Cross-Infectivity of 11 Different Legume Species by 15 Native Rhizobia Isolated from African Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Origin of the Legumes
2.3. Authentication of Bacterial Isolates
2.4. Bacteria Isolation from Root Nodule
2.5. Naming of the Isolates
2.6. Effectiveness of Rhizobial Isolates Under Glasshouse Conditions
2.7. 15N/14N Isotopic Analysis
2.8. Amount of N-Fixed in Shoots
2.9. Statistical Analysis
3. Results
3.1. Plant Nodulation
3.2. Nodule Number and Nodule DM of Test Legumes
3.2.1. Cowpea (Vigna unguiculata)
3.2.2. Bambara Groundnut (Vigna subterranea)
3.2.3. Kersting’s Groundnut (Macrotyloma geocarpum)
3.2.4. Common Bean (Phaseolus vulgaris)
3.2.5. Soybean (Glycine max)
3.2.6. Winged Bean (Psophocarpus tetragonolobus)
3.2.7. Velvet Bean (Mucuna pruriens)
3.2.8. Jack Bean (Canavalia ensiformis)
3.2.9. Pigeonpea (Cajanus cajan)
3.2.10. Mungbean (Vigna radiata)
3.3. Shoot DM of Test Legumes Species Inoculated with Different Rhizobial Isolates
3.3.1. Cowpea (Vigna unguiculata)
3.3.2. Bambara Groundnut (Vigna subterranea)
3.3.3. Kersting’s Groundnut (Macrotyloma geocarpum)
3.3.4. Common Bean (Phaseolus vulgaris)
3.3.5. Soybean (Glycine max)
3.3.6. Winged Bean (Psophocarpus tetragonolobus)
3.3.7. Velvet Bean (Mucuna pruriens)
3.3.8. Jack Bean (Canavalia ensiformis)
3.3.9. Pigeonpea (Cajanus cajan)
3.3.10. Mungbean (Vigna radiata)
3.4. Shoot δ15N, %N and N-Fixed of Diverse Legume Species Inoculated with Native Rhizobial Isolates
3.4.1. Cowpea (Vigna unguiculata)
3.4.2. Bambara Groundnut (Vigna subterranea)
3.4.3. Kersting’s Groundnut (Macrotyloma geocarpum)
3.4.4. Common Bean (Phaseolus vulgaris)
3.4.5. Soybean (Glycine max)
3.4.6. Winged Bean (Psophocarpus tetragonolobus)
3.4.7. Velvet Bean (Mucuna pruriens)
3.4.8. Jack Bean (Canavalia ensiformis)
3.4.9. Pigeonpea (Cajanus cajan)
3.4.10. Mungbean (Vigna radiata)
3.5. Relative Symbiotic Effectiveness (RSE) of Isolates
3.5.1. Cowpea (Vigna unguiculata)
3.5.2. Bambara Groundnut (Vigna subterranea)
3.5.3. Kersting’s Groundnut (Macrotyloma geocarpum)
3.5.4. Common Bean (Phaseolus vulgaris)
3.5.5. Soybean (Glycine max)
3.5.6. Velvet Bean (Mucuna pruriens)
3.5.7. Jack Bean (Canavalia ensiformis)
3.5.8. Pigeonpea (Cajanus cajan)
3.5.9. Mungbean (Vigna radiata)
4. Discussion
4.1. Nodulation and Shoot Dry Matter Accumulation Induced by Rhizobial Isolates
4.2. N2 Fixation and %Relative Symbiotic Effectiveness of Rhizobial Isolates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| %RSE | Percent relative symbiotic effectiveness |
| N2 | Nitrogen |
| DM | Dry matter |
References
- Shahzad, F.; Shafee, M.; Abbas, F.; Babar, S.; Tariq, M.; Ahmad, Z. Isolation and biochemical characterization of Rhizobium meliloti from root nodules of Alfalfa (Medico sativa). J. Anim. Plant Sci. 2012, 22, 522–524. [Google Scholar]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A promising source of plant growth-promoting molecules and their non-legume interactions: Examining applications and mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume–rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- Monib, A.W.; Niazi, P.; Barai, S.M.; Sawicka, B.; Baseer, A.Q.; Nikpay, A.; Fahmawi, S.M.S.; Singh, D.; Alikhail, M.; Thea, B. Nitrogen Cycling Dynamics: Investigating Volatilization and its Interplay with N2 Fixation. J. Res. Appl. Sci. Biotechnol. 2024, 3, 17–31. [Google Scholar] [CrossRef]
