Organic and Inorganic Phosphorus Inputs Shape Wheat Productivity and Soil Bioavailability: A Microbial and Enzymatic Perspective from Long-Term Field Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Design
2.2. Soil Sampling and Analysis
2.3. Measurement of Soil Bioavailable Phosphorus Components
2.4. Analysis of Soil Extracellular Enzyme Activities
2.5. Vector Analysis of Soil Extracellular Enzyme Activities
2.6. Soil DNA Extraction, High-Throughput Sequencing, and Co-Occurrence Analyses
2.7. Statistical Analysis
3. Results
3.1. Soil Chemical Properties and Microbial Biomass
3.2. Variation in Crop Yield
3.3. Soil P Fractions
3.4. Soil Extracellular Enzyme Activities, Stoichiometry, and Vector Characteristics
3.5. Soil Microbial Community and Associations with Soil Properties
3.6. Microbial Co-Occurrence Networks
3.7. Effects of Soil Properties and Microbial Community Structure on Crop Yield
4. Discussion
4.1. Effects of Organic and Inorganic P Sources on Soil P Fractions and Wheat Yield
4.2. Microbial and Enzymatic Mediation of Phosphorus Turnover Under Fertilization
4.3. Integrated Effects of P Fractions, Enzyme Activities, and Microbial Communities on Wheat Yield
4.4. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.S.A.; Abbott, L.K.; Solaiman, Z.M.; Mawson, P.R.; Waite, I.S.; Jenkins, S.N. Complementary Effect of Zoo Compost with Mineral Nitrogen Fertilisation Increases Wheat Yield and Nutrition in a Low-Nutrient Soil. Pedosphere 2022, 32, 339–347. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, K.L.; Zhao, X.Q.; Zhang, H.Q.; Li, D.; Li, J.J.; Shen, R.F. Balanced Fertilization over Four Decades Has Sustained Soil Microbial Communities and Improved Soil Fertility and Rice Productivity in Red Paddy Soil. Sci. Total Environ. 2021, 793, 148664. [Google Scholar] [CrossRef]
- Qu, X.Y.; Yao, W.; Ji, H.J.; Xu, Y.; Jia, R.; Chen, X.J.; Li, H.J.; Sánchez-Rodríguez, A.R.; Shen, Y.J.; Yang, Y.D.; et al. Optimizing Nitrogen Fertilization and Irrigation Strategies to Balance Agroecosystem Services in the Wheat-Maize Double Cropping System: A 21-Year Field Study. Field Crops Res. 2025, 322, 109706. [Google Scholar] [CrossRef]
- Khan, A.; Yang, X.; Sun, B.; Zhang, S.; He, B. Responses of Crop and Soil Phosphorus Fractions to Long-Term Fertilization Regimes in a Loess Soil in Northwest China. Agronomy 2023, 13, 3072. [Google Scholar] [CrossRef]
- Turner, B.L.; Lambers, H.; Condron, L.M.; Cramer, M.D.; Leake, J.R.; Richardson, A.E.; Smith, S.E. Soil Microbial Biomass and the Fate of Phosphorus during Long-Term Ecosystem Development. Plant Soil 2013, 367, 225–234. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Wang, Z.; Long, A.; Ji, X.; Gong, X.; Jiang, Y.; Qi, H. Straw Return Increased Maize Phosphorus Uptake and Grain Yield by Alleviating Rhizosphere Soil Microbial Metabolism Limitation: Insights from Ecoenzymatic Stoichiometry. Plant Soil 2025. [Google Scholar] [CrossRef]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; Van Der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-Induced Nitrogen–Phosphorus Imbalances Alter Natural and Managed Ecosystems across the Globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef]
- Averill, C.; Waring, B. Nitrogen Limitation of Decomposition and Decay: How Can It Occur? Glob. Change Biol. 2018, 24, 1417–1427. [Google Scholar] [CrossRef]
- Li, S.L.; Cui, Y.X.; Moorhead, D.L.; Dijkstra, F.A.; Sun, L.F.; Xia, Z.Q.; Gao, Y.; Ma, Q.; Yu, W.T. Phosphorus Limitation Regulates the Responses of Microbial Carbon Metabolism to Long-Term Combined Additions of Nitrogen and Phosphorus in a Cropland. Soil Biol. Biochem. 2025, 200, 109614. [Google Scholar] [CrossRef]
- Schneider, T.; Keiblinger, K.M.; Schmid, E.; Sterflinger-Gleixner, K.; Ellersdorfer, G.; Roschitzki, B.; Richter, A.; Eberl, L.; Zechmeister-Boltenstern, S.; Riedel, K. Who Is Who in Litter Decomposition? Metaproteomics Reveals Major Microbial Players and Their Biogeochemical Functions. ISME J. 2012, 6, 1749–1762. [Google Scholar] [CrossRef]
- Barrow, N.J. Evaluation and Utilization of Residual Phosphorus in Soils. In The Role of Phosphorus in Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1980; pp. 333–359. ISBN 978-0-89118-249-8. [Google Scholar]
- Feng, G.; Gai, J.P.; Feng, X.H.; Li, H.G.; Zhang, L.; Yi, K.K.; Lv, J.; Zhu, Y.Y.; Tang, L.; Li, Y.L. Strategies for Improving Fertilizer Phosphorus Use Efficiency in Chinese Cropping Systems. Front. Agric. Sci. Eng. 2019, 6, 341–347. [Google Scholar] [CrossRef]
- Sattari, S.Z.; Bouwman, A.F.; Giller, K.E.; van Ittersum, M.K. Residual Soil Phosphorus as the Missing Piece in the Global Phosphorus Crisis Puzzle. Proc. Natl. Acad. Sci. USA 2012, 109, 6348–6353. [Google Scholar] [CrossRef]
- Yahaya, S.M.; Mahmud, A.A.; Abdullahi, M.; Haruna, A. Recent Advances in the Chemistry of Nitrogen, Phosphorus and Potassium as Fertilizers in Soil: A Review. Pedosphere 2023, 33, 385–406. Available online: https://www.sciencedirect.com/science/article/pii/S1002016024001139 (accessed on 1 September 2025).
