Evaluation of Biofilm Inhibitory Activity of Probiotics and Postbiotics Using In Vitro Biofilm Model of Canine Periodontal Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Biofilm Bacterial Collection
2.2. Culture Conditions
2.3. Preparation of Yeast-Derived Postbiotics and Probiotic Bacterial Strains
2.4. Preparation of Canine Artificial Saliva
2.5. Collection of Saliva from Healthy Dogs
2.6. Preliminary Screening of Inhibitory Activity of Yeast-Derived Postbiotics and Probiotic Bacterial Strains
2.7. Production of Polymicrobial Biofilm
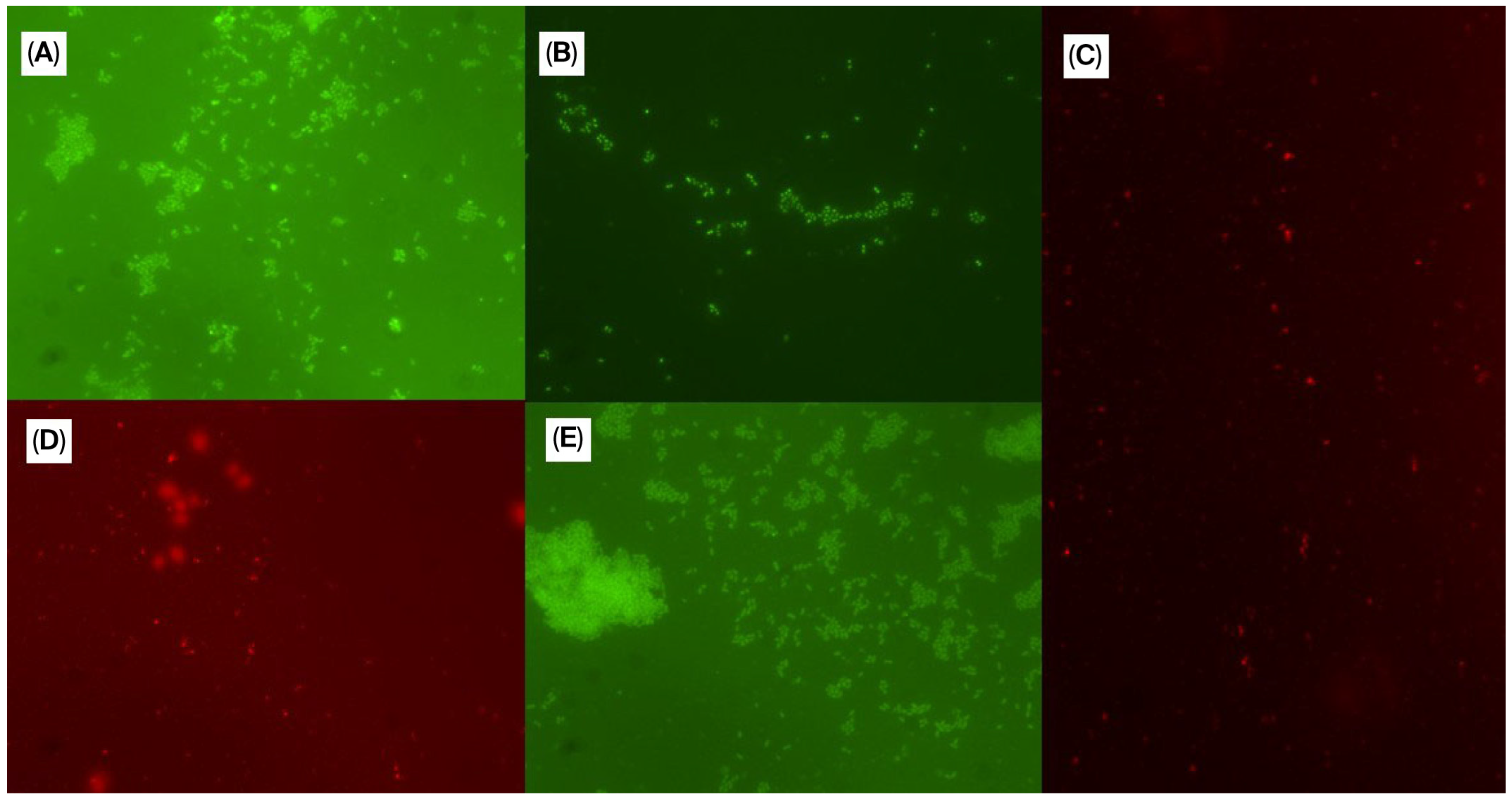
2.8. Biofilm Evaluation Based on Fluorescence In Situ Hybridization (FISH)
2.9. Determination of Minimum Biofilm Inhibitory Concentration (MBIC) and Minimum Biofilm Eradication Concentration (MBEC) of Yeast-Derived Postbiotics and Probiotic Bacterial Strains Using Crystal Violet Method and FISH
2.10. Inhibition and Eradication Potential of Dual Combinations of Yeast-Derived Postbiotics and Probiotic Bacterial Strains
2.11. Inhibitory Activity of Postbiotics and Probiotics in the Presence of Canine Saliva
2.12. Evaluation of Hemolytic Potential of Yeast-Derived Postbiotics and Probiotic Bacterial Strains
2.13. Statistical Analysis
3. Results
3.1. Preliminary Screening Results of Inhibitory Activity of Yeast-Derived Postbiotics and Probiotics
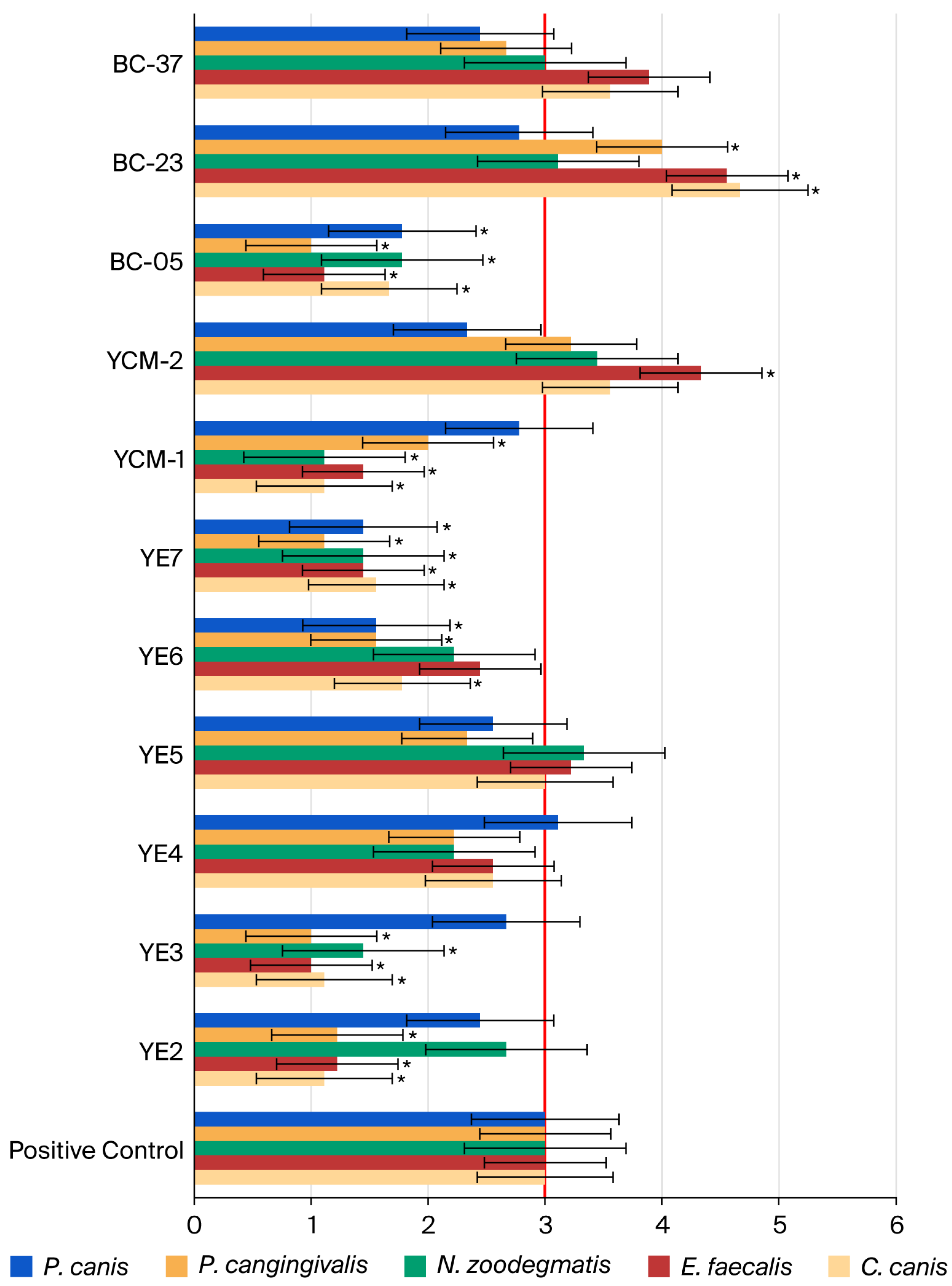
3.2. Results of Minimum Biofilm Inhibitory Concentration (MBIC) and Minimum Biofilm Eradication Concentration (MBEC)
3.3. Inhibition and Eradication Potential of Dual Combinations of Yeast-Derived Postbiotics and a Probiotic Bacterial Strain
3.4. Determination of Inhibitory Activity of Yeast-Derived Postbiotics and Probiotic Bacterial Strain in the Presence of Canine Saliva
3.5. Determination of Hemolytic Potential of Yeast-Derived Postbiotics and Probiotic Bacterial Strain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stella, J.L.; Bauer, A.E.; Croney, C.C. A Cross-Sectional Study to Estimate Prevalence of Periodontal Disease in a Population of Dogs (Canis familiaris) in Commercial Breeding Facilities in Indiana and Illinois. PLoS ONE 2018, 13, e0191395. [Google Scholar] [CrossRef]
- Kačírová, J.; Mad’ar, M.; Štrkolcová, G.; Mad’ari, A.; Nemcová, R. Dental Biofilm as Etiological Agent of Canine Periodontal Disease. In Bacterial Biofilms; Dincer, S., Sümengen Özdenefe, M., Arkut, A., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-899-0. [Google Scholar]
