Effects of Bacillus coagulans (GBI-30, 6086) Supplementation on the Fecal Characteristics and Microbiota of Healthy Adult Dogs Subjected to an Abrupt Diet Change
Abstract
1. Introduction
2. Materials and Methods
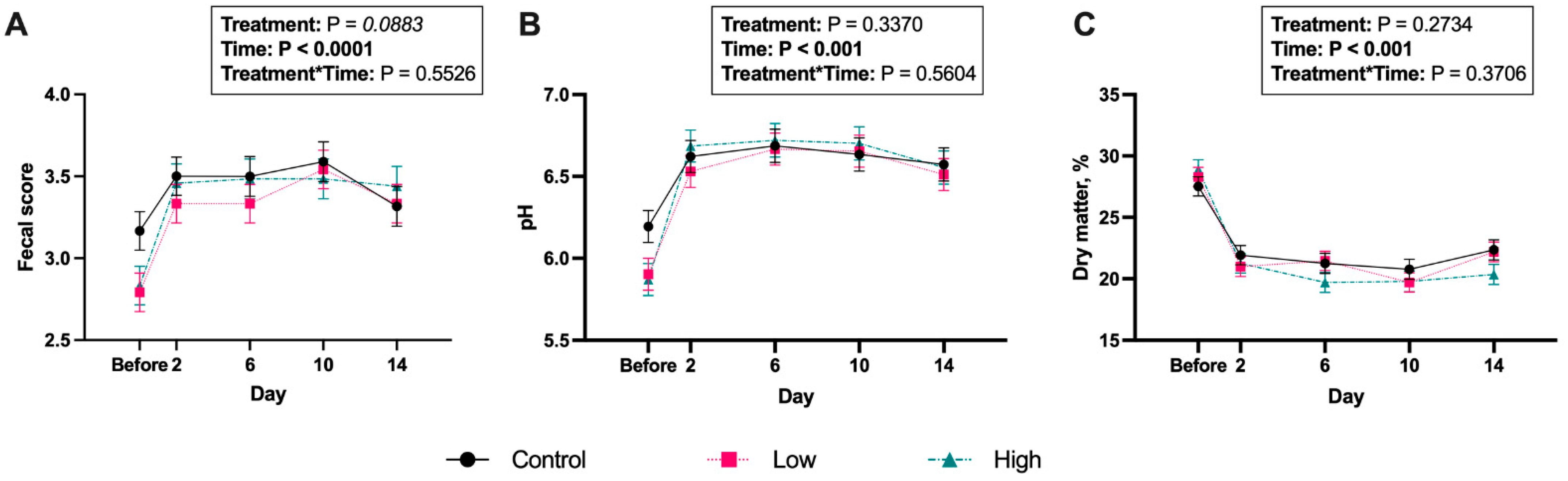
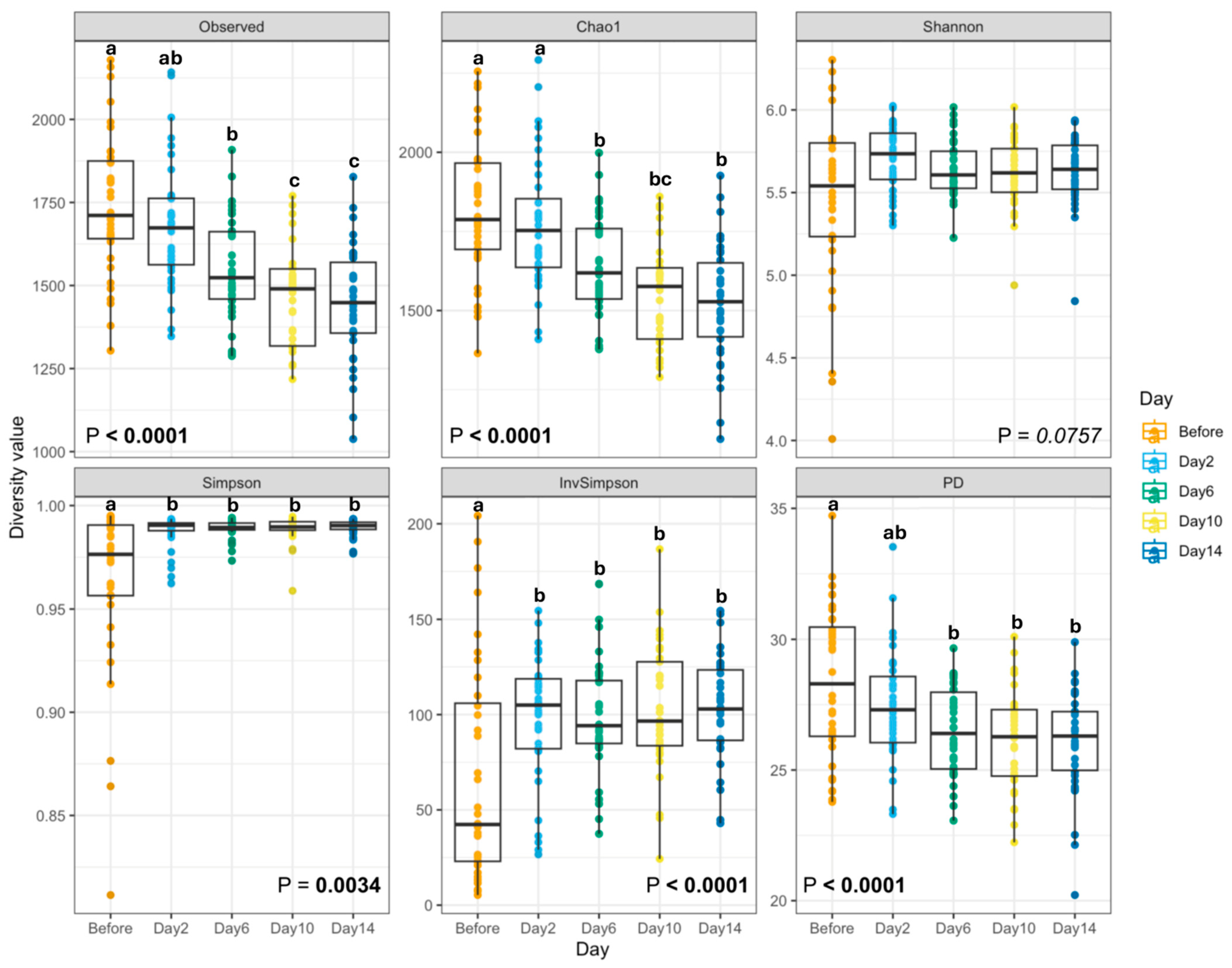
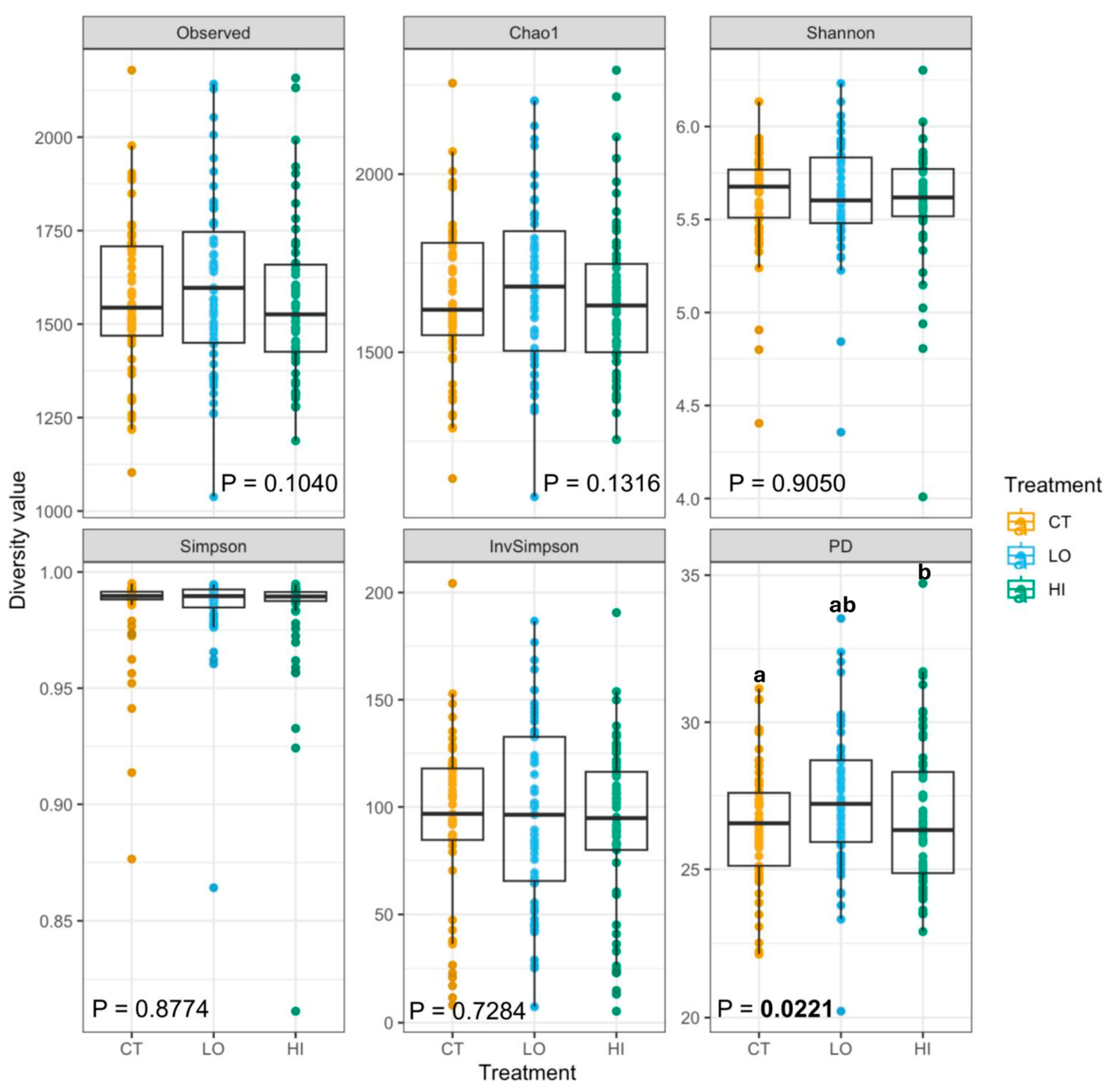
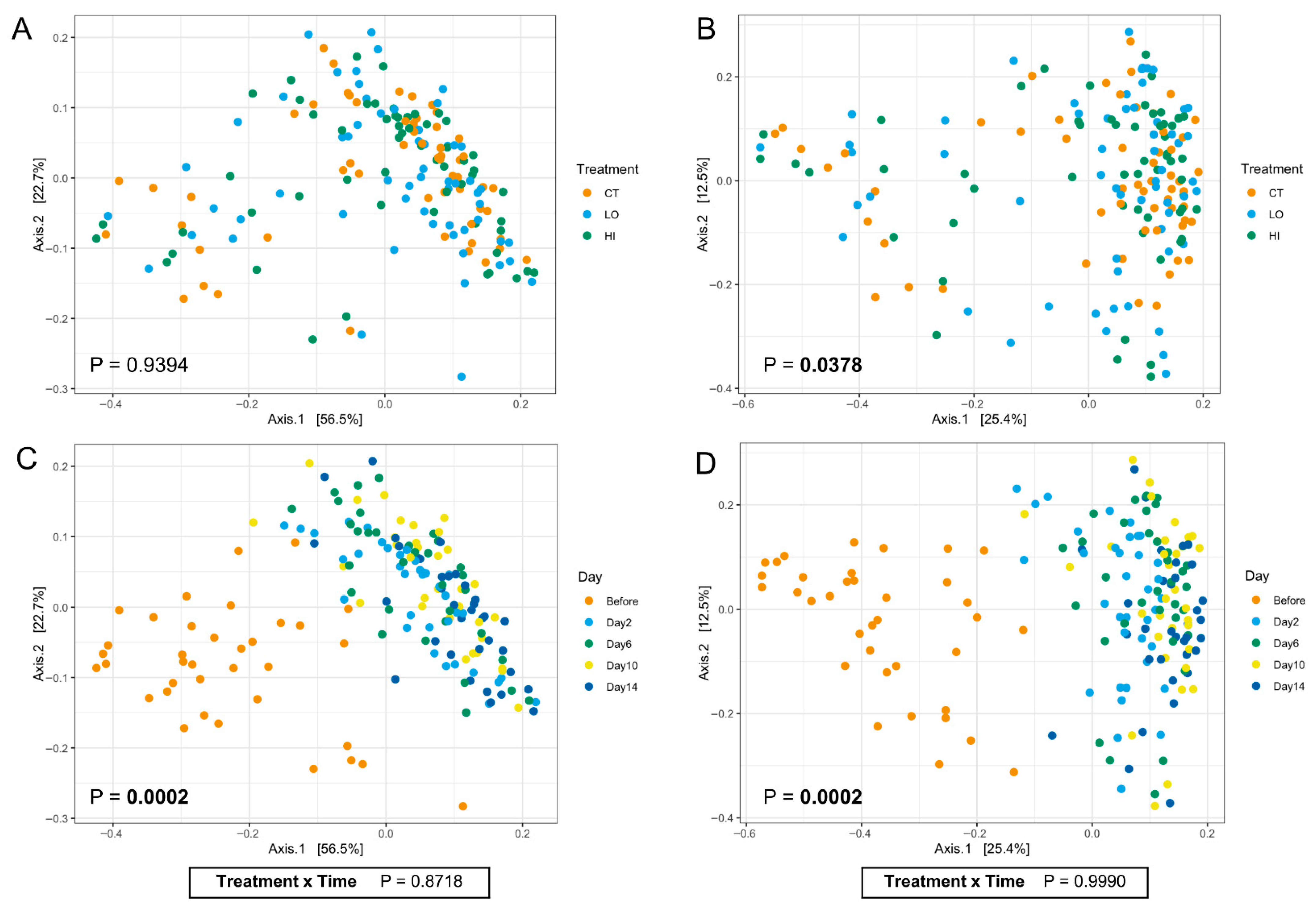
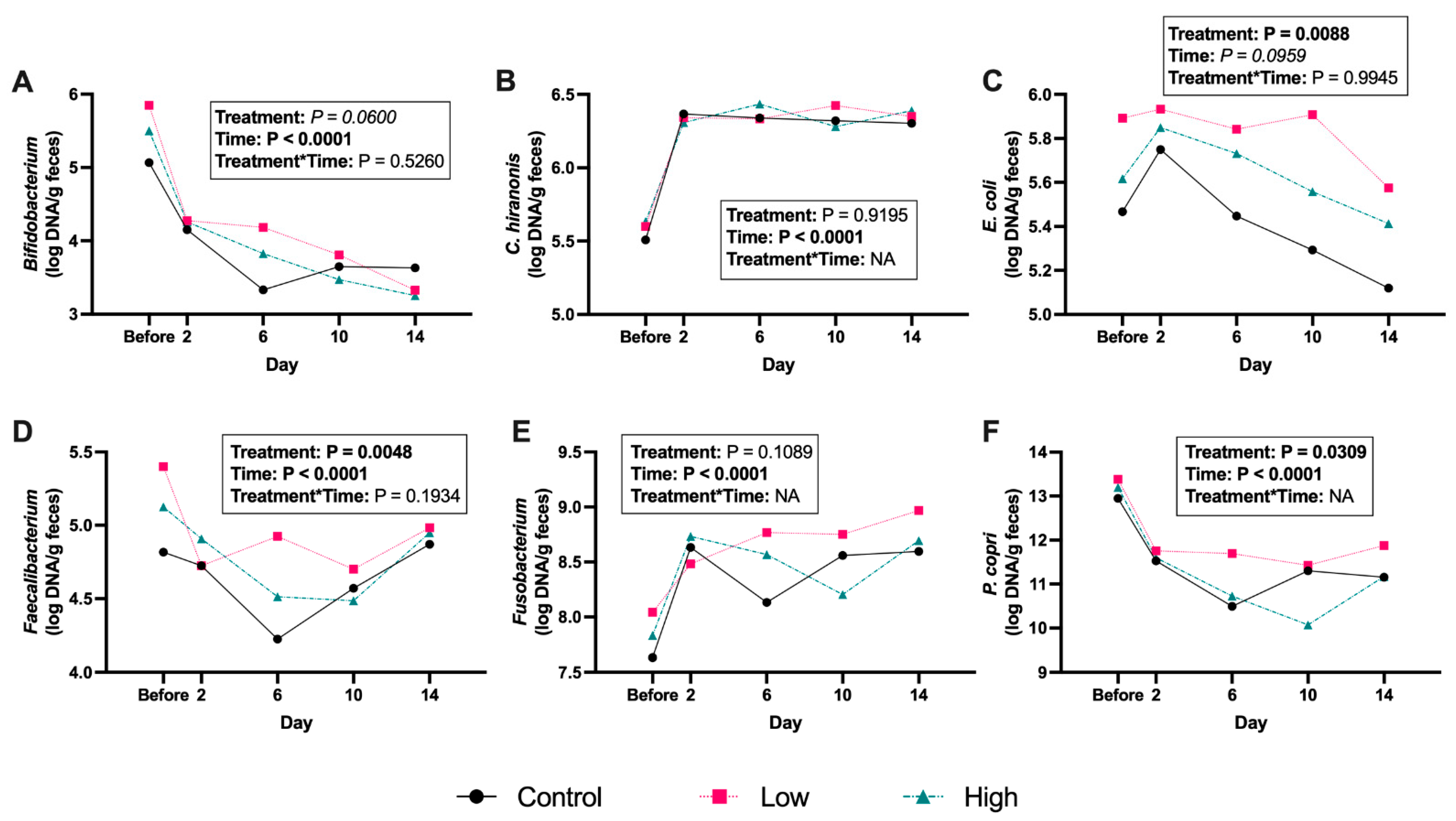
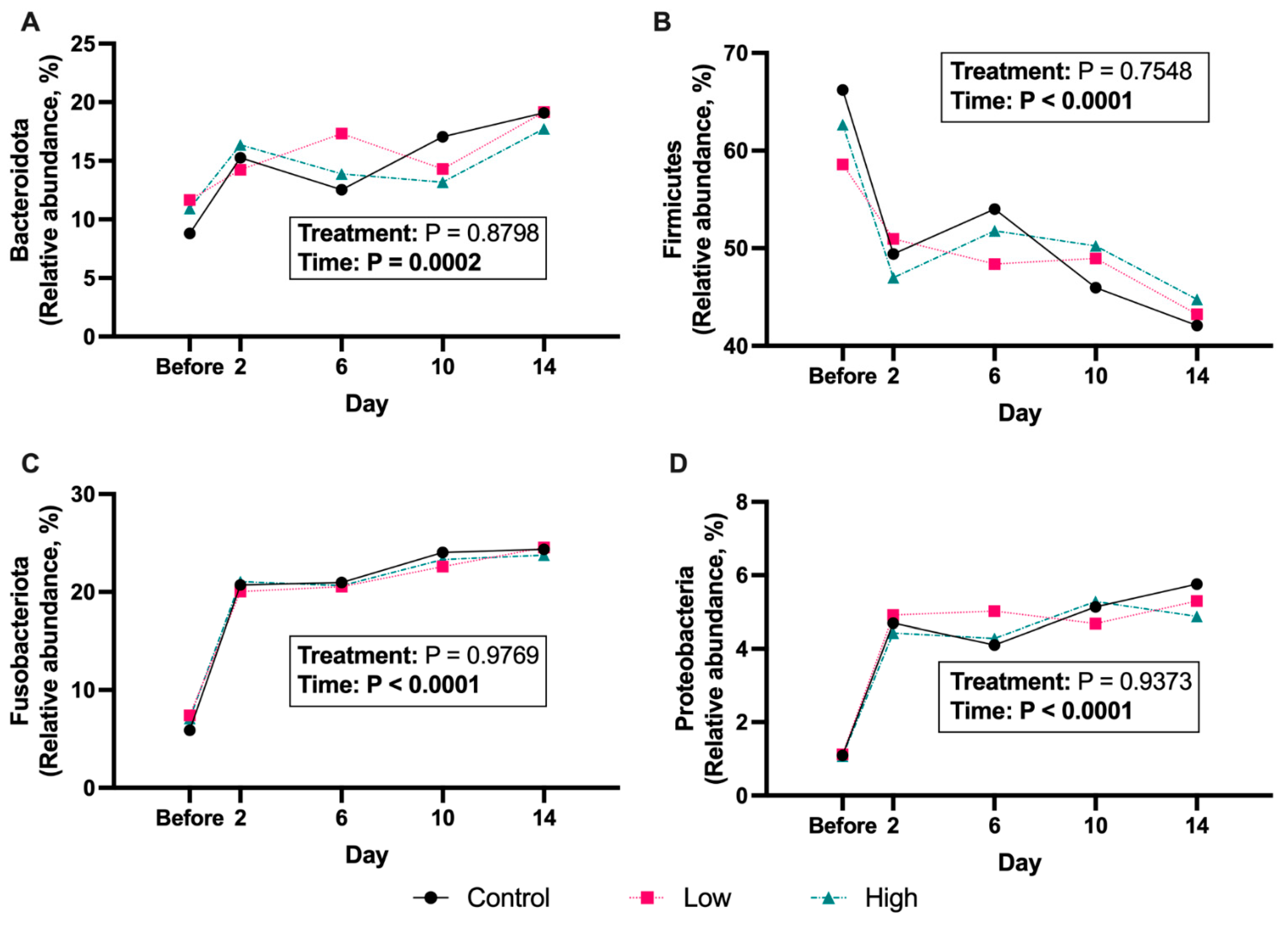
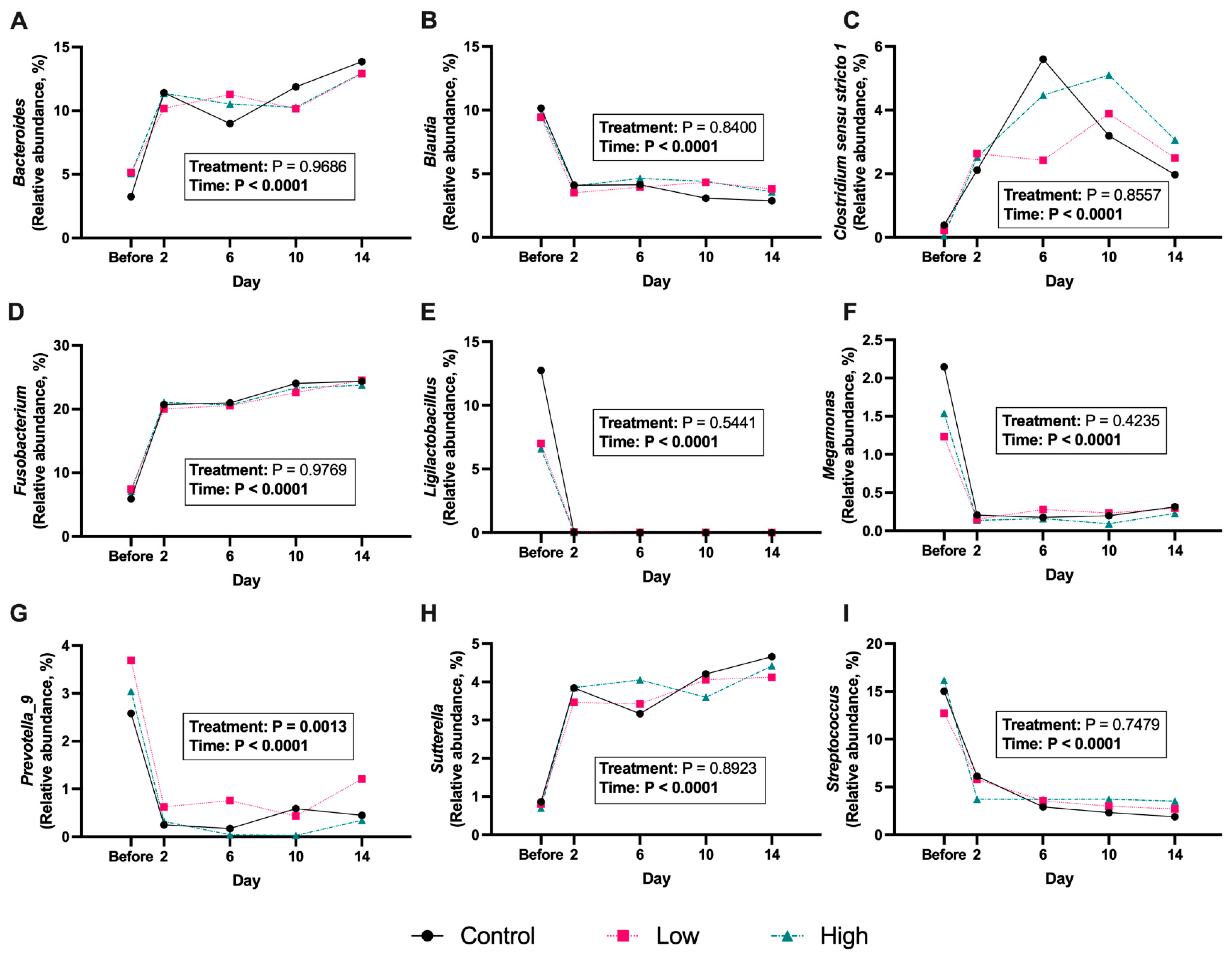
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHF | acid-hydrolyzed fat |
| CFU | colony-forming units |
| CP | crude protein |
| DM | dry matter |
| GI | gastrointestinal |
| NFE | nitrogen-free extract |
| OM | organic matter |
| PD | phylogenetic diversity |
| qPCR | quantitative polymerase chain reaction |
| SCFA | short-chain fatty acids |
| TDF | total dietary fiber |
References
- Wilson, S.M.; Kang, Y.; Wren, J.F.; Menton, J.F.; Vinay, E.; Millette, M.; Kelly, M.R.; Xie, Z.; Miller, M.J.; Swanson, K.S. Effects of Bacillus coagulans (GBI-30, 6086) supplementation on apparent total tract nutrient digestibility and the fecal characteristics and metabolites, immunity, and microbiota of healthy adult dogs. J. Anim. Sci. 2025, in press. [Google Scholar]
- Jensen, A.P.; Bjørnvad, C.R. Clinical effect of probiotics in prevention or treatment of gastrointestinal disease in dogs: A systematic review. J. Vet. Intern. Med. 2019, 33, 1849–1864. [Google Scholar] [CrossRef]
- Mounika, B.; Anil Kumar, B.; Gopala Reddy, A.; Ashok Kumar, D.; Madhuri, G. Effect of probiotic formulation containing Bacillus spp. on diarrhoea in dogs. J. Pharm. Innov. 2019, 8, 81–85. [Google Scholar]
- Merenstein, D.J.; Tancredi, D.J.; Karl, J.P.; Krist, A.H.; Lenoir-Wijnkoop, I.; Reid, G.; Roos, S.; Szajewska, H.; Sanders, M.E. Is there evidence to support probiotic use for healthy people? Adv. Nutr. 2024, 15, 100265. [Google Scholar] [CrossRef]
- Shane, A.L.; Cabana, M.D.; Vidry, S.; Merenstein, D.; Hummelen, R.; Ellis, C.L.; Heimbach, J.T.; Hempel, S.; Lynch, S.V.; Sanders, M.E.; et al. Guide to designing, conducting, publishing and communicating results of clinical studies involving probiotic applications in human participants. Gut Microbes 2010, 1, 243–253. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Jha, A.R.; Oba, P.M.; Yotis, S.M.; Shmalberg, J.; Honaker, R.W.; Swanson, K.S. Longitudinal fecal microbiome and metabolite data demonstrate rapid shifts and subsequent stabilization after an abrupt dietary change in healthy adult dogs. Anim. Microbiome 2022, 4, 46. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Carroll, M.Q.; Miller, M.J.; Rabot, R.; Swanson, K.S. Supplementation of yeast cell wall fraction tends to improve intestinal health in adult dogs undergoing an abrupt diet transition. Front. Vet. Sci. 2020, 7, 597939. [Google Scholar] [CrossRef]