- Zheng, X.Q.; Wei, L.; Lv, W.; Zhang, H.Q.; Zhang, Y.; Zhang, H.Y.; Zhang, H.L.; Zhu, Z.K.; Ge, T.D.; Zhang, W.J. Long-Term Bioorganic and Organic Fertilization Improved Soil Quality and Multifunctionality under Continuous Cropping in Watermelon. Agric. Ecosyst. Environ. 2024, 359, 108721. [Google Scholar] [CrossRef]
- Krause, H.-M.; Mueller, R.C.; Lori, M.; Mayer, J.; Mäder, P.; Hartmann, M. Organic Cropping Systems Alter Metabolic Potential and Carbon, Nitrogen and Phosphorus Cycling Capacity of Soil Microbial Communities. Soil Biol. Biochem. 2025, 203, 109737. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Zhang, X.; Liu, X.; Liu, P.; Chen, L. Enzymatic Stoichiometry Reveals the Metabolic Limitations of Soil Microbes under Nitrogen and Phosphorus Addition in Chinese Fir Plantations. Microorganisms 2024, 12, 1716. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, T.Y.; Peñuelas, J.; Sardans, J.; Tan, W.F.; Wei, X.M.; Cui, Y.X.; Cui, Q.L.; Wu, C.F.; Liu, L.F.; et al. Crop Residue Return Sustains Global Soil Ecological Stoichiometry Balance. Glob. Change Biol. 2023, 29, 2203–2226. [Google Scholar] [CrossRef] [PubMed]
- Nevins, C.J.; Lacey, C.; Armstrong, S. The Synchrony of Cover Crop Decomposition, Enzyme Activity, and Nitrogen Availability in a Corn Agroecosystem in the Midwest United States. Soil Tillage Res. 2020, 197, 104518. [Google Scholar] [CrossRef]
- Doménech-Pascual, A.; Rodriguez, L.C.; Han, X.; Casas-Ruiz, J.P.; Ferriol-Ciurana, J.; Donhauser, J.; Jordaan, K.; Allison, S.D.; Frossard, A.; Priemé, A.; et al. Soil Functions Are Shaped by Aridity through Soil Properties and the Microbial Community Structure. Appl. Soil Ecol. 2025, 213, 106313. [Google Scholar] [CrossRef]
- Li, P.F.; Liu, M.; Ma, X.Y.; Wu, M.; Jiang, C.Y.; Liu, K.; Liu, J.; Li, Z.P. Responses of Microbial Communities to a Gradient of Pig Manure Amendment in Red Paddy Soils. Sci. Total Environ. 2020, 705, 135884. [Google Scholar] [CrossRef]
- Yu, Y.J.; Wu, M.; Petropoulos, E.; Zhang, J.W.; Nie, J.; Liao, Y.L.; Li, Z.P.; Lin, X.G.; Feng, Y.Z. Responses of Paddy Soil Bacterial Community Assembly to Different Long-Term Fertilizations in Southeast China. Sci. Total Environ. 2019, 656, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Shahid, M.; Tripathi, R.; Mohanty, S.; Kumar, A.; Bhattacharyya, P.; Lal, B.; Gautam, P.; Raja, R.; Panda, B.B.; et al. Variation of Functional Diversity of Soil Microbial Community in Sub-Humid Tropical Rice-Rice Cropping System under Long-Term Organic and Inorganic Fertilization. Ecol. Indic. 2017, 73, 536–543. [Google Scholar] [CrossRef]
