Metagenomic Analysis of Distribution Characteristics and Driving Mechanisms of Antibiotic Resistance Genes, Virulence Factors, and Microbial Communities in Rice Seedling Cultivation Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Analysis of Soil Properties
2.3. DNA Extraction, Metagenomics Sequencing and Data Analysis
2.4. ARGs and VFs Analysis
2.5. Statistical Analysis
3. Results
3.1. Soil Properties
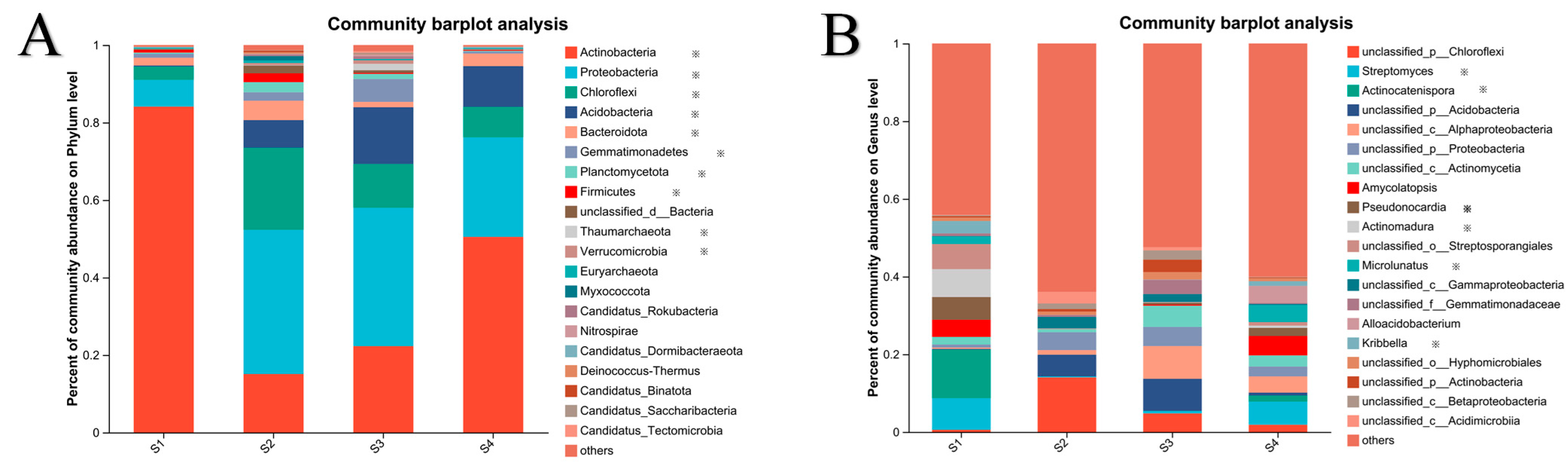
3.2. Microbial Diversity and Community Composition
3.3. Abundance and Composition of ARGs and VFs
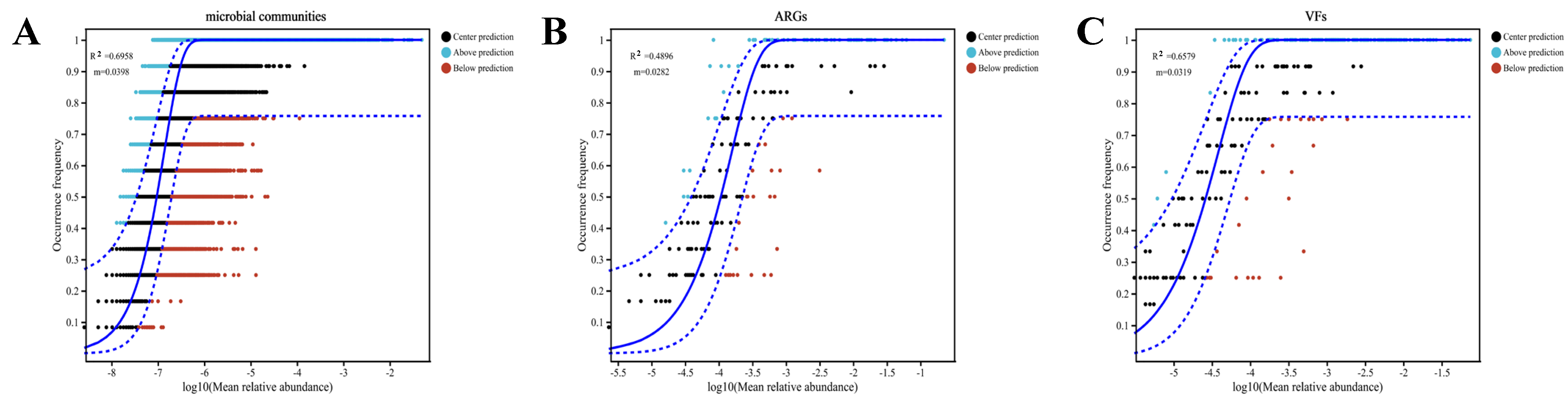
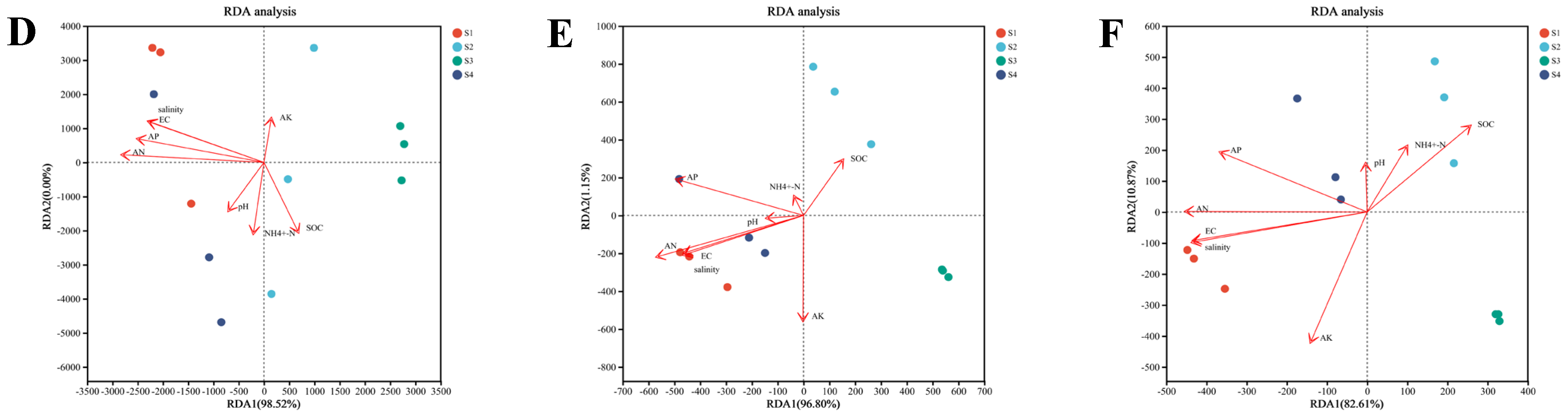
3.4. Assembly Processes and Environmental Drivers of Microbial Taxa, ARGs and VFs
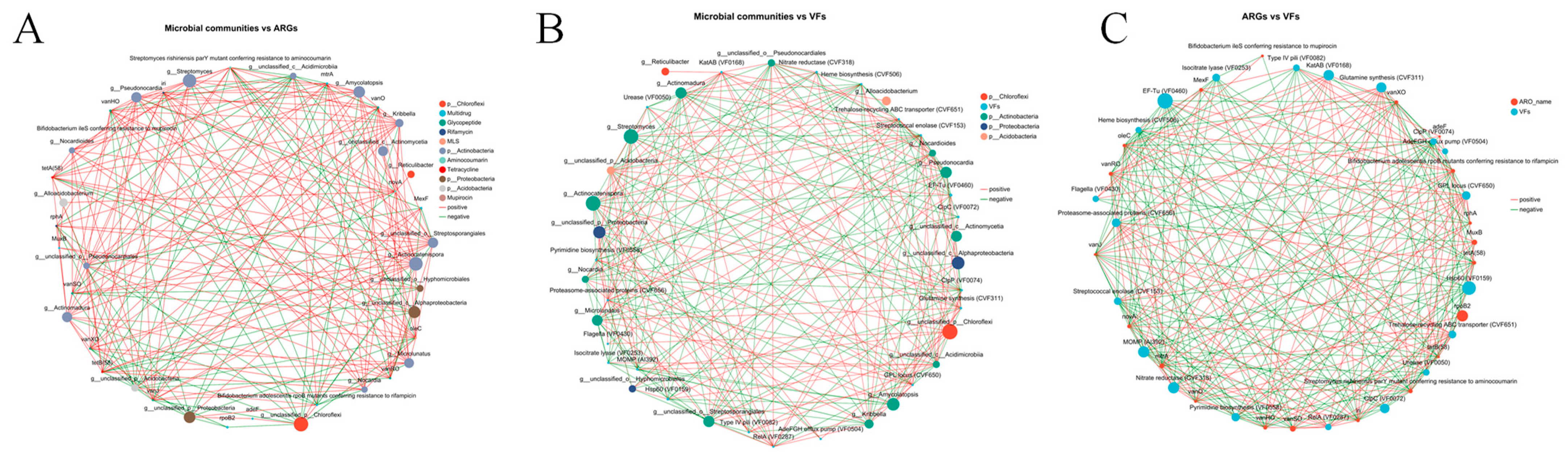
3.5. Relationships Between Microbial Taxa, ARGs and VFs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuppusamy, S.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M.; Yoon, Y.E.; Lee, Y.B. Veterinary antibiotics (VAs) contamination as a global agroecological issue: A critical view. Agric. Ecosyst. Environ. 2018, 257, 47–59. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, L.; Zhu, L.; Wang, J.; Xing, B. Antibiotic resistance in agricultural soils: Source, fate, mechanism and attenuation strategy. Crit. Rev. Environ. Sci. Technol. 2022, 52, 847–889. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Fang, W.; Manaia, C.M.; Virta, M.; Sheng, H.; Liping, M.; Zhang, T.; Topp, E. Antibiotic resistance genes in the human-impacted environment: A one health perspective. Pedosphere 2019, 29, 273–282. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wang, T.; Xu, N.; Lu, T.; Hong, W.; Penuelas, J.; Gillings, M.; Wang, M.; Gao, W.; et al. Assessment of global health risk of antibiotic resistance genes. Nat. Commun. 2022, 13, 1553. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, A.H.; Jennings, M.P. Discovery of virulence factors of pathogenic bacteria. Curr. Opin. Chem. Biol. 2008, 12, 93–101. [Google Scholar] [CrossRef]
- Liang, J.S.; Mao, G.N.; Yin, X.L.; Ma, L.P.; Liu, L.; Bai, Y.H.; Zhang, T.; Qu, J.H. Identification and quantification of bacterial genomes carrying antibiotic resistance genes and virulence factor genes for aquatic microbiological risk assessment. Water Res. 2020, 168, 115160. [Google Scholar] [CrossRef]
