Potential Biomarkers and Underlying Pathogenesis of Mycoplasma synoviae Infection: Insights from Metabolomics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. MS Strain and Culture Conditions
2.2. Pathogenicity Test
2.3. qPCR Assay
2.4. Untargeted Metabolomics
2.5. Metabolite Extraction
2.6. UPLC-MS/MS Analysis
2.7. Data Quality Control and Preprocessing
2.8. Image and Statistical Analyses
3. Results
3.1. Pathogenicity of MS Strains
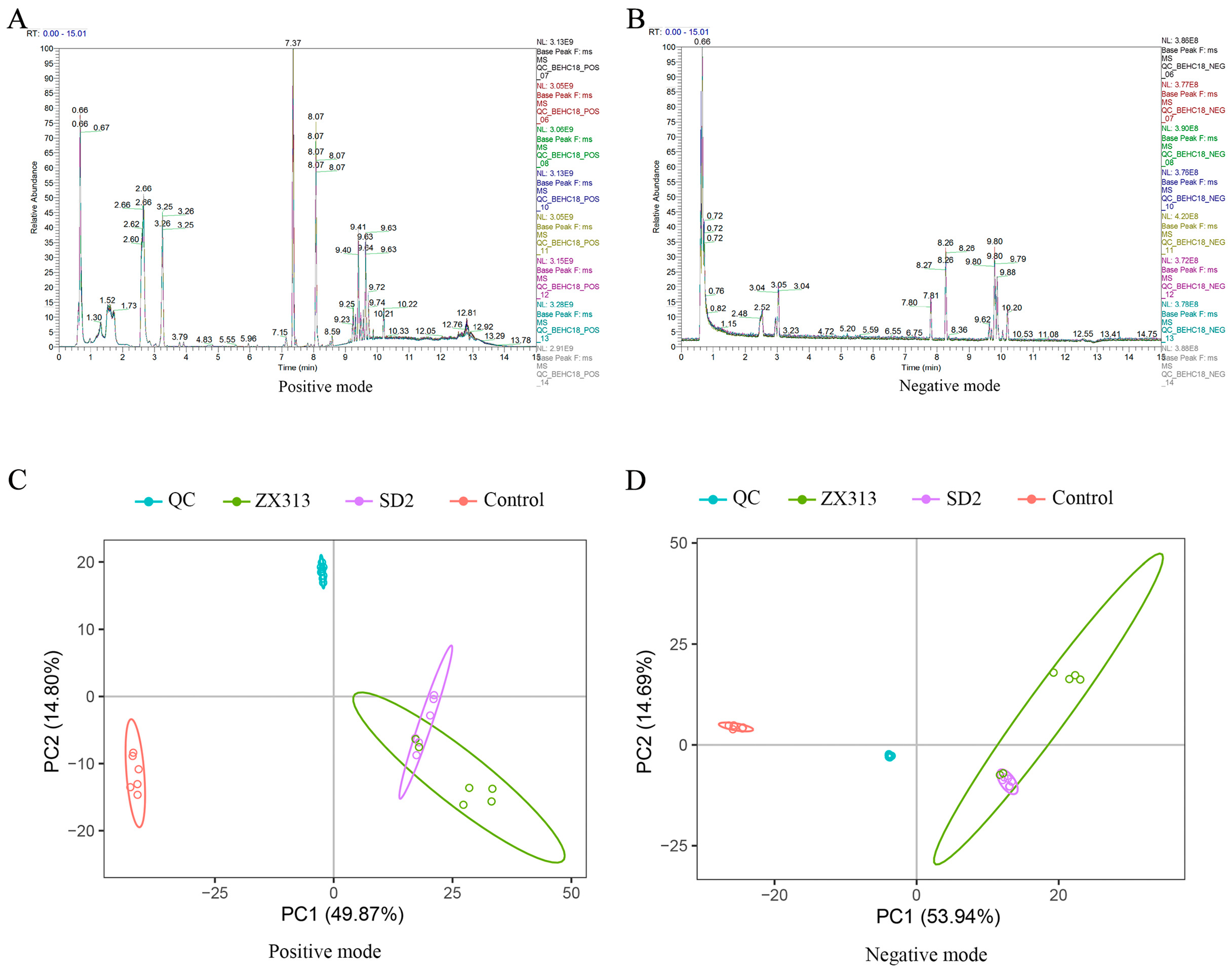
3.2. Overview of Metabolic Changes After MS Infection
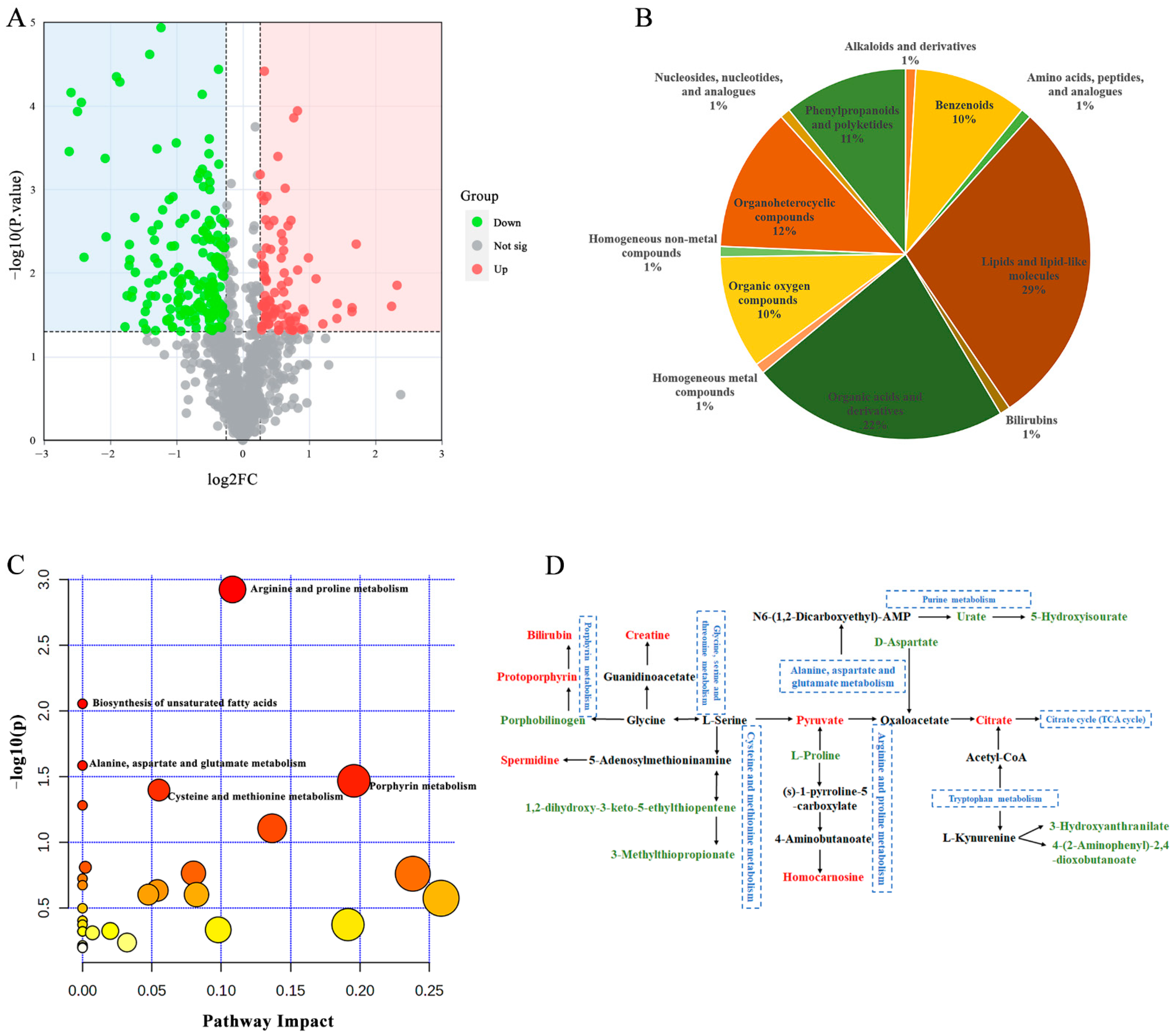
3.3. MS Infection Disrupts Host Metabolism
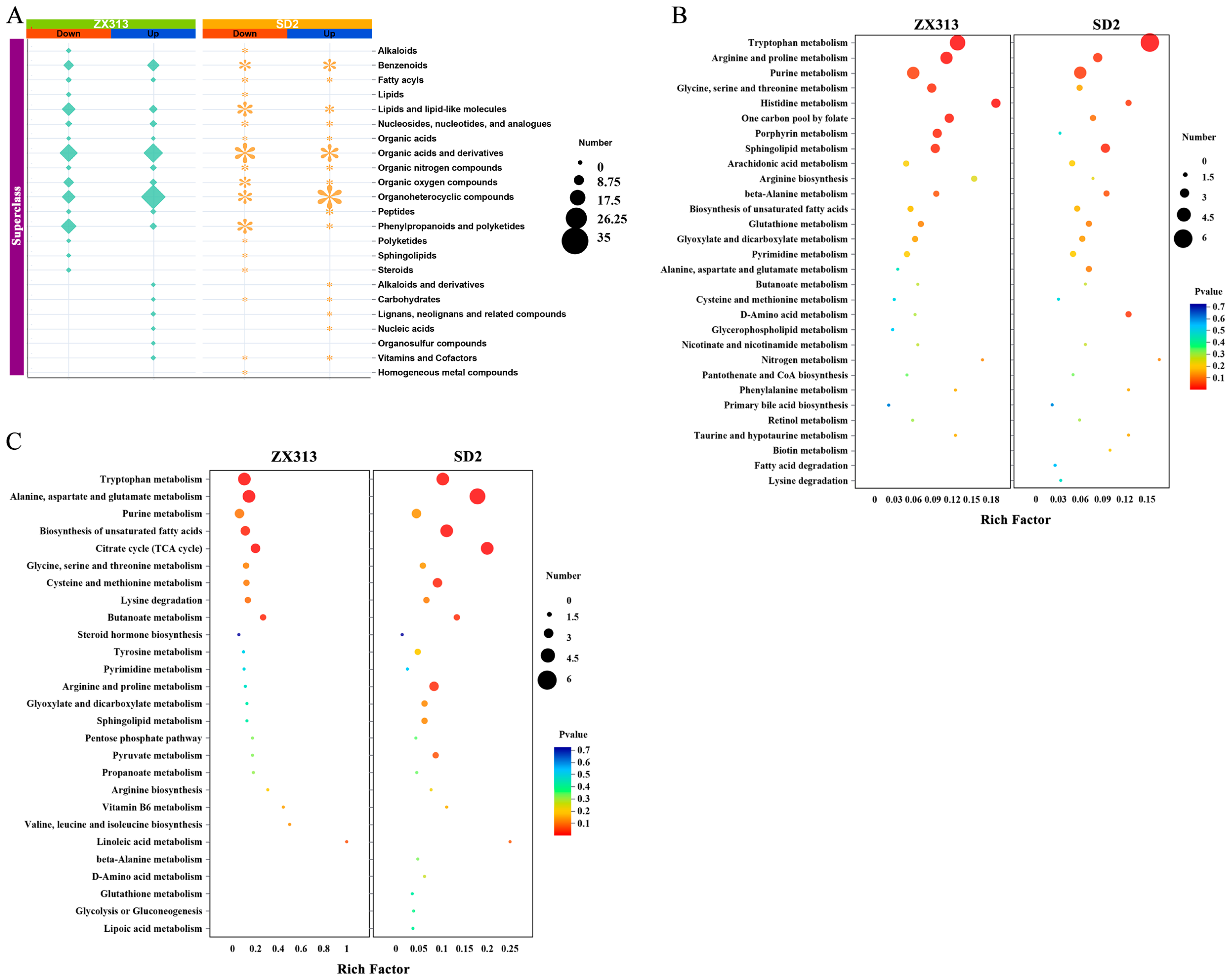
3.4. Metabolites and Pathway Analysis with Different Virulent MS Infections
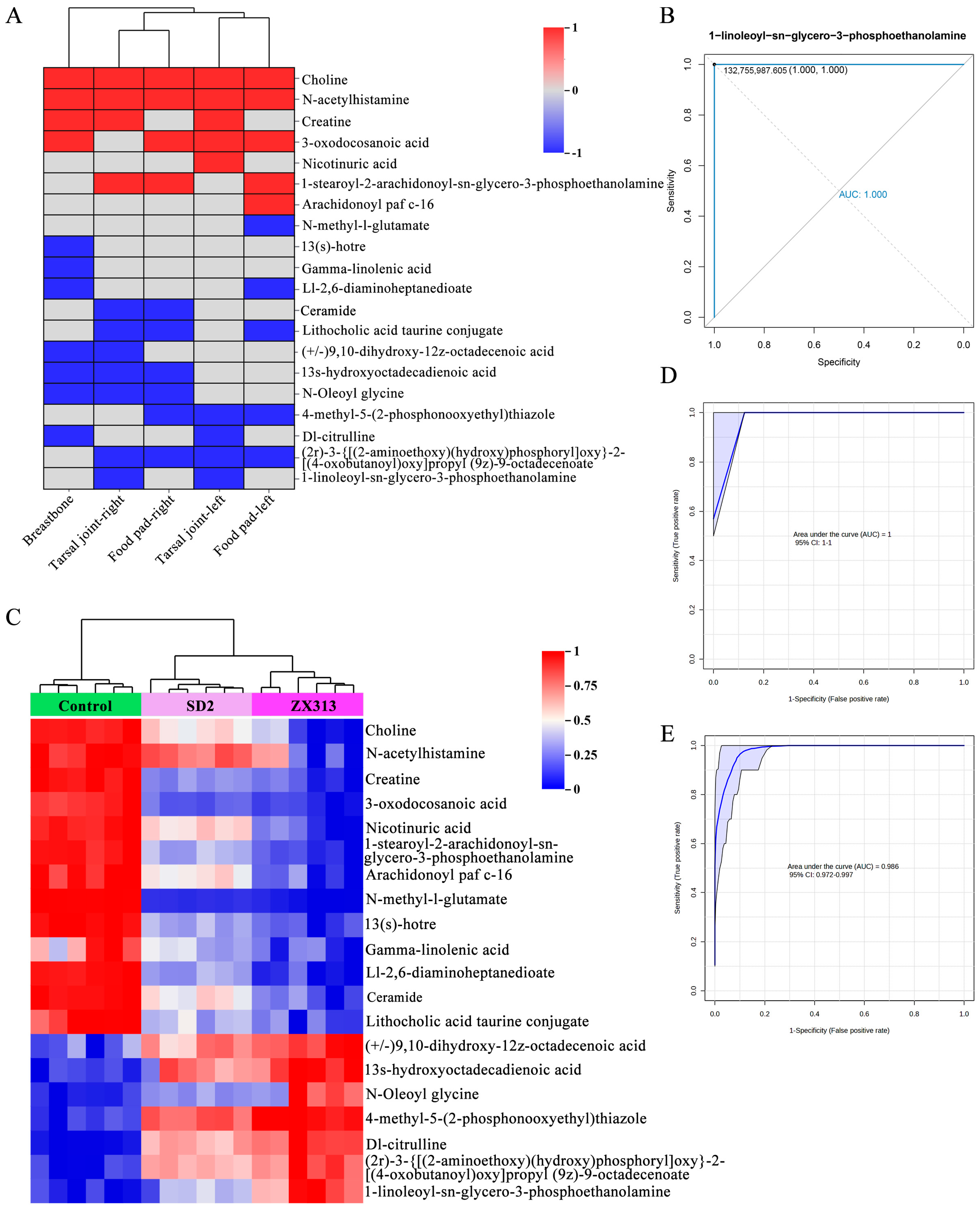
3.5. Metabolites in Relation to the Severity of MS Infections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MS | Mycoplasma synoviae |
| SDM | Significantly differentially abundant metabolites |
| NAD | Nicotinamide adenine dinucleotide |
| CCU | Color-changing unit |
| SPF | Specific pathogen-free |
| WPI | Weeks post-infection |
| QC | Quality control |
| AGC | Automatic gain control |
| PCA | Principal component analysis |
| ROC | Receiver operating characteristic |
| BPC | Base peak chromatograms |
| MP | Mycoplasma pneumoniae |
| PPARα | Peroxisome proliferator-activated receptor α |
| CPT1 | Carnitine palmitoyltransferase 1 |
References
- Zhu, L.; Shahid, M.A.; Markham, J.; Browning, G.F.; Noormohammadi, A.H.; Marenda, M.S. Comparative genomic analyses of Mycoplasma synoviae vaccine strain MS-H and its wild-type parent strain 86079/7NS: Implications for the identification of virulence factors and applications in diagnosis of M. synoviae. Avian Pathol. 2019, 48, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Kleven, S.H. Mycoplasmas in the etiology of multifactorial respiratory disease. Poult. Sci. 1998, 77, 1146–1149. [Google Scholar] [CrossRef]
- Feberwee, A.; de Wit, S.; Dijkman, R. Clinical expression, epidemiology, and monitoring of Mycoplasma gallisepticum and Mycoplasma synoviae: An update. Avian Pathol. 2022, 51, 2–18. [Google Scholar] [CrossRef]
