Molecular Epidemiology and Seroepidemiology of Oz Virus Infection in Ticks and Wild Boars in Ibaraki Prefecture, Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection from Vegetation and RNA Extraction
2.2. Collection of Wild Boar Serum and RNA Extraction
2.3. Detection of OZV RNA
2.4. Sequence Analyses
2.5. NT-Ab Against OZV
3. Results
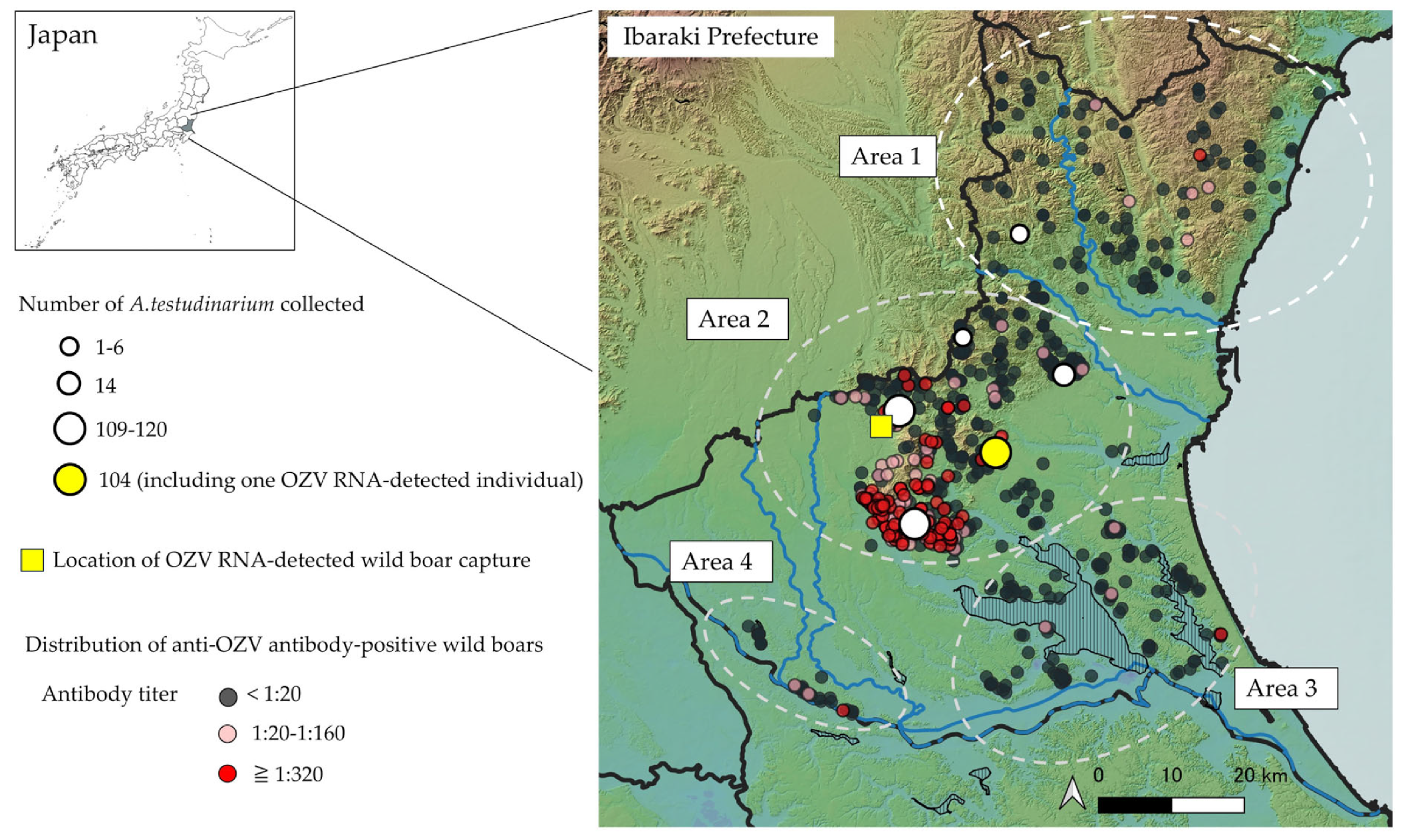
3.1. Detection of OZV RNA from Ticks
3.2. Detection of OZV RNA in Wild Boar Serum
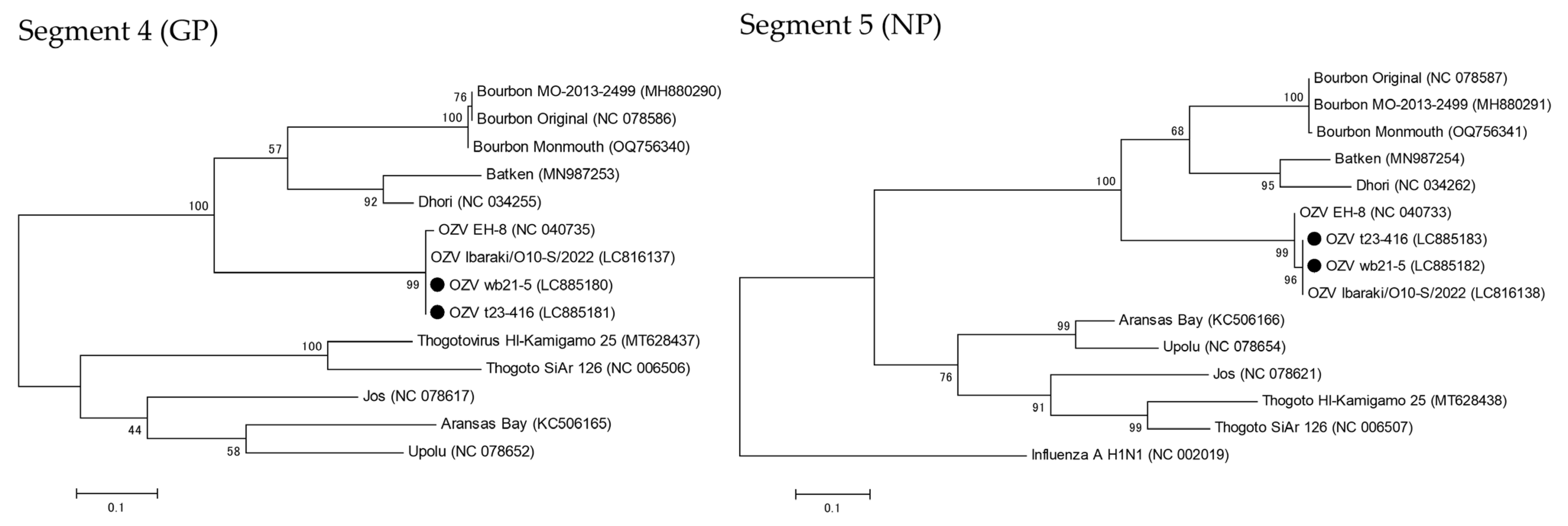
3.3. Sequence and Phylogenetic Analyses of OZV Strains
3.4. Seroepidemiology Against OZV in Wild Boars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ejiri, H.; Lim, C.K.; Isawa, H.; Fujita, R.; Murota, K.; Sato, T.; Kobayashi, D.; Kan, M.; Hattori, M.; Kimura, T.; et al. Characterization of a novel thogotovirus isolated from Amblyomma testudinarium ticks in Ehime, Japan: A significant phylogenetic relationship to Bourbon virus. Virus Res. 2018, 249, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Matsuu, A.; Tatemoto, K.; Ishijima, K.; Nishino, A.; Inoue, Y.; Park, E.; Tamatani, H.; Seto, J.; Higashi, H.; Fukui, Y.; et al. Oz Virus Infection in 6 Animal Species, Including Macaques, Bears, and Companion Animals, Japan. Emerg. Infect. Dis. 2025, 31, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.B.; Shimoda, H.; Ishijima, K.; Yonemitsu, K.; Minami, S.; Supriyono; Kuroda, Y.; Tatemoto, K.; Mendoza, M.V.; Kuwata, R.; et al. Zoonotic Infection with Oz Virus, a Novel Thogotovirus. Emerg. Infect. Dis. 2022, 28, 436–439. [Google Scholar] [CrossRef]
- Osawa, S.; Miyazaki, K.; Mine, S.; Hirata, Y.; Terada, N.; Ozawa, M.; Kikuchi, K.; Uchida, A.; Hirose, K.; Imakawa, Y.; et al. Fulminant Viral Myocarditis Associated with Thogotovirus. N. Engl. J. Med. 2025, 393, 924–926. [Google Scholar] [CrossRef]
- QGIS 3.34, Open Source Geographic Information System. Available online: https://www.qgis.org/ (accessed on 28 January 2025).