- Xiao, K.Q.; Li, B.; Ma, L.P.; Bao, P.; Zhou, X.; Zhang, T.; Zhu, Y.G. Metagenomic profiles of antibiotic resistance genes in paddy soils from South China. FEMS Microbiol. Ecol. 2016, 92, fiw023. [Google Scholar] [CrossRef] [PubMed]
- Braga, L.P.P.; Alves, R.F.; Dellias, M.T.F.; Navarrete, A.A.; Basso, T.O.; Tsai, S.M. Vinasse fertirrigation alters soil resistome dynamics: An analysis based on metagenomic profiles. Biodata Min. 2017, 10, 17. [Google Scholar] [CrossRef]
- Wang, F.H.; Qiao, M.; Su, J.Q.; Chen, Z.; Zhou, X.; Zhu, Y.G. High throughput profiling of antibiotic resistance genes in urban park soils with reclaimed water irrigation. Environ. Sci. Technol. 2014, 48, 9079–9085. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, M.; Stedtfeld, R.D.; Sheng, H.; Fan, J.; Liu, M.; Chai, B.L.; Teotonio, S.C.; Li, H.; Li, Z.P.; et al. Long-term effect of different fertilization and cropping systems on the soil antibiotic resistome. Environ. Sci. Technol. 2018, 52, 13037–13046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Hu, H.W.; Chen, Q.L.; Singh, B.K.; Yan, H.; Chen, D.; He, J.Z. Transfer of antibiotic resistance from manure-amended soils to vegetable microbiomes. Environ. Int. 2019, 130, 104912. [Google Scholar] [CrossRef]
- Song, D.; Tang, X.; Tariq, A.; Pan, K.; Li, D. Regional distribution and migration potential of antibiotic resistance genes in croplands of Qinghai Tibet plateau. Environ. Res. 2023, 231, 116233. [Google Scholar] [CrossRef] [PubMed]
- Ekman, J.; Goldwater, A.; Bradbury, M.; Matthews, J.; Rogers, G. Persistence of human pathogens in manure-amended Australian soils used for production of leafy vegetables. Agriculture 2020, 11, 14. [Google Scholar] [CrossRef]
- Sanz, C.; Casado, M.; Navarro-Martin, L.; Cañameras, N.; Carazo, N.; Matamoros, V.; Bayona, J.M.; Piña, B. Implications of the use of organic fertilizers for antibiotic resistance gene distribution in agricultural soils and fresh food products. A plot-scale study. Sci. Total Environ. 2022, 815, 151973. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.H.; Wang, J.; Zhu, L.; Conkle, J.L.; Yang, R. Soil types influence the characteristic of antibiotic resistance genes in greenhouse soil with long-term manure application. J. Hazard. Mater. 2020, 392, 122334. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Qiu, W.; Zhang, W.; Liu, J.; Yang, Z.; Wu, Z.; Ge, J. Understanding how various forms of phosphorus stress affect microbiome functions and boost plant disease resistance: Insights from metagenomic analysis. Sci. Total Environ. 2023, 904, 166899. [Google Scholar] [CrossRef]