- Xu, B.; Liu, R.; Ding, M.; Zhang, J.; Sun, H.; Liu, C.; Lu, F.; Zhao, S.; Pan, Q.; Zhang, X. Interaction of Mycoplasma synoviae with chicken synovial sheath cells contributes to macrophage recruitment and inflammation. Poult. Sci. 2020, 99, 5366–5377. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Q.; Yan, Z.; Zhou, Q.; Cao, Y.; Chen, F.; Wei, X. Transcriptional profiling of the chicken tracheal and splenic response to virulent Mycoplasma synoviae. Poult. Sci. 2022, 101, 101660. [Google Scholar] [CrossRef]
- Ma, X.; Wang, L.; Li, J.; Guo, Y.; He, S. The pathogenicity and immune effects of different generations of Mycoplasma synoviae on chicken embryos. Br. Poult. Sci. 2024, 65, 19–27. [Google Scholar] [CrossRef]
- Liu, R.; Xu, B.; Yu, S.; Zhang, J.; Sun, H.; Liu, C.; Lu, F.; Pan, Q.; Zhang, X. Integrated Transcriptomic and Proteomic Analyses of the Interaction Between Chicken Synovial Fibroblasts and Mycoplasma synoviae. Front. Microbiol. 2020, 11, 576. [Google Scholar] [CrossRef]
- Zhong, L.; Wu, C.; Liao, L.; Wu, Y. Mycoplasma synoviae induce spleen tissue damage and inflammatory response of chicken through oxidative stress and apoptosis. Virulence 2023, 15, 2283895. [Google Scholar] [CrossRef]
- Ma, J.; Xu, C.; Zhou, Y.; Jiang, N.; Xue, M.; Cao, J.; Li, S.; Fan, Y. Metabolomics in rare minnow (Gobiocypris rarus) after infection by attenuated and virulent grass carp reovirus genotype II. Fish Shellfish Immunol. 2023, 138, 108840. [Google Scholar] [CrossRef] [PubMed]
- Buzatto, A.Z.; Sarkar, I.; van Drunen Littel-van den Hurk, S.; Li, L. Comprehensive Lipidomic and Metabolomic Analysis for Studying Metabolic Changes in Lung Tissue Induced by a Vaccine against Respiratory Syncytial Virus. ACS Infect. Dis. 2020, 6, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Lam, S.M.; Fan, X.; Cao, W.-J.; Wang, S.-Y.; Tian, H.; Chua, G.H.; Zhang, C.; Meng, F.-P.; Xu, Z.; et al. Omics-Driven Systems Interrogation of Metabolic Dysregulation in COVID-19 Pathogenesis. Cell Metab. 2020, 32, 188–202. [Google Scholar] [CrossRef]