- Wang, X.S.; Wang, X.N.; Wu, F.; Zhang, J.W.; Ai, S.H.; Liu, Z.T. Microbial Community Composition and Degradation Potential of Petroleum-Contaminated Sites under Heavy Metal Stress. J. Hazard. Mater. 2023, 457, 131814. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial Interactions: From Networks to Models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Cui, Y.X.; Fang, L.C.; Guo, X.B.; Wang, X.; Zhang, Y.J.; Li, P.F.; Zhang, X.C. Ecoenzymatic Stoichiometry and Microbial Nutrient Limitation in Rhizosphere Soil in the Arid Area of the Northern Loess Plateau, China. Soil Biol. Biochem. 2018, 116, 11–21. [Google Scholar] [CrossRef]
- Tang, S.; Ma, Q.X.; Marsden, K.A.; Chadwick, D.R.; Luo, Y.; Kuzyakov, Y.; Wu, L.H.; Jones, D.L. Microbial Community Succession in Soil Is Mainly Driven by Carbon and Nitrogen Contents Rather than Phosphorus and Sulphur Contents. Soil Biol. Biochem. 2023, 180, 109019. [Google Scholar] [CrossRef]
- Gu, S.S.; Hu, Q.L.; Cheng, Y.Q.; Bai, L.Y.; Liu, Z.H.; Xiao, W.J.; Gong, Z.H.; Wu, Y.N.; Feng, K.; Deng, Y.; et al. Application of Organic Fertilizer Improves Microbial Community Diversity and Alters Microbial Network Structure in Tea (Camellia sinensis) Plantation Soils. Soil Tillage Res. 2019, 195, 104356. [Google Scholar] [CrossRef]
- Alleman, A.B.; Mohammed, Y.A.; McVay, K.A.; Khan, Q.A.; Carr, P.; Miller, J.; Miller, Z.; Torrion, J.; Lamb, P.; Mus, F.; et al. Drivers of Diazotroph Community Structure and Co-Occurrence in a Northern Great Plains Pulse Crop Rotation System. Appl. Soil Ecol. 2021, 157, 103737. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of Microbial Biomass Phosphorus in Soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Glanville, H.C.; Harris, M.; Emmett, B.A.; Pingree, M.R.A.; de Sosa, L.L.; Cerdá-Moreno, C.; Jones, D.L. A Novel Biologically-Based Approach to Evaluating Soil Phosphorus Availability across Complex Landscapes. Soil Biol. Biochem. 2015, 88, 110–119. [Google Scholar] [CrossRef]
- Wu, H.; Xiang, W.; Chen, L.; Ouyang, S.; Xiao, W.; Li, S.; Forrester, D.I.; Lei, P.; Zeng, Y.; Deng, X.; et al. Soil Phosphorus Bioavailability and Recycling Increased with Stand Age in Chinese Fir Plantations. Ecosystems 2020, 23, 973–988. [Google Scholar] [CrossRef]