- Chen, Q.L.; An, X.L.; Zheng, B.X.; Ma, Y.B.; Su, J.Q. Long-term organic fertilization increased antibiotic resistome in phyllosphere of maize. Sci. Total Environ. 2018, 645, 1230–1237. [Google Scholar] [CrossRef]
- Wu, J.; Guo, S.; Li, K.; Li, Z.; Xu, P.; Jones, D.L.; Wang, J.; Zou, J. Effect of fertilizer type on antibiotic resistance genes by reshaping the bacterial community and soil properties. Chemosphere 2023, 336, 139272. [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agro-Chemistrical Analysis, 3rd ed; Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Y.; Cao, W.; Xing, M.; Xu, X.; Wang, Z.; Sun, J. Metagenomic analysis reveals antibiotic resistance genes and virulence factors in the saline-alkali soils from the Yellow River Delta, China. Environ. Res. 2022, 214, 113823. [Google Scholar] [CrossRef]
- Yu, T.B.; Cheng, L.; Zhang, Q.; Yang, J.D.; Zhang, H.D.; Zeng, Z.H.; Yang, Y.D. Characterization of antibiotic resistance genes and virulence factors in organic managed tea plantation soils in southwestern China by metagenomics. Front. Microbiol. 2025, 16, 1580450. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.B. Vegan: Community Ecology Package; R Package Version 2.0–10; ScienceOpen, Inc.: Berlin, Germany, 2013. [Google Scholar]
- Ning, D.L.; Deng, Y.; Tiedje, J.M.; Zhou, J.Z. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef] [PubMed]
- Tshikantwa, T.S.; Ullah, M.W.; He, F.; Yang, G. Current trends and potential applications of microbial interactions for human welfare. Front. Microbiol. 2018, 9, 1156. [Google Scholar] [CrossRef]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of soil microbial diversity through sustainable agricultural practices and its evaluation by-omicsapproaches: A perspective for the environment, food quality and human safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Wen, J.; Mo, F.; Liu, Y. Continuous manure application strengthens the associations between soil microbial function and crop production: Evidence from a 7-year multisite field experiment on the Guanzhong plain. Agric. Ecosyst. Environ. 2022, 338, 108082. [Google Scholar] [CrossRef]
- Shu, X.Y.; He, J.; Zhou, Z.H.; Xia, L.L.; Hu, Y.F.; Zhang, Y.L. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- Wu, T.; Chellemi, D.O.; Graham, J.H.; Martin, K.J.; Rosskopf, E.N. Comparison of soil bacterial communities under diverse agricultural land management and crop production practices. Microb. Ecol. 2008, 55, 293–310. [Google Scholar] [CrossRef]