- Huang, J.; Mondul, A.M.; Weinstein, S.J.; Derkach, A.; Moore, S.C.; Sampson, J.N.; Albanes, D. Prospective serum metabolomic profiling of lethal prostate cancer. Int. J. Cancer 2019, 145, 3231–3243. [Google Scholar] [CrossRef]
- Bhargava, P.; Anthony, D.C. Metabolomics in multiple sclerosis disease course and progression. Mult. Scler. 2020, 26, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Klose, S.M.; De Souza, D.P.; Disint, J.F.; Andrews, D.M.; Underwood, G.J.; Morrow, C.J.; Marenda, M.S.; Noormohammadi, A.H. Reversion of mutations in a live mycoplasma vaccine alters its metabolism. Vaccine 2025, 41, 3358–3366. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Q.; Li, Y.; Wang, Z.; Hou, B.; Zhou, N.; Cui, W.; Hu, S.; Xiao, Y.; Zhang, W.; et al. Plasma metabolomics of Mycoplasma synoviae infection in SPF White Leghorn hens by liquid chromatography-tandem mass spectrometry. Vet. Res. 2025, 56, 65. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, W.; Sun, Q.; Zhong, Q.; Yan, Z.; Zhou, Q.; Cao, Y.; Chen, F.; Zhang, X. Epidemiological investigations and multilocus sequence typing of Mycoplasma synoviae isolates from chicken farms in China. Poult. Sci. 2023, 102, 102006. [Google Scholar] [CrossRef]
- Biernat-Sudolska, M.; Rojek-Zakrzewska, D.; Bilska-Wilkosz, A. In-vitro activity of lipoic acid against Ureaplasma urealyticum and Ureaplasma parvum isolated from women with infections of the urogenital tract. A pilot study. Acta Biochim. Pol. 2020, 67, 623–628. [Google Scholar] [CrossRef]
- Wei, X.; Zhong, Q.; Wang, D.; Yan, Z.; Liang, H.; Zhou, Q.; Chen, F. Epidemiological investigations and multilocus sequence typing of Mycoplasma gallisepticum collected in China. Poult. Sci. 2023, 102, 102930. [Google Scholar] [CrossRef]
- Sprygin, A.V.; Andreychuk, D.B.; Kolotilov, A.N.; Volkov, M.S.; Runina, I.A.; Mudrak, N.S.; Borisov, A.V.; Irza, V.N.; Drygin, V.V.; Perevozchikova, N.A. Development of a duplex real-time TaqMan PCR assay with an internal control for the detection of Mycoplasma gallisepticum and Mycoplasma synoviae in clinical samples from commercial and backyard poultry. Avian Pathol. 2010, 39, 99–109. [Google Scholar] [CrossRef]
