Abstract
Essential oils (EOs) from oregano (Origanum vulgare), rosemary (Salvia rosmarinus), clove (Syzygium aromaticum), thyme (Thymus vulgaris), cinnamon (Cinnamomum verum), and basil (Ocimum basilicum) possess antifungal properties. This study aimed to evaluate their ability to inhibit the growth of fungi isolated from the rot of banana peel (Musa paradisiaca) to control or reduce fungal growth in bananas. The methodology involved preparing dilutions of EOs and inoculating them onto Potato Dextrose Agar (PDA) medium amended with chloramphenicol to prevent bacterial contamination. Fungal species, including Trichoderma spp., Aspergillus spp., Penicillium spp., and Fusarium spp., were isolated, purified, and characterized macroscopically and microscopically. Their growth was assessed ex vivo and the inhibition percentage was measured in vitro. The ex vivo analysis revealed that the severity of fungal infection, ranked from highest to lowest, was as follows: Penicillium spp., Trichoderma spp., Fusarium spp., and Aspergillus spp. The results showed that rosemary and basil oils did not inhibit fungal growth, whereas clove oil, cinnamon, and oregano were effective against the four tested fungi at 800, 400, and 200 ppm, respectively. These findings suggest that certain EOs, including clove, cinnamon, and oregano, have strong antifungal potential and could serve as eco-friendly alternatives to synthetic fungicides in banana postharvest disease management.
1. Introduction
Banana (Musa paradisiaca), a staple food in tropical regions and in many developing countries, plays an essential role as a pillar for the economic growth and social development of local communities, due to its ability to maintain consistent production throughout the year [1]. Ecuador’s banana production is vital for its economy and food security [2]. The main banana-producing provinces are Guayas, El Oro, and Los Ríos, with El Oro bananas notable for their quality, while Guayas and Los Ríos are the major producers [2,3].
In production, various factors, both external and internal, affect the quality of the fruit during the postharvest phase. External factors include environmental conditions such as relative humidity and temperature, and among internal factors, metabolic changes and the presence of fungal pathogens are highlighted [2,4].
It is important to emphasize that fungal pathogens, in particular diseases associated with Fusarium spp., Penicillium spp., Trichoderma spp., and Aspergillus spp., represent the main causes of banana wilt [5,6]. These diseases manifest as a reduction in the firmness of the superficial tissues in the areas of the rachis and the banana crown, accompanied by changes in leaf coloration and cracks at the base of the pseudo-stem [7]. This causes internal rot in the fingers of the banana, eventually resulting in crown rot and, in more severe cases, the death of the fruit [8,9].
Traditionally, these diseases have been managed using synthetic fungicides. However, the growing interest in organic farming and the concerns about toxic residues in food have prompted the search for more sustainable alternatives [10]. In this context, EOs derived from plants such as Origanum vulgare, Salvia rosmarinus, Syzygium aromaticum, Thymus vulgaris, and Cinnamomum verum have emerged as promising alternatives [11]. These oils not only possess antimicrobial properties, but have also been shown to extend the shelf life of fruits by providing fungitoxic effects and enhancing resistance to postharvest diseases [12,13].
EOs contain bioactive compounds such as terpenes and phenols, which are documented to have antimicrobial effects. These compounds can inhibit the growth of fungal pathogens, including those responsible for postharvest rot in bananas [14]. Previous studies have demonstrated the antifungal efficacy of EOs such as oregano, rosemary, and clove, although the results vary depending on the concentration, the type of fungus, and the storage conditions [15,16]. However, despite these promising results, there is limited research specifically evaluating the antifungal activity of these oils against Fusarium spp., Penicillium spp., Trichoderma spp., and Aspergillus spp. in bananas, especially under real postharvest conditions.
This investigation focuses on assessing the antifungal effectiveness of commercially available EOs against fungal pathogens isolated from banana peels. Existing research has helped in the development of sustainable alternatives to synthetic fungicides, promoting eco-friendly agricultural practices, and enhancing postharvest management [17]. Understanding the composition of the EOs used is crucial to identifying the bioactive compounds responsible for observed antifungal effects. The EOs of oregano and thyme share similar volatile compounds [18], such as the carvacrol, thymol, and p-cymene, and rosemary contains alpha-pinene, 1.8 cineol, camphor, and verbenone [19,20]. Clove oil is characterized by its content of eugenol, acetyl eugenol, and α and β caryophyllenes, and basil contains estragole, linalool, eugenol, and methyl cinnamate [19]. Cinnamon oil is rich in cinnamaldehyde and eugenol, which have demonstrated significant antifungal and bioactive properties [21].
Although EOs from oregano, basil, thyme, clove, cinnamon, and rosemary have a potent antifungal effects, their phytotoxicity must be carefully monitored. The excessive use or misdosage of these oils can cause damage to plants, such as chlorosis, necrosis, and reduced root growth, especially at concentrations above 1%. It is recommended to use safe concentrations of these oils and conduct preliminary tolerance tests on specific plant species before their large-scale application [11,22].
The objective of this study is to evaluate the potential of EOs as an ecological alternative for controlling fungal diseases in bananas, promoting sustainable agriculture and organic production. Specifically, this study evaluates the antifungal efficacy of these oils against Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp. isolated from Musa paradisiaca through in vitro and ex vivo experiments.
Fusarium a filamentous genus known for its ability to adapt to diverse environmental conditions, is a major cause of vascular wilt in bananas, blocking the transport of water and nutrients, and reducing both the quality and quantity of fruit production [23]. In Ecuador is where one can generally find the species oxysporum, verticillioides, and solani, which are studied to innovate strategies to avoid damage to bananas [3,24].
Penicillium is distributed in various environments, and some species have antagonistic activity against pathogens that can cause deterioration of the fruit postharvest [25]. P. citrinum, P. expansum, and P. digitatum are frequently isolated from banana surfaces, and EOs like mandarin have been shown to inhibit their growth [26,27].
Trichoderma is a genus of filamentous, cosmopolitan fungi known for its biocontrol properties [28,29]. Trichoderma species produce antibiotics and secondary metabolites that inhibit phytopathogenic fungi [30]. India distributes certified species of Trichoderma asperellum, T. atroviride, T. gamsii, T. hamatum, T. harzianum, T. polysporum, T. virens, and T. viride, and with genetic engineering, has made significant improvements to their application in industrial processes [28,31].
Aspergillus spp. including species such as A. niger, A. flavus, and A. parasiticus, are common contaminants in bananas during storage and transportation [32]. These fungi produce a variety of enzymes that decompose plant tissues and can also produce harmful aflatoxins, which pose a risk to human health [14,32]. Aspergillus parasiticus is similar to A. flavus, and this species can also produce aflatoxin and affect the quality and safety of bananas [33].
In light of the above, the objective of the current study was to evaluate the potential of oregano, rosemary, clove, cinnamon, and basil EOs as an ecological alternative for controlling fungal diseases in bananas (Musa paradisiaca), promoting sustainable agriculture and organic production [34]. Specifically, this study evaluates the antifungal efficacy of these oils against Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp. isolated from Musa paradisiaca through in vitro and ex vivo analysis. By promoting sustainable agriculture and reducing reliance on chemical fungicides, this research supports the development of more eco-friendly, effective strategies for postharvest disease management.
2. Materials and Methods
In this research, we used Musa paradisiaca (Ecuadorian bananas, which were harvested with export in mind). A sample of bananas was taken and exposed to an ambient temperature of approximately 25 °C until signs of rot were observed. Bananas that showed at least 50% of the symptoms of the presence of fungi in their peels were selected [35].
2.1. Isolation and Purification of Microorganisms
The isolation of pathogenic fungi from infected Musa paradisiaca peels was carried out using Potato Dextrose Agar (PDA) medium (Difco™, Detroit, MI, USA), widely recognized for its ability to promote fungal growth [36]. To inhibit bacterial contamination, chloramphenicol (Merck, Quito, Ecuador) was added at a final concentration of 0.5 g/L following the agar diffusion method [37]. The antibiotic was incorporated to prevent bacterial contamination during the fungal isolation process using the agar diffusion method. The prepared medium was then poured into sterile Petri dishes and allowed to solidify at room temperature.