- Bastos, T.S.; Souza, C.M.M.; Legendre, H.; Richard, N.; Pilla, R.; Suchodolski, J.S.; De Oliveira, S.G.; Lesaux, A.A.; Félix, A.P. Effect of yeast Saccharomyces cerevisiae as a probiotic on diet digestibility, fermentative metabolites, and composition and functional potential of the fecal microbiota of dogs submitted to an abrupt dietary change. Microorganisms 2023, 11, 506. [Google Scholar] [CrossRef]
- Golnari, M.; Bahrami, N.; Milanian, Z.; Rabbani Khorasgani, M.; Asadollahi, M.A.; Shafiei, R.; Fatemi, S.S. Isolation and characterization of novel Bacillus strains with superior probiotic potential: Comparative analysis and safety evaluation. Sci. Rep. 2024, 14, 1457. [Google Scholar] [CrossRef]
- Vreeland, R.H.; Rosenzweig, W.D.; Powers, D.W. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 2000, 407, 897–900. [Google Scholar] [CrossRef]
- Cartman, S.; La Ragione, R.; Woodward, M. Bacterial spore formers as probiotics for poultry. Food Sci. Technol. Bull. Funct. Foods 2007, 4, 21–30. [Google Scholar] [CrossRef]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, F.; Minamoto, Y.; Suchodolski, J.S.; Galiazzo, G.; Vecchiato, C.G.; Pinna, C.; Biagi, G.; Pietra, M. Effect of an extruded animal protein-free diet on fecal microbiota of dogs with food-responsive enteropathy. J. Vet. Intern. Med. 2018, 32, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; La Ragione, R.M.; Nunez, A.; Cutting, S.M. Immunostimulatory activity of Bacillus spores. FEMS Immunol. Med. Microbiol. 2008, 53, 195–203. [Google Scholar] [CrossRef]
- Urdaci, M.C.; Pinchuk, I. Antimicrobial activity of Bacillus probiotics. In Bacterial Spore Formers: Probiotics and Emerging Applications; Ricca, E., Henriques, A.O., Cutting, S.M., Eds.; Horizon Bioscience: Gurgaon, India, 2004; pp. 171–182. [Google Scholar]
- Rai, A.K.; Sanjukta, S.; Chourasia, R.; Bhat, I.; Bhardwaj, P.K.; Sahoo, D. Production of bioactive hydrolysate using protease, β-glucosidase and α-amylase of Bacillus spp. isolated from kinema. Bioresour. Technol. 2017, 235, 358–365. [Google Scholar] [CrossRef]
- Cai, D.; Rao, Y.; Zhan, Y.; Wang, Q.; Chen, S. Engineering Bacillus for efficient production of heterologous protein: Current progress, challenge and prospect. J. Appl. Microbiol. 2019, 126, 1632–1642. [Google Scholar] [CrossRef]