- Niemiec, B.A. Periodontal Disease. Top. Companion Anim. Med. 2008, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.M. Gene Sequence Analyses of the Healthy Oral Microbiome in Humans and Companion Animals: A Comparative Review. J. Vet. Dent. 2016, 33, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sanguansermsri, P.; Nobbs, A.H.; Jenkinson, H.F.; Surarit, R. Interspecies Dynamics among Bacteria Associated with Canine Periodontal Disease. Mol. Oral Microbiol. 2018, 33, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pereira Dos Santos, J.D.; Cunha, E.; Nunes, T.; Tavares, L.; Oliveira, M. Relation between Periodontal Disease and Systemic Diseases in Dogs. Res. Vet. Sci. 2019, 125, 136–140. [Google Scholar] [CrossRef]
- Clarke, D.E.; Kelman, M.; Perkins, N. Effectiveness of a Vegetable Dental Chew on Periodontal Disease Parameters in Toy Breed Dogs. J. Vet. Dent. 2011, 28, 230–235. [Google Scholar] [CrossRef]
- Do, K.-H.; Park, H.-E.; Kang, M.-S.; Kim, J.-T.; Yeu, J.-E.; Lee, W.-K. Effects of Weissella cibaria CMU on Halitosis and Calculus, Plaque, and Gingivitis Indices in Beagles. J. Vet. Dent. 2019, 36, 135–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Guo, Q. Probiotic Species in the Management of Periodontal Diseases: An Overview. Front. Cell. Infect. Microbiol. 2022, 12, 806463. [Google Scholar] [CrossRef]
- Florit-Ruiz, A.; Rago, L.; Rojas, A.; Guzelkhanova, B.; Pont-Beltran, A.; Lamelas, A.; Solaz-Fuster, M.C.; Martinez-Blanch, J.F.; López, M.E.; García-Lainez, G.; et al. Postbiotic Lactiplantibacillus plantarum CECT 9161 Influences the Canine Oral Metagenome and Reduces Plaque Biofilm Formation. Animals 2025, 15, 1615. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Belstrøm, D. Effect of Synbiotic and Postbiotic Supplements on Dental Caries and Periodontal Diseases—A Comprehensive Review. Int. J. Environ. Res. Public Health 2025, 22, 72. [Google Scholar] [CrossRef]
- Di Stefano, M.; Santonocito, S.; Polizzi, A.; Mauceri, R.; Troiano, G.; Lo Giudice, A.; Romano, A.; Mascitti, M.; Isola, G. A Reciprocal Link between Oral and Gut Microbiota during Periodontitis: The Potential Role of Probiotics in Reducing Dysbiosis-Induced Inflammation. Int. J. Mol. Sci. 2023, 24, 1084. [Google Scholar] [CrossRef]