- Zafar, M. Molecular and biochemical characterization of rhizobia from chickpea (Cicer arietinum). Pak. J. Agric. Sci. 2017, 54, 373–381. [Google Scholar] [CrossRef]
- Robertson, G.P.; Groffman, P. Nitrogen transformations. In Soil Microbiology, Ecology and Biochemistry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 407–438. [Google Scholar]
- Kumari, B.; Ram, M.; Mallaiah, K. Studies on nodulation, biochemical analysis and protein profiles of Rhizobium isolated from Indigofera species. Malays. J. Microbiol. 2010, 6, 133–139. [Google Scholar]
- Wu, J.; Yan, S. Natural Nitrogen Boosters: The Symbiotic Relationship Between Legumes and Rhizobia. Mol. Soil. Biol. 2024, 15, 74–86. [Google Scholar] [CrossRef]
- Latif, S.; Khan, S.; Naveed, M.; Mustafa, G.; Bashir, T.; Mumtaz, A.S. The diversity of Rhizobia, Sinorhizobia and novel non-Rhizobial Paenibacillus nodulating wild herbaceous legumes. Arch. Microbiol. 2013, 195, 647–653. [Google Scholar] [CrossRef]
- Porter, S.S.; Dupin, S.E.; Denison, R.F.; Kiers, E.T.; Sachs, J.L. Host-imposed control mechanisms in legume–rhizobia symbiosis. Nat. Microbiol. 2024, 9, 1929–1939. [Google Scholar] [CrossRef]
- Shumilina, J.; Soboleva, A.; Abakumov, E.; Shtark, O.Y.; Zhukov, V.A.; Frolov, A. Signaling in Legume–Rhizobia Symbiosis. Int. J. Mol. Sci. 2023, 24, 17397. [Google Scholar] [CrossRef]
- Bedi, M.K.; Naglot, A. Characterization of Rhizobium isolated from root nodules of Trifolium alexandrinum. J. Agric. Technol. 2011, 7, 1705–1723. [Google Scholar]
- Willems, A. The taxonomy of rhizobia: An overview. Plant Soil 2006, 287, 3–14. [Google Scholar] [CrossRef]
- Singha, B.; Das, P.; Mazumder, P. Morphological and biochemical characterization of rhizobia isolated from root nodule of Crotolaria junceae L. grown in Assam. Int. J. Sci. Res. 2015, 4, 1928–1931. [Google Scholar]
- Abdullahi, A.; Abubakar, F. Sustainable Way of Improving Grain Legumes Productivityagainst Failure of Introduced Rhizobia Inoculant. Biosci. J. 2022, 10, 167–180. [Google Scholar]
- Osei, O.; Abaidoo, R.C.; Ahiabor, B.D.; Boddey, R.M.; Rouws, L.F. Bacteria related to Bradyrhizobium yuanmingense from Ghana are effective groundnut micro-symbionts. Appl. Soil Ecol. 2018, 127, 41–50. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H.J. Methods in Legume-Rhizobium Technology; Springer: New York, NY, USA, 1994. [Google Scholar]
- Broughton, W.; Dilworth, M. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of the Root-Nodule Bacteria; Blackwell Scientific Publishers: Oxford, UK, 1970. [Google Scholar]
- Ibny, F.Y.; Jaiswal, S.K.; Mohammed, M.; Dakora, F.D. Symbiotic effectiveness and ecologically adaptive traits of native rhizobial symbionts of Bambara groundnut (Vigna subterranea L. Verdc.) in Africa and their relationship with phylogeny. Sci. Rep. 2019, 9, 12666. [Google Scholar] [CrossRef]
- Purcino, H.; Festin, P.; Elkan, G. Identification of effective strains of Bradyrhizobium for Arachis pintoi. Trop. Agric. 2000, 77, 226–231. [Google Scholar]
- Mariotti, A.; Germon, J.; Hubert, P.; Kaiser, P.; Letolle, R.; Tardieux, A.; Tardieux, P. Experimental determination of nitrogen kinetic isotope fractionation: Some principles; illustration for the denitrification and nitrification processes. Plant Soil 1981, 62, 413–430. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, B.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2008. [Google Scholar]