- De Clerck, E.; Rodriguez-Diaz, M.; Forsyth, G.; Lebbe, L.; Logan, N.A.; DeVos, P. Polyphasic characterization of Bacillus coagulans strains, illustrating heterogeneity within this species, and emended description of the species. Syst. Appl. Microbiol. 2004, 27, 50–60. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Banerjee, A.; Liu, G.H.; Thamchaipenet, A. Genome-based reclassification of Bacillus acidicola, Bacillus pervagus and the genera Heyndrickxia, Margalitia, and Weizmannia. Int. J. Syst. Evol. Microbiol. 2023, 73, 005961. [Google Scholar] [CrossRef]
- Hung, A.T.; Lin, S.-Y.; Yang, T.-Y.; Chou, C.-K.; Liu, H.-C.; Lu, J.-J.; Wang, B.; Chen, S.-Y.; Lien, T.-F. Effects of Bacillus coagulans ATCC 7050 on growth performance, intestinal morphology, and microflora composition in broiler chickens. Anim. Prod. Sci. 2012, 52, 874–879. [Google Scholar] [CrossRef]
- Gu, S.B.; Zhao, L.N.; Wu, Y.; Li, S.C.; Sun, J.R.; Huang, J.F.; Li, D.D. Potential probiotic attributes of a new strain of Bacillus coagulans CGMCC 9951 isolated from healthy piglet feces. World J. Microbiol. Biotechnol. 2015, 31, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Nyangale, E.P.; Farmer, S.; Cash, H.A.; Keller, D.; Chernoff, D.; Gibson, G.R. Bacillus coagulans GBI-30, 6086 modulates Faecalibacterium prausnitzii in older men and women. J. Nutr. 2015, 145, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shao, Y.; Song, B.; Zhen, W.; Wang, Z.; Guo, Y.; Shahid, M.S.; Nie, W. Effects of Bacillus coagulans supplementation on the growth performance and gut health of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Sci. Biotechnol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, H.; Yu, Y.; Zhang, R.; Wu, Y.; Yue, M.; Yang, C. Effects of Bacillus coagulans on growth performance, antioxidant capacity, immunity function, and gut health in broilers. Poult. Sci. 2021, 100, 101168. [Google Scholar] [CrossRef]
- Acuff, H.L.; Aldrich, C.G. Evaluation of graded levels of Bacillus coagulans GBI-30, 6086 on apparent nutrient digestibility, stool quality, and intestinal health indicators in healthy adult dogs. J. Anim. Sci. 2021, 99, skab137. [Google Scholar] [CrossRef]
- Laflamme, D.P. Development and validation of a body condition score system for dogs: A clinical tool. Canine Pract. 1997, 25, 10–15. [Google Scholar]
- AAFCO. Official Publication 2023; Association of American Feed Control Officials: Champaign, IL, USA, 2023. [Google Scholar]