- Zeng, S.; Peng, O.; Hu, F.; Xia, Y.; Geng, R.; Zhao, Y.; He, Y.; Xu, Q.; Xue, C.; Cao, Y.; et al. Metabolomic analysis of porcine intestinal epithelial cells during swine acute diarrhea syndrome coronavirus infection. Front. Cell. Infect. Microbiol. 2022, 12, 1079297. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Xu, Y.; Ma, L.; Du, M.; Li, P.; Yin, Z.; Xu, H.; Wu, X. Stereoselective toxicity mechanism of neonicotinoid dinotefuran in honeybees: New perspective from a spatial metabolomics study. Sci. Total Environ. 2022, 809, 151116. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Y.; Wang, G.; Zheng, X.; Hao, H. Gut Microbial Metabolites of Aromatic Amino Acids as Signals in Host-Microbe Interplay. Trends Endocrinol. Metab. 2020, 31, 818–834. [Google Scholar]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Du, L.; Yuan, H.; Shan, J.; Wang, S.; Ye, J.; Lin, L. Integration of lipidomics and metabolomics reveals plasma and urinary profiles associated with pediatric Mycoplasma pneumoniae infections and its severity. Biomed. Chromatogr. 2024, 38, e5817. [Google Scholar] [CrossRef] [PubMed]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms Mediating the Regulation of Peroxisomal Fatty Acid Beta-Oxidation by PPARalpha. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar]
- Rottem, S. Interaction of mycoplasmas with host cells. Physiol. Rev. 2003, 83, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luu, L.D.W.; Wang, X.; Cui, X.; Huang, X.; Fu, J.; Zhu, X.; Li, Z.; Wang, Y.; Tai, J. Metabolomic analysis reveals potential biomarkers and the underlying pathogenesis involved in Mycoplasma pneumoniae pneumonia. Emerg. Microbes Infect. 2022, 11, 593–605. [Google Scholar] [CrossRef]
- Nair, M.S.; Yao, D.; Chen, C.; Pieters, M. Serum metabolite markers of early Mycoplasma hyopneumoniae infection in pigs. Vet. Res. 2019, 50, 98. [Google Scholar]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]
- Fadiel, A.; Eichenbaum, K.D.; El Semary, N.; Epperson, B. Mycoplasma genomics: Tailoring the genome for minimal life requirements through reductive evolution. Front. Biosci.-Landmark 2007, 12, 2020–2028. [Google Scholar] [CrossRef]