- Khandual, S. Flavonoids as signaling molecules and regulators of root nodule development. Dyn. Soil Dyn. Plant 2007, 1, 83–94. [Google Scholar]
- Singla, P.; Garg, N. Plant flavonoids: Key players in signaling, establishment, and regulation of rhizobial and mycorrhizal endosymbioses. In Mycorrhiza-Function, Diversity, State of the Art; Springer: Berlin/Heidelberg, Germany, 2017; pp. 133–176. [Google Scholar]
- Ghantasala, S.; Roy Choudhury, S. Nod factor perception: An integrative view of molecular communication during legume symbiosis. Plant Mol. Biol. 2022, 110, 485–509. [Google Scholar] [CrossRef]
- Liu, C.-W.; Murray, J.D. The role of flavonoids in nodulation host-range specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef]
- Gyogluu, C.; Mohammed, M.; Jaiswal, S.K.; Kyei-Boahen, S.; Dakora, F.D. Assessing host range, symbiotic effectiveness, and photosynthetic rates induced by native soybean rhizobia isolated from Mozambican and South African soils. Symbiosis 2018, 75, 257–266. [Google Scholar] [CrossRef]
- Zhang, J.J.; Yu, T.; Lou, K.; Mao, P.H.; Wang, E.T.; Chen, W.F.; Chen, W.X. Genotypic alteration and competitive nodulation of Mesorhizobium muleiense against exotic chickpea rhizobia in alkaline soils. Syst. Appl. Microbiol. 2014, 37, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckmann, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Pueppke, S.G.; Broughton, W.J. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant-Microbe Interact. 1999, 12, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Woomer, P.; Huising, J.; Giller, K.E.; Baijukya, F.P.; Kantengwa, S.; Vanlauwe, B.; Boahen, S.; Wolf, J.d.; Franke, L.; Abaidoo, R.C. N2Africa Final Report of the First Phase: 2009–2013; N2Africa: Wageningen, The Netherlands, 2014. [Google Scholar]
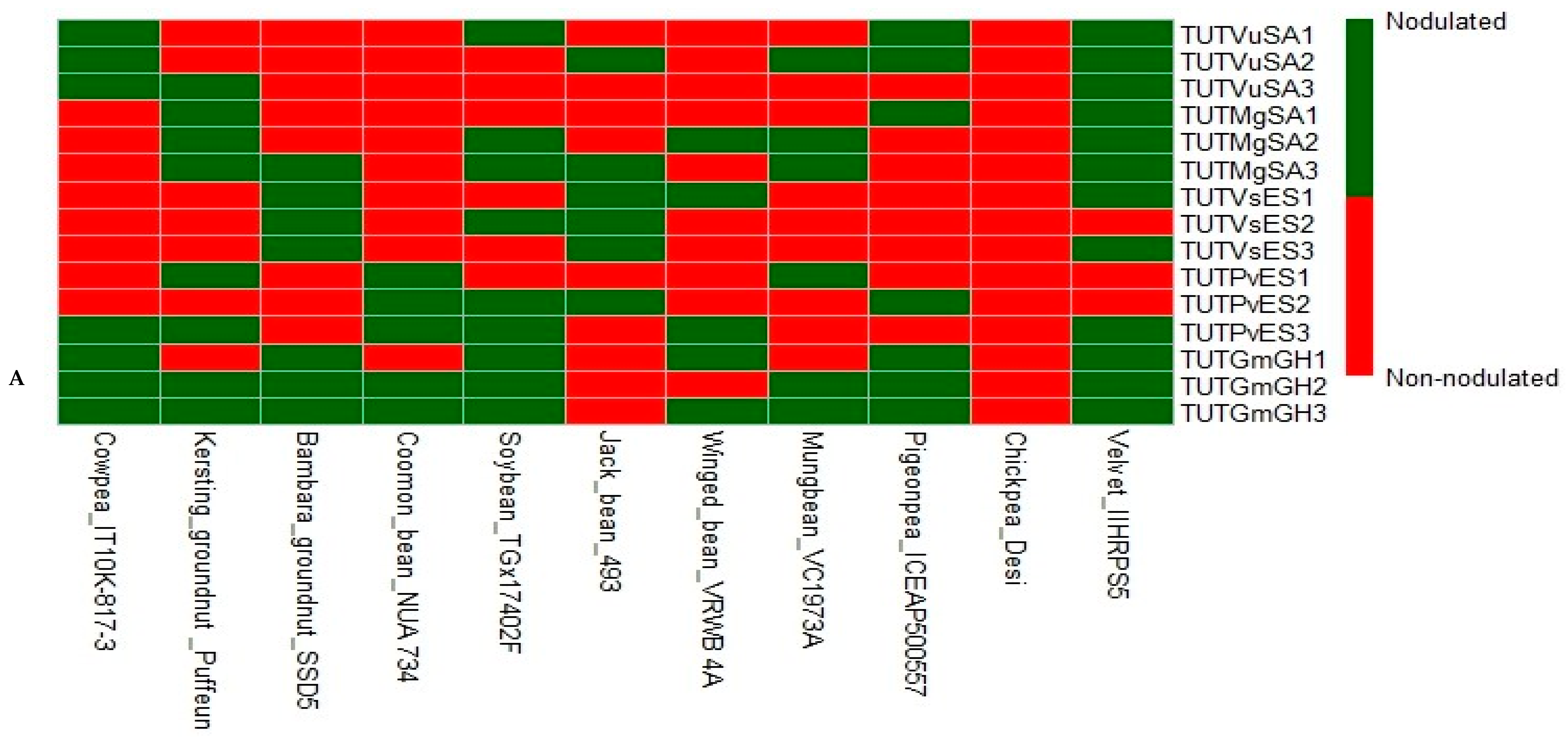

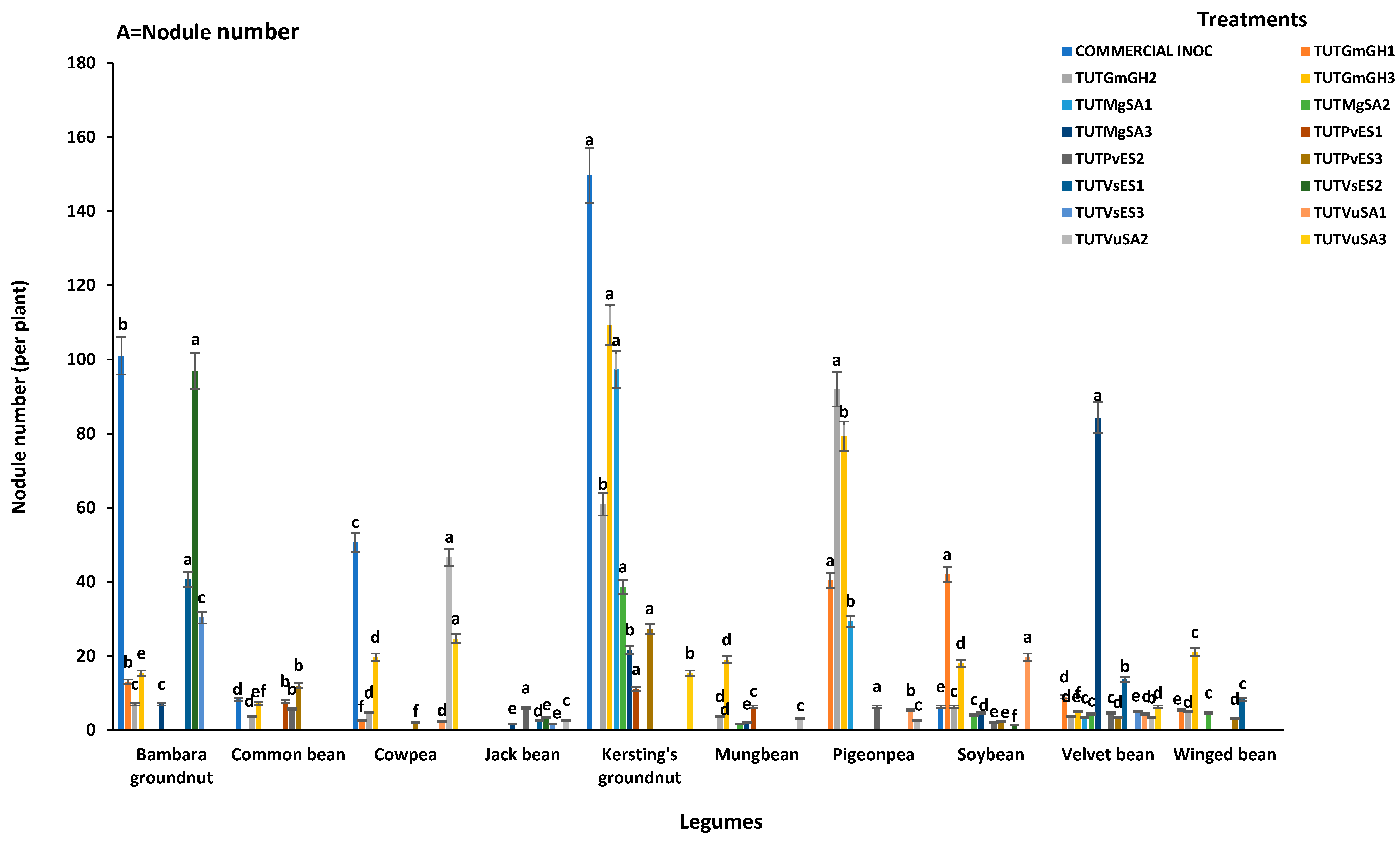

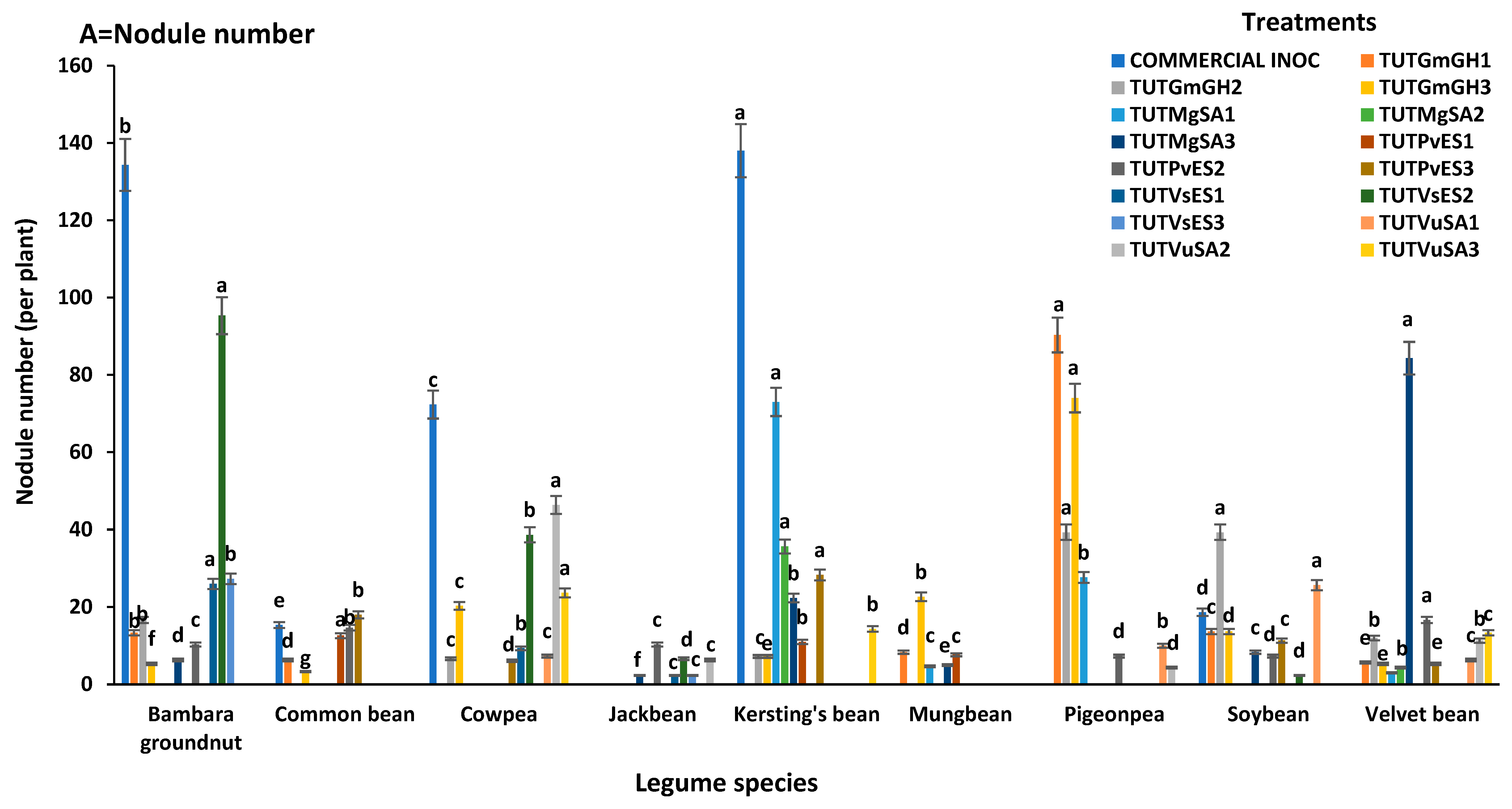

| Legumes | Treatments | Shoot DM | δ15N | %N | N-Fixed | RSE |
|---|---|---|---|---|---|---|
| g.plant−1 | ‰ | % | mg.plant−1 | % | ||
| Cowpea cv. IT10K-817-3 | TUTVuSA1 | 3.40 ± 0.20 a | 2.58 ± 0.05 c | 0.84 ± 0.14 d | 22.72 ± 1.29 de | 88.77 ± 0.33 c |
| TUTVuSA2 | 3.50 ± 0.30 a | 0.99 ± 0.15 ef | 1.62 ± 0.23 cd | 46.38 ± 3.50 c | 91.38 ± 0.54 b | |
| TUTVuSA3 | 2.43 ± 0.09 b | 0.36 ± 0.01 f | 3.66 ± 0.55 a | 128.21 ± 9.30 a | 67.10 ± 0.77 d | |
| TUTGmGH2 | 0.43 ± 0.03 d | 4.96 ± 0.23 a | 0.94 ± 0.14 d | 20.37 ± 1.40 de | 37.83 ± 0.54 e | |
| TUTGmGH3 | 1.43 ± 0.03 c | 1.23 ± 0.03 e | 2.69 ± 0.43 b | 31.85 ± 1.82 d | 37.34 ± 0.11 e | |
| TUTVsES1 | 0.63 ± 0.09 d | 3.72 ± 0.56 b | 0.94 ± 0.14 d | 5.68 ± 0.65 f | 16.45 ± 0.10 g | |
| TUTVsES2 | 1.13 ± 0.03 cd | 2.03 ± 0.11 cd | 1.23 ± 0.20 d | 11.91 ± 0.36 ef | 29.50 ± 0.21 f | |
| COMMERCIAL INOC | 3.73 ± 0.49 a | 1.32 ± 0.67 de | 2.27 ± 0.32 bc | 78.82 ± 6.05 b | 97.39 ± 0.58 a | |
| NITRATE | 3.83 ± 0.32 a | 0.68 ± 0.34 ef | 2.24 ± 0.33 bc | ND | ND | |
| F-STATISTIC | 37.05 *** | 38.52 *** | 10.05 *** | 108.73 *** | 7.33 *** | |
| Bambara groundnut LR.SSD5 | TUTVsES1 | 3.43 ± 0.49 a | 1.51 ± 0.43 a | 2.99 ± 0.42 ab | 126.89 ± 25.44 a | 85.11 ± 0.31 b |
| TUTVsES2 | 2.53 ± 0.28 bc | 0.53 ± 0.07 b | 1.85 ± 0.26 bc | 43.53 ± 5.49 bc | 62.78 ± 0.18 d | |
| TUTVsES3 | 3.13 ± 0.54 ab | 0.28 ± 0.07 b | 1.87 ± 0.28 bc | 57.36 ± 0.43 b | 77.67 ± 0.23 c | |
