Tackling Conifer Needle Cast and Ash Dieback with Host-Derived Microbial Antagonists Exhibiting Plant Growth-Promoting Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacteria
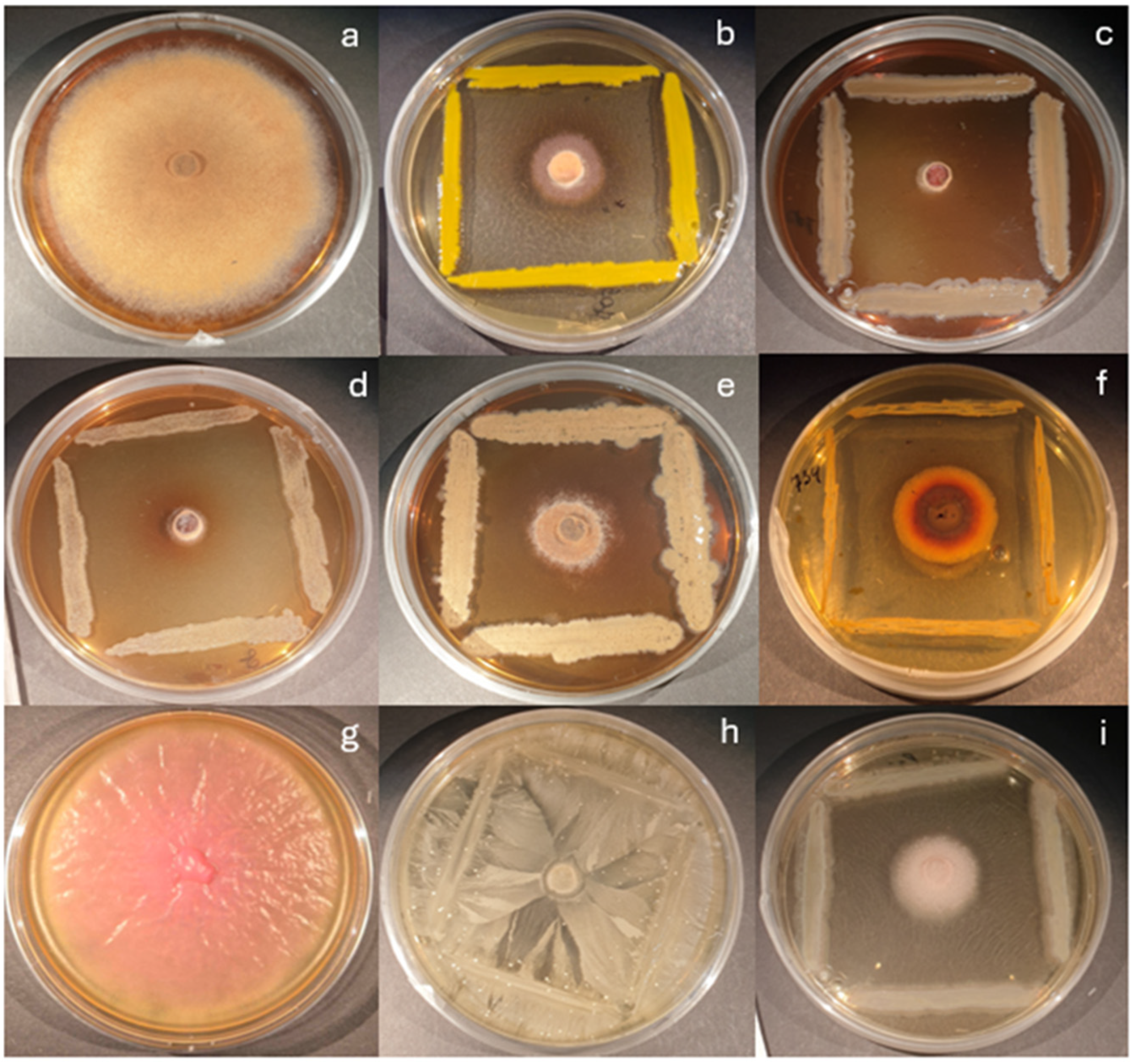
2.2. Dual Culture Antagonism Assay
2.3. Plant Growth-Promoting Traits (PGPTs)
2.4. Statistical Analysis
3. Results
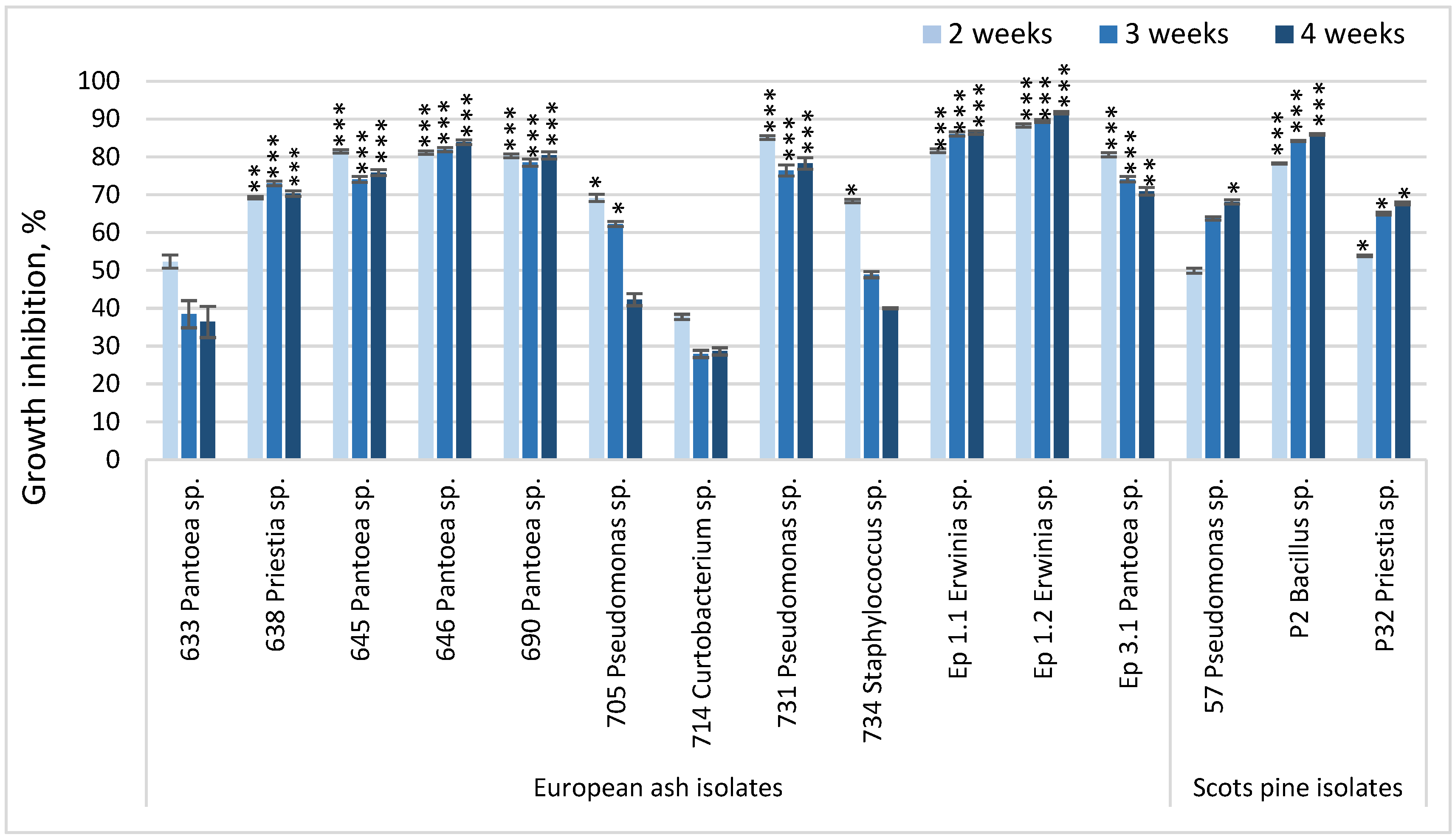
3.1. Antagonistic Activity in Dual Culture Assay
3.2. Bacterial Identification
3.3. Plant Growth-Promoting Traits in Isolated Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobrowolska, D.; Hein, S.; Oosterbaan, A.; Wagner, S.; Clark, J.; Skovsgaard, J.P. A Review of European Ash (Fraxinus excelsior L.): Implications for Silviculture. Forestry 2011, 84, 133–148. [Google Scholar] [CrossRef]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European Ash (Fraxinus excelsior) Dieback—A Conservation Biology Challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Brichta, J.; Vacek, S.; Vacek, Z.; Cukor, J.; Mikeska, M.; Bílek, L.; Šimůnek, V.; Gallo, J.; Brabec, P. Importance and Potential of Scots Pine (Pinus sylvestris L.) in 21st Century. Cent. Eur. For. J. 2023, 69, 3–20. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Beaton, J.K.; Bellamy, P.E.; Broome, A.; Chetcuti, J.; Eaton, S.; Ellis, C.J.; Gimona, A.; Harmer, R.; Hester, A.J.; et al. Ash Dieback in the UK: A Review of the Ecological and Conservation Implications and Potential Management Options. Biol. Conserv. 2014, 175, 95–109. [Google Scholar] [CrossRef]
- Jansons, A.; Neimane, U.; Polmanis, K.; Zaluma, A.; Gaitnieks, T.; Baumanis, I. Cumulative Effect of Needle Cast on Scots Pine Saplings. For. Stud. 2016, 65, 5–15. [Google Scholar] [CrossRef]
- Enderle, R.; Stenlid, J.; Vasaitis, R. An Overview of Ash (Fraxinus spp.) and the Ash Dieback Disease in Europe. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2019, 14, 1–12. [Google Scholar] [CrossRef]
- Aučina, A.; Bačkaitis, J.; Danusevičius, J.; Malinauskas, A.; Paičius, J.; Račinskas, J.; Riepšas, E.; Suchockas, V.; Žiogas, A. Miško Želdintojo Žinynas; Eugrimas: Vilnius, Lithuania, 2016. [Google Scholar]
- Lazarev, V. Praćenje Infekcionog Perioda Lophodermium Vrsta u Uslovima Bosne. Šumarstvo I Prerada Drv. 1981, 1, 77–83. [Google Scholar]
- Lazarev, V.; Karadžić, D.; Marković, M.; Pap, P.; Poljaković-Pajnik, L. The Most Frequent Lophodermium Spp. on Scots Pine and Austrian Pine and Their Role in the Appearance of Other Fungi on the Needles. Acta Silv. Lignaria Hung. 2007, 3, 53–59. [Google Scholar] [CrossRef]