- Marx, M.-C.; Wood, M.; Jarvis, S.C. A Microplate Fluorimetric Assay for the Study of Enzyme Diversity in Soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The Effects of Long Term Nitrogen Deposition on Extracellular Enzyme Activity in an Acer Saccharum Forest Soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector Analysis of Ecoenzyme Activities Reveal Constraints on Coupled C, N and P Dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Hu, X.-F.; Wan, S.; Wang, H.; Liang, C.; Chen, F.-S. Litter Manipulation Effects on Microbial Communities and Enzymatic Activities Vary with Soil Depth in a Subtropical Chinese Fir Plantation. For. Ecol. Manag. 2021, 480, 118641. [Google Scholar] [CrossRef]
- Adams, R.I.; Miletto, M.; Taylor, J.W.; Bruns, T.D. Dispersal in Microbes: Fungi in Indoor Air Are Dominated by Outdoor Air and Show Dispersal Limitation at Short Distances. ISME J. 2013, 7, 1262–1273, Erratum in ISME J. 2013, 7, 1460. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.B.; Chen, C.; Zhang, J.X.; Dai, Y.; Zhang, X.J.; Xie, S.G. Operational Performance, Biomass and Microbial Community Structure: Impacts of Backwashing on Drinking Water Biofilter. Environ. Sci. Pollut. Res. 2015, 22, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xia, H.; Jiang, C.C.; Riaz, M.; Yang, L.; Chen, Y.F.; Fan, X.P.; Xia, X.G. 14 Year Applications of Chemical Fertilizers and Crop Straw Effects on Soil Labile Organic Carbon Fractions, Enzyme Activities and Microbial Community in Rice-Wheat Rotation of Middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-Bacterial Diversity and Microbiome Complexity Predict Ecosystem Functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Shetty, S.A.; Hugenholtz, F.; Lahti, L.; Smidt, H.; de Vos, W.M. Intestinal Microbiome Landscaping: Insight in Community Assemblage and Implications for Microbial Modulation Strategies. FEMS Microbiol. Rev. 2017, 41, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Z.Y.; Liu, W.H.; Li, H.F.; Wang, Z.H.; Liu, J.S. Phosphorus Fertilizer Input Threshold Shifts Bacterial Community Structure and Soil Multifunctionality to Maintain Dryland Wheat Production. Soil Tillage Res. 2024, 243, 106174. [Google Scholar] [CrossRef]
- Singh, J.; Singh, G.; Gupta, N. Balancing Phosphorus Fertilization for Sustainable Maize Yield and Soil Test Phosphorus Management: A Long-Term Study Using Machine Learning. Field Crops Res. 2023, 304, 109169. [Google Scholar] [CrossRef]
- Chen, G.L.; Xiao, L.; Yue, K.; Wang, Y.; Wang, S.Q.; Zhu, Y.Y.; Kai, L. Optimizing Phosphate Application to Improve Soil Quality and Reduce Phosphorus Loss in Rice-Wheat Rotation. Agric. Ecosyst. Environ. 2025, 378, 109310. [Google Scholar] [CrossRef]