| TUTMgSA3 | 1.50 ± 0.10 cd | 0.39 ± 0.04 b | 2.33 ± 0.34 a–c | 28.90 ± 1.97 b–d | 37.22 ± 0.39 e | |
| TUTPvES2 | 0.77 ± 0.03 d | 0.31 ± 0.11 b | 1.89 ± 0.27 bc | 12.16 ± 0.5 d | 19.11 ± 0.56 g | |
| TUTGmGH1 | 1.13 ± 0.03 d | 0.23 ± 0.05 b | 1.66 ± 0.23 c | 16.54 ± 0.85 cd | 28.05 ± 0.66 f | |
| TUTGmGH2 | 1.13 ± 0.03 d | 0.65 ± 0.09 b | 1.35 ± 0.21 c | 13.74 ± 0.52 cd | 28.04 ± 0.88 f | |
| TUTGmGH3 | 1.43 ± 0.09 cd | 0.18 ± 0.08 b | 1.36 ± 0.26 c | 15.22 ± 1.35 cd | 35.48 ± 0.76 ef | |
| COMMERCIAL INOC | 3.83 ± 0.58 a | 0.46 ± 0.07 b | 3.15 ± 0.78 a | 107.51 ± 16.06 a | 95.04 ± 0.54 a | |
| NITRATE | 4.03 ± 1.27 a | 0.43 ± 0.12 b | 1.95 ± 0.28 bc | ND | ND | |
| F-STATISTIC | 9.44 *** | 5.85 *** | 3.11 * | 19.28 *** | 5.98 *** | |
| Kersting’s groundnut LR.Puffeun | TUTMgSA1 | 1.20 ± 0.75 c | 0.96 ± 0.13 c | 1.54 ± 0.22 bc | 65.37 ± 1.71 c | 54.55 ± 0.89 cd |
| TUTMgSA2 | 1.97 ± 0.09 a | 0.83 ± 0.04 cd | 1.00 ± 0.15 c | 39.00 ± 3.60 d | 89.54 ± 0.88 a | |
| TUTMgSA3 | 1.83 ± 0.15 b | 0.21 ± 0.01 g | 2.94 ± 0.42 a | 82.01 ± 1.53 b | 83.18 ± 0.60 b | |
| TUTVuSA3 | 1.33 ± 0.15 c | 0.48 ± 0.09 ef | 1.29 ± 0.20 bc | 33.11 ± 0.64 de | 60.45 ± 0.98 c | |
| TUTPvES1 | 0.87 ± 0.09 d | 0.23 ± 0.05 g | 1.52 ± 0.21 bc | 10.46 ± 0.46 fg | 39.55 ± 0.78 f | |
| TUTPvES3 | 0.97 ± 0.07 d | 0.29 ± 0.10 fg | 1.87 ± 0.28 b | 15.99 ± 1.18 f | 44.10 ± 0.92 d | |
| TUTGmGH1 | 0.77 ± 0.07 d | 1.26 ± 0.06 b | 0.99 ± 0.14 c | 5.95 ± 0.04 g | 35.00 ± 0.67 g | |
| TUTGmGH3 | 1.00 ± 0.06 c | 0.61 ± 0.03 de | 0.98 ± 0.16 c | 31.08 ± 1.71 e | 45.45 ± 0.87 e | |
| COMMERCIAL INOC | 1.97 ± 0.78 a | 0.64 ± 0.03 de | 1.50 ± 0.21 bc | 97.62 ± 5.72 a | 89.55 ± 0.77 a | |
| NITRATE | 2.20 ± 0.36 a | 1.67 ± 0.15 a | 0.92 ± 0.14 c | ND | ND | |
| F-STATISTIC | 25.28 *** | 31.01 *** | 7.55 *** | 204.73 ** | 6.59 *** | |
| Common bean cv. NUA 734 | TUTPvES1 | 4.70 ± 2.60 a | 3.80 ± 0.12 b | 2.93 ± 0.43 a | 50.72 ± 12.59 cd | 145.51 ± 1.00 a |
| TUTPvES2 | 3.33 ± 0.12 c | 3.98 ± 0.02 b | 2.23 ± 0.33 ab | 65.77 ± 2.24 c | 103.10. ± 0.88 c | |
| TUTPvES3 | 2.77 ± 0.18 d | 2.30 ± 0.02 c | 3.01 ± 0.43 a | 90.21 ± 8.05 b | 85.76 ± 0.81 d | |
| TUTGmGH2 | 2.43 ± 0.12 d | 3.51 ± 0.06 b | 2.48 ± 0.34 ab | 51.14 ± 0.95 cd | 75.23 ± 0.79 e | |
| TUTGmGH3 | 2.27 ± 0.07 d | 4.06 ± 0.52 a | 1.56 ± 0.22 b | 46.19 ± 0.56 c–e | 70.28 ± 0.74 ef | |
| COMMERCIAL INOC | 4.30 ± 0.96 b | 4.08 ± 0.00 a | 2.93 ± 0.43 a | 171.87 ± 15.81 a | 133.13 ± 0.93 b | |
| NITRATE | 3.23 ± 0.44 c | 3.97 ± 0.10 b | 1.24 ± 0.20 b | ND | ND | |
| F-STATISTIC | 11.01 *** | 15.19 *** | 3.15 * | 33.24 *** | 7.57 *** | |
| Soybean cv. TGX1740-2F | TUTGmGH1 | 3.20 ± 0.78 a | 1.50 ± 0.00 b–d | 3.69 ± 0.54 a | 52.14 ± 3.78 cd | 233.58 ± 3.66 a |
| TUTGmGH2 | 2.47 ± 0.57 a | 0.23 ± 0.10 g | 2.72 ± 0.50 a–c | 119.32 ± 19.94 a | 180.29 ± 2.65 d | |
| TUTGmGH3 | 2.53 ± 1.39 a | 0.27 ± 0.07 fg | 2.15 ± 0.31 b–d | 107.88 ± 25.30 ab | 184.67 ± 3.06 c | |
| TUTVuSA1 | 1.27 ± 0.07 b | 1.11 ± 0.04 c–e | 3.17 ± 0.44 ab | 37.60 ± 5.64 cd | 92.70 ± 3.01 g | |
| TUTMgSA3 | 0.77 ± 0.07 b | 3.11 ± 0.12 a | 2.03 ± 0.29 b–e | 18.28 ± 3.58 d | 82.48 ± 1.77 h | |
| TUTVsES2 | 1.73 ± 0.42 b | 2.11 ± 0.03 b | 1.15 ± 0.16 de | 15.17 ± 3.56 d | 126.28 ± 1.98 e | |
| TUTPvES2 | 1.53 ± 0.07 b | 0.06 ± 0.02 g | 2.49 ± 0.36 bc | 34.16 ± 0.05 cd | 111.68 ± 1.82 f | |
| TUTPvES3 | 0.93 ± 0.15 b | 3.48 ± 0.59 a | 1.64 ± 0.30 c–e | 13.68 ± 3.33 d | 67.88 ± 1.07 i | |