The medium was prepared by dissolving 39 g of PDA in 1 L of distilled water, sterilized by autoclaving, poured into sterile Petri dishes, and left to solidify at room temperature [38]. Thirty banana samples with visible symptoms were collected, and approximately 20 g of peel was extracted per fruit. The peels were washed twice with sterile distilled water to remove superficial contaminants. Subsequently, the fragments were transferred to Erlenmeyer flasks containing 200 mL of a 0.05% (v/v) Tween 80 aqueous solution (Merck, Quito, Ecuador), and vortexed for 2 min to facilitate spore release.
Four serial dilutions (0.1%) were prepared from the suspension to ensure homogeneity [39]. From each dilution, 0.1 mL was inoculated onto PDA plates supplemented with 0.05% chloramphenicol and incubated at 25 ± 2 °C and 75 ± 5% relative humidity under a photoperiod of 12 h light/12 h dark [40]. Emerging colonies were monitored daily, individually isolated using a sterile loop, and subcultured weekly until pure cultures were obtained for macroscopic and microscopic morphological analyses [41].
2.2. Morphological Identification
Once pure fungi were obtained, macroscopic analysis was conducted to assess the characteristics of the fungal colonies, including their shape, color, and texture. For the identification of the causative agents affecting the banana, the upper and lower surfaces of the Petri dishes were observed macroscopically, considering the morphological similarities obtained through direct comparisons. The pure cultures were examined in triplicate weekly after inoculation with the isolated pathogens [42]. Their macroscopic characteristics were recorded and compared with bibliographic information from books and descriptive guides of fungal morphology to identify the genus of the pathogen [39,43]. Aspects such as the colony shape, elevation, edges and appearance of pure fungi were considered.
Microscopic examination was performed using conventional methods to observe the fungal reproductive structures, including hyphae, conidia, and spores. To visualize the microscopic and specialized structures, a piece of adhesive tape was used to collect the aerial mycelium of the fungus, and it was mounted on a microscope slide [39]. The plate was examined using a compound microscope (Motic Instruments Inc., Xiamen, China), equipped with a digital camera (Euromex Microscopes, Arnhem, The Netherlands), with 40× and 60× objective lenses, this analysis was performed in triplicate weekly. The evaluation was based on the observation of hyphae, mycelium, spores, and other microscopic structures present.
2.3. Molecular Identification Through DNA Sequencing
Molecular identification of fungi isolated from Musa paradisiaca peel was performed through DNA sequencing. Genomic DNA was extracted from pure fungal colonies using the commercial Invitrogen kit (Novogene, CA, USA), following the manufacturer’s instructions. The quality and quantity of the extracted DNA were evaluated using spectrophotometry with a Nanodrop spectrophotometer (Thermo Fisher Scientific, MA, USA) and by performing 1% agarose gel electrophoresis to assess DNA integrity [37].
The ribosomal DNA fragment corresponding to the Internal Transcribed Spacer (ITS) region was amplified using the universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [44]. The PCR amplification was carried out under optimal conditions in a thermocycler, and the amplified products were visualized on a 1.5% agarose gel stained with ethidium bromide under UV light to confirm successful amplification of the ITS region [37].
Purified amplicons were sent to Macrogen Inc. (Seoul, Republic of Korea) for sequencing. The obtained sequences were analyzed using BioEdit software, version 7.0 [45], for alignment and subsequent comparison with public databases, such as NCBI GenBank (https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 10 April 2025)), using the BLASTn algorithm. The fungal species were identified based on a ≥98% similarity to deposited sequences in the database.
Phylogenetic analysis was conducted by aligning ITS sequences using the ClustalW algorithm. Phylogenetic trees were constructed employing both the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) and the Neighbor-Joining approach, implemented in MEGA version 11 [46]. Pairwise, identity matrices and genetic distances were calculated based on the Tamura-Nei substitution model. The reliability of clade groupings was assessed through 1000 bootstrap replicates. This protocol is consistent with prior studies that support the effectiveness of ITS sequences for fungal identification and phylogenetic inference [11,47,48].
2.4. Ex Vivo Fungal Activity
After completing the macroscopic and microscopic characterization of the pathogens, the ex vivo antifungal activity was assessed. The pathogens were isolated and purified from banana peel samples and included Trichoderma spp., Aspergillus spp., Penicillium spp., and Fusarium spp. [49]. These fungi were selected from a total of 15 due to their relevance in postharvest diseases of bananas during storage.
The growth index was calculated based on the development of these fungi on fruit, with 20 samples used for each fungus and with four replicates for each treatment. The inoculum concentration was adjusted to 106 conidia/mL to maintain constant infection [14]. A volume of 100 µL of the standardized fungal inoculum was applied, and the samples were maintained at approximately 13 ± 1 °C and 92 ± 3% relative humidity. The fungal growth diameter was measured weekly for up to 5 weeks post-inoculation, providing a comprehensive timeline of the pathogen’s progression and activity. The severity of infection was classified based on the growth diameter of the fungal colonies, with all of the purified pathogens considered in the analysis [43]. This classification provides valuable data on their interaction with the banana peel under controlled conditions [49]. A one-way ANOVA was applied to identify statistically significant differences in species growth, followed by Tukey’s HSD post hoc test.
2.5. In Vitro Antifungal Activity with Essential Oils
The antifungal efficacy of EOs derived from six species—oregano (Origanum vulgare), rosemary (Salvia rosmarinus), clove (Syzygium aromaticum), thyme (Thymus vulgaris), cinnamon (Cinnamomum verum), and basil (Ocimum basilicum)—was assessed in vitro using a method of incorporation into the medium [49,50,51]. Antifungal activity was assessed under controlled laboratory conditions at 25 ± 2 °C and 75 ± 5% relative humidity, with a photo period of 12 h of light and 12 h of darkness, and monitored daily. Essential oils (batch 20230516) supplied by Green Harmony were used, a company specialized in the production and distribution of pure and natural oils.
EOs were obtained using steam distillation, a widely accepted method for isolating plant-derived bioactive compounds. Oregano oil was extracted from dried leaves, cinnamon oil from dried bark, and clove oil from dried flower buds. For rosemary, basil, and thyme, EOs were derived from fresh leaves and flowers. All oils were purchased from certified commercial suppliers. Specifically, oregano EO originated from Turkey, cinnamon from Sri Lanka, clove from Indonesia, thyme from Japan, basil from India, and rosemary from Spain. It is worth noting that the geographic origin of EOs plays a critical role in determining their chemical composition and biological activity [52,53].
Cinnamon EOs consist mainly of cinnamaldehyde (60–70%) and eugenol (5–15%), both known for their antimicrobial and antioxidant effects. Basil EOs are characterized by their high contents of eugenol (50–70%), linalool (10–15%), and methyl chavicol (5–15%), which are compounds with antimicrobial, anti-inflammatory, and antioxidant properties. Oregano EOs are predominantly composed of carvacrol (60–80%), a phenolic compound recognized for its antimicrobial and antioxidant properties. It also contains thymol (5–10%), p-cymene (5–10%), and γ-terpinene (2–5%), which act together to inhibit microbial growth.
Clove EOs are distinguished by their high eugenol content (70–85%), followed by acetyl eugenol (5–15%) and β-caryophyllene (5–12%), all of which contribute to their antifungal and antibacterial effectiveness. The EOs of thyme contain thymol (30–50%) and carvacrol (10–20%), along with p-cymene (15–25%) and γ-terpinene (10–15%), which are responsible for their antifungal properties. The EOs of rosemary are mainly made up of 1,8-cineole (20–50%), carnosol (5–15%), rosmarinic acid (2–5%), and α-pinene (10–20%), compounds known for their antioxidant, antimicrobial, and potential neuroprotective effects.