- Soldi, E.; Tiley, A.; O’hanlon, R.; Murphy, B.R.; Hodkinson, T.R. Ash Dieback and Other Pests and Pathogens of Fraxinus on the Island of Ireland. Biol. Environ. 2022, 122, 85–122. [Google Scholar] [CrossRef]
- Kowalski, T. Chalara Fraxinea sp. Nov. Associated with Dieback of Ash (Fraxinus excelsior) in Poland. For. Pathol. 2006, 36, 264–270. [Google Scholar] [CrossRef]
- Nielsen, L.R.; McKinney, L.V.; Hietala, A.M.; Kjær, E.D. The Susceptibility of Asian, European and North American Fraxinus Species to the Ash Dieback Pathogen Hymenoscyphus fraxineus Reflects Their Phylogenetic History. Eur. J. For. Res. 2017, 136, 59–73. [Google Scholar] [CrossRef]
- Stocks, J.J.; Metheringham, C.L.; Plumb, W.J.; Lee, S.J.; Kelly, L.J.; Nichols, R.A.; Buggs, R.J.A. Genomic Basis of European Ash Tree Resistance to Ash Dieback Fungus. Nat. Ecol. Evol. 2019, 3, 1686–1696. [Google Scholar] [CrossRef]
- Jansons, A.; Zeltiņš, P.; Donis, J.; Neimane, U. Long-Term Effect of Lophodermium Needle Cast on the Growth of Scots Pine and Implications for Financial Outcomes. Forests 2020, 11, 718. [Google Scholar] [CrossRef]
- Stenström, E.; Arvidsson, B. Fungicidal Control of Lophodermium seditiosum on Pinus sylvestris Seedlings in Swedish Forest Nurseries. Scand. J. For. Res. 2001, 16, 147–154. [Google Scholar] [CrossRef]
- Hrabětová, M.; Černý, K.; Zahradník, D.; Havrdová, L. Efficacy of Fungicides on Hymenoscyphus fraxineus and Their Potential for Control of Ash Dieback in Forest Nurseries. For. Pathol. 2016, 47, e12311. [Google Scholar] [CrossRef]
- Okorski, A.; Pszczółkowska, A.; Oszako, T.; Nowakowska, J.A. Current Possibilities and Prospects of Using Fungicides in Forestry. For. Res. Pap. 2015, 76, 191–206. [Google Scholar] [CrossRef]
- Al Raish, S.M.; Sourani, O.M.; Abu-Elsaoud, A.M. Plant Growth-Promoting Microorganisms as Biocontrol Agents: Mechanisms, Challenges, and Future Prospects. Appl. Microbiol. 2025, 5, 44. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Petkova, M.; Marcheva, M.; Petrova, A.L.; Slavova, V.; Shilev, S. Plant Growth-Promoting and Biocontrol Characteristics of Four Bacillus Strains and Evaluation of Their Effects on Wheat (Tr. Aestivum L.). Int. J. Plant Biol. 2025, 16, 1. [Google Scholar] [CrossRef]
- Ghadamgahi, F.; Tarighi, S.; Taheri, P.; Saripella, G.V.; Anzalone, A.; Kalyandurg, P.B.; Catara, V.; Ortiz, R.; Vetukuri, R.R. Plant Growth-Promoting Activity of Pseudomonas aeruginosa FG106 and Its Ability to Act as a Biocontrol Agent against Potato, Tomato and Taro Pathogens. Biology 2022, 11, 140. [Google Scholar] [CrossRef]