| COMMERCIAL INOC | 3.17 ± 2.32 a | 0.89 ± 0.19 d–f | 1.26 ± 0.18 de | 73.01 ± 26.88 bc | 231.39 ± 4.04 b | |
| NITRATE | 1.37 ± 0.03 b | 1.59 ± 0.21 bc | 0.98 ± 0.23 e | ND | ND | |
| F-STATISTIC | 7.44 *** | 31.56 *** | 5.99 *** | 8.21 *** | 6.45 *** | |
| Winged bean cv.VRWB 4A | TUTMgSA2 | 1.43 ± 0.09 c | 2.05 ± 0.18 a | 1.72 ± 0.24 c | 21.36 ± 0.70 e | ND |
| TUTVsES1 | 2.03 ± 0.12 b | 1.87 ± 0.06 a | 2.42 ± 0.34 a–c | 41.70 ± 2.98 c | ND | |
| TUTPvES3 | 1.90 ± 0.12 b | 2.01 ± 0.09 a | 2.26 ± 0.32 a–c | 35.08 ± 1.80 d | ND | |
| TUTGmGH1 | 2.93 ± 0.17 a | 1.86 ± 0.01 a | 3.24 ± 0.47 ab | 85.87 ± 0.72 a | ND | |
| TUTGmGH2 | 2.03 ± 0.12 b | 0.51 ± 0.23 c | 3.39 ± 0.54 a | 56.90 ± 0.43 b | ND | |
| TUTGmGH3 | 1.77 ± 0.18 bc | 1.30 ± 0.03 b | 1.99 ± 0.28 bc | 32.45 ± 1.76 d | ND | |
| F-STATISTIC | 13.86 *** | 21.78 *** | 3.16 * | 193.25 *** | ||
| Velvet bean cv. IIHR PS 1 | TUTVuSA1 | 2.67 ± 0.12 de | 0.97 ± 0.04 ab | 1.33 ± 0.19 a | 31.35 ± 0.99 h | ND |
| TUTVuSA2 | 4.43 ± 0.20 a | 0.66 ± 0.13 b–e | 1.21 ± 0.17 a | 46.28 ± 2.10 b | ND | |
| TUTVuSA3 | 4.33 ± 0.23 a | 0.18 ± 0.05 fg | 1.36 ± 0.20 a | 50.12 ± 2.81 a | ND | |
| TUTMgSA1 | 3.30 ± 0.15 bc | 0.33 ± 0.05 e–g | 1.27 ± 0.18 a | 36.31 ± 1.28 fg | ND | |
| TUTMgSA2 | 3.43 ± 0.07 bc | 0.77 ± 0.17 | 1.38 ± 0.20 a | 40.31 ± 0.98 de | ND | |
| TUTMgSA3 | 2.67 ± 0.09 de | 0.62 ± 0.02 b–e | 1.17 ± 0.17 a | 27.51 ± 0.48 i | ND | |
| TUTVsES1 | 3.10 ± 0.17 b–d | 0.25 ± 0.08 e–g | 1.38 ± 0.20 a | 38.51 ± 0.93 d–f | ND | |
| TUTVsES3 | 3.03 ± 0.18 cd | 1.06 ± 0.03 a | 1.19 ± 0.17 a | 32.79 ± 0.31 | ND | |
| TUTPvES2 | 2.33 ± 0.19 d | 0.86 ± 0.18 a–c | 1.23 ± 0.18 a | 22.58 ± 0.43 j | ND | |
| TUTPvES3 | 3.63 ± 0.15 b | 0.60 ± 0.12 b–e | 1.30 ± 0.19 a | 40.64 ± 1.08 de | ND | |
| TUTGmGH1 | 4.23 ± 0.20 a | 0.49 ± 0.28 c–f | 1.22 ± 0.17 a | 44.68 ± 1.03 bc | ND | |
| TUTGmGH2 | 3.60 ± 0.17 b | 0.07 ± 0.01 g | 1.32 ± 0.19 a | 42.38 ± 0.98 cd | ND | |
| TUTGmGH3 | 3.50 ± 0.12 bc | 0.38 ± 0.10 d–g | 1.22 ± 0.17 a | 37.78 ± 0.19 ef | ND | |
| F-STATISTIC | 16.21 *** | 6.37 *** | 0.16 ns | 37.74 *** | ||
| Jack bean cv. Accession 493 | TUTVuSA2 | 4.60 ± 0.29 a | 1.13 ± 0.06 d | 1.25 ± 0.21 a | 45.58 ± 1.62 a | ND |
| TUTMgSA3 | 2.80 ± 0.10 de | 3.07 ± 0.04 b | 1.44 ± 0.21 a | 33.83 ± 1.07 b | ND | |
| TUTVsES1 | 2.53 ± 0.09 e | 3.38 ± 0.08 a | 1.58 ± 00.22 a | 35.37 ± 0.94 b | ND | |
| TUTVsES2 | 3.53 ± 0.09 b | 2.82 ± 0.08 c | 1.51 ± 0.22 a | 45.81 ± 1.32 a | ND | |
| TUTPvES2 | 2.00 ± 0.10 f | 3.32 ± 0.06 a | 1.59 ± 0.27 a | 28.56 ± 0.12 c | ND | |
| TUTVsES3 | 3.23 ± 0.09 bc | 2.83 ± 0.03 c | 1.62 ± 0.22 a | 44.08 ± 0.32 a | ND | |
| F-STATISTIC | 38.61 *** | 193.69 *** | 0.40 ns | 48.87 *** | ||
| Pigeonpea cv. ICEAP500557 | TUTVuSA1 | 0.87 ± 0.03 b | 0.76 ± 0.08 c | 1.12 ± 0.16 b | 8.82 ± 0.83 c | ND |
| TUTVuSA2 | 0.97 ± 0.09 ab | 0.76 ± 0.22 c | 1.19 ± 0.23 b | 9.11 ± 1.61 c | ND | |
| TUTMgSA1 | 0.77 ± 0.09 b | 3.03 ± 0.12 a | 1.32 ± 0.19 b | 9.85 ± 1.02 bc | ND | |
| TUTGmGH1 | 0.97 ± 0.09 ab | 1.36 ± 0.03 b | 1.26 ± 0.19 b | 11.26 ± 0.02 bc | ND | |
| TUTGmGH2 | 1.17 ± 0.07 a | 0.16 ± 0.04 d | 1.53 ± 0.21 b | 15.99 ± 1.42 a | ND | |
| TUTGmGH3 | 0.70 ± 0.10 b | 1.20 ± 0.07 b | 2.47 ± 0.35 a | 12.75 ± 0.33 b | ND | |
| F-STATISTIC | 4.28 * | 74.99 *** | 4.81 * | 6.91 ** | ||