To prepare the solutions, each EO was diluted in a 0.05% Tween 80 solution to form a homogeneous emulsion, at concentrations of 200, 400, 600, 800, and 1000 ppm [54]. These emulsions were added to cooled PDA medium before solidification. The pathogens were inoculated to determine the most effective concentration. Visual assessments were carried out every 24 h, considering three independent replicates and four subsamples per replicate for each microorganism. The mycelial growth inhibition percentages were calculated to assess the antifungal efficacy of the applied essential oils.
A negative control was included, consisting of PDA medium supplemented with 0.05% Tween 80 but without essential oils (EOs), allowing for the establishment of a baseline fungal growth reference in the absence of treatment. This facilitated the evaluation of the antifungal efficacy of the tested EOs. The experimental design followed a 6 × 5 mixed-factor model, where the independent variables were six different types of EOs and five concentration levels, while the dependent variable was the percentage of mycelial growth inhibition. Statistical analyses were performed to identify the optimal concentration of each EO capable of significantly inhibiting fungal growth. The findings provide meaningful insights into the potential of plant-derived essential oils as natural antifungal agents, supporting their future use in biocontrol strategies and sustainable agriculture.
3. Results
3.1. Morphological Identification
Figure 1 shows the pure fungi isolated from banana peel rot on selective medium (PDA + Chloramphenicol) and stored in PDA at approximately 25 °C, with macroscopic images of the front and reverse sides of Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp. Figure 2 presents the aerial mycelium of the fungi. These observations provide visual information that complements the morphological analysis and microscopic images observed under 40× and 60× magnifications.
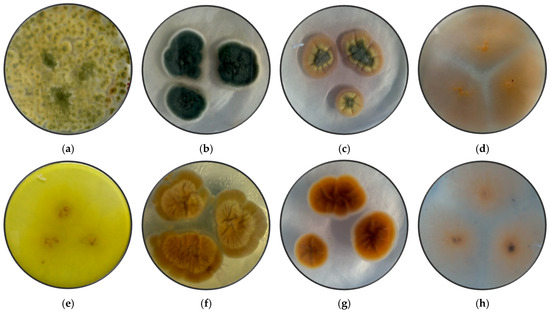
Figure 1.
Macroscopic images of the front side of (a) Trichoderma spp, (b) Penicillium spp., (c) Aspergillus spp., and (d) Fusarium spp., and the reverse side of (e) Trichoderma spp., (f) Penicillium spp., (g) Aspergillus spp., (h) Fusarium spp.
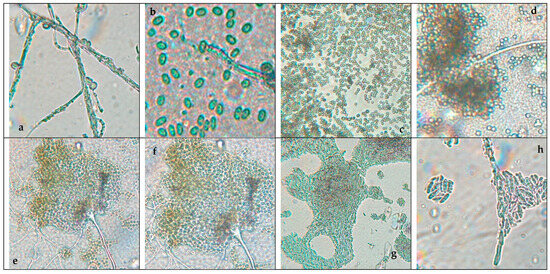
Figure 2.
Microscopic images of (a) Trichoderma spp, (c) Penicillium spp., (e) Aspergillus spp., and (g) Fusarium spp. at 40× magnification and (b) Trichoderma spp., (d) Penicillium spp., (f) Aspergillus spp., and (h) Fusarium spp. at 60× magnification.
3.2. Molecular Identification Through DNA Sequencing
Figure 3 shows the phylogenetic tree constructed from ITS-region sequences of fungal isolates recovered from Musa paradisiaca, aligned against GenBank reference sequences. The analysis revealed consistent clustering by genus and species, supported by high bootstrap values confirming the molecular identification of the isolates.
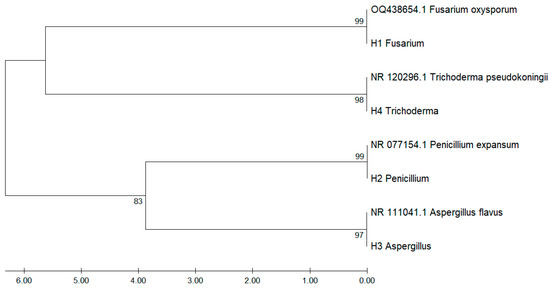
Figure 3.
Phylogenetic tree based on ITS-region sequences of fungal isolates associated with Musa paradisiaca.
Isolate H1 clustered closely with Fusarium oxysporum (OQ438654.1), with 99% bootstrap support, while H4 grouped with Trichoderma pseudokoningii (NR_120296.1) had 98% bootstrap support. Similarly, H2 aligned with Penicillium expansum (NR_077154.1) and H3 with Aspergillus flavus (NR_111041.1), with bootstrap values of 99% and 97%, respectively.
The phylogenetic tree also revealed two main clades: one comprising Fusarium and Trichoderma, and the other comprising Penicillium and Aspergillus, supported by a bootstrap value of 83%. These findings highlight the utility of ITS sequencing in accurately discriminating fungal pathogens in tropical crops such as banana.
Table 1 presents a summary of the results from sequencing the ITS region of fungi isolated from banana peels. It lists the identified fungal organisms, the genetic fragments utilized for sequencing, and the percentage similarity derived from a comparison with the database. The sequences exhibited a high degree of similarity (≥98%), which supports the reliability of the fungal genera and species identification in the analyzed samples.

Table 1.
The sequencing results for fungi isolated from the samples of Musa paradisiaca.
The pairwise identity matrix derived from ITS sequence alignment (Table 2) revealed high similarity levels between the fungal isolates and GenBank reference sequences. Isolate H1 exhibited 99.6% identity with Fusarium oxysporum (OQ438654.1), while H4 showed 99.7% identity with Trichoderma pseudokoningii (NR_120296.1). Likewise, H2 matched Penicillium expansum (NR_077154.1) with 99.8% similarity, and H3 shared 99.2% identity with Aspergillus flavus (NR_111041.1).

Table 2.
Pairwise genetic distance matrix of ITS sequences among fungal isolates and GenBank reference strains.
The lowest identity values were observed between different genera, with genetic distances exceeding 10,000 units, highlighting clear taxonomic differentiation. This matrix quantitatively supports the phylogenetic proximity observed in the dendrogram and confirms molecular identification at the species level.
3.3. Fungal Activity Ex Vivo
The severity of fungal infection in banana samples was assessed through ex vivo analysis, in which 20 banana samples were monitored over a 6-week period. Fungal growth was evaluated for various species, including Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp., with Figure 4 providing a visual representation of this growth over time.
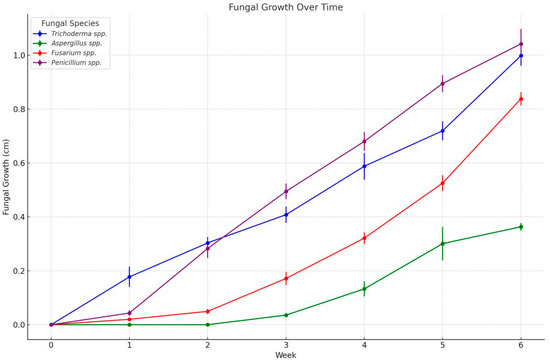
Figure 4.
Fungal growth (cm) during 6 weeks in 20 banana samples inoculated with Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp. stored at 13 °C and 95% HR approximately.
Regarding the progression of fungal growth on Musa paradisiaca peel samples over a six-week storage period, the analysis utilized an ANOVA approach to examine the impact of different treatments and concentrations on fungal development. Statistically significant differences were observed among treatments (p < 0.05), indicating that both the type of treatment and its concentration had a notable effect on fungal growth.
In addition, a one-way ANOVA revealed highly significant differences in growth among the four fungal species evaluated (Penicillium, Trichoderma, Fusarium, and Aspergillus) (F = 48.25, p < 0.001). These differences underscore the varying capacity of these fungi to colonize postharvest banana tissue.