- Ye, J.; Wang, Y.; Jia, X.; Wu, Z.; Wang, H. Improvement of soil acidification in tea plantations by long-term use of organic fertilizers and its effect on tea yield and quality. Front. Plant Sci. 2022, 13, 1055900. [Google Scholar] [CrossRef]
- Bloem, E.; Albihn, A.; Elving, J.; Hermann, L.; Lehmann, L.; Sarvi, M.; Schaaf, T.; Schick, J.; Turtola, E.; Ylivainio, K. Contamination of organic nutrient sources with potentially toxic elements, antibiotics and pathogen microorganisms in relation to P fertilizer potential and treatment options for the production of sustainable fertilizers: A review. Sci. Total Environ. 2017, 607–608, 225–242. [Google Scholar] [CrossRef]
- Sun, Y.M.; Qiu, T.L.; Gao, M.; Shi, M.M.; Zhang, H.F.; Wang, X.M. Inorganic and organic fertilizers application enhanced antibiotic resistome in greenhouse soils growing vegetables. Ecotoxicol. Environ. Saf. 2019, 179, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.R.; Zhang, X.; Li, N.; Yang, Z.Z.; Li, B.X.; Zhang, X.L.; Li, H.G. Prioritized regional management for antibiotics and heavy metals in animal manure across China. J. Hazard. Mater. 2024, 461, 132706. [Google Scholar] [CrossRef]
- Zhu, L.; Lian, Y.; Lin, D.; Huang, D.; Yao, Y.; Ju, F.; Wang, M. Insights into microbial contamination in multi-type manure-amended soils: The profile of human bacterial pathogens, virulence factor genes and antibiotic resistance genes. J. Hazard. Mater. 2022, 437, 129356. [Google Scholar] [CrossRef]
- Xiao, R.H.; Huang, D.L.; Du, L.; Song, B.; Yin, L.S.; Chen, Y.S.; Gao, L.; Li, R.J.; Huang, H.; Zeng, G.G. Antibiotic resistance in soil-plant systems: A review of the source, dissemination, influence factors, and potential exposure risks. Sci. Total Environ. 2023, 869, 161855. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.L.V.; Cheng, A.A. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Q.; Zhang, X.X.; Wang, C.H.; Li, M.; Chen, J.; Xiong, J.B. Responses of sediment resistome, virulence factors and potential pathogens to decades ofantibiotics pollution in a shrimp aquafarm. Sci. Total Environ. 2021, 794, 148760. [Google Scholar] [CrossRef] [PubMed]
