. Allergy Clin. Immunol. 2024, 154, 1260–1276.e9. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.; Berthon, B.S.; Rogers, G.B.; Baines, K.J.; Leong, L.E.; Gibson, P.G.; Williams, E.J.; Wood, L.G. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine 2019, 46, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Cabana, M.D.; McKean, M.; Caughey, A.B.; Fong, L.; Lynch, S.; Wong, A.; Leong, R.; Boushey, H.A.; Hilton, J.F. Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. Pediatrics 2017, 140, e20163000. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, M.M.; Maiello, N.; Allegorico, A.; Iavarazzo, L.; Capasso, M.; Capristo, C.; Ciprandi, G. Lactobacillus reuteri DSM 17938 plus vitamin D3 as ancillary treatment in allergic children with asthma. Ann. Allergy Asthma Immunol. 2016, 117, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Komulainen, M.; Saros, L.; Vahlberg, T.; Nermes, M.; Jartti, T.; Laitinen, K. Maternal fish oil and/or probiotics intervention: Allergic diseases in children up to two years old. Pediatr. Allergy Immunol. 2023, 34, e14004. [Google Scholar] [CrossRef]
- Huang, C.F.; Chie, W.-C.; Wang, I.-J. Efficacy of Lactobacillus Administration in School-Age Children with Asthma: A Randomized, Placebo-Controlled Trial. Nutrients 2018, 10, 1678. [Google Scholar] [CrossRef]
- Drago, L.; Cioffi, L.; Giuliano, M.; Pane, M.; Amoruso, A.; Schiavetti, I.; Reid, G.; Ciprandi, G.; PROPAM Study Group. The Probiotics in Pediatric Asthma Management (PROPAM) Study in the Primary Care Setting: A Randomized, Controlled, Double-Blind Trial with Ligilactobacillus salivarius LS01 (DSM 22775) and Bifidobacterium breve B632 (DSM 24706). J. Immunol. Res. 2022, 2022, 3837418. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Ma, T.; Xu, N.; Jin, H.; Zhao, F.; Kwok, L.-Y.; Zhang, H.; Zhang, S.; Sun, Z. Adjunctive Probiotics Alleviates Asthmatic Symptoms via Modulating the Gut Microbiome and Serum Metabolome. Microbiol. Spectr. 2021, 9, e0085921. [Google Scholar] [CrossRef]
- Liao, H.; Tao, L.; Zhao, J.; Qin, J.; Zeng, G.-C.; Cai, S.-W.; Li, Y.; Zhang, J.; Chen, H.-G. Clostridium butyricum in combination with specific immunotherapy converts antigen-specific B cells to regulatory B cells in asthmatic patients. Sci. Rep. 2016, 6, 20481. [Google Scholar] [CrossRef]
- Liu, D.; Hu, L.; Yang, Y.; Wang, Y.; Li, Y.; Su, J.; Wang, G.; Gong, S. Saccharomyces boulardii alleviates allergic asthma by restoring gut microbiota and metabolic homeostasis via up-regulation of METTL3 in an m6A-dependent manner. Immunol. Lett. 2024, 267, 106853. [Google Scholar] [CrossRef]
- Satia, I.; Cusack, R.; Stevens, C.; Schlatman, A.; Wattie, J.; Mian, F.; Killian, K.J.; O’Byrne, P.M.; Bienenstock, J.; Forsythe, P.; et al. Limosilactobacillus reuteri DSM-17938 for preventing cough in adults with mild allergic asthma: A double-blind randomized placebo-controlled cross-over study. Clin. Exp. Allergy 2021, 51, 1133–1143. [Google Scholar] [CrossRef]
- Lai, Y.; Qiu, R.; Zhou, J.; Ren, L.; Qu, Y.; Zhang, G. Fecal Microbiota Transplantation Alleviates Airway Inflammation in Asthmatic Rats by Increasing the Level of Short-Chain Fatty Acids in the Intestine. Inflammation 2025, 48, 1538–1552. [Google Scholar] [CrossRef]
- Sun, B.; He, X.; Zhang, W. Findings on the Relationship Between Intestinal Microbiome and Vasculitis. Front. Cell. Infect. Microbiol. 2022, 12, 908352. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Nie, R.; Wang, C.; Luan, H.; Ma, X.; Gui, Y.; Zeng, X.; Yuan, H. A two sample mendelian randomization analysis investigates causal effects between gut microbiome and immune related Vasculitis. Sci. Rep. 2024, 14, 18810. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Yan, B.; Zhao, J.-M.; Yuan, L.-P. Effect of gut microbiota from Henoch-Schonlein Purpura patients on acid-sensitive ion channel 3 expression and intestinal motility in germ-free rats. BMC Pediatr. 2021, 21, 536. [Google Scholar] [CrossRef]