- Burcham, D.C.; Abarrientos, N.V.; Wong, J.Y.; Ali, M.I.M.; Fong, Y.K.; Schwarze, F.W.M.R. Field Evaluation of Trichoderma spp. as a Biological Control Agent to Prevent Wood Decay on Benin Mahogany (Khaya grandifoliola) and Rain Tree (Samanea saman) in Singapore. Biol. Control 2017, 114, 114–124. [Google Scholar] [CrossRef]
- Drenkhan-Maaten, T.; Hanso, S.; Hanso, M. Effect of the Stump Treatment with Phlebiopsis gigantea against Heterobasidion Root Rot in Estonia. Balt. For. 2008, 14, 16–25+93. [Google Scholar]
- Haidar, R.; Roudet, J.; Bonnard, O.; Dufour, M.C.; Corio-Costet, M.F.; Fert, M.; Gautier, T.; Deschamps, A.; Fermaud, M. Screening and Modes of Action of Antagonistic Bacteria to Control the Fungal Pathogen Phaeomoniella chlamydospora Involved in Grapevine Trunk Diseases. Microbiol. Res. 2016, 192, 172–184. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621, https://doi.org/10.1038/s41579-020-0412-1; Erratum in Nat. Rev. Microbiol. 2021, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103, Erratum in Microbiome 2020, 8, 119. [Google Scholar] [CrossRef]
- Gardner, J.M.; Feldman, A.W.; Zablotowiczt, R.M. Identity and Behavior of Xylem-Residing Bacteria in Rough Lemon Roots of Florida Citrus Treest. Appl. Environ. Microbiol. 1982, 43, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Eevers, N.; Gielen, M.; Sánchez-López, A.; Jaspers, S.; White, J.C.; Vangronsveld, J.; Weyens, N. Optimization of isolation and cultivation of bacterial endophytes through addition of plant extract to nutrient media. Microb. Biotechnol. 2015, 8, 707–715. [Google Scholar] [CrossRef]
- Muhtari, K.; Sailaja, I.; Shekhawat, B.K.; Kaura, S.; Mehta, S. Identification of Bacterial Endophytes Isolated from Selected Medicinal Plants. Int. J. Biomed. Clin. Anal. 2024, 4, 24–40. [Google Scholar] [CrossRef]
- Vaitiekūnaitė, D.; Striganavičiūtė, G.; Beniušytė, E.; Sirgedaitė-šėžienė, V.; Augustauskaitė, M. Putative Biocontrol Agents for European Forest Pathogens Found in English Oak (Quercus robur L.) Endosphere. Zemdirbyste 2023, 110, 79–86. [Google Scholar] [CrossRef]
- Castiblanco, L.F.; Sundin, G.W. New Insights on Molecular Regulation of Biofilm Formation in Plant-Associated Bacteria. J. Integr. Plant Biol. 2016, 58, 362–372. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, S. Identification and Characterization of the Phosphate-Solubilizing Bacterium Pantoea sp. S32 in Reclamation Soil in Shanxi, China. Front. Microbiol. 2019, 10, 02171. [Google Scholar] [CrossRef]