- Moxham, G. The WALTHAM faeces scoring system—A tool for veterinarians and pet owners: How does your pet rate? Walth. Focus 2001, 11, 24–25. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Rockville, Maryland, 2006. [Google Scholar]
- American Association of Cereal Chemists (AACC). Approved Methods, 8th ed.; American Association of Cereal Chemists: Eagan, MN, USA, 1983. [Google Scholar]
- Budde, E.F. The Determination of Fat in Baked Biscuit Type of Dog Foods. J. Assoc. Off. Agric. Chem. 1952, 35, 799–805. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; De Vries, J.W.; Fruda, I. Determination of insoluble and soluble dietary fiber in foods and food products: Collaborative study. J. AOAC Int. 1992, 75, 360–367. [Google Scholar] [CrossRef]
- Ras, V.; Botha, G.; Aron, S.; Lennard, K.; Allali, I.; Claassen-Weitz, S.; Mwaikono, K.S.; Kennedy, D.; Holmes, J.R.; Rendon, G.; et al. Using a multiple-delivery-mode training approach to develop local capacity and infrastructure for advanced bioinformatics in Africa. PLoS Comput. Biol. 2021, 17, e1008640. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Wong, J.; Heiner, C.; Oh, S.; Theriot, C.M.; Gulati, A.S.; Mcgill, S.K.; Dougherty, M.K. High-throughput amplicon sequencing of the full-length 16S rRNA gene with single-nucleotide resolution. Nucleic Acids Res. 2019, 47, e103. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, Q.; Cole, J.R.; Rosen, G.L. Using the RDP classifier to predict taxonomic novelty and reduce the search space for finding novel organisms. PLoS ONE 2012, 7, e32491. [Google Scholar] [CrossRef]
- Wright, E.S. DECIPHER: Harnessing local sequence context to improve protein multiple sequence alignment. BMC Bioinform. 2015, 16, 322. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Li, Q.; Lauber, C.L.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S.S. Effects of the dietary protein and carbohydrate ratio on gut microbiomes in dogs of different body conditions. mBio 2017, 8. [Google Scholar] [CrossRef]
- Nery, J.; Goudez, R.; Biourge, V.; Tournier, C.; Leray, V.; Martin, L.; Thorin, C.; Nguyen, P.; Dumon, H. Influence of dietary protein content and source on colonic fermentative activity in dogs differing in body size and digestive tolerance1. J. Anim. Sci. 2012, 90, 2570–2580. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef]
- Schauf, S.; de la Fuente, G.; Newbold, C.J.; Salas-Mani, A.; Torre, C.; Abecia, L.; Castrillo, C. Effect of dietary fat to starch content on fecal microbiota composition and activity in dogs1. J. Anim. Sci. 2018, 96, 3684–3698. [Google Scholar] [CrossRef] [PubMed]