- Lane, A.N.; Fan, T.W.-M. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Warner, D.F.; Evans, J.C.; Mizrahi, V. Nucleotide Metabolism and DNA Replication. In Molecular Genetics of Mycobacteria, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Klement, M.L.R.; Öjemyr, L.; Tagscherer, K.E.; Widmalm, G.; Wieslander, Å. A processive lipid glycosyltransferase in the small human pathogen Mycoplasma pneumoniae: Involvement in host immune response. Mol. Microbiol. 2007, 65, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Tengfei, Z.; Wenting, Z.; Qin, L.; Yunqing, G.; Xiaoyi, C.; Huabin, S.; Xinguo, Z.; Qingping, L. Mechanistic insights of magnolol antimicrobial activity against Mycoplasma using untargeted metabolomic analyses. Front. Cell. Infect. Microbiol. 2023, 13, 1325347. [Google Scholar] [CrossRef]
- Ravid, T.; Tsaba, A.; Gee, P.; Rasooly, R.; Medina, E.A.; Goldkorn, T. Ceramide accumulation precedes caspase-3 activation during apoptosis of A549 human lung adenocarcinoma cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L1082–L1092. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed]
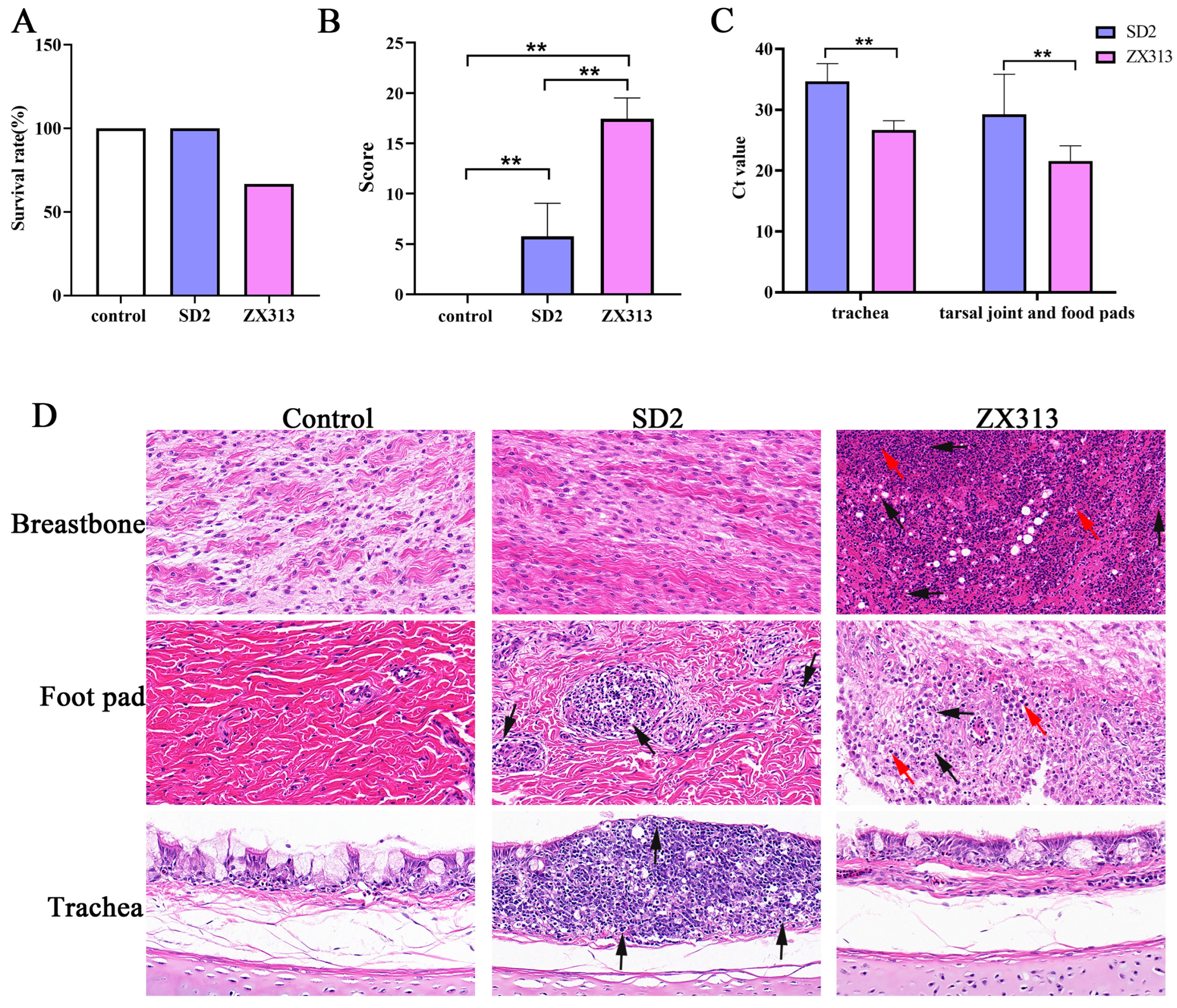

| Tissue | Lesion Degree | Score |
|---|---|---|
| tarsal joint, foot pad, and breastbone | no obvious swelling, no inflammatory mucus, no cheesy material | 0 |
| no obvious swelling, a small amount of inflammatory mucus, no cheesy material | 1 | |
| slight swelling, large amount of inflammatory mucus, no cheesy material | 2 | |
| obviously swollen, a large amount of inflammatory mucus, and a small amount of cheesy material | 3 | |
| obviously swollen, a large amount of inflammatory mucus and cheesy material | 4 |
| Number | Metabolite_Name | Metabolite Formula | Actual.RT | Database Annotation | Super Class | ZX313 Infection | SD2 Infection | |||
|---|---|---|---|---|---|---|---|---|---|---|
| KEGG | HMDB | Fold Change | p Value | Fold Change | p Value | |||||
| 1 | Deoxycholate | C24H40O4 | 8.783 | C04483 | HMDB0000626 | Steroids | 0.0120 | 6.95 × 10−13 | 0.0135 | 7.06 × 10−13 |
| 2 | 8-oxoguanine | C5H3N5O2 | 4.329 | / | HMDB0041820 | Organoheterocyclic compounds | 28.2861 | 4.14 × 10−6 | 51.9779 | 9.03 × 10−7 |