- Dehner, C.; Fine, R.; Kriegel, M.A. The microbiome in systemic autoimmune disease: Mechanistic insights from recent studies. Curr. Opin. Rheumatol. 2019, 31, 201–207. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Lin, X.; Bian, X.; Jing, R.; Frelinger, A.; Zhang, A. Comparison and Analysis of Gut Microbiota in Children with IgA Vasculitis with Different Clinical Symptoms. Front. Pediatr. 2021, 9, 800677. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Li, X.-A.; Law, B.; U, K.I.; Pan, B.Q.; Lei, C.; Hsiao, W.W. Correlation of gut microbial compositions to the development of kawasaki disease vasculitis in children. Future Microbiol. 2020, 15, 591–600. [Google Scholar] [CrossRef]
- Jena, P.K.; Wakita, D.; Gomez, A.C.; Carvalho, T.T.; Atici, A.E.; Aubuchon, E.; Narayanan, M.; Lee, Y.; Fishbein, M.C.; Takasato, Y.; et al. Intestinal Microbiota Contributes to the Development of Cardiovascular Inflammation and Vasculitis in Mice. Circ. Res. 2025, 136, e53–e72. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, C.; Zhao, L.; Sheng, D.; Chen, B.; Zhao, G.; Wang, Q.; Zhang, L. Taxonomic and functional shifts of gut microbiome in immunoglobulin A vasculitis children and their mothers. Front. Pediatr. 2024, 12, 1356529. [Google Scholar] [CrossRef]
- Hu, X.; Fan, R.; Song, W.; Qing, J.; Yan, X.; Li, Y.; Duan, Q.; Li, Y. Landscape of intestinal microbiota in patients with IgA nephropathy, IgA vasculitis and Kawasaki disease. Front. Cell. Infect. Microbiol. 2022, 12, 1061629. [Google Scholar] [CrossRef]
- Shimizu, J.; Kubota, T.; Takada, E.; Takai, K.; Fujiwara, N.; Arimitsu, N.; Ueda, Y.; Wakisaka, S.; Suzuki, T.; Suzuki, N. Relative abundance of Megamonas hypermegale and Butyrivibrio species decreased in the intestine and its possible association with the T cell aberration by metabolite alteration in patients with Behcet’s disease (210 characters). Clin. Rheumatol. 2019, 38, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Fu, S.; Yang, R.; Yang, K.; Lei, W.; Yang, Y.; Zhang, Q.; Zhao, Y.; Yu, J.; Yu, L.; et al. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J. Inflamm. 2023, 20, 33. [Google Scholar] [CrossRef]
- Sato, W.; Ishibashi, K.-I.; Yamanaka, D.; Adachi, Y.; Ohno, N. Effects of Natural and Chemically Defined Nutrients on Candida albicans Water-soluble Fraction (CAWS) Vasculitis in Mice. Med. Mycol. J. 2017, 58, E47–E62. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.S.; Ryu, H.-M.; Sayeed, H.M.; Byun, H.-O.; Jung, J.-Y.; Kim, H.-A.; Suh, C.-H.; Sohn, S. Eubacterium rectale Attenuates HSV-1 Induced Systemic Inflammation in Mice by Inhibiting CD83. Front. Immunol. 2021, 12, 712312. [Google Scholar] [CrossRef]
- Wang, F.; Qian, F.; Zhang, Q.; Zhao, J.; Cen, J.; Zhang, J.; Zhou, J.; Luo, M.; Jia, C.; Rong, X.; et al. The reduced SCFA-producing gut microbes are involved in the inflammatory activation in Kawasaki disease. Front. Immunol. 2023, 14, 1124118. [Google Scholar] [CrossRef]
- Saravanan, C.; Gopinath, N.K.; Ganesan, R.; Thirumurugan, D. Challenges and limitations in using bacterial metabolites as immunomodulators. Front. Cell. Infect. Microbiol. 2025, 15, 1535394. [Google Scholar] [CrossRef]
- Pagliai, G.; Dinu, M.; Fiorillo, C.; Becatti, M.; Turroni, S.; Emmi, G.; Sofi, F. Modulation of gut microbiota through nutritional interventions in Behçet’s syndrome patients (the MAMBA study): Study protocol for a randomized controlled trial. Trials 2020, 21, 511. [Google Scholar] [CrossRef]
- Cao, J.; Wu, C.; Wang, K.; Hu, H.; Duan, J.; Zhao, B.; Xiong, J.; Liu, M.; Cui, J.; Ji, X.; et al. Metagenomic profiling reveals dominance of gram-positive bacteria in the gut microbiome shifts associated with immunoglobulin A vasculitis (Henoch-Schonlein Purpura). Clin. Transl. Immunol. 2021, 10, e1342. [Google Scholar] [CrossRef]
- Dominguez-Villar, M.; Hafler, D.A. Regulatory T cells in autoimmune disease. Nat. Immunol. 2018, 19, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Bu, X.; Wang, J.; Yin, Z.; Pan, W.; Liu, L.; Jin, H.; Liu, Q.; Zheng, L.; Sun, H.; Gao, Y.; et al. Human Amniotic Mesenchymal Stem Cells Alleviate aGVHD after allo-HSCT by Regulating Interactions between Gut Microbiota and Intestinal Immunity. Stem Cell Rev. Rep. 2023, 19, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
| Terms | Definition |
|---|---|
| MICROBIOTA | Microorganisms, composed of bacteria, fungi, virus, protozoa and archaea, inhabiting a defined environment. |