| Mungbean cv. VC1973A | TUTMgSA2 | 0.90 ± 0.12 b | 1.45 ± 0.28 c | 1.49 ± 0.23 b | 10.09 ± 0.41 b | ND |
| TUTMgSA3 | 0.90 ± 0.00 b | 2.07 ± 0.02 b | 1.26 ± 0.20 b | 9.59 ± 0.49 b | ND | |
| TUTPvES1 | 0.77 ± 0.09 b | 2.29 ± 0.29 b | 1.52 ± 0.22 b | 11.08 ± 0.25 b | ND | |
| TUTGmGH1 | 0.87 ± 0.09 b | 4.16 ± 0.13 a | 1.42 ± 0.21 b | 9.59 ± 0.44 b | ND | |
| TUTGmGH3 | 1.17 ± 0.03 a | 1.28 ± 0.04 c | 3.36 ± 0.51 a | 34.26 ± 1.00 a | ND | |
| F-STATISTIC | 3.67 * | 36.52 *** | 8.52 ** | 348.88 *** |
| Legumes | Treatments | Shoot DM | δ15N | N | N-Fixed | RSE |
|---|---|---|---|---|---|---|
| g.plant−1 | ‰ | % | mg.plant−1 | % | ||
| Cowpea cv. IT10K-866-1 | TUTVuSA1 | 3.93 ± 0.68 a | 2.58 ± 0.05 c | 0.71 ± 0.04 h | 39.62 ± 1.44 c | 161.73 ± 1.93 a |
| TUTVuSA2 | 3.60 ± 0.21 b | 3.63 ± 0.08 b | 0.98 ± 0.06 fg | 32.74 ± 4.17 d | 148.15 ± 1.45 b | |
| TUTVuSA3 | 2.93 ± 0.43 c | 1.26 ± 0.04 d | 2.02 ± 0.01 d | 49.46 ± 1.47 b | 120.58 ± 1.63 c | |
| TUTGmGH1 | 2.03 ± 0.07 de | 1.10 ± 0.04 d | 2.56 ± 0.03 b | 51.15 ± 1.99 b | 83.54 ± 1.22 e | |
| TUTGmGH2 | 0.63 ± 0.09 h | 0.38 ± 0.20 e | 1.99 ± 0.08 d | 12.74 ± 1.24 f | 25.93 ± 1.07 h | |
| TUTGmGH3 | 0.43 ± 0.07 h | 1.80 ± 0.15 cd | 2.20 ± 0.03 c | 8.86 ± 1.41 f | 17.70 ± 1.42 i | |
| TUTVsES1 | 0.87 ± 0.03 gh | 4.96 ± 0.24 a | 0.82 ± 0.08 gh | 27.97 ± 1.41 d | 35.80 ± 0.69 g | |
| TUTPvES3 | 1.10 ± 0.10 fh | 2.20 ± 0.11 c | 1.34 ± 0.08 e | 20.76 ± 1.59 e | 60.49 ± 1.48 f | |
| COMMERCIAL INOC | 2.70 ± 0.12 cd | 0.34 ± 0.00 e | 3.18 ± 0.01 a | 90.43 ± 4.17 a | 111.10 ± 1.51 d | |
| NITRATE | 2.43 ± 0.12 cd | 2.08 ± 0.71 c | 0.84 ± 0.10 gh | ND | ND | |
| F-STATISTIC | 71.80 *** | 31.66 *** | 216.17 ** | 135.81 ** | 6.98 *** | |
| Bambara groundnut LR. SSD8 | TUTVsES1 | 3.83 ± 0.43 a | 0.62 ± 0.03 ab | 2.26 ± 0.34 ab | 73.25 ± 6.31 a | 126.40 ± 2.65 b |
| TUTVsES2 | 3.97 ± 0.13 a | 0.63 ± 0.13 ab | 2.06 ± 0.31 b | 71.75 ± 2.13 a | 131.02 ± 2.23 a | |
| TUTVsES3 | 2.50 ± 0.31 c | 0.69 ± 0.13 a | 2.95 ± 0.42 a | 69.71 ± 9.81 a | 82.51 ± 1.45 de | |
| TUTMgSA3 | 0.83 ± 0.03 e | 0.23 ± 0.05 c | 1.88 ± 0.34 b | 13.21 ± 0.87 de | 27.39 ± 1.32 e | |
| TUTPvES2 | 0.73 ± 0.03 e | 0.65 ± 0.23 ab | 1.29 ± 0.21 b | 8.12 ± 0.16 e | 24.09 ± 1.21 f | |
| TUTGmGH1 | 3.07 ± 0.09 bc | 0.20 ± 0.09 c | 1.41 ± 0.19 b | 38.31 ± 1.71 b | 101.32 ± 1.49 c | |
| TUTGmGH2 | 2.53 ± 0.32 c | 0.69 ± 0.04 b | 1.36 ± 0.20 b | 24.10 ± 1.91 cd | 83.50 ± 2.12 de | |
| TUTGmGH3 | 2.63 ± 0.07 bc | 0.29 ± 0.04 bc | 1.84 ± 0.25 b | 42.93 ± 1.09 b | 86.80 ± 1.97 d | |
| COMMERCIAL INOC | 3.87 ± 0.09 a | 0.40 ± 0.01 a–c | 1.89 ± 0.27 b | 63.78 ± 0.10 a | 127.72 ± 2.22 b | |
| NITRATE | 3.03 ± 0.47 bc | 0.32 ± 0.17 a–c | 1.77 ± 0.31 b | ND | ND | |
| F-STATISTIC | 28.93 *** | 3.07 * | 2.79 * | 42.04 *** | 7.47 *** | |
| Kersting’s groundnut LR. Dowie | TUTMgSA1 | 3.20 ± 0.10 a | 0.88 ± 0.12 c | 1.51 ± 0.22 b–d | 48.09 ± 6.08 a | 177.78 ± 4.56 a |
| TUTMgSA2 | 2.93 ± 0.15 a | 0.77 ± 0.32 c | 1.07 ± 0.15 d | 31.13 ± 3.17 b | 162.78 ± 3.76 b | |
| TUTMgSA3 | 2.10 ± 0.17 b | 0.43 ± 0.07 c | 2.97 ± 0.42 a | 54.41 ± 11.46 a | 116.67 ± 3.81 c | |
| TUTVuSA3 | 0.97 ± 0.09 h–j | 0.58 ± 0.10 c | 1.14 ± 0.16 cd | 10.80 ± 0.62 c | 53.89 ± 3.42 f | |
| TUTPvES1 | 1.00 ± 0.06 h–j | 0.37 ± 0.13 c | 1.87 ± 0.28 bc | 18.73 ± 2.98 bc | 55.56 ± 2.77 f | |
| TUTPvES3 | 0.87 ± 0.09 h–j | 0.38 ± 0.07 c | 1.99 ± 0.30 b | 17.70 ± 4.28 bc | 48.33 ± 2.81 g | |