- Oliveira, M.; Tavares, M.; Gomes, D.; Touret, T.; São Braz, B.; Tavares, L.; Semedo-Lemsaddek, T. Virulence Traits and Antibiotic Resistance among Enterococci Isolated from Dogs with Periodontal Disease. Comp. Immunol. Microbiol. Infect. Dis. 2016, 46, 27–31. [Google Scholar] [CrossRef]
- Cunha, E.; Rebelo, S.; Carneiro, C.; Tavares, L.; Carreira, L.M.; Oliveira, M. A Polymicrobial Biofilm Model for Testing the Antimicrobial Potential of a Nisin-Biogel for Canine Periodontal Disease Control. BMC Vet. Res. 2020, 16, 469. [Google Scholar] [CrossRef]
- Tong, Z.; Dong, L.; Zhou, L.; Tao, R.; Ni, L. Nisin Inhibits Dental Caries-Associated Microorganism In Vitro. Peptides 2010, 31, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Cunha, E.; Freitas, F.B.; Braz, B.S.; Silva, J.M.D.; Tavares, L.; Veiga, A.S.; Oliveira, M. Polyphasic Validation of a Nisin-Biogel to Control Canine Periodontal Disease. Antibiotics 2020, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Bexiga, R.; Nunes, S.F.; Carneiro, C.; Cavaco, L.M.; Bernardo, F.; Vilela, C.L. Biofilm Forming Ability Profiling of Staphylococcus aureus and Staphylococcus epidermidis Mastitis Isolates. Vet. Microbiol. 2006, 118, 133–140. [Google Scholar] [CrossRef]
- Walcher, M.; Skvoretz, R.; Montgomery-Fullerton, M.; Jonas, V.; Brentano, S. Description of an Unusual Neisseria meningitidis Isolate Containing and Expressing Neisseria gonorrhoeae-Specific 16S rRNA Gene Sequences. J. Clin. Microbiol. 2013, 51, 3199–3206. [Google Scholar] [CrossRef]
- Costa, P.M.; Oliveira, M.; Bica, A.; Vaz-Pires, P.; Bernardo, F. Antimicrobial Resistance in Enterococcus spp. and Escherichia coli Isolated from Poultry Feed and Feed Ingredients. Vet. Microbiol. 2007, 120, 122–131. [Google Scholar] [CrossRef]
- Mark, W.J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a Human Oral Microbiome at the Micron Scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef]
- Alm, E.W.; Oerther, D.B.; Larsen, N.; Stahl, D.A.; Raskin, L. The Oligonucleotide Probe Database. Appl. Environ. Microbiol. 1996, 62, 3557–3559. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; McTeague, M.; Vu, A.; Liu, J.Y.; Finegold, S.M. Rapid Identification of Gram-Positive Anaerobic Coccal Species Originally Classified in the Genus Peptostreptococcus by Multiplex PCR Assays Using Genus- and Species-Specific Primers. Microbiology 2003, 149, 1719–1727. [Google Scholar] [CrossRef]