- Soborg, D.A.; Hendriksen, N.B.; Kilian, M.; Kroer, N. Widespread occurrence of bacterial human virulence determinants in soil and freshwater environments. Appl. Environ. Microbiol. 2013, 79, 5488–5497. [Google Scholar] [CrossRef]
- Martínez, J.L.; Coque, T.M.; Baquero, F. What is a resistance gene? Ranking risk in resistomes. Nat. Rev. Microbiol. 2015, 13, 116–123. [Google Scholar] [CrossRef]
- Li, Y.; Kong, F.G.; Li, S.; Wang, J.; Hu, J.R.; Chen, S. Insights into the driving factors of vertical distribution of antibiotic resistance genes in long-term fertilized soils. J. Hazard. Mater. 2023, 456, 131706. [Google Scholar] [CrossRef]
- Xie, W.Y.; Yuan, S.T.; Xu, M.G.; Yang, X.P.; Shen, Q.R.; Zhang, W.W. Long-term effects of manure and chemical fertilizers on soil antibiotic resistome. Soil. Biol. Biochem. 2018, 122, 111–119. [Google Scholar] [CrossRef]
- Yin, Y.; Zhu, D.; Yang, G.; Su, J.; Duan, G. Diverse antibiotic resistance genes and potential pathogens inhabit in the phyllosphere of fresh vegetables. Sci. Total Environ. 2022, 815, 152851. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Martiny, J.B.; Allison, S.D. Effects of dispersal and selection on stochastic assembly in microbial communities. ISME J. 2017, 11, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Zhao, J.Y.; Liu, Y.; Huang, S.J.; Zhao, C.Y.; Jiang, Z.K. Decipher soil resistance and virulence gene risks in conventional and organic farming systems. J. Hazard. Mater. 2024, 468, 133788. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guo, S.; Lin, H.Y.; Li, K.J.; Li, Z.T.; Wang, J.Y. Uncovering the prevalence and drivers of antibiotic resistance genes in soils across different land-use types. J. Environ. Manag. 2023, 344, 118920. [Google Scholar] [CrossRef]
- Shen, C.; He, M.; Zhang, J.; Liu, J.; Wang, Y. Response of soil antibiotic resistance genes and bacterial communities to fresh cattle manure and organic fertilizer application. J. Environ. Manag. 2024, 349, 119453. [Google Scholar] [CrossRef]
- Li, T.T.; Li, R.C.; Cao, Y.F.; Tao, C.Y.; Deng, X.H.; Ou, Y.N. Soil antibiotic abatement associates with the manipulation of soil microbiome via long-term fertilizer application. J. Hazard. Mater. 2022, 439, 129704. [Google Scholar] [CrossRef]


| Treatments | S1 | S2 | S3 | S4 |
|---|---|---|---|---|
| AN 1 (mg/kg) | 112.85 ± 2.92 a | 59.72 ± 1.30 c | 53.70 ± 2.83 c | 103.08 ± 2.36 b |
| AK 2 (mg/kg) | 247.05 ± 2.24 a | 177.08 ± 3.06 c | 246.34 ± 0.97 a | 209.91 ± 0.60 b |
| AP 3 (mg/kg) | 76.27 ± 2.05 a | 64.45 ± 3.0 b | 32.65 ± 2.58 d | 54.07 ± 2.79 c |