- van der Bom, F.J.T.; Williams, A.; Borrell, A.K.; Raymond, N.; Bell, M.J. Phosphorus Management Is Key to Effective Deployment of Root Ideotypes in Complex Soil Environments. Plant Soil 2023, 489, 323–340. [Google Scholar] [CrossRef]
- Spohn, M.; Kuzyakov, Y. Phosphorus Mineralization Can Be Driven by Microbial Need for Carbon. Soil Biol. Biochem. 2013, 61, 69–75. [Google Scholar] [CrossRef]
- Crain, G.M.; McLaren, J.R.; Brunner, B.; Darrouzet-Nardi, A. Biologically Available Phosphorus in Biocrust-Dominated Soils of the Chihuahuan Desert. Soil Syst. 2018, 2, 56. [Google Scholar] [CrossRef]
- Li, J.Q.; Marschner, P. Phosphorus Pools and Plant Uptake in Manure-Amended Soil. J. Soil Sci. Plant Nutr. 2019, 19, 175–186. [Google Scholar] [CrossRef]
- Ablimit, R.; Li, W.; Zhang, J.; Gao, H.; Zhao, Y.; Cheng, M.; Meng, X.; An, L.; Chen, Y. Altering Microbial Community for Improving Soil Properties and Agricultural Sustainability during a 10-Year Maize-Green Manure Intercropping in Northwest China. J. Environ. Manag. 2022, 321, 115859. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Ju, W.L.; Moorhead, D.L.; Shen, G.T.; Cui, Y.X.; Fang, L.C. Soil Aggregate Development and Associated Microbial Metabolic Limitations Alter Grassland Carbon Storage Following Livestock Removal. Soil Biol. Biochem. 2023, 177, 108907. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Sun, C.X.; Chen, Z.H.; Zhang, G.N.; Chen, L.J.; Wu, Z.J. Stoichiometric Analyses of Soil Nutrients and Enzymes in a Cambisol Soil Treated with Inorganic Fertilizers or Manures for 26 years. Geoderma 2019, 353, 382–390. [Google Scholar] [CrossRef]
- Kumari, A.; Kapoor, K.K.; Kundu, B.S.; Mehta, R.K. Identification of Organic Acids Produced during Rice Straw Decomposition and Their Role in Rock Phosphate Solubilization. Plant Soil Environ. 2008, 54, 72–77. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Rodrigues, M.; Coelho, M.J.A.; Gasperini, A.M.; Sartor, L.R.; Pavinato, P.S. Changes in Soil Phosphorus Lability Promoted by Phosphate Sources and Cover Crops. Soil Tillage Res. 2018, 179, 20–28. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.-M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of Nitrogen and Phosphorus Addition on Microbial Community Composition and Element Cycling in a Grassland Soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Zhu, Z.K.; Ge, T.D.; Luo, Y.; Liu, S.L.; Xu, X.L.; Tong, C.L.; Shibistova, O.; Guggenberger, G.; Wu, J.S. Microbial Stoichiometric Flexibility Regulates Rice Straw Mineralization and Its Priming Effect in Paddy Soil. Soil Biol. Biochem. 2018, 121, 67–76. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, K.L.; Zhao, X.Q.; Gao, G.-F.; Wu, Y.H.; Shen, R.F. Microbial Keystone Taxa Drive Crop Productivity through Shifting Aboveground-Belowground Mineral Element Flows. Sci. Total Environ. 2022, 811, 152342. [Google Scholar] [CrossRef]