- Moinard, A.; Payen, C.; Ouguerram, K.; André, A.; Hernandez, J.; Drut, A.; Biourge, V.C.; Suchodolski, J.S.; Flanagan, J.; Nguyen, P.; et al. Effects of high-fat diet at two energetic levels on fecal microbiota, colonic barrier, and metabolic parameters in dogs. Front. Vet. Sci. 2020, 7, 566282. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Cochrane, C.-Y.; Jewell, D.E. Varying protein levels influence metabolomics and the gut microbiome in healthy adult dogs. Toxins 2020, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Portune, K.J.; Beaumont, M.; Davila, A.-M.; Tomé, D.; Blachier, F.; Sanz, Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin. Trends Food Sci. Technol. 2016, 57, 213–232. [Google Scholar] [CrossRef]
- Yamamura, R.; Inoue, K.Y.; Nishino, K.; Yamasaki, S. Intestinal and fecal pH in human health. Front. Microbiomes 2023, 2, 1192316. [Google Scholar] [CrossRef]
- Simpson, J.W. Diet and large intestinal disease in dogs and cats. J. Nutr. 1998, 128, S2717–S2722. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Enumeration of amino acid fermenting bacteria in the human large intestine: Effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol. Ecol. 1998, 25, 355–368. [Google Scholar] [CrossRef]
- Diether, N.E.; Willing, B.P. Microbial fermentation of dietary protein: An important factor in diet–microbe–host interaction. In Microorganisms; 2019; Volume 7. [Google Scholar]
- Shah, P.; Swiatlo, E. A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 2008, 68, 4–16. [Google Scholar] [CrossRef]
- Richter, J.F.; Pieper, R.; Zakrzewski, S.S.; Günzel, D.; Schulzke, J.D.; Van Kessel, A.G. Diets high in fermentable protein and fibre alter tight junction protein composition with minor effects on barrier function in piglet colon. Br. J. Nutr. 2014, 111, 1040–1049. [Google Scholar] [CrossRef]
- Llewellyn, S.R.; Britton, G.J.; Contijoch, E.J.; Vennaro, O.H.; Mortha, A.; Colombel, J.-F.; Grinspan, A.; Clemente, J.C.; Merad, M.; Faith, J.J. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 2018, 154, 1037–1046.e1032. [Google Scholar] [CrossRef]
- Shade, A. Diversity is the question, not the answer. ISME J. 2017, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.R.M. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Dowd, S.E.; Suchodolski, J.S.; Middelbos, I.S.; Vester, B.M.; Barry, K.A.; Nelson, K.E.; Torralba, M.; Henrissat, B.; Coutinho, P.M.; et al. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011, 5, 639–649. [Google Scholar] [CrossRef]