- National Land Information Division Home Page. Available online: https://nlftp.mlit.go.jp/ksj/index.html (accessed on 31 January 2025).
- Peng, R.; Zhang, S.; Cui, Y.; Shi, Y.; Gao, G.F.; Qi, J. Structures of human-infecting Thogotovirus fusogens support a common ancestor with insect baculovirus. Proc. Natl. Acad. Sci. USA 2017, 114, E8905–E8912. [Google Scholar] [CrossRef]
- Babar, M.M.; Zaidi, N.U.S. Protein sequence conservation and stable molecular evolution reveals influenza virus nucleoprotein as a universal druggable target. Infect. Genet. Evol. 2015, 34, 200–210. [Google Scholar] [CrossRef]
- Biggerstaff, B. PooledInfRate, Version 4.0: A Microsoft® Office Excel© Add-In to Compute Prevalence Estimates from Pooled Samples; Centers for Disease Control and Prevention: Fort Collins, CO, USA, 2009. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kiyasu, Y.; Osawa, S.; Tsutsumi, N.; Terada, N.; Nagata, N. Distribution of ticks and their possession of spotted fever group Rickettsia in Ibaraki prefecture. J. Infect. Chemother. 2024, 30, 590–596. [Google Scholar] [CrossRef]
- Shimada, M.; Doi, K.; Kawabata, H.; Yamauchi, T.; Ando, S.; Kobayashi, Y.; Hirose, Y.; Shuto, F.; Fujiwara, Y.; Saitou, M.; et al. A review of 49 tick bite cases that occurred from 2020–2022 at Japanese Red Cross Ashikaga Hospital in Tochigi Prefecture—Trends in 40 cases of Amblyomma testudinarium bites. Med Èntomol. Zool. 2023, 74, 53–56. [Google Scholar] [CrossRef]
- Jackson, K.C.; Gidlewski, T.; Root, J.J.; Bosco-Lauth, A.M.; Lash, R.R.; Harmon, J.R.; Brault, A.C.; Panella, N.A.; Nicholson, W.L.; Komar, N. Bourbon Virus in Wild and Domestic Animals, Missouri, USA, 2012–2013. Emerg. Infect. Dis. 2019, 25, 1752–1753. [Google Scholar] [CrossRef] [PubMed]
- Haig, D.A.; Woodall, J.P.; Danskin, D. Thogoto Virus: A Hitherto Undescribed Agent Isolated from Ticks in Kenya. J. Gen. Microbiol. 1965, 38, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Srihongse, S.; Albanese, M.; Casals, J. Characterization of Thogoto virus isolated from ticks (Rhipicephalus bursa) in Western Sicily, Italy. Am. J. Trop. Med. Hyg. 1974, 23, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Wood, O.L.; Lee, V.H.; Ash, J.S.; Casals, J. Crimean-congo hemorrhagic fever, Thogoto, dugbe, and Jos viruses isolated from ixodid ticks in Ethiopia. Am. J. Trop. Med. Hyg. 1978, 27, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.A.; Hoogstraal, H.; Omar, F.M. A serological survey for Thogoto virus in humans, domestic mammals, and rats in Egypt. J. Egypt. Public Health Assoc. 1979, 54, 1–8. [Google Scholar]