- Grilo, M.L.; Pereira, A.; Sousa-Santos, C.; Robalo, J.I.; Oliveira, M. Climatic Alterations Influence Bacterial Growth, Biofilm Production and Antimicrobial Resistance Profiles in Aeromonas spp. Antibiotics 2021, 10, 1008. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Mendonça, D.A.; Bakker, M.; Cruz-Oliveira, C.; Neves, V.; Jiménez, M.A.; Defaus, S.; Cavaco, M.; Veiga, A.S.; Cadima-Couto, I.; Castanho, M.A.R.B.; et al. Penetrating the Blood–Brain Barrier with New Peptide–Porphyrin Conjugates Having Anti-HIV Activity. Bioconjug. Chem. 2021, 32, 1067–1077. [Google Scholar] [CrossRef]
- Butrungrod, W.; Chaiyasut, C.; Makhamrueang, N.; Peerajan, S.; Chaiyana, W.; Sirilun, S. Postbiotic Metabolite of Lactiplantibacillus plantarum PD18 against Periodontal Pathogens and Their Virulence Markers in Biofilm Formation. Pharmaceutics 2023, 15, 1419. [Google Scholar] [CrossRef] [PubMed]
- Sachelarie, L.; Scrobota, I.; Romanul, I.; Iurcov, R.; Potra Cicalau, G.I.; Todor, L. Probiotic Therapy as an Adjuvant in the Treatment of Periodontal Disease: An Innovative Approach. Medicina 2025, 61, 126. [Google Scholar] [CrossRef] [PubMed]
- You, I.; Mahiddine, F.Y.; Park, H.; Kim, M.J. Lactobacillus acidophilus Novel Strain, MJCD175, as a Potential Probiotic for Oral Health in Dogs. Front. Vet. Sci. 2022, 9, 946890. [Google Scholar] [CrossRef]
- Niemiec, B.A.; Gawor, J.; Tang, S.; Prem, A.; Krumbeck, J.A. The Bacteriome of the Oral Cavity in Healthy Dogs and Dogs with Periodontal Disease. Am. J. Vet. Res. 2022, 83, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.M.; Shahirfar, H.S.; Mobaiyen, H.M. Effect of Consumption of Fermented Milk with Lactobacillus casei and Lactobacillus plantarum Isolated from Ligvan Cheese against E. coli O157:H7 Induced Infections in BALB/C Mice. Adv. Biores. 2012, 3, 34–38. [Google Scholar]
- Jalali, S.; Mojgani, N.; Haghighat, S.; Sanjabi, M.R.; Sarem-Nezhad, S. Investigation of Antimicrobial and Antioxidant Properties of Postbiotics Produced by Lactobacillus rhamnosus and Limosilactobacillus reuteri and Their Potential Application in Surface Decontamination of Red Meat. LWT—Food Sci. Technol. 2024, 209, 116758. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, B.; Wuri, G.; Lan, H.; Wang, R.; Sun, Y.; Zhao, W.; Hung, W.L.; Zhang, M. From Biofilm to Breath: The Role of Lacticaseibacillus paracasei ET-22 Postbiotics in Combating Oral Malodor. J. Agric. Food Chem. 2024, 72, 27203–27214. [Google Scholar] [CrossRef]