- Middelbos, I.S.; Vester Boler, B.M.; Qu, A.; White, B.A.; Swanson, K.S.; Fahey, G.C. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS ONE 2010, 5, e9768. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Liu, H.; Brown, M.A.; Qiao, S. Dietary protein and gut microbiota composition and function. Curr. Protein Pept. Sci. 2019, 20, 145–154. [Google Scholar] [CrossRef]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key bacterial families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) are related to the digestion of protein and energy in dogs. PeerJ 2017, 5, e3019. [Google Scholar] [CrossRef]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The fecal microbiome and metabolome differs between dogs fed bones and raw food (BARF) diets and dogs fed commercial diets. PLoS ONE 2018, 13, e0201279. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee Ying, S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.M.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef]
- Giaretta, P.R.; Rech, R.R.; Guard, B.C.; Blake, A.B.; Blick, A.K.; Steiner, J.M.; Lidbury, J.A.; Cook, A.K.; Hanifeh, M.; Spillmann, T.; et al. Comparison of intestinal expression of the apical sodium-dependent bile acid transporter between dogs with and without chronic inflammatory enteropathy. J. Vet. Intern. Med. 2018, 32, 1918–1926. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S rRNA gene pyrosequencing reveals bacterial dysbiosis in the duodenum of dogs with idiopathic inflammatory bowel disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; Alshawaqfeh, M.K.; et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef]
- Lebas, M.; Garault, P.; Carrillo, D.; Codoñer, F.M.; Derrien, M. Metabolic Response of Faecalibacterium prausnitzii to Cell-Free Supernatants from Lactic Acid Bacteria. Microorganisms 2020, 8, 1528. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Y.; Imre, K.; Arslan-Acaroz, D.; Istanbullugil, F.R.; Fang, Y.; Ros, G.; Zhu, K.; Acaroz, U. Mechanisms of Probiotic Bacillus against Enteric Bacterial Infections. One Health Adv. 2023, 1, 21. [Google Scholar] [CrossRef]
- Kaga, C.; Kakiyama, S.; Hokkyo, A.; Ogata, Y.; Shibata, J.; Nagahara, T.; Nakazawa, M.; Nakagawa, T.; Tsujimoto, H.; Chambers, J.K.; et al. Characterization of faecal microbiota and serum inflammatory markers in dogs diagnosed with chronic enteropathy or small-cell lymphoma: A pilot study. Sci. Rep. 2024, 14, 19387. [Google Scholar] [CrossRef] [PubMed]