- Lledó, L.; Giménez-Pardo, C.; Gegúndez, M.I. Epidemiological Study of Thogoto and Dhori Virus Infection in People Bitten by Ticks, and in Sheep, in an Area of Northern Spain. Int. J. Environ. Res. Public Health 2020, 17, 2254. [Google Scholar] [CrossRef]
- Lee, V.H.; Kemp, G.E.; Madbouly, M.H.; Moore, D.L.; Causey, O.R.; Casals, J. Jos, a new tick-borne virus from Nigeria. Am. J. Vet. Res. 1974, 35, 1165–1167. [Google Scholar] [CrossRef]
- L’vov, D.N.; Dzharkenov, A.F.; Aristova, V.A.; Kovtunov, A.I.; Gromashevskiĭ, V.L.; Vyshemirskiĭ, O.I.; Galkina, I.V.; Larichev, V.F.; Butenko, A.M.; L’vov, D.K. The isolation of Dhori viruses (Orthomyxoviridae, Thogotovirus) and Crimean-Congo hemorrhagic fever virus (Bunyaviridae, Nairovirus) from the hare (Lepus europaeus) and its ticks Hyalomma marginatum in the middle zone of the Volga delta, Astrakhan region, 2001. Vopr. Virusol. 2002, 47, 32–36. [Google Scholar]
- Da Silva, E.V.; Da Rosa, A.P.; Nunes, M.R.; Diniz, J.A.; Tesh, R.B.; Cruz, A.C.; Vieira, C.M.; Vasconcelos, P.F. Araguari virus, a new member of the family Orthomyxoviridae: Serologic, ultrastructural, and molecular characterization. Am. J. Trop. Med. Hyg. 2005, 73, 1050–1058. [Google Scholar] [CrossRef]
- Iashkulov, K.B.; Shchelkanov, M.I.u.; L’vov, S.S.; Dzhambinov, S.D.; Galkina, I.V.; Fediakina, I.T.; Bushkieva, B.T.s; Morozova, T.N.; Kireev, D.E.; Akanina, D.S.; et al. Isolation of influenza virus A (Orthomyxoviridae, Influenza A virus), Dhori virus (Orthomyxoviridae, Thogotovirus), and Newcastle’s disease virus (Paromyxoviridae, Avulavirus) on the Malyi Zhemchuzhnyi Island in the north-western area of the Caspian Sea. Vopr. Virusol. 2008, 53, 34–38. [Google Scholar]
- Doi, K.; Kato, T.; Tabata, I.; Hayama, S.I. Mapping the Potential Distribution of Ticks in the Western Kanto Region, Japan: Predictions Based on Land-Use, Climate, and Wildlife. Insects 2021, 12, 1095. [Google Scholar] [CrossRef]
- Shimada, M.; Doi, K.; Yamauchi, T.; Kawabata, H.; Ando, S.; Abe, T.; Kobayashi, Y.; Hirose, Y.; Fujiwara, Y.; Saitou, M.; et al. Preliminary report on the relationship between recent tick bite cases caused by Amblyomma testudinarium and ticks collected from wild boar and deer in Ashikaga City, Tochigi Prefecture, Japan. J. Acarol. Soc. Jpn. 2022, 31, 75–83. [Google Scholar] [CrossRef]
- Lange, R.E.; Dupuis, A.P., 2nd; Ciota, A.T. Diversification of Bourbon Virus in New York State. Microorganisms 2023, 11, 1590. [Google Scholar] [CrossRef]
- Popczyk, B.; Klich, D.; Nasiadka, P.; Nieszała, A.; Gadkowski, K.; Sobczuk, M.; Balcerak, M.; Kociuba, P.; Olech, W.; Purski, L. Over 300 km Dispersion of Wild Boar during Hot Summer, from Central Poland to Ukraine. Animals 2024, 14, 170. [Google Scholar] [CrossRef]