Tukey’s Honest Significant Difference (HSD) post hoc test (α = 0.05) was conducted to identify pairwise differences. The results showed that Penicillium and Trichoderma exhibited the highest growth rates, with no significant difference between them (p > 0.05), while both displayed significantly greater growth compared to Fusarium and Aspergillus. Among all of the fungi, Aspergillus spp. showed the lowest growth, differing significantly from the other species (p < 0.001). These findings highlight the variability in fungal infections in bananas and emphasize the importance of effective treatment strategies to control postharvest decay.
Assumptions of normality and homogeneity of variances were verified through residual diagnostic plots and statistical tests. The Shapiro–Wilk test confirmed the normal distribution of residuals (p > 0.05), while Levene’s test indicated homogeneity of variances across groups (p > 0.05). Additionally, visual inspection of standard deviations revealed consistent dispersion among treatment groups, supporting the validity of the ANOVA results.
3.4. In Vitro Antifungal Activity with Essential Oils
Figure 5, Figure 6, Figure 7 and Figure 8 show the in vitro growth of Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp., respectively, in PDA medium with 200 ppm, 400 ppm, 600 ppm, 800 ppm, and 1000 ppm of oregano, basil, cinnamon, rosemary, thyme, and clove EOs.
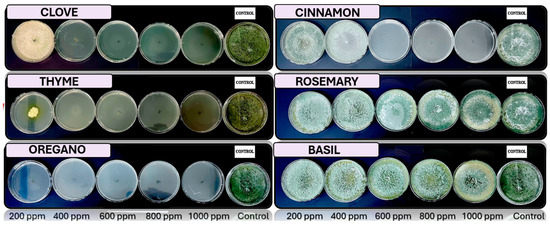
Figure 5.
In vitro analysis of Trichoderma spp. in PDA medium supplied with basil, cinnamon, clove, oregano, rosemary, and thyme essential oils at various concentrations of 200, 400, 600, 800, and 1000 ppm (n = 12).

Figure 6.
In vitro analysis of Penicillium spp. in PDA medium supplied with basil, cinnamon, clove, oregano, rosemary, and thyme essential oils at various concentrations of 200, 400, 600, 800, and 1000 ppm (n = 12).

Figure 7.
In vitro analysis of Aspergillus spp. in PDA medium supplied with basil, cinnamon, clove, oregano, rosemary, and thyme essential oils at various concentrations of 200, 400, 600, 800, and 1000 ppm (n = 12).
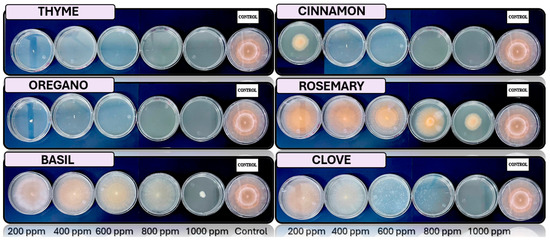
Figure 8.
In vitro analysis of Fusarium spp. in PDA medium supplied with basil, cinnamon, clove, oregano, rosemary, and thyme essential oils at various concentrations of 200, 400, 600, 800, and 1000 ppm (n = 12).
In this study, the antifungal efficacy of six essential oils (EOs) was evaluated in vitro against Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp. at five concentration levels ranging from 200 to 1000 ppm. Fungal inhibition was recorded as the absence of visible mycelial growth and denoted by “−”, whereas growth was indicated by “+”. Table 3 presents a detailed overview of these inhibitory effects.

Table 3.
Evaluation of in vitro antifungal activity of Trichoderma spp., Penicillium spp., Aspergillus spp., and Fusarium spp. upon using essential oils of oregano, rosemary, clove, thyme, cinnamon, and basil.
These results exhibit a clear concentration-dependent inhibition pattern. Cinnamon, oregano, and thyme oils demonstrated the highest antifungal efficacy, each achieving 23 out of 25 possible inhibitions across all tested fungi and concentrations. Conversely, basil oil displayed the weakest performance, with only 16 inhibitory responses.
Among the fungal genera, Fusarium spp. were the most sensitive, exhibiting consistent inhibition by most oils at concentrations of 400 ppm and above. Trichoderma spp. and Penicillium spp. showed moderate susceptibility, while Aspergillus spp. appeared comparatively more resistant, maintaining growth at lower concentrations for several oils.
These findings underscore the potential of cinnamon, oregano, and thyme essential oils as effective natural alternatives for the control of fungal pathogens in Musa paradisiaca, supporting their possible application in postharvest disease management strategies.
The analysis of fungal growth inhibition revealed a clear concentration-dependent response to the application of essential oils (EOs). As shown in Figure 9, the inhibition percentages increased progressively with EO concentration, reaching statistically relevant thresholds. At the lowest tested concentration (200 ppm), the average inhibition was approximately 13%, while at 400 ppm it increased to 33%. A marked rise in antifungal activity was observed at 600 ppm, achieving 77% inhibition, with subsequent increases at 800 ppm (85%) and 1000 ppm (89%).
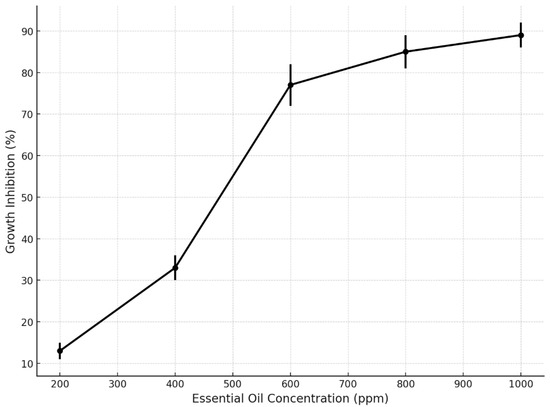
Figure 9.
Fungal growth inhibition according to essential oil concentration.
4. Discussion
4.1. Morphological Identification
In Figure 1 and Figure 2, the results of the macroscopic and microscopic analysis are presented and detailed.
Trichoderma spp. grow rapidly, with colonies that vary in color (white, green, yellow, or orange) and texture (cottony, velvety, or granular) depending on growth conditions. Microscopically, they produce branched conidiophores with small oval or cylindrical conidia that form in chains or clusters. A characteristic odor may be present but is not a reliable feature [55]. Fusarium spp. have networks of filaments (hyphae) and conidia (asexual and sexual spores or ascospores). Under a microscope, the phialides are generally thin, with a bottle shape that can be simple or branched and short or long, and they can have different characteristics depending on the species [56].
Aspergillus spp. are characterized by a prominent columella and highly organized plumose structures, where conidia emerge symmetrically. This distinct arrangement differentiates Aspergillus spp. from Penicillium and Fusarium, which have simpler conidiophore structures. Penicillium spp. can cause stains and discoloration on the peel of the banana, affecting its appearance and quality. Expansum can affect bananas during storage, causing rotting and loss of firmness, while digitatum is more common in citrus fruits but can affect bananas if they are stored in wet and warm storage conditions, causing rotting and stains on the peel [57]. To prevent the presence of this fungus, the fruit should be handled properly during storage and transport.
Trichoderma spp., including T. asperellum, T. harzianum, and T. koningii, are characterized by ellipsoidal conidia grouped together and thin, septate hyphae [58]. The colonies exhibit rapid growth and are typically green, with a circular shape, rough surface, regular edges, and slightly elevated contours [59]. The texture varies from fuzzy to cottony, reflecting their adaptability to different growth conditions.
Penicillium spp. display septate hyphae of variable length, forming structures that resemble plumes. The colonies range in color from white to green or blue, with a velvety surface and well-defined edges. The characteristic brush-like arrangement of conidia is a defining feature of this genus, enabling clear morphological differentiation from other fungi [25].
Aspergillus spp. are distinguished by septate hyphae and conidiophores bearing long, ellipsoidal conidia arranged in characteristic plumose structures. The colonies exhibit a range of colors, typically yellow to green, with a rugged surface and a texture resembling tufts. The edges are regular or slightly wavy, and the colony elevation often shows a cratiform pattern [33].