- Endres, J.R.; Clewell, A.; Jade, K.A.; Farber, T.; Hauswirth, J.; Schauss, A.G. Safety Assessment of a Proprietary Preparation of a Novel Probiotic, Bacillus coagulans, as a Food Ingredient. Food Chem. Toxicol. 2009, 47, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Nascarella, M.; Pressman, P.; Hayes, A.W.; Dhawan, G.; Kapoor, R.; Calabrese, V.; Agathokleous, E. Hormesis Determines Lifespan. Ageing Res. Rev. 2024, 94, 102181. [Google Scholar] [CrossRef]
- Spinosa, M.R.; Braccini, T.; Ricca, E.; De Felice, M.; Morelli, L.; Pozzi, G.; Oggioni, M.R. On the fate of ingested Bacillus spores. Res. Microbiol. 2000, 151, 361–368. [Google Scholar] [CrossRef]
- Fakhry, S.; Sorrentini, I.; Ricca, E.; De Felice, M.; Baccigalupi, L. Characterization of spore forming Bacilli isolated from the human gastrointestinal tract. J. Appl. Microbiol. 2008, 105, 2178–2186. [Google Scholar] [CrossRef]
- Casula, G.; Cutting, S.M. Bacillus probiotics: Spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 2002, 68, 2344–2352. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Moir, A.; Smith, D.A. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 1990, 44, 531–553. [Google Scholar] [CrossRef]
- Setlow, P. Spore germination. Curr. Opin. Microbiol. 2003, 6, 550–556. [Google Scholar] [CrossRef]
- Jadamus, A.; Vahjen, W.; Simon, O. Growth behaviour of a spore forming probiotic strain in the gastrointestinal tract of broiler chicken and piglets. Arch. Tierernahr. 2001, 54, 1–17. [Google Scholar] [CrossRef]
- Tam, N.K.M.; Uyen, N.Q.; Hong, H.A.; Duc, L.H.; Hoa, T.T.; Serra, C.R.; Henriques, A.O.; Cutting, S.M. The intestinal life cycle of Bacillus subtilis and close relatives. J. Bacteriol. 2006, 188, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Colom, J.; Freitas, D.; Simon, A.; Brodkorb, A.; Buckley, M.; Deaton, J.; Winger, A.M. Presence and germination of the probiotic Bacillus subtilis DE111 in the human small intestinal tract: A randomized, crossover, double-blind, and placebo-controlled study. Front. Microbiol. 2021, 12, 715863. [Google Scholar] [CrossRef] [PubMed]
- Sonenshein, A.L. Control of sporulation initiation in Bacillus subtilis. Curr. Opin. Microbiol. 2000, 3, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Piggot, P.J.; Hilbert, D.W. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 2004, 7, 579–586. [Google Scholar] [CrossRef]
- Hill, R.C.; Ellison, G.W.; Burrows, C.F.; Bauer, J.E.; Carbia, B. Ileal cannulation and associated complications in dogs. Lab. Anim. Sci. 1996, 46, 77–80. [Google Scholar]
- Mawby, D.I.; Mathew, A.G.; Mears, E.A.; Moyers, T.D.; Krahwinkel, D.J. Complications of ileal cannulation in cats. Lab. Anim. Sci. 1999, 49, 406–410. [Google Scholar]