- Prajapati, K.B.; Modi, H.A. Isolation and Characterization of Potassium Solubilizing Bacteria from Ceramic Industry Soil; IOP Publishing Ltd.: Bristol, UK, 2012; Volume 1, pp. 8–14. [Google Scholar]
- Striganavičiūtė, G.; Vaitiekūnaitė, D.; Šilanskienė, M.; Sirgedaitė-Šėžienė, V. Rooting for Success: The Role of Microorganisms in Promoting Growth and Resilience in Black Alder Seedlings. Environ. Microbiol. Rep. 2024, 16, e70060. [Google Scholar] [CrossRef]
- Vaitiekūnaitė, D.; Kuusienė, S.; Beniušytė, E. Oak (Quercus robur) Associated Endophytic Paenibacillus Sp. Promotes Poplar (Populus spp.) Root Growth in Vitro. Microorganisms 2021, 9, 1151. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Vaitiekūnaitė, D.; Snitka, V. Differentiation of Closely Related Oak-Associated Gram-Negative Bacteria by Label-Free Surface Enhanced Raman Spectroscopy (Sers). Microorganisms 2021, 9, 1969. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Li, X.; Zhao, L.; Zhang, H.; Jia, Q.; Yao, B.; Zhang, Z. Physicochemical Properties and Antibiosis Activity of the Pink Pigment of Erwinia persicina Cp2. Agriculture 2022, 12, 1641. [Google Scholar] [CrossRef]
- Rakhalaru, P.; Mampholo, B.M.; Mamphogoro, T.P.; Thantsha, M.S. Endophytic and Epiphytic Microorganisms as Biocontrol Agents: Mechanisms, Applications, and Metagenomic Approaches in Tomato Cultivation. Molecules 2025, 30, 3816. [Google Scholar] [CrossRef]
- Ulrich, K.; Becker, R.; Behrendt, U.; Kube, M.; Ulrich, A. A Comparative Analysis of Ash Leaf-Colonizing Bacterial Communities Identifies Putative Antagonists of Hymenoscyphus fraxineus. Front. Microbiol. 2020, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Ulrich, K.; Behrendt, U.; Kube, M.; Ulrich, A. Analyzing Ash Leaf-Colonizing Fungal Communities for Their Biological Control of Hymenoscyphus fraxineus. Front. Microbiol. 2020, 11, 590944. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, M.; Nguyen, D.; Witzell, J.; Cleary, M. Mycobiome of Fraxinus Excelsior With Different Phenotypic Susceptibility to Ash Dieback. Front. For. Glob. Change 2021, 4, 580514. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Sheikh, I.; Kumar, V.; Yadav, A.N.; Dhaliwal, H.S.; Saxena, A.K. Alleviation of Drought Stress and Plant Growth Promotion by Pseudomonas libanensis EU-LWNA-33, a Drought-Adaptive Phosphorus-Solubilizing Bacterium. Proc. Natl. Acad. Sci. India Sect. B-Biol. Sci. 2020, 90, 785–795. [Google Scholar] [CrossRef]