- Tomičić, Z.; Šarić, L.; Tomičić, R. Potential Future Applications of Postbiotics in the Context of Ensuring Food Safety and Human Health Improvement. Antibiotics 2025, 14, 674. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Liu, Y.; Li, X.; Du, J.; Guo, L.; Liu, Y. Reuterin Isolated from the Probiotic Lactobacillus reuteri Promotes Periodontal Tissue Regeneration by Inhibiting Cx43-Mediated Intercellular Transmission of Endoplasmic Reticulum Stress. J. Periodontal Res. 2024, 59, 552–564. [Google Scholar] [CrossRef]
- Torres, S.M.F.; Furrow, E.; Souza, C.P.; Granick, J.L.; de Jong, E.P.; Griffin, T.J.; Wang, X. Salivary Proteomics of Healthy Dogs: An In-Depth Catalog. PLoS ONE 2018, 13, e0191307. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Kouidhi, B.; Fdhila, K.; Elabed, H.; Ben Slama, R.; Mahdouani, K.; Bakhrouf, A.; Chaieb, K. Anti-Bacterial and Anti-Biofilm Activity of Probiotic Bacteria against Oral Pathogens. Microb. Pathog. 2016, 97, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, I.; Saeed, M.; Khan, W.A.; Khaliq, A.; Chughtai, M.F.J.; Iqbal, R.; Tehseen, S.; Naz, S.; Liaqat, A.; Mehmood, T.; et al. In Vitro Probiotic Potential and Safety Evaluation (Hemolytic, Cytotoxic Activity) of Bifidobacterium Strains Isolated from Raw Camel Milk. Microorganisms 2020, 8, 354. [Google Scholar] [CrossRef]
- Golnari, M.; Bahrami, N.; Milanian, Z.; Rabbani Khorasgani, M.; Asadollahi, M.A.; Shafiei, R.; Fatemi, S.S. Isolation and Characterization of Novel Bacillus Strains with Superior Probiotic Potential: Comparative Analysis and Safety Evaluation. Sci. Rep. 2024, 14, 1457. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Dannenfelser, R.M. In Vitro Hemolysis: Guidance for the Pharmaceutical Scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef]
| Probiotic/Postbiotic | Stock/Suspension Concentration | Maximum Concentration per Well in a Microplate |
|---|---|---|
| YE-2 | 100% | 50% |
| YE-3 | 100% | 50% |
| YE-4 | 100% | 50% |
| YE-5 | 100% | 50% |
| YE-6 | 100% | 50% |
| YE-7 | 100% | 50% |
| YCW-1 | 25% | 12.5% |
| YCW-2 | 10% | 5% |
| BC-23 | 109 CFU/mL | 109 CFU/mL |
| BC-05 | 109 CFU/mL | 109 CFU/mL |
| BC-37 | 109 CFU/mL | 109 CFU/mL |
| Bacterial Species | Sequence | Fluorophore | Reference |
|---|---|---|---|