| 3 | Glycoursodeoxycholic acid | C26H43NO5 | 8.326 | / | HMDB0000708 | Lipids and lipid-like molecules | 0.0231 | 1.72 × 10−12 | 0.0641 | 8.30 × 10−12 |
| 4 | Hippurate | C9H9NO3 | 3.821 | C01586 | HMDB0000714 | Benzenoids | 24.1298 | 2.14 × 10−3 | 29.2069 | 2.02 × 10−4 |
| 5 | Triethanolamine | C6H15NO3 | 0.697 | C06771 | HMDB0032538 | Organic nitrogen compounds | 24.4627 | 9.16 × 10−5 | 17.8228 | 2.34 × 10−6 |
| 6 | beta-Muricholate | C24H40O5 | 8.288 | C17726 | HMDB0000415 | Lipids and lipid-like molecules | 0.0380 | 8.91 × 10−11 | 0.0639 | 7.24 × 10−11 |
| 7 | Lupulone | C26H38O4 | 8.907 | C10706 | HMDB0030041 | Organic oxygen compounds | 0.0488 | 5.26 × 10−15 | 0.0469 | 5.14 × 10−15 |
| 8 | Gluconolactone | C6H10O6 | 0.624 | C00198 | HMDB0000150 | Organic oxygen compounds | 0.0433 | 4.03 × 10−11 | 0.0769 | 5.33 × 10−11 |
| 9 | Patuletin | C16H12O8 | 0.604 | C10118 | HMDB0030802 | Phenylpropanoids and polyketides | 0.0584 | 1.30 × 10−11 | 0.0795 | 1.93 × 10−11 |
| 10 | Dodecanedioic acid | C12H22O4 | 5.978 | C02678 | HMDB0000623 | Lipids and lipid-like molecules | 16.0433 | 7.76 × 10−4 | 11.5431 | 1.27 × 10−3 |
| 11 | Kynurenic acid | C10H7NO3 | 3.08 | C01717 | HMDB0000715 | Organoheterocyclic compounds | 8.0333 | 1.94 × 10−3 | 13.1842 | 1.76 × 10−3 |
| 12 | 3-(2-hydroxyphenyl)propanoate | C9H10O3 | 2.857 | C01198 | HMDB0033752 | Phenylpropanoids and polyketides | 0.2075 | 1.74 × 10−8 | 0.0662 | 4.62 × 10−16 |
| 13 | Hexanoic acid | C6H12O2 | 3.29 | C01585 | HMDB0000535 | Lipids and lipid-like molecules | 0.1500 | 1.21 × 10−2 | 0.1222 | 1.01 × 10−2 |
| 14 | Corticosterone | C21H30O4 | 6.91 | C02140 | HMDB0001547 | Lipids and lipid-like molecules | 0.1475 | 1.14 × 10−13 | 0.1267 | 2.39 × 10−13 |
| 15 | Daidzein | C15H10O4 | 5.816 | C10208 | HMDB0003312 | Phenylpropanoids and polyketides | 0.1408 | 7.18 × 10−14 | 0.1640 | 6.81 × 10−12 |
| 16 | Phenyllactate | C9H10O3 | 3.029 | C05607 | HMDB0000748 | Phenylpropanoids and polyketides | 0.1396 | 6.07 × 10−15 | 0.1835 | 1.05 × 10−16 |
| 17 | Acetophenone | C8H8O | 3.031 | C07113 | HMDB0033910 | Organic oxygen compounds | 0.1396 | 6.07 × 10−15 | 0.1835 | 1.05 × 10−16 |