| NH4+-N 4 (mg/kg) | 143.28 ± 3.22 c | 219.19 ± 0.74 b | 214.63 ± 4.00 b | 340.65 ± 4.37 a |
| SOC 5 (g/kg) | 132.46 ± 4.21 d | 435.69 ± 11.00 b | 347.30 ± 4.93 c | 512.36 ± 0.88 a |
| pH 6 | 6.31 ± 0.18 c | 6.45 ± 0.04 b | 6.55 ± 0.11 b | 7.27 ± 0.14 a |
| EC 7 (μS/cm) | 1922.67 ± 143.18 a | 707.00 ± 16.82 c | 497.33 ± 6.43 d | 847.33 ± 18.34 b |
| Salinity 8 (g/kg) | 1.02 ± 0.06 a | 0.36 ± 0.01 c | 0.26 ± 0.01 d | 0.45 ± 0.02 b |
| Samples | Sobs | Ace | Chao | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|
| S1 | 16,859.33 ± 52.70 d | 16,859.33 ± 52.70 d | 16,859.33 ± 52.70 d | 5.92 ± 0.01 b | 0.016 ± 0.0006 c | 1 |
| S2 | 19,107.00 ± 106.93 a | 19,107.00 ± 106.93 a | 19,107.00 ± 106.93 a | 5.17 ± 0.03 c | 0.034 ± 0.0008 a | 1 |
| S3 | 17,922.33 ± 5.03 c | 17,922.33 ± 5.03 c | 17,922.33 ± 5.03 c | 5.01 ± 0.02 d | 0.029 ± 0.0004 b | 1 |
| S4 | 18,498.00 ± 120.62 b | 18,498.00 ± 120.62 b | 18,498.00 ± 120.62 b | 6.18 ± 0.01 a | 0.010 ± 0.0002 d | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Wu, C.; Yao, Z.; Li, X.; Chi, H.; Wu, T.; Du, X. Metagenomic Analysis of Distribution Characteristics and Driving Mechanisms of Antibiotic Resistance Genes, Virulence Factors, and Microbial Communities in Rice Seedling Cultivation Soils. Microorganisms 2025, 13, 2419. https://doi.org/10.3390/microorganisms13112419
Zhong Y, Wu C, Yao Z, Li X, Chi H, Wu T, Du X. Metagenomic Analysis of Distribution Characteristics and Driving Mechanisms of Antibiotic Resistance Genes, Virulence Factors, and Microbial Communities in Rice Seedling Cultivation Soils. Microorganisms. 2025; 13(11):2419. https://doi.org/10.3390/microorganisms13112419
Chicago/Turabian StyleZhong, Yu, Chanchan Wu, Zhipeng Yao, Xinyang Li, Hai Chi, Tao Wu, and Xinglin Du. 2025. "Metagenomic Analysis of Distribution Characteristics and Driving Mechanisms of Antibiotic Resistance Genes, Virulence Factors, and Microbial Communities in Rice Seedling Cultivation Soils" Microorganisms 13, no. 11: 2419. https://doi.org/10.3390/microorganisms13112419
APA StyleZhong, Y., Wu, C., Yao, Z., Li, X., Chi, H., Wu, T., & Du, X. (2025). Metagenomic Analysis of Distribution Characteristics and Driving Mechanisms of Antibiotic Resistance Genes, Virulence Factors, and Microbial Communities in Rice Seedling Cultivation Soils. Microorganisms, 13(11), 2419. https://doi.org/10.3390/microorganisms13112419