Fusarium spp. are characterized by fusiform, cylindrical conidia arranged in chains or clusters, supported by septate hyphae. The colonies appear pink, red, or orange, with a cottony or velvety texture, diffuse edges, and a flat or slightly elevated surface. These macroscopic and microscopic features distinguish Fusarium spp. from other genera [24].
This macroscopic and microscopic analysis of the fungal colonies, in combination with DNA sequencing, revealed distinct characteristics that facilitated the accurate identification of the species. The following discussion outlines the macroscopic features observed for Fusarium oxysporum, Penicillium expansum, Trichoderma pseudokoringii, and Aspergillus flavus.
Fusarium oxysporum exhibited typical characteristics of the Fusarium genus, including pink to orange colonies with a cottony texture and regular edges. The colony surface was flat or slightly elevated, which is consistent with the morphological description in [60]. The conidia, arranged in chains, were observable under the microscope. The macroscopic features, including the color and texture, provided reliable indicators for the identification of Fusarium oxysporum when compared to other species.
Penicillium expansum showed green colonies with white edges and a powdery, flat surface, which are characteristic of many Penicillium species, particularly those associated with decaying fruits and vegetables [61]. The white edge and powdery surface were key distinguishing features that aligned with the macroscopic description of Penicillium expansum. This appearance supports its identification, as it may have similar macroscopic features to other fungi, but differs in terms of details such as colony color or edge definition.
Trichoderma pseudokoringii include green colonies with a rough, granular surface and slightly elevated, regular edges. These features, common in many Trichoderma species [62], were consistent with the macroscopic description of T. pseudokoringii. The growing of the colonies and the distinctive texture helped confirm its identification. The rough surface and elevated edges serve as distinguishing features when compared to other fungi [30].
Aspergillus flavus exhibited yellow-green colonies with a rough surface and a cratiform elevation, consistent with the known morphological traits of A. flavus [33,63]. The coloration and rough texture of the colonies made it easy to differentiate A. flavus from other Aspergillus species. Its cratiform elevation, a distinct feature, further facilitated its identification. The yellow-green color and texture are particularly indicative of the species.
4.2. Molecular Identification Through DNA Sequencing
The DNA sequencing of fungi isolated from Musa paradisiaca samples provided highly reliable identification of the species through the ITS region, with all isolates showing a percentage identity above 98% [37]. These results validate the initial morphological analysis and emphasize the precision of molecular techniques in fungal taxonomy.
Fusarium oxysporum was identified with a 99.26% identity in the ITS region, confirming its classification within the Fusarium genus. This high similarity aligns with the macroscopic features previously observed, such as the pink-to-orange colonies with a cottony texture and regular edges. These combined findings provide strong evidence of its presence in the samples analyzed.
Penicillium expansum showed the highest percentage identity (99.68%) among the isolates, reflecting its genetic distinction. The molecular data corroborates its characteristic macroscopic traits, such as green colonies with white edges and a powdery, flat surface. The DNA sequencing and morphological analysis solidify its identification and highlight its significance in Musa paradisiaca samples and its association with fruit decay.
Trichoderma pseudokoringii exhibited a 99.24% identity, supporting its classification, which aligns with the observation of its rapid growth and green colonies with a granular texture. This genetic match enhances the confidence in its identification, particularly given the importance of Trichoderma species in biological control and agricultural systems.
Aspergillus flavus was identified with a 99.34% identity, confirming its classification. The sequencing results are consistent with their distinctive morphologies, such as yellow-green colonies with a rough surface and cratiform elevation. This high genetic identity underscores the reliability of ITS sequencing for distinguishing A. flavus, a species of economic and health significance due to its ability to produce aflatoxins.
The pairwise distance matrix derived from ITS sequences (Table 1 and Table 2) revealed significant genetic divergence among the fungal isolates analyzed. The lowest genetic distance was recorded between Fusarium oxysporum (OQ438654.1) and its corresponding local isolate, H1 (0.004), indicating a high degree of sequence similarity. In contrast, the highest divergence was observed between Penicillium expansum (NR_077154.1) and Trichoderma pseudokoningii (NR_120296.1), with values exceeding 16.4 units, reflecting distinct phylogenetic separation.
These findings align with the clustering patterns observed in the phylogenetic tree (Figure 3), further validating the internal transcribed spacer (ITS) region as a robust molecular marker for delineating taxonomic relationships among filamentous fungi. Moreover, the distance matrix (Table 2) reinforces the bootstrap-supported clade assignments, particularly between Penicillium and Aspergillus species, which exhibited moderate genetic distances.
4.3. Ex Vivo Fungal Activity
The inhibition rate was evaluated by measuring fungal growth from 20 inoculations of each fungus to assess the severity of the pathogens. The ex vivo growth of fungal species isolated from Musa paradisiaca was monitored over six weeks, as shown in Figure 4. The data demonstrates distinct growth patterns among the fungal species (Trichoderma pseudokoringii, Aspergillus flavus, Fusarium oxysporum, and Penicillium expansum), highlighting their varying rates of colonization and adaptation.
Trichoderma pseudokoringii exhibited the slowest growth among the species, with minimal increases in fungal colony size during the first three weeks. By week 6, the colony size had reached approximately 0.4 cm. The initial slow growth may reflect the competitive nature of Trichoderma spp. as a biological control agent, requiring time to adapt and establish in the substrate [62]. However, its accelerated growth in later weeks suggests its ability to utilize the available nutrients efficiently once adapted.
Aspergillus flavus displayed moderate growth, showing a consistent increase in colony size throughout the six weeks and reaching approximately 0.8 cm by the end of the observation period. This growth pattern aligns with its documented ability to colonize plant materials, particularly in nutrient-rich environments such as stored grains and fruits [64,65]. The steady growth of A. flavus’s indicates its adaptability and efficiency in utilizing Musa paradisiaca as a substrate.
Fusarium oxysporum demonstrated a growth rate between that of Trichoderma pseudokoringii and Aspergillus flavus. By week 6, the colony size was approximately 0.6 cm. The intermediate growth rate is consistent with the ecological behavior of Fusarium spp., which thrives under specific conditions but does not dominate rapidly in environments with competing organisms [55,60].
Penicillium expansum showed the highest growth rate among the species, reaching over 1 cm by week 6. This rapid growth reflects its opportunistic nature and strong ability to colonize decaying organic matter, including fruits like Musa paradisiaca [26,61,66]. A powdery texture and efficiency in nutrient utilization are characteristic of P. expansum, enabling its dominance in the substrate.
4.4. In Vitro Antifungal Activity with Essential Oils
The in vivo experiments with EOs at different concentrations (200, 400, 600, 800, and 1000 ppm) demonstrated distinct antifungal efficacy across fungal species (Trichoderma pseudokoringii, Penicillium expansum, Aspergillus flavus, and Fusarium oxysporum). These results emphasize the importance of EOs as natural antifungal agents in agricultural and food preservation applications.
Clove and cinnamon EOs contain active compounds such as eugenol and cinnamaldehyde, which have demonstrated significant antimicrobial properties. These compounds interact with fungal cell membranes, increasing their permeability and causing the leakage of essential components, which leads to fungal cell death [14].
Furthermore, cinnamaldehyde in cinnamon oil inhibits the production of intracellular enzymes such as amylases and proteases, resulting in the degradation of the cell wall and a high degree of cellular lysis. This action contributes to the efficacy of cinnamon oil in inhibiting the growth of various pathogens.
EOs also affect fungal spore germination. For instance, studies have shown that clove oil inhibits the germination of F. oxysporum and F. solani spores at concentrations of 125 µL/L, suggesting its potential as a preventive biofungicide. Likewise, thyme oil has been shown to inhibit the germination of Colletotrichum acutatum spores, significantly reducing the spread of this pathogen [67]. These findings support the efficacy of clove and cinnamon EOs as natural antimicrobial agents, offering sustainable alternatives for controlling fungal pathogens in the postharvest management of Musa paradisiaca.