- van der Bom, F.; Nunes, I.; Raymond, N.S.; Hansen, V.; Bonnichsen, L.; Magid, J.; Nybroe, O.; Jensen, L.S. Long-Term Fertilisation Form, Level and Duration Affect the Diversity, Structure and Functioning of Soil Microbial Communities in the Field. Soil Biol. Biochem. 2018, 122, 91–103. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone Taxa as Drivers of Microbiome Structure and Functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative Metagenomic, Phylogenetic and Physiological Analyses of Soil Microbial Communities across Nitrogen Gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guan, D.W.; Zhou, B.K.; Zhao, B.S.; Ma, M.C.; Qin, J.; Jiang, X.; Chen, S.F.; Cao, F.M.; Shen, D.L.; et al. Influence of 34-Years of Fertilization on Bacterial Communities in an Intensively Cultivated Black Soil in Northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Guo, Z.B.; Wan, S.X.; Hua, K.K.; Yin, Y.; Chu, H.Y.; Wang, D.Z.; Guo, X.S. Fertilization Regime Has a Greater Effect on Soil Microbial Community Structure than Crop Rotation and Growth Stage in an Agroecosystem. Appl. Soil Ecol. 2020, 149, 103510. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.; Ma, M.; Guan, D.; Li, J.; Chen, S.; Cao, F.; Shen, D.; et al. Thirty Four Years of Nitrogen Fertilization Decreases Fungal Diversity and Alters Fungal Community Composition in Black Soil in Northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Keystone Taxa Predict Compositional Change in Microbial Communities. Environ. Microbiol. 2018, 20, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.P.; Yang, T.J.; Bao, Y.Z.; He, P.P.; Yang, K.M.; Mei, X.L.; Wei, Z.; Xu, Y.C.; Shen, Q.R.; Banerjee, S. Network Analysis and Subsequent Culturing Reveal Keystone Taxa Involved in Microbial Litter Decomposition Dynamics. Soil Biol. Biochem. 2021, 157, 108230. [Google Scholar] [CrossRef]
- Banerjee, S.; Kirkby, C.A.; Schmutter, D.; Bissett, A.; Kirkegaard, J.A.; Richardson, A.E. Network Analysis Reveals Functional Redundancy and Keystone Taxa amongst Bacterial and Fungal Communities during Organic Matter Decomposition in an Arable Soil. Soil Biol. Biochem. 2016, 97, 188–198. [Google Scholar] [CrossRef]