- Lv, L.; Luo, J.; Ahmed, T.; Zaki, H.E.M.; Tian, Y.; Shahid, M.S.; Chen, J.; Li, B. Beneficial Effect and Potential Risk of Pantoea on Rice Production. Plants 2022, 11, 2608. [Google Scholar] [CrossRef]
- Soliman, M.A. Paenibacillus polymyxa and Bacillus aryabhattai as Biocontrol Agents against Ralstonia Solanacearum in Vitro and in Planta. J. Plant Prot. Pathol. 2020, 11, 197–203. [Google Scholar] [CrossRef]
- Shahid, M.; Zeyad, M.T.; Syed, A.; Singh, U.B.; Mohamed, A.; Bahkali, A.H.; Elgorban, A.M.; Pichtel, J. Stress-Tolerant Endophytic Isolate Priestia aryabhattai BPR-9 Modulates Physio-Biochemical Mechanisms in Wheat (Triticum aestivum L.) for Enhanced Salt Tolerance. Int. J. Environ. Res. Public Health 2022, 19, 10883. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Y.; Zhao, Q.; Chen, M.; Zhang, Y.; Gao, C.; Jia, Z.; Song, S.; Guan, J.; Shang, Z. Volatile Organic Compounds Produced by Bacillus aryabhattai AYG1023 against Penicillium Expansum Causing Blue Mold on the Huangguan Pear. Microbiol. Res. 2024, 278, 127531. [Google Scholar] [CrossRef]
- Goyal, T.; Mukherjee, A.; Chouhan, G.K.; Gaurav, A.K.; Kumar, D.; Abeysinghe, S.; Verma, J.P. Impact of Bacterial Volatiles on the Plant Growth Attributes and Defense Mechanism of Rice Seedling. Heliyon 2024, 10, e29692. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, D.; Wang, Y.; Zhu, X.; Chen, L.; Duan, Y. Evaluation of Bacillus aryabhattai Sneb517 for Control of Heterodera glycines in Soybean. Biol. Control 2020, 142, 104147. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Podolich, O.; Koskimäki, J.J.; Hohtola, E.; Hohtola, A. Role of Origin and Endophyte Infection in Browning of Bud-Derived Tissue Cultures of Scots Pine (Pinus sylvestris L.). Plant Cell Tissue Organ Cult. 2008, 95, 47–55. [Google Scholar] [CrossRef]
- Izumi, H.; Anderson, I.C.; Killham, K.; Moore, E.R.B. Diversity of Predominant Endophytic Bacteria in European Deciduous and Coniferous Trees. Can. J. Microbiol. 2008, 54, 173–179. [Google Scholar] [CrossRef]
- Bizjak, T.; Sellstedt, A.; Gratz, R.; Nordin, A. Presence and Activity of Nitrogen-Fixing Bacteria in Scots Pine Needles in a Boreal Forest: A Nitrogen-Addition Experiment. Tree Physiol. 2023, 43, 1354–1364. [Google Scholar] [CrossRef]
- Kim, C.-K.; Eo, J.-K.; Eom, A.-H. Diversity and Seasonal Variation of Endophytic Fungi Isolated from Three Conifers in Mt. Taehwa, Korea. Mycobiology 2013, 41, 82–85. [Google Scholar] [CrossRef]