Trichoderma pseudokoringii displayed a response to cinnamon and clove oils, which were most effective at higher concentrations (800–1000 ppm), showing significant inhibition of fungal growth, probably due to the phenolic compounds, such as cinnamaldehyde and eugenol, that disrupt fungal cell membranes [62]. Basil and rosemary oils exhibited moderate inhibition at all concentrations, while oregano and thyme oils showed minimal effects. These findings align with previous studies highlighting the potential of Trichoderma pseudokoringii as a biological control agent when combined with natural compounds [68].
The growth of Penicillium expansum was highly sensitive to cinnamon and clove oils, with complete inhibition observed at 800–1000 ppm. Basil and rosemary oils provided moderate inhibition across all concentrations, while thyme and oregano oils had limited effects. The pronounced sensitivity of Penicillium expansum to phenolic compounds suggests their potential use in controlling postharvest diseases in fruits and vegetables [69].
Aspergillus flavus showed significant growth inhibition when treated with cinnamon and clove oils, particularly at higher concentrations (800–1000 ppm). Thyme and oregano oils provided moderate inhibition, while basil and rosemary oils were less effective. The susceptibility of Aspergillus flavus to these oils underscores their utility in controlling fungal contamination and reducing aflatoxin production [69].
Fusarium oxysporum demonstrated varied responses to EOs. Cinnamon and clove oils showed moderate inhibition at concentrations above 600 ppm, while basil and rosemary oils exhibited consistent but limited effects across all concentrations. Oregano and thyme oils had minimal inhibitory activity. These results suggest that Fusarium oxysporum may require combined treatments, such as the integration of EOs with chemical fungicides or other biological agents, to achieve effective control [70].
Figure 9 shows an inhibition curve that reveals a clear concentration-dependent antifungal effect of essential oils, with inhibition rates increasing notably from 200 to 1000 ppm. A significant threshold was observed at 600 ppm, where the inhibition exceeded 75%, reaching nearly 90% at the highest concentration. These results highlight the effectiveness of EOs in fungal suppression, with reduced variability at higher doses, confirming their potential as natural antifungal agents for postharvest protection in Musa paradisiaca.
Cinnamon and clove oils exhibited the highest antifungal activity across all species, particularly at higher concentrations. Their effectiveness is attributed to their high phenolic content, which disrupts fungal cell structures and inhibits growth [71]. Basil and rosemary oils demonstrated moderate antifungal activity, suggesting their potential for combined use with other antifungal agents. Thyme and oregano oils exhibited limited antifungal effects [72,73], particularly against Fusarium oxysporum and Trichoderma pseudokoringii, highlighting the need for higher concentrations or combinations with other treatments.
5. Conclusions
Our findings showed that the characterization of fungi by order of severity identified Penicillium expansum, Trichoderma pseudokoringii, Fusarium oxysporum, and Aspergillus flavus as the fungi affecting Musa paradisiaca during the postharvest period. The in vitro analysis of essential oil efficacy revealed that Penicillium expansum was controlled by 400 ppm of cinnamon, oregano, and thyme essential oils. Trichoderma pseudokoringii was inhibited by 200 ppm of oregano, 400 ppm of clove and thyme, and 600 ppm of cinnamon essential oils. Fusarium oxysporum was most effectively managed with 200 ppm of cinnamon and thyme and 400 ppm of oregano essential oils. Aspergillus flavus was controlled by 200 ppm of oregano, and 400 ppm of cinnamon, clove, and thyme essential oils.
These findings suggest that essential oils, from cinnamon, clove, oregano, and thyme are promising natural alternatives to synthetic fungicides. They offer an eco-friendly solution for managing fungal infections in bananas. Future research should focus on optimizing their application in agricultural systems and investigating their synergistic effects with other control methods to enhance their antifungal efficacy
Author Contributions
M.D.R.M. and J.R. have significantly contributed to the development and writing of this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge the support of DECAB—Escuela Politécnica Nacional. During the preparation of this manuscript/study, the authors used Quillbot (https://quillbot.com, accessed on 24 March 2025) for the purposes of minor language editing, such as grammar, spelling, and style refinement. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galan, V.; Rangel, A.; Lopez, J.; Hernandez, J.B.P.; Sandoval, J.; Rocha, H.S. Propagación del banano: Técnicas tradicionales, nuevas tecnologías e innovaciones. Rev. Bras. Frutic. 2018, 40, 1–22. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Ruales, J. Post-Harvest Alternatives in Banana Cultivation. Agronomy 2024, 14, 2109. [Google Scholar] [CrossRef]
- Magdama, F.; Monserrate-Maggi, L.; Serrano, L.; García Onofre, J.; Jiménez-Gasco, M. del M. Genetic Diversity of Fusarium oxysporum f. sp. cubense, the Fusarium Wilt Pathogen of Banana, in Ecuador. Plants 2020, 9, 1133. [Google Scholar] [CrossRef]
- Capa Benítez, L.B.; Alaña Castillo, T.P.; Benítez Narváez, R.M. Importancia de la producción de banano orgánico. Caso: Provincia de El Oro, Ecuador. Rev. Univ. Soc. 2016, 8, 64–71. Available online: https://rus.ucf.edu.cu/index.php/rus/article/view/412 (accessed on 4 August 2024).
- Mata Anchundia, D.; Suatunce Cunuhay, P.; Poveda Morán, R. Análisis económico del banano orgánico y convencional en la provincia Los Ríos, Ecuador. Avances 2021, 23, 419–430. Available online: https://www.redalyc.org/journal/6378/637869393005/html/ (accessed on 12 August 2024).
- Aguilar-Anccota, R.; Arévalo-Quinde, C.G.; Morales-Pizarro, A.; Galecio-Julca, M.; Aguilar-Anccota, R.; Arévalo-Quinde, C.G.; Morales-Pizarro, A.; Galecio-Julca, M. Hongos asociados a la necrosis de haces vasculares en el cultivo de banano orgánico: Síntomas, aislamiento e identificación, y alternativas de manejo integrado. Sci. Agropecu. 2021, 12, 249–256. [Google Scholar] [CrossRef]
- Guzmán Piedrahita, Ó.A. El nematodo barrenador (Radopholus similis [Cobb] Thorne) del banano y plátano. Luna Azul 2011, 33, 137–153. Available online: https://revistasojs.ucaldas.edu.co/index.php/lunazul/article/view/1210 (accessed on 4 May 2025).
- Ruiz Medina, M.D.; Ruales, J. Essential Oils as an Antifungal Alternative to Control Several Species of Fungi Isolated from Musa paradisiaca: Part III. Microorganisms 2025, 13, 1663. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Ruales, J. Essential Oils as an Antifungal Alternative for the Control of Various Species of Fungi Isolated from Musa paradisiaca: Part I. Microorganisms 2025, 13, 1827. [Google Scholar] [CrossRef]
- Castellanos, D.; Algecira, N.; Villota, C. Aspectos relevantes en el almacenamiento de banano en empaques con atmósferas modificadas. Rev. Iberoam. Tecnol. Postcosecha 2011, 12, 114–134. Available online: https://www.redalyc.org/pdf/813/81320900002.pdf (accessed on 7 May 2025).
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Viollon, C.; Chaumont, J.P. Antifungal properties of essential oils and their main components against Cryptococcus neoformans. Mycopathologia 1994, 128, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Aulakh, J.; Regmi, A.; Fulton, J.; Alexander, C. Postharvest losses of fruits and vegetables in developing countries: A review of the literature. Front. Pharmacol. 2022, 13, 872127. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Gómez-García, R.; Martínez-Ávila, G.C.G.; Medina-Herrera, N.; González-Hernández, M.D. Unlocking Essential Oils’ Potential as Sustainable Food Additives: Current State and Future Perspectives for Industrial Applications. Sustainability 2025, 17, 2053. [Google Scholar] [CrossRef]
- Zambonelli, A.; Guerrini, L. Essential oils and their antifungal properties. Fungal Biol. Rev. 2021, 35, 21–32. [Google Scholar] [CrossRef]
- Mesa, V.A.M.; Marín, P.A.; Ocampo, O.; Calle, J.; Monsalve, Z. Fungicidas a partir de extractos vegetales: Una alternativa en el manejo integrado de hongos fitopatógenos. RIA Rev. Investig. Agropecu. 2019, 45, 23–30. Available online: https://www.redalyc.org/journal/864/86458941001/html/ (accessed on 7 February 2025).