- Zhong, Y.Q.W.; Hu, J.H.; Xia, Q.M.; Zhang, S.L.; Li, X.; Pan, X.Y.; Zhao, R.P.; Wang, R.W.; Yan, W.M.; Shangguan, Z.P.; et al. Soil Microbial Mechanisms Promoting Ultrahigh Rice Yield. Soil Biol. Biochem. 2020, 143, 107741, Erratum in Soil Biol. Biochem. 2020, 150, 107894. [Google Scholar] [CrossRef]
- Alamgir, M.; McNeill, A.; Tang, C.; Marschner, P. Changes in Soil P Pools during Legume Residue Decomposition. Soil Biol. Biochem. 2012, 49, 70–77. [Google Scholar] [CrossRef]
- Wu, Q.C.; Chen, Y.; Dou, X.H.; Liao, D.X.; Li, K.Y.; An, C.C.; Li, G.H.; Dong, Z. Microbial Fertilizers Improve Soil Quality and Crop Yield in Coastal Saline Soils by Regulating Soil Bacterial and Fungal Community Structure. Sci. Total Environ. 2024, 949, 175127. [Google Scholar] [CrossRef]
- Bogati, K.A.; Sewerniak, P.; Walczak, M. Unraveling the Effect of Soil Moisture on Microbial Diversity and Enzymatic Activity in Agricultural Soils. Microorganisms 2025, 13, 1245. [Google Scholar] [CrossRef]
- Ren, J.H.; Liu, X.L.; Yang, W.P.; Yang, X.X.; Li, W.G.; Xia, Q.; Li, J.H.; Gao, Z.Q.; Yang, Z.P. Rhizosphere Soil Properties, Microbial Community, and Enzyme Activities: Short-Term Responses to Partial Substitution of Chemical Fertilizer with Organic Manure. J. Environ. Manag. 2021, 299, 113650. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil Beneficial Bacteria and Their Role in Plant Growth Promotion: A Review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Leininger, S.; Urich, T.; Schloter, M.; Schwark, L.; Qi, J.; Nicol, G.W.; Prosser, J.I.; Schuster, S.C.; Schleper, C. Archaea Predominate among Ammonia-Oxidizing Prokaryotes in Soils. Nature 2006, 442, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.K.; Delgado-Baquerizo, M.; Guo, X.S.; Wang, D.Z.; Zhu, Y.; Chu, H.Y. Biodiversity of Key-Stone Phylotypes Determines Crop Production in a 4-Decade Fertilization Experiment. ISME J. 2021, 15, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, D.; Sale, P.; Tang, C. Effect of Soil Phosphorus Availability and Residue Quality on Phosphorus Transfer from Crop Residues to the Following Wheat. Plant Soil 2017, 416, 361–375. [Google Scholar] [CrossRef]
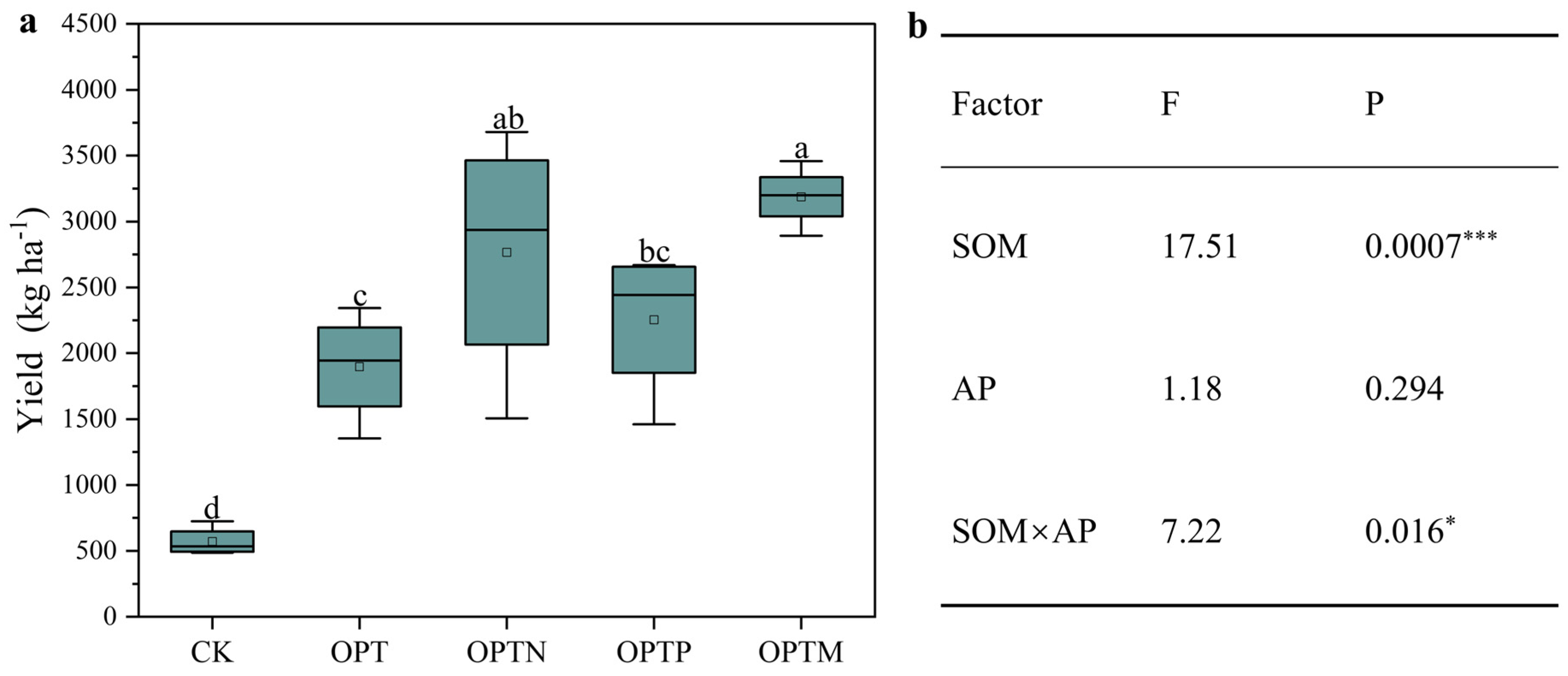
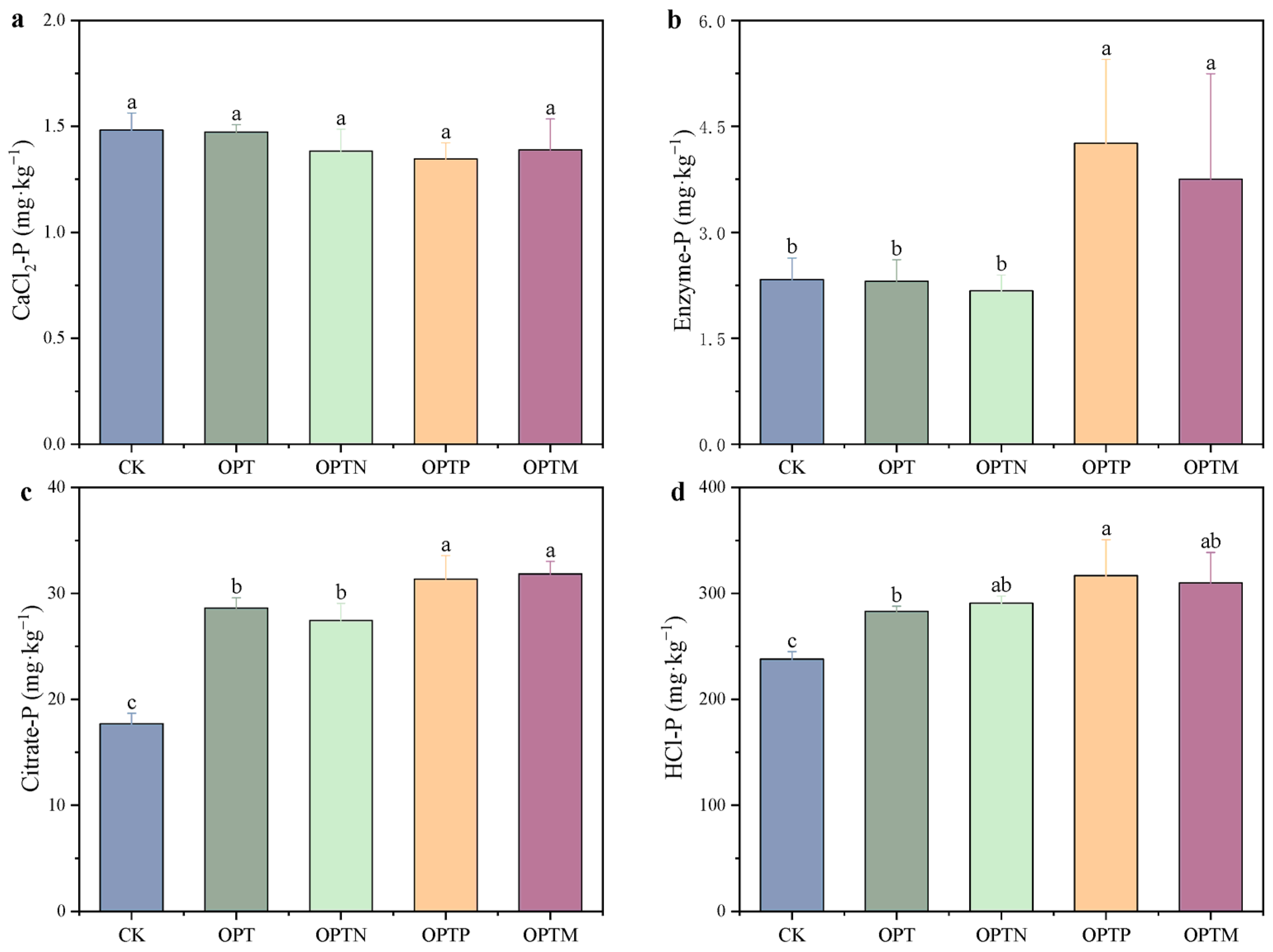
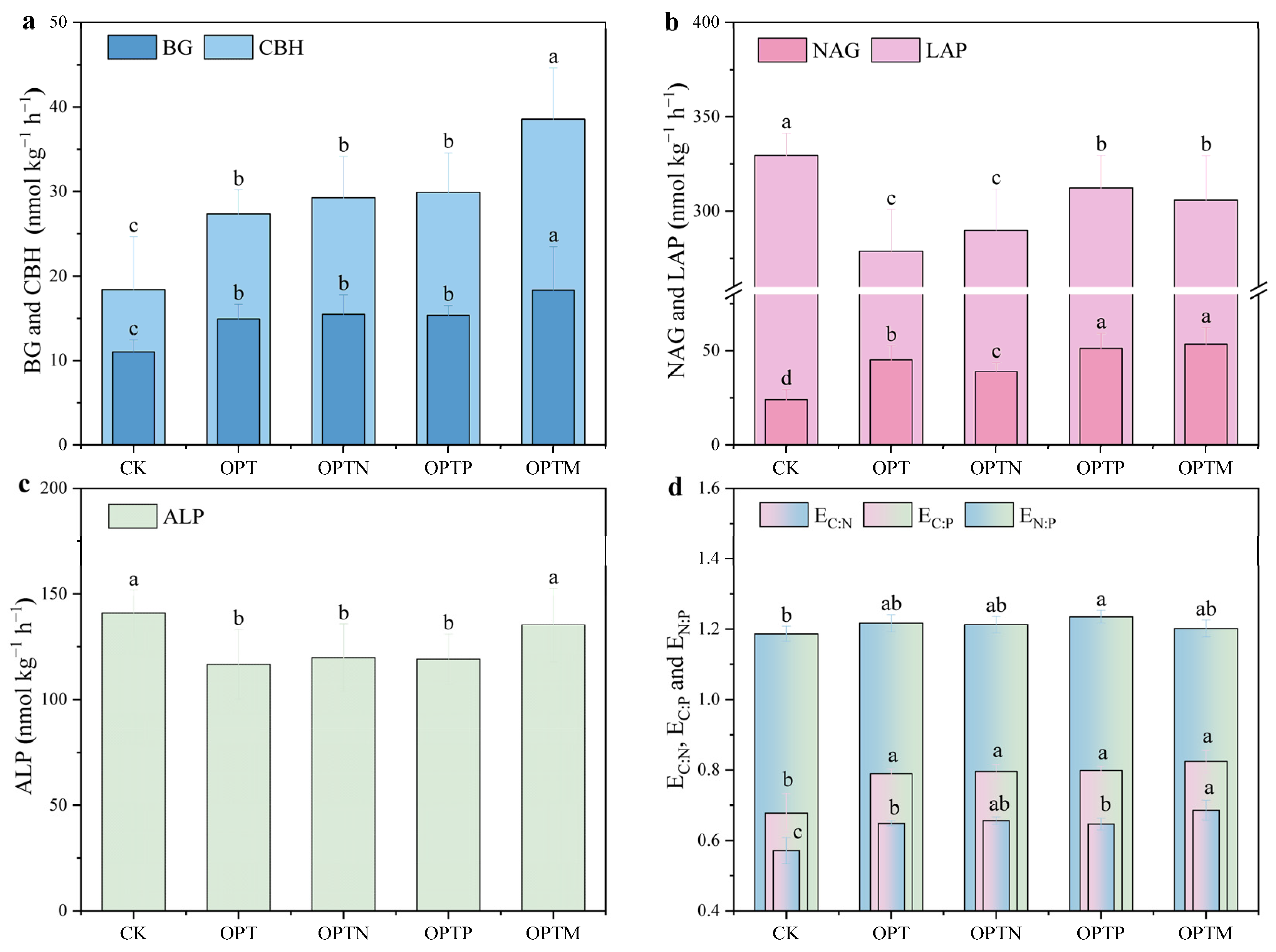
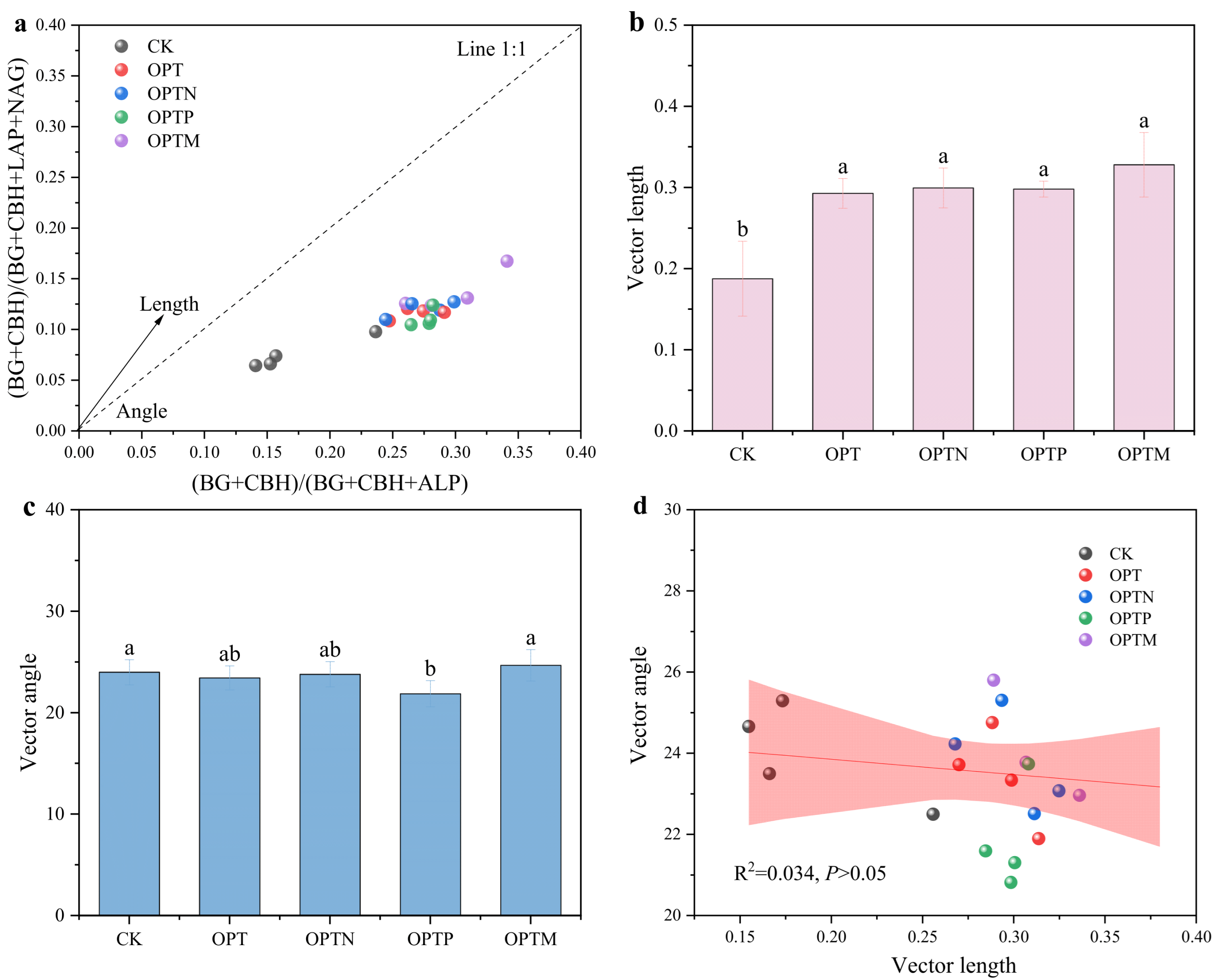
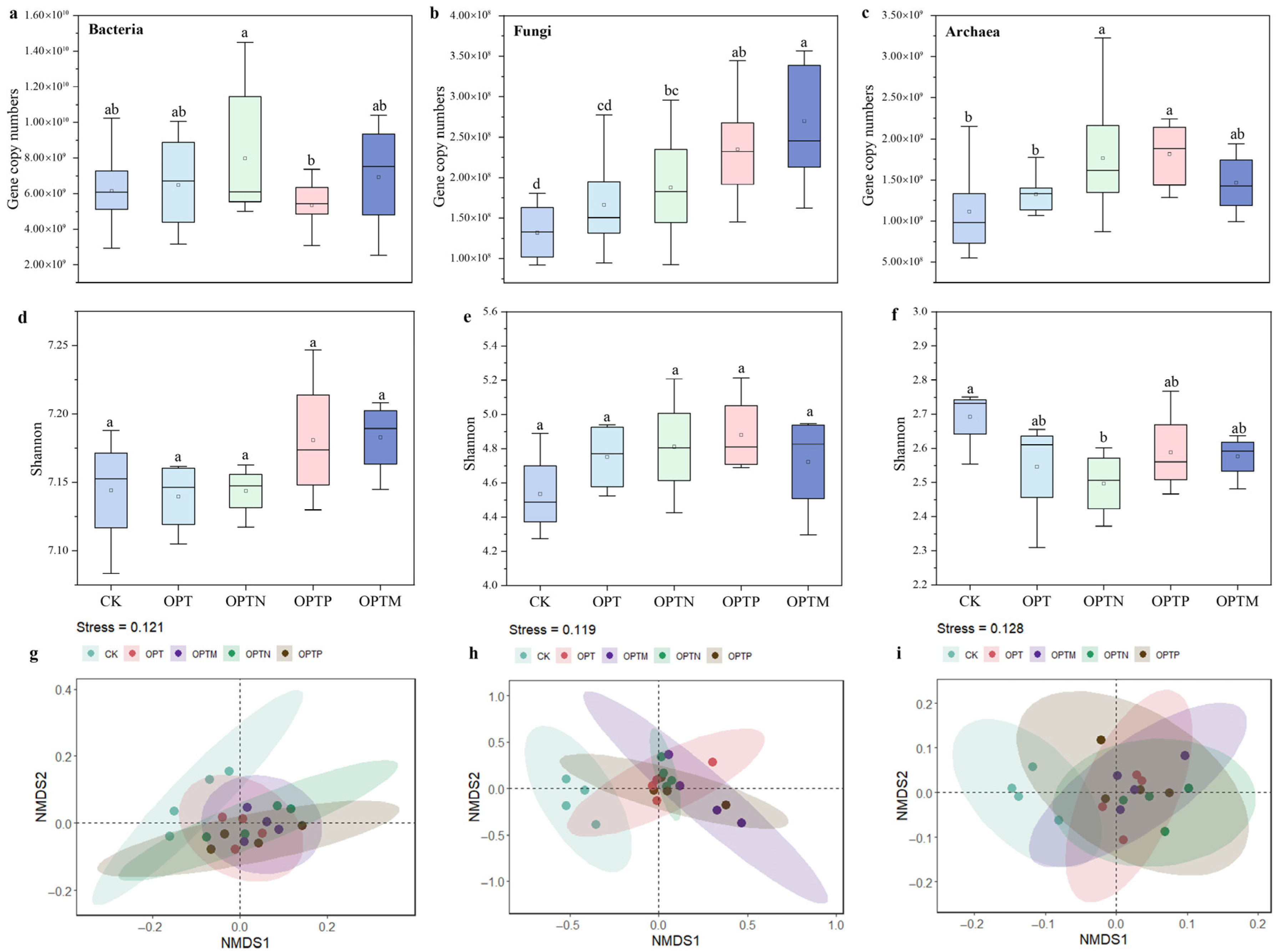
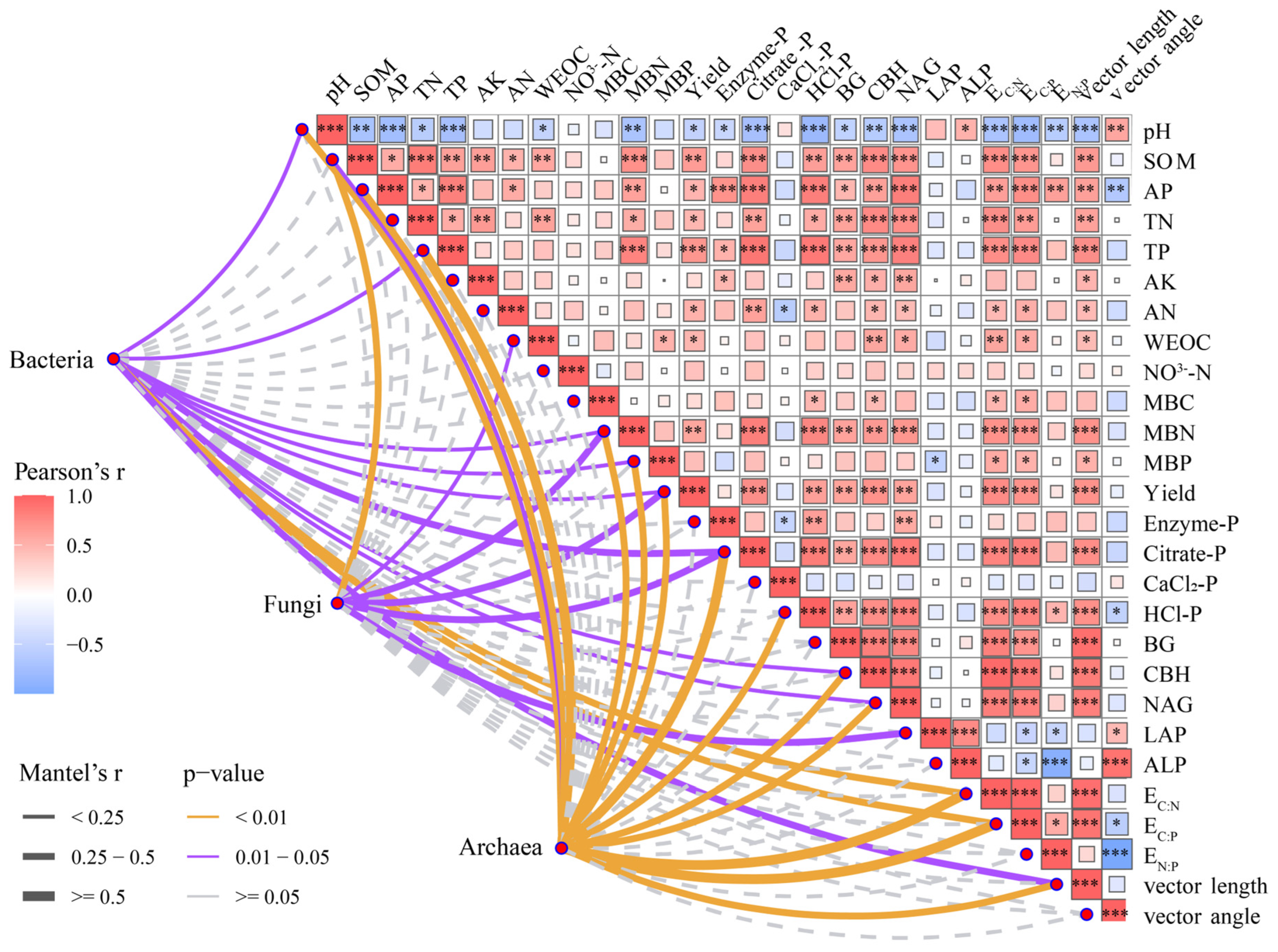


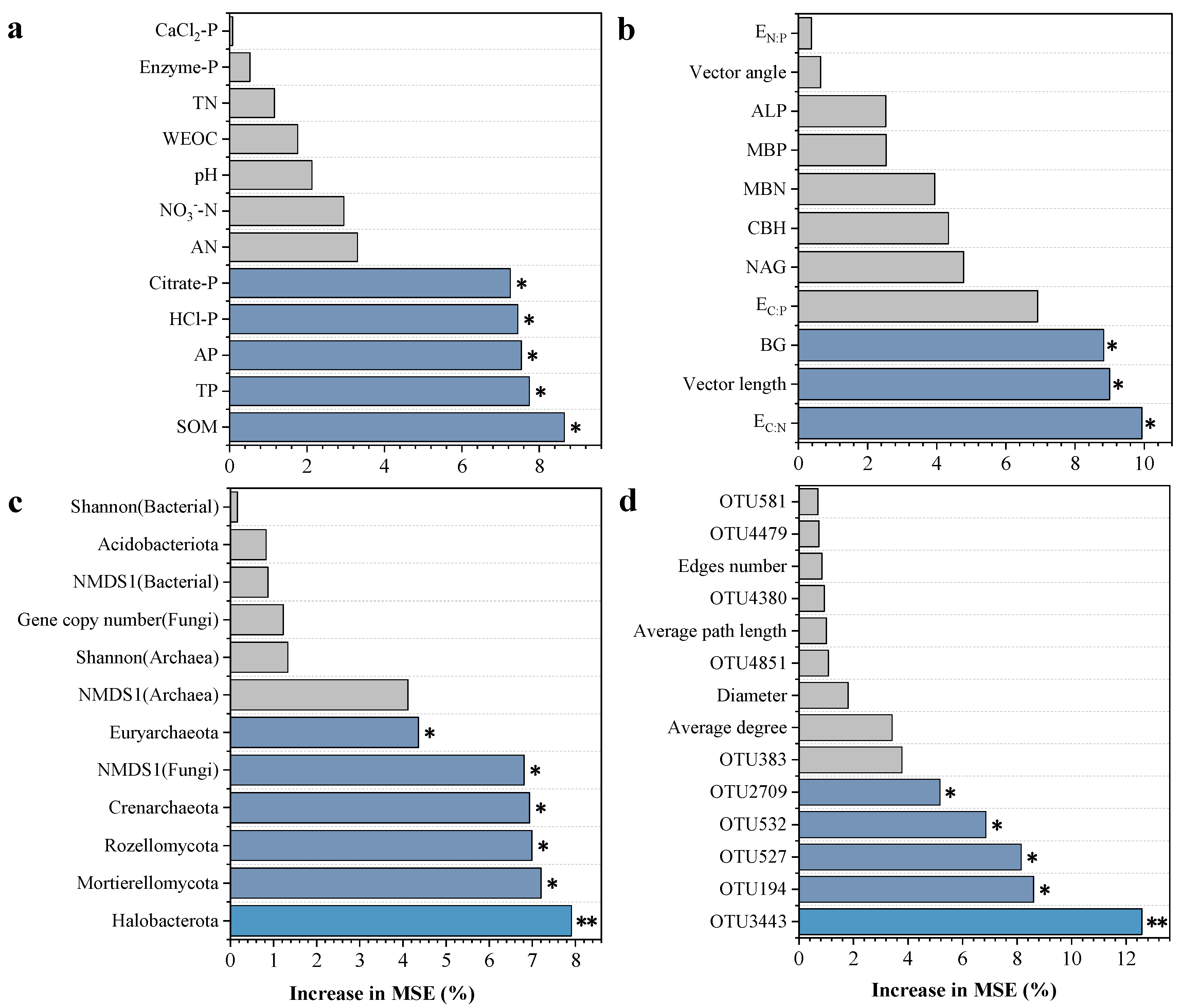
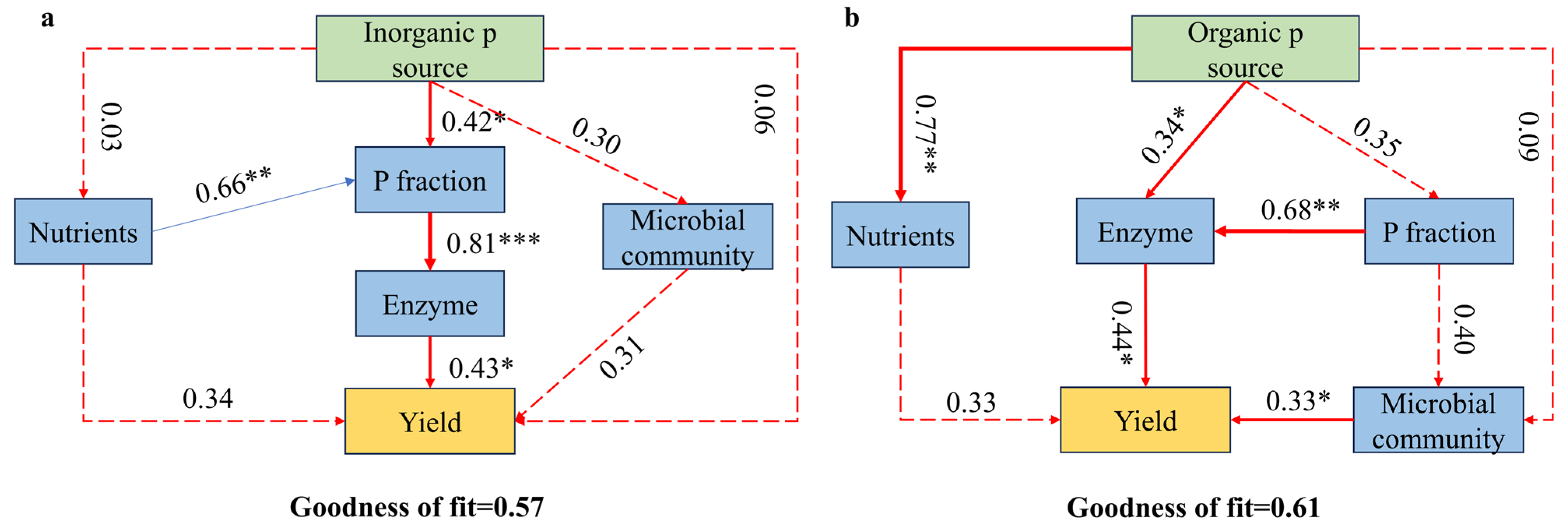
| Parameter | CK | OPT | OPTN | OPTP | OPTM |
|---|---|---|---|---|---|