| Neisseria sp. | 5′-CGGGTGAGTAACATATCGG-3′ | Rhodamine | [18] |
| E. faecalis | 5′-TTATCCCCCTCTGATGGG-3′ | Fluorescein | [19] |
| Corynebacterium sp. | 5′-CCGGAATTTCACAGACGACG-3′ | Fluorescein | [20] |
| Porphyromonas sp. | 5′-TGTCAGTCGCAGTATGGCAA-3′ | Fluorescein | [21] |
| Peptostreptococcus sp. | 5′-TGCGCAAGCATGAAA-3′ | Rhodamine | [22] |
| Score | Qualitative Assessment Level | Definition |
|---|---|---|
| 1 | Lower | Bacterial quantity clearly lower than the positive control |
| 2 | Slightly lower | Bacterial quantity lower than the positive control (up to approximately half of the bacterial quantity observed in the positive control) |
| 3 | Reference level | Bacterial quantity equivalent to the positive control |
| 4 | Slightly higher | Bacterial quantity higher than the positive control (up to approximately twice the reference level) |
| 5 | Higher | Bacterial quantity clearly higher than the positive control (more than twice the reference level) |
| Combination | Concentrations of Components in Microplate Well |
|---|---|
| YE-3 + YE-7 | 25%/25% |
| YE-3 + YCW-1 | 25%/6.25% |
| YE-3 + YCW-2 | 25%/2.5% |
| YE-3 + BC-05 | 25%/109 CFU/mL |
| YE-7 + YCW-1 | 25%/6.25% |
| YE-7 + YCW-2 | 25%/2.5% |
| YE-7 + BC-05 | 25%/109 CFU/mL |
| YCW-1 + YCW-2 | 6.25%/2.5% |
| YCW-1 + BC-05 | 6.25%/109 CFU/mL |
| YCW-2 + BC-05 | 2.5%/109 CFU/mL |
| Compound | Concentrations (%) of Postbiotic Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50.00% | 25.00% | 12.50% | 6.25% | 3.13% | 1.56% | 0.78% | 0.39% | 0.20% | 0.10% | |
| YE-2 | 16.86 | 15.34 | 7.68 | 3.99 | 2.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| YE-3 | 6.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| YE-4 | 21.66 | 15.79 | 17.49 | 13.90 | 6.37 | 2.47 | 0.00 | 0.00 | 0.00 | 0.00 |
| YE-5 | 14.27 | 13.60 | 12.15 | 9.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| YE-6 | 22.55 | 20.89 | 16.52 | 14.24 | 4.83 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| YE-7 | 13.63 | 14.62 | 12.76 | 15.02 | 8.07 | 3.78 | 0.00 | 0.00 | 0.00 | 0.00 |
| Concentrations (%) of Postbiotic Compound | ||||||||||
| 12.50% | 6.25% | 3.13% | 1.56% | 0.78% | 0.39% | 0.20% | 0.10% | 0.05% | 0.02% | |
| YCW-1 | 19.45 | 18.62 | 14.29 | 10.26 | 4.36 | 3.53 | 2.82 | 3.75 | 4.09 | 6.11 |
| Concentrations (%) of Postbiotic Compound | ||||||||||
| 5.00% | 2.50% | 1.25% | 0.63% | 0.31% | 0.16% | 0.08% | 0.04% | 0.02% | 0.01% | |