| TUTGmGH2 | 1.43 ± 0.15 d–f | 2.46 ± 0.37 a | 0.95 ± 0.13 d | 13.94 ± 3.32 c | 79.44 ± 2.12 ef | |
| TUTGmGH3 | 1.50 ± 0.10 c–e | 0.28 ± 0.07 c | 1.52 ± 0.21 b–d | 22.71 ± 2.81 bc | 83.33 ± 2.65 e | |
| COMMERCIAL INOC | 1.70 ± 0.10 cd | 1.27 ± 0.28 bc | 1.12 ± 0.16 cd | 18.86 ± 2.18 bc | 94.44 ± 1.97 d | |
| NITRATE | 1.80 ± 0.15 c | 2.07 ± 0.79 ab | 0.85 ± 0.12 d | ND | ND | |
| F-STATISTIC | 49.86 *** | 5.73 *** | 7.06 *** | 9.67 *** | 8.01 *** | |
| Common bean cv. NUA 721 | TUTPvES1 | 3.10 ± 0.12 ab | 4.31 ± 0.66 ab | 2.78 ± 0.40 ab | 85.37 ± 9.28 ab | 139.01 ± 1.77 b |
| TUTPvES2 | 2.80 ± 0.17 bc | 1.13 ± 0.22 c | 2.47 ± 0.35 a–c | 70.14 ± 14.22 bc | 125.56 ± 1.87 d | |
| TUTPvES3 | 3.03 ± 0.12 ab | 2.82 ± 0.42 bc | 3.22 ± 0.46 ab | 69.53 ± 6.02 bc | 135.87 ± 1.32 c | |
| TUTGmGH2 | 2.20 ± 0.21 de | 4.98 ± 0.70 a | 1.51 ± 0.21 cd | 35.33 ± 9.22 d | 98.65 ± 1.90 e | |
| TUTGmGH3 | 2.10 ± 0.15 df | 5.64 ± 0.78 a | 2.12 ± 0.30 bc | 64.86 ± 11.34 bc | 94.17 ± 1.66 ef | |
| COMMERCIAL INOC | 3.30 ± 0.21 a | 5.10 ± 0.73 a | 3.02 ± 0.42 ab | 98.03 ± 6.90 a | 147.99 ± 1.84 a | |
| NITRATE | 2.23 ± 0.09 de | 4.68 ± 0.65 ab | 0.86 ± 0.15 d | ND | ND | |
| F-STATISTIC | 20.62 *** | 6.29 ** | 6.01 ** | 9.55 *** | 5.78 ** | |
| Soybean cv. TGX1937-1F | TUTGmGH1 | 2.47 ± 0.15 ab | 1.58 ± 0.22 bc | 3.82 ± 0.55 a | 77.48 ± 7.82 a | 119.32 ± 1.41 b |
| TUTGmGH2 | 2.80 ± 0.15 a | 0.43 ± 0.19 de | 2.39 ± 0.34 b–e | 67.66 ± 12.49 a | 135.27 ± 1.54 a | |
| TUTGmGH3 | 2.07 ± 0.22 bc | 0.39 ± 0.06 de | 2.04 ± 0.28 d–f | 43.13 ± 9.78 bc | 100.00 ± 1.11 c | |
| TUTVuSA1 | 1.97 ± 0.09 c | 1.43 ± 0.20 bc | 3.55 ± 0.52 ab | 68.98 ± 6.93 a | 95.17 ± 1.45 d | |
| TUTMgSA3 | 1.23 ± 0.09 d–f | 3.79 ± 0.56 a | 1.20 ± 0.17 ef | 14.56 ± 1.18 d | 59.42 ± 0.87 f | |
| TUTVsES2 | 1.43 ± 0.09 d | 2.05 ± 0.29 b | 1.19 ± 0.17 ef | 17.36 ± 3.56 d | 69.08 ± 0.27 e | |
| TUTPvES2 | 1.37 ± 0.09 de | 1.09 ± 0.15 cd | 3.31 ± 0.50 a–c | 45.80 ± 9.30 b | 66.18 ± 1.42 ef | |
| TUTPvES3 | 0.93 ± 0.15 ef | 0.15 ± 0.06 e | 2.81 ± 0.46 a–d | 26.47 ± 5.77 b–d | 44.93 ± 0.77 g | |
| COMMERCIAL INOC | 1.93 ± 0.09 c | 1.16 ± 0.18 cd | 1.12 ± 0.16 f | 21.86 ± 4.20 cd | 93.24 ± 0.56 de | |
| NITRATE | 2.07 ± 0.18 bc | 1.99 ± 0.29 b | 1.14 ± 0.18 f | ND | ND | |
| F-STATISTIC | 16.72 *** | 17.53 *** | 7.43 *** | 11.19 *** | 8.22 *** | |
| Velvet bean cv. IIHR PS 2 | TUTVuSA1 | 3.77 ± 0.20 ab | 0.61 ± 0.11 ab | 1.29 ± 0.18 a | 47.81 ± 4.34 ab | 103.86 ± 3.11 b |
| TUTVuSA2 | 2.73 ± 0.09 e | 0.51 ± 0.07 a–d | 1.15 ± 0.18 a | 31.16 ± 4.35 b–d | 75.21 ± 2.02 f | |
| TUTVuSA3 | 4.00 ± 0.21 a | 0.83 ± 0.12 a | 1.29 ± 0.19 a | 52.34 ± 10.37 a | 110.19 ± 2.76 a | |
| TUTMgSA1 | 3.37 ± 0.12 b–d | 0.18 ± 0.05 cd | 1.23 ± 0.18 a | 41.10 ± 4.77 a–c | 92.84 ± 1.79 c | |
| TUTMgSA2 | 2.97 ± 0.29 c–e | 0.52 ± 0.09 a–d | 1.30 ± 0.19 a | 39.70 ± 9.69 a–c | 81.82 ± 1.82 d | |
| TUTMgSA3 | 3.40 ± 0.17 b–d | 0.70 ± 0.10 ab | 1.26 ± 0.18 a | 42.89 ± 6.39 a–c | 93.66 ± 1.54 c | |
| TUTVsES1 | 2.90 ± 0.12 de | 0.15 ± 0.06 d | 1.41 ± 0.20 a | 40.58 ± 4.34 a–c | 79.89 ± 1.44 e | |
| TUTVsES2 | 2.20 ± 0.20 f | 0.69 ± 0.10 ab | 1.54 ± 0.21 a | 55.84 ± 7.90 a | 60.61 ± 0.86 g | |
| TUTPvES2 | 3.43 ± 0.07 bc | 0.90 ± 0.22 a | 1.20 ± 0.20 a | 24.90 ± 4.87 cd | 57.02 ± 0.65 h | |
| TUTPvES3 | 3.60 ± 0.17 ab | 0.35 ± 0.06 b–d | 1.35 ± 0.19 a | 46.59 ± 7.17 ab | 94.49 ± 1.89 c | |
| NITRATE | 3.63 ± 0.15 ab | 0.55 ± 0.20 a–c | 1.11 ± 0.16 a | ND | ND | |