- Sun, M.; Liang, C.; Fu, X.; Liu, G.; Zhong, Y.; Wang, T.; Tang, G.; Li, P. Nematocidal Activity and Biocontrol Efficacy of Endophytic Bacillus Velezensis Pt-RP9 from Pinus tabuliformis against Pine Wilt Disease Caused by Bursaphelenchus xylophilus. Biol. Control 2024, 196, 105579. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, S.-I.; Ahn, Y.S. Biocontrol of Bacillus Velezensis CE 100 against Fusarium oxysporum Causing Damping-off in Picea abies (L.) Karst. Seedling. Trends Agric. Life Sci. 2023, 61, 51–61. [Google Scholar] [CrossRef]
- Naik, S.; Palys, S.; Di Falco, M.; Tsang, A.; Périnet, P.; Ramanan, U.S.; Dayanandan, S. Isolation and Characterization of Bacillus velezensis EB14, an Endophytic Bacterial Strain Antagonistic to Poplar Stem Canker Pathogen Sphaerulina musiva and Its Interactions with the Endophytic Fungal Microbiome in Poplar. PhytoFrontiersTM 2021, 1, 229–238. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Zhou, Y.; Wang, J.; Jiang, Y.; Shen, N.; Wang, Y.; Yang, L.; Jiang, M. Unlocking the Strength of Plant Growth Promoting Pseudomonas in Improving Crop Productivity in Normal and Challenging Environments: A Review. J. Plant Interact. 2022, 17, 220–238. [Google Scholar] [CrossRef]
- Tozlu, E.; Karagöz, K.; Emel Babagil, G.; Dizikisa, T.; Kotan, R. Effect of Some Plant Growth Promoting Bacteria on Yield, Yield Components of Dry Bean (Phaseolus vulgaris L. Cv. Aras 98). J. Fac. Agric. 2012, 43, 101–106. [Google Scholar]
- Devi, R.; Kaur, T.; Kour, D.; Yadav, A.N. Microbial Consortium of Mineral Solubilizing and Nitrogen Fixing Bacteria for Plant Growth Promotion of Amaranth (Amaranthus hypochondrius L.). Biocatal. Agric. Biotechnol. 2022, 43, 102404. [Google Scholar] [CrossRef]
- Devi, S.; Kiesewalter, H.T.; Kovács, R.; Frisvad, J.C.; Weber, T.; Larsen, T.O.; Kovács, Á.T.; Ding, L. Depiction of Secondary Metabolites and Antifungal Activity of Bacillus velezensis DTU001. Synth. Syst. Biotechnol. 2019, 4, 142–149. [Google Scholar] [CrossRef]
- Torres, M.; Llamas, I.; Torres, B.; Toral, L.; Sampedro, I.; Béjar, V. Growth Promotion on Horticultural Crops and Antifungal Activity of Bacillus velezensis XT1. Appl. Soil Ecol. 2020, 150, 103453. [Google Scholar] [CrossRef]
- Kwak, Y.; Park, G.S.; Shin, J.H. High Quality Draft Genome Sequence of the Type Strain of Pseudomonas lutea OK2T, a Phosphate-Solubilizing Rhizospheric Bacterium. Stand. Genom. Sci. 2016, 11, 51. [Google Scholar] [CrossRef]