| pH | 8.01 ± 0.03 a | 7.75 ± 0.04 b | 7.67 ± 0.05 b | 7.67 ± 0.11 b | 7.70 ± 0.22 b |
| SOM (g kg−1) | 22.23 ± 0.73 c | 26.57 ± 1.93 b | 27.45 ± 2.24 b | 26.92 ± 1.34 b | 33.16 ± 2.35 a |
| TN (g kg−1) | 1.23 ± 0.10 c | 1.42 ± 0.20 b | 1.39 ± 0.03 bc | 1.39 ± 0.10 bc | 1.63 ± 0.04 a |
| TP (g kg−1) | 0.67 ± 0.02 c | 0.99 ± 0.05 b | 1.03 ± 0.15 ab | 1.15 ± 0.14 a | 1.09 ± 0.04 ab |
| AK (mg kg−1) | 82.64 ± 3.74 b | 86.44 ± 4.82 b | 79.72 ± 8.58 b | 94.32 ± 23.91 ab | 111.21 ± 12.84 a |
| AP (mg kg−1) | 5.77 ± 5.84 d | 48.69 ± 8.85 c | 39.89 ± 6.07 c | 92.63 ± 9.03 a | 62.84 ± 9.53 b |
| AN (mg kg−1) | 80.30 ± 13.34 b | 95.14 ± 21.95 ab | 97.04 ± 24.22 ab | 111.03 ± 6.04 ab | 123.03 ± 19.79 a |
| WEOC (mg kg−1) | 151.90 ± 6.54 b | 167.86 ± 18.05 ab | 164.67 ± 11.16 ab | 157.79 ± 7.67 b | 180.74 ± 7.89 a |
| NO3−–N (mg kg−1) | 1.09 ± 0.07 a | 1.08 ± 0.05 a | 1.23 ± 0.24 a | 1.39 ± 0.55 a | 1.33 ± 0.15 a |
| MBC (mg kg−1) | 261.89 ± 75.36 a | 320.22 ± 65.78 a | 304.03 ± 87.39 a | 314.10 ± 91.94 a | 307.69 ± 59.11 a |
| MBN (mg kg−1) | 17.71 ± 8.20 b | 38.84 ± 6.59 a | 39.30 ± 11.71 a | 40.28 ± 9.97 a | 43.19 ± 6.24 a |
| MBP (mg kg−1) | 0.35 ± 0.11 b | 2.41 ± 0.48 a | 2.30 ± 0.80 a | 0.66 ± 0.25 b | 2.05 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Gan, Y.; Zhang, F.; Fu, X.; Xiong, L.; Xia, Y.; Zhu, D.; Fan, X. Organic and Inorganic Phosphorus Inputs Shape Wheat Productivity and Soil Bioavailability: A Microbial and Enzymatic Perspective from Long-Term Field Trials. Microorganisms 2025, 13, 2434. https://doi.org/10.3390/microorganisms13112434
Zhang Z, Gan Y, Zhang F, Fu X, Xiong L, Xia Y, Zhu D, Fan X. Organic and Inorganic Phosphorus Inputs Shape Wheat Productivity and Soil Bioavailability: A Microbial and Enzymatic Perspective from Long-Term Field Trials. Microorganisms. 2025; 13(11):2434. https://doi.org/10.3390/microorganisms13112434
Chicago/Turabian StyleZhang, Zhiyi, Yafen Gan, Fulin Zhang, Xihao Fu, Linhuan Xiong, Ying Xia, Dandan Zhu, and Xianpeng Fan. 2025. "Organic and Inorganic Phosphorus Inputs Shape Wheat Productivity and Soil Bioavailability: A Microbial and Enzymatic Perspective from Long-Term Field Trials" Microorganisms 13, no. 11: 2434. https://doi.org/10.3390/microorganisms13112434
APA StyleZhang, Z., Gan, Y., Zhang, F., Fu, X., Xiong, L., Xia, Y., Zhu, D., & Fan, X. (2025). Organic and Inorganic Phosphorus Inputs Shape Wheat Productivity and Soil Bioavailability: A Microbial and Enzymatic Perspective from Long-Term Field Trials. Microorganisms, 13(11), 2434. https://doi.org/10.3390/microorganisms13112434