| YCW-2 | 12.91 | 10.03 | 6.91 | 3.47 | 2.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Concentrations (CFU/mL) of Probiotic Bacterial Strains | ||||||||||
| 109 | 108 | 107 | 106 | 105 | 104 | 103 | 102 | 101 | 100 | |
| BC-23 | 6.47 | 2.35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BC-05 | 16.11 | 2.70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BC-37 | 13.69 | 4.69 | 1.39 | 3.40 | 2.37 | 0.37 | 1.01 | 0 | 0 | 0 |
| Postbiotic–Probiotic Components | % of Inhibition ± SD | % of Eradication ± SD |
|---|---|---|
| YE-3 + YE-7 | 71.57 ± 32.92 | 0.00 |
| YE-3 + YCW-1 | 0.00 | 0.00 |
| YE-3 + YCW-2 | 0.00 | 0.00 |
| YE-3 + BC-05 | 0.00 | 0.00 |
| YE-7 + YCW-1 | 0.00 | 0.00 |
| YE-7 + YCW-2 | 41.11 ± 22.36 | 0.00 |
| YE-7 + BC-05 | 33.14 ± 25.93 | 0.00 |
| YCW-1 + YCW-2 | 0.00 | 0.00 |
| YCW1 + BC-05 | 17.21 ± 34.37 | 0.00 |
| YCW-2 + BC-05 | 25.03 ± 25.07 | 0.00 |
| Postbiotic/Probiotic | Hemolytic Activity (%) ± SD | |
|---|---|---|
| 4 h | 24 h | |
| YE-3 | 65.13 ± 44.82 | 83.94 ± 19.89 |
| YE-7 | 72.54 ± 10.88 | 73.29 ± 18.12 |
| YCW-1 | 24.97 ± 18.64 | 31.34 ± 7.20 |
| YCW-2 | 61.09 ± 40.80 | 62.19 ± 11.91 |
| BC-05 | 6.80 ± 4.59 | 17.88 ± 13.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adib Lesaux, A.; Cunha, E.; Ballet, N.; Oliveira, M. Evaluation of Biofilm Inhibitory Activity of Probiotics and Postbiotics Using In Vitro Biofilm Model of Canine Periodontal Disease. Microorganisms 2025, 13, 2472. https://doi.org/10.3390/microorganisms13112472
Adib Lesaux A, Cunha E, Ballet N, Oliveira M. Evaluation of Biofilm Inhibitory Activity of Probiotics and Postbiotics Using In Vitro Biofilm Model of Canine Periodontal Disease. Microorganisms. 2025; 13(11):2472. https://doi.org/10.3390/microorganisms13112472
Chicago/Turabian StyleAdib Lesaux, Achraf, Eva Cunha, Nathalie Ballet, and Manuela Oliveira. 2025. "Evaluation of Biofilm Inhibitory Activity of Probiotics and Postbiotics Using In Vitro Biofilm Model of Canine Periodontal Disease" Microorganisms 13, no. 11: 2472. https://doi.org/10.3390/microorganisms13112472
APA StyleAdib Lesaux, A., Cunha, E., Ballet, N., & Oliveira, M. (2025). Evaluation of Biofilm Inhibitory Activity of Probiotics and Postbiotics Using In Vitro Biofilm Model of Canine Periodontal Disease. Microorganisms, 13(11), 2472. https://doi.org/10.3390/microorganisms13112472