| MICROBIOME | Generally, microbiota, its genes, gene products and activities in niches in a habitat. |
| DYSBIOSIS | There is no consensus that defines dysbiosis despite a high frequency of usage in microbiome studies. It is often described as a state, in which alterations to the microbiota of hosts and its functional components may be correlated with undermined host immunity and increasing susceptibility to diseases. Dysbiosis usually features: (i) impaired microbial diversity; (ii) loss of beneficial commensal bacteria; (iii) thriving of pathogens. |
| DISEASE DRIVER | Causal factors that initiate or promote disease development or progression. |
| BIOMARKER | A subset of markers that are objectively measurable and evaluated as indicators of biological process, pathogenic processes, or responses to therapeutic interventions. |
| PATHWAY | Biological mechanisms or networks through which disease processes unfold, often involving multiple molecular interactions. |
| Disease | Estimated Global Prevalence | Primary Etiology | Autoimmune Features | Major Host Susceptibility Genes | Gut Microbiome-Targeted Therapies Tested Clinically | Supporting Literature |
|---|---|---|---|---|---|---|
| Rheumatoid Arthritis (RA) | ~17·6 million ([47] Black, R. J. et al., 2023) | Joint-targeted autoantibodies | Yes | HLA alleles, PTPN22, PADI4, STAT4, CTLA4, IL2RA, etc. | High-fiber diet; Probiotics (e.g., L. casei); FMT | [45,46,47,48,49,50] |
| Inflammatory Bowel Disease (IBD) | ~4.9 million ([51] Wang, R. et al., 2023) | Aberrant immune response to gut commensals | Yes | NOD2, IL23R, HLA, etc. | FMT; Probiotics (e.g., Bifidobacterium, Lactobacillus); Prebiotics (e.g., FOS); Diet (e.g., low-FODMAP, EEN, Mediterranean diet) | [51,52,53,54,55] |
| Psoriasis | at least 100 million ([56] WHO, 2016) | Skin-targeted inflammation | Yes | NFKB1, ZFYVE28, IL23R, IL12B, etc. | Probiotics (e.g., B. infantis, Bacillus genus, etc.); Prebiotics (e.g., fructooligosaccharides, SXRG84, etc.) | [56,57,58] |
| Systemic Lupus Erythematosus (SLE) | ~3.41 million ([59] Tian, J. et al., 2023) | Multi-organ autoantibody production | Yes | TYK2, STAT1, IRF5, STAT4, etc. | FMT; Probiotics (e.g., L. helveticus, B. infantis, B. bifidum); Diet (e.g., inulin and FOS) | [59,60,61,62,63] |
| Asthma | ~260 million ([64] Oh, J. et al., 2025) | Allergic/immune dysregulation in the respiratory tract | No | ORMDL3, IL33, TSLP, etc. | Probiotics (e.g., L. salivarius, B. brev, C. butyricum, etc.); Diet (e.g., inulin) | [64,65] |
| Vasculitis | varies widely by type; average < 50 cases per million people for a single type ([66] Watts, R.A. et al., 2022) | Vessel-targeted autoimmunity | Yes | KD-associated genes: ITPKC, CASP3, ORAI1, BLK, etc. BD-associated genes IL-10A, CPVL, STAT4, TNFAIP3, etc. | Dietary modulation | [66,67,68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.J.; Maddirala, N.R.; Saint Fleur, A.; Zhou, F.; Yu, D.; Wei, F.; Zhang, Y. Gut Microbiome and Immune System Crosstalk in Chronic Inflammatory Diseases: A Narrative Review of Mechanisms and Therapeutic Opportunities. Microorganisms 2025, 13, 2516. https://doi.org/10.3390/microorganisms13112516
Feng JJ, Maddirala NR, Saint Fleur A, Zhou F, Yu D, Wei F, Zhang Y. Gut Microbiome and Immune System Crosstalk in Chronic Inflammatory Diseases: A Narrative Review of Mechanisms and Therapeutic Opportunities. Microorganisms. 2025; 13(11):2516. https://doi.org/10.3390/microorganisms13112516
Chicago/Turabian StyleFeng, Jefferson J., Nikhil R. Maddirala, Ashley Saint Fleur, Fenfen Zhou, Di Yu, Feng Wei, and Yongrong Zhang. 2025. "Gut Microbiome and Immune System Crosstalk in Chronic Inflammatory Diseases: A Narrative Review of Mechanisms and Therapeutic Opportunities" Microorganisms 13, no. 11: 2516. https://doi.org/10.3390/microorganisms13112516
APA StyleFeng, J. J., Maddirala, N. R., Saint Fleur, A., Zhou, F., Yu, D., Wei, F., & Zhang, Y. (2025). Gut Microbiome and Immune System Crosstalk in Chronic Inflammatory Diseases: A Narrative Review of Mechanisms and Therapeutic Opportunities. Microorganisms, 13(11), 2516. https://doi.org/10.3390/microorganisms13112516