- Mannaa, M.; Park, A.R.; Park, J.; Jeon, H.W.; Jung, H.; Jeon, H.S.; Han, G.; Kim, J.-C.; Seo, Y.-S. Eco-Friendly Biocontrol of Pine Wilt Disease: Enhancing Tree Defense with Bacillus subtilis JCK-1398 for Sustainable Forest Management. Sci. Total Environ. 2024, 955, 177233. [Google Scholar] [CrossRef]
- Choub, V.; Yim, E.Y.; Choi, S.I.; Won, S.J.; Moon, J.H.; Yun, J.Y.; Ajuna, H.B.; Ahn, Y.S. Effective Biocontrol Efficacy of Bacillus subtilis CV21 against Cherry Leaf Spot Disease Caused by Alternaria Phytopathogens and Growth Promotion of Flowering Cherry (Prunus sargentii Rehder) Seedlings. Biol. Control 2024, 197, 105603. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Jung, H.; Koo, B.K.; Han, J.A.; Lee, H.S. Exploiting Bacterial Genera as Biocontrol Agents: Mechanisms, Interactions and Applications in Sustainable Agriculture. J. Plant Biol. 2023, 66, 485–498. [Google Scholar] [CrossRef]
- Fan, B.; Wang, C.; Song, X.; Ding, X.; Wu, L.; Wu, H.; Gao, X.; Borriss, R. Bacillus velezensis FZB42 in 2018: The Gram-Positive Model Strain for Plant Growth Promotion and Biocontrol. Front. Microbiol. 2018, 9, 02491. [Google Scholar] [CrossRef]
- Yang, X.; Hong, C. Biological Control of Phytophthora Blight by Pseudomonas protegens Strain 14D5. Eur. J. Plant Pathol. 2020, 156, 591–601. [Google Scholar] [CrossRef]
- Pusey, P.L.; Stockwell, V.O.; Reardon, C.L.; Smits, T.H.M.; Duffy, B. Antibiosis Activity of Pantoea agglomerans Biocontrol Strain E325 against Erwinia amylovora on Apple Flower Stigmas. Phytopathology 2011, 101, 1234–1241. [Google Scholar] [CrossRef]

| Bacteria Number | Closest NCBI Match | Accession No. * | Fragment Length, bp | % Identity | Query Coverage, % | |
|---|---|---|---|---|---|---|
| European ash antagonistic bacteria | 633 | Pantoea agglomerans, NCTC9381 | NR_114735.1 | 1467 | 98.98 | 100 |
| 638 | Priestia aryabhattai, B8W22 | NR_115953.1 | 1488 | 100.00 | 100 | |
| 645 | Pantoea agglomerans, NCTC9381 | NR_114735.1 | 1470 | 98.98 | 100 | |
| 646 | Pantoea allii, BD 390 | NR_115258.1 | 1467 | 98.98 | 100 | |
| 690 | Pantoea vagans, LMG 24199 | NR_116115.1 | 625 | 97.76 | 100 | |
| 705 | Pseudomonas baltica, MBT-2 | NR_181571.1 | 1466 | 99.66 | 100 | |
| 714 | Curtobacterium flaccumfaciens, BCCM/LMG 3645 | NR_025467.1 | 1021 | 100.00 | 100 | |
| 731 | Pseudomonas libanensis, CIP 105460 | NR_024901.1 | 1467 | 99.86 | 100 | |
| 734 | Staphylococcus haemolyticus, JCM 2416 | NR_113345.1 | 1464 | 99.93 | 100 | |
| Ep 1-1 | Erwinia rhapontici, DSM 4484 | NR_041976.1 | 1468 | 99.73 | 100 | |
| Ep 1-2 | Erwinia rhapontici, DSM 4484 | NR_041976.1 | 1466 | 99.73 | 100 | |