| 18 | Sinapinic acid | C11H12O5 | 4.742 | C00482 | HMDB0032616 | Phenylpropanoids and polyketides | 0.1066 | 1.47 × 10−7 | 0.3501 | 2.98 × 10−9 |
| 19 | Dambonitol | C8H16O6 | 3.051 | / | HMDB0033942 | Organic oxygen compounds | 6.40533 | 2.10 × 10−6 | 4.6094 | 1.98 × 10−4 |
| 20 | Dl-4-hydroxyphenyllactic acid | C9H10O4 | 1.255 | C03672 | HMDB0000755 | Phenylpropanoids and polyketides | 0.1834 | 4.17 × 10−8 | 0.1844 | 3.89 × 10−8 |
| Number | Metabolite_Name | Metabolite Formula | Actual.RT | Database Annotation | Super Class | Fold Change | p-Value | |
|---|---|---|---|---|---|---|---|---|
| KEGG | HMDB | |||||||
| 1 | Thromboxane b2 | C20H34O6 | 6.914 | C05963 | HMDB0003252 | Lipids and lipid-like molecules | 0.1661 | 1.97 × 10−6 |
| 2 | Sinapinic acid | C11H12O5 | 4.742 | C00482 | HMDB0032616 | Phenylpropanoids and polyketides | 0.3045 | 2.60 × 10−3 |
| 3 | 3-(2-hydroxyphenyl)propanoate | C9H10O3 | 2.857 | C01198 | HMDB0033752 | Phenylpropanoids and polyketides | 3.1363 | 1.53 × 10−2 |
| 4 | 4-hydroxy-1h-indole-3-acetonitrile | C10H8N2O | 3.36 | / | HMDB0038462 | Organoheterocyclic compounds | 0.3254 | 1.35 × 10−3 |
| 5 | 1,2-dihydroxy-3-keto-5-methylthiopentene | C6H10O3S | 2.506 | C15606 | HMDB0012134 | Organic oxygen compounds | 0.3538 | 1.04 × 10−2 |
| 6 | Nicotinuric acid | C8H8N2O3 | 3.02 | C05380 | HMDB0003269 | Organic acids and derivatives | 2.6766 | 1.86 × 10−2 |
| 7 | Methyl jasmonate | C13H20O3 | 8.515 | C11512 | HMDB0036583 | Fatty acyls | 0.3779 | 6.14 × 10−6 |
| 8 | 20-hydroxy-(5z,8z,11z,14z)-eicosatetraenoic acid | C20H32O3 | 8.881 | C14748 | HMDB0005998 | Lipids and lipid-like molecules | 0.4255 | 5.40 × 10−7 |
| 9 | Hexanoylcarnitine | C13H25NO4 | 4.525 | / | HMDB0000705 | Fatty Acyls | 2.3119 | 1.29 × 10−2 |
| 10 | 4-vinylphenol sulfate | C8H8O4S | 4.719 | / | HMDB0062775 | Organic acids and derivatives | 0.4328 | 4.82 × 10−5 |
| 11 | Androstanolone | C19H30O2 | 8.814 | C03917 | HMDB0002961 | Lipids and lipid-like molecules | 0.4824 | 8.10 × 10−5 |
| 12 | Pyrogallol-2-o-glucuronide | C12H14O9 | 2.785 | / | HMDB0060017 | Organic oxygen compounds | 0.4990 | 8.63 × 10−4 |