- Barrera Necha, L.L.; García Barrera, L.J. Actividad antifúngica de aceites esenciales y sus compuestos sobre el crecimiento de Fusarium sp. aislado de papaya (Carica papaya). Rev. Científica UDO Agríc 2008, 8, 33–41. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=3094829 (accessed on 2 September 2024).
- López Luengo, M.T. El romero. Planta aromática con efectos antioxidantes. Offarm 2008, 27, 60–63. Available online: https://api.semanticscholar.org/CorpusID:177740524 (accessed on 14 July 2025).
- Flores-Villa, E.; Sáenz-Galindo, A.; Castañeda-Facio, A.O.; Narro-Céspedes, R.I.; Flores-Villa, E.; Sáenz-Galindo, A.; Castañeda-Facio, A.O.; Narro-Céspedes, R.I. Romero (Rosmarinus officinalis L.): Su origen, importancia y generalidades de sus metabolitos secundarios. TIP Rev. Espec. En Cienc. Quím.-Biológicas 2020, 23, 1–17. [Google Scholar] [CrossRef]
- Ruiz, M.; Ávila, J.; Ruales, J. Diseño de un recubrimiento comestible bioactivo para aplicarlo en la frutilla (Fragaria vesca) como proceso de postcosecha. Rev. Iberoam. Tecnol. Postcosecha 2016, 17, 276–287. Available online: https://www.redalyc.org/journal/813/81349041015/html/ (accessed on 7 August 2024).
- Suppakul, P.; Miltz, J.; Sonneveld, K.; Bigger, S.W. Antimicrobial properties of basil and its possible application in food packaging. J. Agric. Food Chem. 2003, 51, 3197–3207. [Google Scholar] [CrossRef]
- Román Jeri, C.H. Consideraciones Epidemiológicas para el Manejo de la Marchitez por Fusarium (Fusarium oxysporum f. sp. cubense) del Banano en la Región Central del Perú; CATIE: Turrialba, Costa Rica, 2012; Available online: https://repositorio.catie.ac.cr/handle/11554/926 (accessed on 17 February 2025).
- Tapia, C.; Amaro, J. Género Fusarium. Rev. Chil. Infectol. 2014, 31, 85–86. [Google Scholar] [CrossRef]
- Srinivasan, R.; Prabhu, G.; Prasad, M.; Mishra, M.; Chaudhary, M.; Srivastava, R. Penicillium. In Beneficial Microbes in Agro-Ecology; Amaresan, N., Senthil Kumar, M., Annapurna, K., Kumar, K., Sankaranarayanan, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 651–667. [Google Scholar] [CrossRef]
- Velásquez, M.A.; Álvarez, R.M.; Tamayo, P.J.; Carvalho, C.P. Evaluación in vitro de la actividad fungistática del aceite esencial de mandarina sobre el crecimiento de Penicillium sp. Cienc. Tecnol. Agropecu. 2014, 15, 7–14. [Google Scholar] [CrossRef]
- Kaur, M.; Arora, S. Antifungal Potential of Essential Oils: A Review. In Phytochemicals in Human Health; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Schuster, A.; Schmoll, M. Biology and Biotechnology of Trichoderma. Appl. Microbiol. Biotechnol. 2010, 87, 787–799. [Google Scholar] [CrossRef]
- Brotman, Y.; Kapuganti, J.G.; Viterbo, A. Trichoderma. Curr. Biol. 2010, 20, R390–R391. [Google Scholar] [CrossRef]
- Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; Alarcón, A.; Hernández-Melchor, D.J.; Ferrera-Cerrato, R.; Alarcón, A. Trichoderma: Importancia agrícola, biotecnológica y sistemas de fermentación para producir biomasa y enzimas de interés industrial. Chil. J. Agric. Amp Anim. Sci. 2019, 35, 98–112. [Google Scholar] [CrossRef]
- Palou, L. Control integrado no contaminante de enfermedades de poscosecha (CINCEP): Nuevo paradigma para el sector español de los cítricos. Levante Agríc. 2011, 406, 173–183. Available online: http://hdl.handle.net/20.500.11939/8254 (accessed on 14 December 2024).
- Chang, P.K.; Horn, B.W.; Abe, K.; Gomi, K. Aspergillus: Introduction. In Encyclopedia of Food Microbiology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 77–82. [Google Scholar] [CrossRef]
- Rokas, A. Aspergillus. Curr. Biol. 2013, 23, R187–R188. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.A.; Bicas, J.L. Antifungal activity of essential oils of tea tree, oregano, thyme, and cinnamon, and their components. Braz. J. Food Technol. 2024, 27, 1–15. [Google Scholar] [CrossRef]
- Vargas-Fernández, J.P.; Wang-Wong, A.; Muñoz-Fonseca, M. Microorganismos asociados a la enfermedad conocida como pudrición suave del fruto de banano (Musa sp.) y alternativas de control microbiológicas y químicas a nivel in vitro. Agron. Costarric. 2022, 46, 61–76. [Google Scholar] [CrossRef]
- Suárez, L.Y.; Rangel, A.L. Aislamiento de microorganismos para control biológico de Moniliophthora roreri. Acta Agronómica 2013, 62, 370–378. Available online: https://repositorio.unal.edu.co/handle/unal/71314 (accessed on 14 July 2025).
- Salazar, E.; Hernández, R.; Tapia, A.; Gómez-Alpízar, L. Identificación molecular del hongo Colletotrichum spp., aislado de banano (Musa spp.) de la altura en la zona de Turrialba y determinación de su sensibilidad a fungicidas poscosecha. Agron. Costarric. 2012, 36, 53–68. [Google Scholar] [CrossRef]
- Paster, N.; Juven, B.J.; Shaaya, E.; Menasherov, M.; Nitzan, R.; Weisslowicz, H.; Ravid, U. Efecto inhibidor de los aceites esenciales de orégano y tomillo sobre mohos y bacterias transmitidas por los alimentos. Lett. Appl. Microbiol. 1990, 11, 33–37. [Google Scholar] [CrossRef]
- Morales, R.; Henríquez, G. Aislamiento e identificación del moho causante de antracnosis en musa paradisiaca l. (plátano) en cooperativa san carlos, el salvador y aislamiento de mohos y levaduras con capacidad antagonista. Crea Cienc. Rev. Científica 2021, 13, 84–94. Available online: https://www.uees.edu.sv/revistaenlinea/index.php/CreaCiencia/article/view/89 (accessed on 14 July 2025). [CrossRef]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Introducción a la microbiología; Ed. Médica Panamericana: Madrid, Spain, 2007; ISBN 978-950-06-0740-7. [Google Scholar]
- Johanna, S.V.; Natalia, C.G.; Ximena Carolina, P.M. Manual de Microbiología General: Principios Básicos de Laboratorio; Editorial Tadeo Lozano: Bogotá, Colombia, 2014; ISBN 978-958-725-153-1. [Google Scholar]
- Suárez Contreras, L.Y. Identificación molecular de aislamientos de Moniliophthora roreri en huertos de cacao de Norte de Santander, Colombia. Acta Agronómica 2016, 65, 51–57. [Google Scholar] [CrossRef]
- Aguilar Armijos, J.S. Identificación del Hongo Fitopatógeno Phoma spp. Aislado a partir de plantas de uvilla (Physalis peruviana L.) en Localidades de zona Norte y Centro-Norte de la Serranía Ecuatoriana; PUCE: Quito, Ecuador, 2020; Available online: https://repositorio.puce.edu.ec/handle/123456789/20871 (accessed on 28 May 2025).