| F-STATISTIC | 22.32 *** | 3.99 ** | 0.39 ns | 3.68 ** | 6.06 ** | |
| Jack bean cv. Accession 498 | TUTVuSA2 | 3.37 ± 0.09 e | 0.32 ± 0.15 b | 1.23 ± 0.17 b | 41.33 ± 6.04 b | 102.12 ± 3.23 f |
| TUTMgSA3 | 4.33 ± 0.15 bc | 3.43 ± 0.48 a | 1.61 ± 0.23 b | 69.35 ± 7.78 b | 131.21 ± 3.44 b | |
| TUTVsES1 | 4.47 ± 0.18 b | 3.70 ± 0.54 a | 1.60 ± 0.23 b | 72.41 ± 13.54 b | 135.45 ± 2.67 a | |
| TUTVsES2 | 3.67 ± 0.09 de | 3.85 ± 0.53 a | 1.65 ± 0.24 b | 59.98 ± 7.11 b | 111.21 ± 3.02 e | |
| TUTVsES3 | 3.93 ± 0.19 cd | 3.85 ± 0.53 b | 1.65 ± 0.24 b | 80.67 ± 13.74 b | 119.09 ± 2.17 d | |
| TUTGmGH3 | 4.10 ± 0.00 bc | 1.27 ± 0.200 b | 3.43 ± 0.54 a | 140.77 ± 22.08 a | 124.24 ± 1.12 c | |
| NITRATE | 4.87 ± 0.15 a | 3.43 ± 0.49 b | 1.61 ± 0.23 b | ND | ND | |
| F-STATISTIC | 17.97 *** | 8.95 *** | 5.79 ** | 6.59 *** | 8.88 ** | |
| Pigeonpea cv. ICEAP00850 | TUTVuSA1 | 0.83 ± 0.07 c | 1.11 ± 0.15 cd | 2.04 ± 0.29 ab | 17.15 ± 3.30 a | 49.70 ± 0.35 d |
| TUTVsES1 | 1.27 ± 0.07 b | 2.31 ± 0.34 b | 1.22 ± 0.21 bc | 15.28 ± 2.06 a | 37.72 ± 0.76 e | |
| TUTMgSA1 | 1.20 ± 0.06 b | 3.64 ± 0.51 a | 1.53 ± 0.21 a–c | 18.49 ± 3.31 a | 46.11 ± 1.23 d | |
| TUTPvES2 | 1.27 ± 0.03 b | 1.06 ± 0.18 d | 1.69 ± 0.24 a–c | 21.29 ± 2.44 a | 115.57 ± 1.09 a | |
| TUTGmGH2 | 1.27 ± 0.17 b | 2.09 ± 0.29 bc | 1.64 ± 0.23 a–c | 21.47 ± 5.94 a | 67.66 ± 0.91 c | |
| TUTGmGH3 | 0.97 ± 0.09 bc | 1.61 ± 0.24 b–d | 2.34 ± 0.35 a | 22.04 ± 1.14 a | 76.05 ± 0.29 b | |
| NITRATE | 1.67 ± 0.12 a | 1.53 ± 0.29 b–d | 0.98 ± 0.15 c | ND | ND | |
| F-STATISTIC | 9.16 *** | 7.42 *** | 3.06 * | 0.86 ns | 8.66 ** | |
| Mungbean cv. VC6 153 (B-20P) | TUTMgSA2 | 0.77 ± 0.03 c | 2.78 ± 0.45 b | 1.34 ± 0.19 cd | 10.30 ± 1.61 c | 100.00 ± 0.33 d |
| TUTMgSA3 | 0.90 ± 0.00 c | 1.53 ± 0.21 bc | 2.25 ± 0.32 ab | 21.50 ± 2.50 b | 116.88 ± 0.87 c | |
| TUTPvES1 | 1.17 ± 0.12 ab | 2.75 ± 0.55 b | 1.52 ± 0.22 b–d | 17.43 ± 1.70 bc | 151.95 ± 0.76 b | |
| TUTGmGH2 | 1.33 ± 0.09 a | 1.05 ± 0.30 c | 1.55 ± 0.25 b–d | 20.44 ± 2.72 bc | 172.73 ± 0.94 a | |
| TUTGmGH3 | 1.33 ± 0.03 a | 1.33 ± 0.20 c | 2.77 ± 0.49 a | 37.23 ± 7.47 a | 172.73 ± 0.44 a | |
| NITRATE | 0.97 ± 0.07 bc | 4.40 ± 0.74 a | 1.05 ± 0.17 d | ND | ND | |
| F-STATISTIC | 11.53 *** | 7.07 ** | 4.46 ** | 7.64 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msiza, L.J.; Ngmenzuma, T.Y.; Mohammed, M.; Jaiswal, S.K.; Dakora, F.D. Cross-Infectivity of 11 Different Legume Species by 15 Native Rhizobia Isolated from African Soils. Microorganisms 2025, 13, 2463. https://doi.org/10.3390/microorganisms13112463
Msiza LJ, Ngmenzuma TY, Mohammed M, Jaiswal SK, Dakora FD. Cross-Infectivity of 11 Different Legume Species by 15 Native Rhizobia Isolated from African Soils. Microorganisms. 2025; 13(11):2463. https://doi.org/10.3390/microorganisms13112463
Chicago/Turabian StyleMsiza, Lebogang J., Titus Y. Ngmenzuma, Mustapha Mohammed, Sanjay K. Jaiswal, and Felix D. Dakora. 2025. "Cross-Infectivity of 11 Different Legume Species by 15 Native Rhizobia Isolated from African Soils" Microorganisms 13, no. 11: 2463. https://doi.org/10.3390/microorganisms13112463
APA StyleMsiza, L. J., Ngmenzuma, T. Y., Mohammed, M., Jaiswal, S. K., & Dakora, F. D. (2025). Cross-Infectivity of 11 Different Legume Species by 15 Native Rhizobia Isolated from African Soils. Microorganisms, 13(11), 2463. https://doi.org/10.3390/microorganisms13112463