| 13 | Isoliquiritigenin | C15H12O4 | 6.348 | C08650 | HMDB0037316 | Phenylpropanoids and polyketides | 1.9874 | 1.12 × 10−2 |
| 14 | 3-indoxyl sulphate | C8H7NO4S | 3.067 | / | HMDB0000682 | Organic acids and derivatives | 0.5096 | 7.50 × 10−3 |
| 15 | 8-oxoguanine | C5H3N5O2 | 4.329 | / | HMDB0041820 | Organoheterocyclic compounds | 0.5442 | 1.88 × 10−3 |
| 16 | Gluconolactone | C6H10O6 | 0.624 | C00198 | HMDB0000150 | Organic oxygen compounds | 0.5630 | 4.03 × 10−3 |
| 17 | 4-(2-aminophenyl)-2,4-dioxobutanoic acid | C10H9NO4 | 3.813 | C01252 | HMDB0000978 | Organic oxygen compounds | 0.5689 | 2.60 × 10−3 |
| 18 | 9-oxo-10(e),12(e)-octadecadienoic acid | C18H30O3 | 8.721 | C14766 | HMDB0004669 | Lipids and lipid-like molecules | 1.6621 | 4.87 × 10−2 |
| 19 | Glycitein | C16H12O5 | 5.949 | C14536 | HMDB0005781 | Phenylpropanoids and polyketides | 1.6371 | 9.43 × 10−3 |
| 20 | N-acetylproline | C7H11NO3 | 3 | / | HMDB0094701 | Organic acids and derivatives | 0.6249 | 1.40 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Sun, M.; Zhang, K.; Wen, J.; Yan, Z.; Liu, Y.; Wang, L. Potential Biomarkers and Underlying Pathogenesis of Mycoplasma synoviae Infection: Insights from Metabolomics Analysis. Microorganisms 2025, 13, 2427. https://doi.org/10.3390/microorganisms13112427
Wei X, Sun M, Zhang K, Wen J, Yan Z, Liu Y, Wang L. Potential Biomarkers and Underlying Pathogenesis of Mycoplasma synoviae Infection: Insights from Metabolomics Analysis. Microorganisms. 2025; 13(11):2427. https://doi.org/10.3390/microorganisms13112427
Chicago/Turabian StyleWei, Xiaona, Mengyao Sun, Kuan Zhang, Jing Wen, Zhuanqiang Yan, Yangxue Liu, and Lianxiang Wang. 2025. "Potential Biomarkers and Underlying Pathogenesis of Mycoplasma synoviae Infection: Insights from Metabolomics Analysis" Microorganisms 13, no. 11: 2427. https://doi.org/10.3390/microorganisms13112427
APA StyleWei, X., Sun, M., Zhang, K., Wen, J., Yan, Z., Liu, Y., & Wang, L. (2025). Potential Biomarkers and Underlying Pathogenesis of Mycoplasma synoviae Infection: Insights from Metabolomics Analysis. Microorganisms, 13(11), 2427. https://doi.org/10.3390/microorganisms13112427