- Manter, D.; Vivanco, J. Use of the ITS primers, ITS1F and ITS4, to characterize fungal abundance and diversity in mixed-template samples by qPCR and length heterogeneity analysis. J. Microbiol. Methods 2024, 71, 7–14. [Google Scholar] [CrossRef]
- Cervantes, C.; Tarqui, M.; Huiza, P.; Quispe, A. Determinación de Secuencias de ADN en Bioedit. ResearchGate n.d. Available online: https://www.researchgate.net/publication/370895529_Determinacion_de_secuencias_de_ADN_en_Bioedit (accessed on 25 February 2025).
- Tamura, K.; Stecher, G.; Kumar, S. Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Pinto, E.; Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of essential oils: A review of mechanisms and applications. J. Appl. Microbiol. 2018, 124, 1089–1099. [Google Scholar]
- Samarakoon, K.W.; Thong, P.H.; Jeewanthi, R.K.C. Evaluation of antifungal activity of cassia and holy basil essential oils against postharvest banana pathogens. Chem. Pap. 2020, 74, 3113–3121. [Google Scholar] [CrossRef]
- Aguilar-Anccota, R.; Apaza-Apaza, S.; Maldonado, E.; Calle-Cheje, Y.; Rafael-Rutte, R.; Montalvo, K.; More-Yarleque, M.; Chavez, R.; Chuquiscusma, P.; Morales-Pizarro, A. Control in vitro e in vivo de Thielavipsis paradoxa y Collettrichum musae cn biofungicidas en frutos de banano orgánico. Manglar 2024, 21, 57–63. [Google Scholar] [CrossRef]
- dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef]
- Camargo Piñeres, Y.; Zambrano Montenegro, G.; Ortega Cuadros, M.; Gutierrez Montero, D.J.; Yepes Escorcia, J.A. Actividad antifúngica in vitro del aceite esencial de Swinglea glutinosa Merr sobre Colletotrichum sp. patógeno de mango (Mangifera indica L.). Rev. Colomb. Biotecnol. 2021, 23, 62–71. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Quimbita Yupangui, Y.; Ruales, J. Effect of a Protein–Polysaccharide Coating on the Physicochemical Properties of Banana (Musa paradisiaca) During Storage. Coatings 2025, 15, 812. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medina, M.; Ruales, J. Essential Oils as an Antifungal Alternative to Control Fusarium spp., Penicillium spp., Trichoderma spp. and Aspergillus spp. Preprints 2024. [Google Scholar] [CrossRef]
- Aquino-Martínez, J.G.; Vázquez-García, L.M.; Reyes-Reyes, B.G. Biocontrol in vitro e in vivo de Fusarium oxysporum Schlecht. f. sp. dianthi (Prill. y Delacr.) Snyder y Hans. con Hongos Antagonistas Nativos de la Zona Florícola de Villa Guerrero, Estado de México. Rev. Mex. Fitopatol. 2008, 26, 127–137. Available online: https://www.redalyc.org/articulo.oa?id=61226205 (accessed on 5 April 2025).
- Ortiz, E.; Riascos, D.; Angarita, M.; Castro, O.; Rivera, C.; Romero, D.; Puentes, C.; Silva, K.; Hoyos, L. Tópicos taxonómicos para el estudio del género Fusarium. Fitopatología Colombiana 2020, 33, 61–66. Available online: https://www.researchgate.net/publication/342672474_TOPICOS_TAXONOMICOS_PARA_EL_ESTUDIO_DEL_GENERO_Fusarium (accessed on 4 February 2025).
- Smith, J.; Henderson, R. Mycotoxins and Animal Foods, 1st ed.; CRC Press: Glasgow, UK, 1991; Available online: https://api.semanticscholar.org/CorpusID:82247296 (accessed on 28 March 2025).
- Savín-Molina, J.; Hernández-Montiel, L.G.; Ceiro-Catasú, W.; Ávila-Quezada, G.D.; Palacios-Espinosa, A.; Ruiz-Espinoza, F.H.; Romero-Bastidas, M. Caracterización morfológica y potencial de biocontrol de especies de Trichoderma aisladas de suelos del semiárido. Rev. Mex. Fitopatol. 2021, 39, 435–451. [Google Scholar] [CrossRef]
- Cayotopa-Torres, J.; Arevalo, L.; Pichis-García, R.; Olivera-Cayotopa, D.; Rimachi-Valle, M.; Kadir, K.J.M.D. New cadmium bioremediation agents: Trichodermaspecies native to the rhizosphere of cacao trees. Sci. Agropecu. 2021, 24, 155–160. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006; ISBN 978-0-8138-1919-8. [Google Scholar]
- Samson, R.A.; Pitt, J.I. (Eds.) Advances in Penicillium and Aspergillus Systematics; NATO Science Series A: Vol. 102; Springer: New York, NY, USA, 2013; ISBN 978-0-306-42222-5. [Google Scholar] [CrossRef]
- Harman, G.E.; Kubicek, C.P. Trichoderma and Gliocladium: Basic Biology, Taxonomy and Genetics; CRC Press: Vienna, Austria, 2002; 300p, ISBN 978-0429078934. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Pitt, J.I. Mycotoxins: Fumonisins. In Encyclopedia of Food Safety; Motarjemi, Y., Ed.; Academic Press: Waltham, MA, USA, 2014; pp. 299–303. [Google Scholar] [CrossRef]
- Ruiz Medina, M.D.; Quimbita Yupangui, Y.; Artés-Hernández, F.; Ruales, J. Combined Effect of Antifungal Coating and Polyethylene Packaging on the Quality of Banana During Storage. Preprints 2025, 2025071496. [Google Scholar] [CrossRef]
- Abadias, M.; Teixidó, N.; Usall, J.; Viñas, I. Evaluation of alternative strategies to control postharvest blue mould of apple caused by Penicillium expansum. Int. J. Food Microbiol. 2008, 122, 25–31. [Google Scholar] [CrossRef]
- López-Reyes, E.A.; Castañeda-Vildózola, Á.; Sánchez-Pale, J.R.; Contreras-Rendón, A.; Fragoso-Benhumea, J.M.; García-Velasco, R. Diagrammatic scale to quantify the severity of Ascochyta blight in broad bean crops. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2023, 42, 1–8. [Google Scholar] [CrossRef]
- Acurio Vásconez, R.D.; España Imbaquingo, C.K. Aislamiento, caracterización y evaluación de Trichoderma spp. como promotor de crecimiento vegetal en pasturas de Raygrass (Lolium perenne) y trébol blanco (Trifolium repens). GRANJA Rev. Cienc. Vida 2017, 25, 53–61. [Google Scholar] [CrossRef][Green Version]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Allagui, M.B.; Moumni, M.; Romanazzi, G. Antifungal Activity of Thirty Essential Oils to Control Pathogenic Fungi of Postharvest Decay. Antibiotics 2024, 13, 28. [Google Scholar] [CrossRef]
- Farias, A.P.P.; dos S. Monteiro, O.; da Silva, J.K.R.; Figueiredo, P.L.B.; Rodrigues, A.A.C.; Monteiro, I.N.; Maia, J.G.S. Chemical composition and biological activities of two chemotype-oils from Cinnamomum verum J. Presl growing in North Brazil. J. Food Sci. Technol. 2020, 57, 3176–3183. [Google Scholar] [CrossRef] [PubMed]
- Pilozo, G.; Villavicencio-Vásquez, M.; Chóez-Guaranda, I.; Murillo, D.V.; Pasaguay, C.D.; Reyes, C.T.; Maldonado-Estupiñán, M.; Ruiz-Barzola, O.; León-Tamariz, F.; Manzano, P. Chemical, antioxidant, and antifungal analysis of oregano and thyme essential oils from Ecuador: Effect of thyme against Lasiodiplodia theobromae and its application in banana rot. Heliyon 2024, 10, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Vilaplana, R.; Pazmiño, L.; Valencia-Chamorro, S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol. Technol. 2018, 138, 56–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).