| Ep 3-1 | Pantoea agglomerans, DSM 3493 | NR_041978.1 | 1457 | 99.17 | 100 | |
| Scots pine antagonistic bacteria | 57 | Pseudomonas lutea, OK2 16S | NR_029103.1 | 1459 | 98.93 | 100 |
| P2 | Bacillus velezensis, FZB42 | NR_075005.2 | 1468 | 99.93 | 100 | |
| P32 | Priestia megaterium, ATCC 14581 | NR_112636.1 | 1462 | 100.00 | 100 |
| Bacteria Number | Solubilization of Potassium | Solubilization of Inorganic Phosphate | Mineralization of Organic Phosphate | Nitrogen Fixation | Production of Siderophore | IAA Production, µg/mL | Formation of Biofilms | |
|---|---|---|---|---|---|---|---|---|
| European ash antagonistic bacteria | 633 | + * | − | + | − | + | 12.87 | moderate |
| 638 | − | − | + | + | − | 6.76 | weak | |
| 645 | + | − | + | + | + | 28.61 | moderate | |
| 646 | + | − | + | + | + | 44.79 | weak | |
| 690 | + | − | + | + | + | 20.19 | weak | |
| 705 | − | − | + | − | + | 9.12 | weak | |
| 714 | − | − | − | + | − | 7.96 | weak | |
| 731 | + | − | + | + | + | 7.79 | moderate | |
| 734 | − | − | − | − | − | 3.28 | weak | |
| Ep 1-1 | − | − | + | + | + | 5.91 | weak | |
| Ep 1-2 | − | − | + | + | + | 6.17 | weak | |
| Ep 3-1 | − | − | − | − | + | 19.94 | moderate | |
| Scots pine antagonistic bacteria | 57 | + | − | − | − | + | 5.49 | weak |
| P2 | − | − | − | − | + | 6.45 | weak | |
| P32 | − | − | + | + | − | 5.56 | weak |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šilanskienė, M.; Vaitiekūnaitė, D.; Sirgedaitė-Polikaitienė, V. Tackling Conifer Needle Cast and Ash Dieback with Host-Derived Microbial Antagonists Exhibiting Plant Growth-Promoting Traits. Microorganisms 2025, 13, 2517. https://doi.org/10.3390/microorganisms13112517
Šilanskienė M, Vaitiekūnaitė D, Sirgedaitė-Polikaitienė V. Tackling Conifer Needle Cast and Ash Dieback with Host-Derived Microbial Antagonists Exhibiting Plant Growth-Promoting Traits. Microorganisms. 2025; 13(11):2517. https://doi.org/10.3390/microorganisms13112517
Chicago/Turabian StyleŠilanskienė, Milana, Dorotėja Vaitiekūnaitė, and Vaida Sirgedaitė-Polikaitienė. 2025. "Tackling Conifer Needle Cast and Ash Dieback with Host-Derived Microbial Antagonists Exhibiting Plant Growth-Promoting Traits" Microorganisms 13, no. 11: 2517. https://doi.org/10.3390/microorganisms13112517
APA StyleŠilanskienė, M., Vaitiekūnaitė, D., & Sirgedaitė-Polikaitienė, V. (2025). Tackling Conifer Needle Cast and Ash Dieback with Host-Derived Microbial Antagonists Exhibiting Plant Growth-Promoting Traits. Microorganisms, 13(11), 2517. https://doi.org/10.3390/microorganisms13112517