- Fuchs, J.; Lamkiewicz, K.; Kolesnikova, L.; Hölzer, M.; Marz, M.; Kochs, G. Comparative Study of Ten Thogotovirus Isolates and Their Distinct In Vivo Characteristics. J. Virol. 2022, 96, e0155621. [Google Scholar] [CrossRef]

| Tick Species | Larva | Nymph | Adult | Total | |
|---|---|---|---|---|---|
| Haemaphysalis | flava | 22 | 1025 | 189 | 1236 |
| hystrics | 62 | 136 | 27 | 225 | |
| longicornis | 30 | 424 | 9 | 463 | |
| megaspinosa | 0 | 9 | 1 | 10 | |
| Amblyomma | testudinarium | 108 | 272 * | 2 | 382 |
| Ixodes | ovatus | 0 | 5 | 31 | 36 |
| turdus | 74 | 34 | 0 | 108 | |
| Year of Collection | No. of Samples | Neutralization Antibody Titer | GMT † (95% CI ‡) | Positive Rate (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| <1:20 * | 1:20 | 1:40 | 1:80 | 1:160 | ≥1:320 | ||||
| 2019 | 101 | 76 | 2 | 1 | 0 | 5 | 17 | 20.4 (15.7–26.6) | 24.8 (17.4–34.0) |
| 2020 | 155 | 123 | 3 | 0 | 3 | 7 | 19 | 18.3 (15.0–22.3) | 20.6 (15.0–27.7) |
| 2021 | 224 | 153 | 5 | 0 | 32 | 16 | 18 | 23.6 (19.8–28.2) | 32.1 (26.4–38.5) |
| 2022 | 208 | 145 | 6 | 3 | 11 | 16 | 27 | 22.6(18.9–27.1) | 30.2 (24.4–36.8) |
| 2023 | 246 | 194 | 5 | 2 | 4 | 7 | 34 | 18.5 (15.7–21.7) | 21.1 (16.5–26.7) |
| Total | 934 | 691 | 21 | 6 | 50 | 51 | 115 | 20.7 (19.0–22.5) | 26.0 (23.3–28.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osawa, S.; Kimura, H.; Abe, S.; Nagata, N.; Ueno, E.; Ebihara, H.; Kimura, R.; Furuya, T. Molecular Epidemiology and Seroepidemiology of Oz Virus Infection in Ticks and Wild Boars in Ibaraki Prefecture, Japan. Microorganisms 2025, 13, 2421. https://doi.org/10.3390/microorganisms13112421
Osawa S, Kimura H, Abe S, Nagata N, Ueno E, Ebihara H, Kimura R, Furuya T. Molecular Epidemiology and Seroepidemiology of Oz Virus Infection in Ticks and Wild Boars in Ibaraki Prefecture, Japan. Microorganisms. 2025; 13(11):2421. https://doi.org/10.3390/microorganisms13112421
Chicago/Turabian StyleOsawa, Shuichi, Hirokazu Kimura, Sakurako Abe, Noriko Nagata, Eri Ueno, Hideki Ebihara, Ryusuke Kimura, and Tetsuya Furuya. 2025. "Molecular Epidemiology and Seroepidemiology of Oz Virus Infection in Ticks and Wild Boars in Ibaraki Prefecture, Japan" Microorganisms 13, no. 11: 2421. https://doi.org/10.3390/microorganisms13112421
APA StyleOsawa, S., Kimura, H., Abe, S., Nagata, N., Ueno, E., Ebihara, H., Kimura, R., & Furuya, T. (2025). Molecular Epidemiology and Seroepidemiology of Oz Virus Infection in Ticks and Wild Boars in Ibaraki Prefecture, Japan. Microorganisms, 13(11), 2421. https://doi.org/10.3390/microorganisms13112421