- Bezkorovainy, A. Probiotics: Determinants of survival and growth in the gut. Am. J. Clin. Nutr. 2001, 73, 399s–405s. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic gastrointestinal transit and colonization after oral administration: A long journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Jones, J.; Reinke, S.N.; Ali, A.; Palmer, D.J.; Christophersen, C.T. Fecal sample collection methods and time of day impact microbiome composition and short chain fatty acid concentrations. Sci. Rep. 2021, 11, 13964. [Google Scholar] [CrossRef]
| Analyzed Composition | Kibble Diet 1 | Canned Diet 2 |
|---|---|---|
| Dry matter (DM), % | 94.05 | 23.15 |
| -- %, DM -- | ||
| Ash | 8.06 | 10.37 |
| Crude protein | 22.47 | 42.07 |
| Acid-hydrolyzed fat | 16.07 | 29.72 |
| Total dietary fiber | 18.08 | 16.10 |
| Insoluble fiber | 15.58 | 11.82 |
| Soluble fiber | 2.50 | 4.28 |
| Gross energy, kcal/g as-is | 4.71 | 1.35 |
| Gross energy, kcal/g DM | 5.01 | 5.83 |
| Calculated ME, kcal/g 3 as-is | 3.19 | 0.94 |
| Calculated ME, kcal/g 3 DM | 3.39 | 4.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, S.M.; Kang, Y.; Wren, J.F.; Menton, J.F.; Vinay, E.; Millette, M.; Kelly, M.R.; Swanson, K.S. Effects of Bacillus coagulans (GBI-30, 6086) Supplementation on the Fecal Characteristics and Microbiota of Healthy Adult Dogs Subjected to an Abrupt Diet Change. Microorganisms 2025, 13, 2462. https://doi.org/10.3390/microorganisms13112462
Wilson SM, Kang Y, Wren JF, Menton JF, Vinay E, Millette M, Kelly MR, Swanson KS. Effects of Bacillus coagulans (GBI-30, 6086) Supplementation on the Fecal Characteristics and Microbiota of Healthy Adult Dogs Subjected to an Abrupt Diet Change. Microorganisms. 2025; 13(11):2462. https://doi.org/10.3390/microorganisms13112462
Chicago/Turabian StyleWilson, Sofia M., Yifei Kang, Jocelyn F. Wren, John F. Menton, Elena Vinay, Mathieu Millette, Melissa R. Kelly, and Kelly S. Swanson. 2025. "Effects of Bacillus coagulans (GBI-30, 6086) Supplementation on the Fecal Characteristics and Microbiota of Healthy Adult Dogs Subjected to an Abrupt Diet Change" Microorganisms 13, no. 11: 2462. https://doi.org/10.3390/microorganisms13112462
APA StyleWilson, S. M., Kang, Y., Wren, J. F., Menton, J. F., Vinay, E., Millette, M., Kelly, M. R., & Swanson, K. S. (2025). Effects of Bacillus coagulans (GBI-30, 6086) Supplementation on the Fecal Characteristics and Microbiota of Healthy Adult Dogs Subjected to an Abrupt Diet Change. Microorganisms, 13(11), 2462. https://doi.org/10.3390/microorganisms13112462







